Background: Triacylglycerol (TAG) can be formed via an acyl-CoA-dependent or acyl-CoA-independent pathway.
Results: Overexpressing particular flax phospholipid:diacylglycerol acyltransferase (PDAT) genes in yeast and Arabidopsis resulted in an enhanced proportion of α-linolenic acid (ALA) in TAG.
Conclusion: Certain PDATs have the unique ability to efficiently channel ALA into TAG.
Significance: The identified PDATs will benefit future projects aimed at producing oils with enhanced polyunsaturated fatty acid content.
Keywords: Lipid Metabolism, Metabolic Engineering, Molecular Genetics, Plant Molecular Biology, Triacylglycerol, α-Linolenic Acid, Flax, Phospholipid:diacylglycerol Acyltransferases
Abstract
The oil from flax (Linum usitatissimum L.) has high amounts of α-linolenic acid (ALA; 18:3cisΔ9,12,15) and is one of the richest sources of omega-3 polyunsaturated fatty acids (ω-3-PUFAs). To produce ∼57% ALA in triacylglycerol (TAG), it is likely that flax contains enzymes that can efficiently transfer ALA to TAG. To test this hypothesis, we conducted a systematic characterization of TAG-synthesizing enzymes from flax. We identified several genes encoding acyl-CoA:diacylglycerol acyltransferases (DGATs) and phospholipid:diacylglycerol acyltransferases (PDATs) from the flax genome database. Due to recent genome duplication, duplicated gene pairs have been identified for all genes except DGAT2-2. Analysis of gene expression indicated that two DGAT1, two DGAT2, and four PDAT genes were preferentially expressed in flax embryos. Yeast functional analysis showed that DGAT1, DGAT2, and two PDAT enzymes restored TAG synthesis when produced recombinantly in yeast H1246 strain. The activity of particular PDAT enzymes (LuPDAT1 and LuPDAT2) was stimulated by the presence of ALA. Further seed-specific expression of flax genes in Arabidopsis thaliana indicated that DGAT1, PDAT1, and PDAT2 had significant effects on seed oil phenotype. Overall, this study indicated the existence of unique PDAT enzymes from flax that are able to preferentially catalyze the synthesis of TAG containing ALA acyl moieties. The identified LuPDATs may have practical applications for increasing the accumulation of ALA and other polyunsaturated fatty acids in oilseeds for food and industrial applications.
Introduction
Flax (Linum usitatissimum L.), which produces oil containing ∼45 to 65% α-linolenic acid (18:3cisΔ9,12,15; ALA),3 is considered one of the most abundant plant sources of ALA. With a low oxidative stability, ALA can react rapidly with oxygen and polymerize into a soft and durable film upon air exposure, which makes flax oil suitable for domestic and industrial coatings such as linoleum, varnishes, and paints (1). In addition, ALA is an essential fatty acid for humans and a precursor for nutritionally beneficial very long chain omega-3 polyunsaturated fatty acids (VLC-ω-3-PUFA, ≥20 carbons) (2, 3).
Efforts to characterize the molecular basis of high ALA content in flax have focused largely on the characterization of fatty acid desaturases (FAD). Two genes encoding Δ15 desaturases (LuFAD3A and LuFAD3B) have been identified in flax (4). Ethyl methanesulfonate-generated point mutations in LuFAD3A and LuFAD3B led to a reduction in ALA content to ∼1–2%, suggesting that these two genes encoded the main desaturases responsible for the synthesis of ALA in flax (4–6). Generally, there is good correlation between the expression of FAD3 genes with the accumulation of ALA in flax and other plant species (7). However, along with desaturases, many other enzymes can contribute to the flux of ALA to storage lipids. Essentially, ALA has to be efficiently transferred from the desaturation product (sn-2-linolenoyl-phosphatidylcholine, PC) to the substrates for triacylglycerol (TAG) synthesis and this process can take different biochemical routes. ALA synthesized on PC can enter the acyl-CoA pool by either the reverse action of acyl-CoA:lysophosphatidylcholine acyltransferase (LPCAT) (8) or the combined action of phospholipase A2 and long-chain acyl-CoA synthetase. 2-Linolenoyl-sn-PC can also be potentially converted into 2-linolenoyl-sn-diacylglycerol (DAG) by the catalytic action of phospholipase C or phospholipase D (9) together with phosphatidic acid phosphatase. In addition, the enzyme phosphatidylcholine:diacylglycerol cholinephosphotransferase catalyzes the transfer of a phosphocholine head group from PC to DAG in a symmetrical reaction (10), which could potentially produce DAG enriched in ALA for the synthesis of TAG.
TAG can be formed via an acyl-CoA-dependent or acyl-CoA-independent process. The final step of the acyl-CoA-dependent pathway, also known as the Kennedy pathway (11), is catalyzed by acyl-CoA:diacylglycerol acyltransferase (DGAT), which uses acyl-CoA as acyl donor to convert DAG to TAG. At least four different types of DGAT have been identified in plants (12–15). DGAT1 has been proposed to exert dominant control over TAG accumulation in many oilseeds (16–19). DGAT2 was first identified in the oleaginous fungus Umbelopsis (formerly Mortierella) ramanniana and shows no sequence homology to DGAT1 (15). DGAT1 and DGAT2 appear to localize to different subdomains of endoplasmic reticulum and have been suggested to have nonredundant functions in the production of TAG (20). The third type of DGAT (DGAT3) is a soluble enzyme isolated from developing peanut cotyledons and it differs from DGAT1 and DGAT2, which are membrane-bound (13). Another soluble enzyme with DGAT activity known as defective in cuticular ridge (DCR) has been identified in Arabidopsis thaliana (21).
The acyl-CoA-independent pathway of TAG synthesis is characterized by the enzymatic action of phospholipid:diacylglycerol acyltransferase (PDAT) that transfers the fatty acyl moiety from the sn-2 position of a phospholipid to the sn-3 position of sn-1, 2-DAG (22). A gene encoding a PDAT was first reported in yeast (22). Two homologs of yeast PDAT have been identified in Arabidopsis named PDAT1 (At5g13640) and PDAT2 (At3g4480) (23) and three putative PDAT genes have been identified in the castor bean genome (24).
The existence of specialized acyltransferases has been observed in other plant species. DGAT2 from tung tree (Vernicia fordii) and castor bean (Ricinus communis) display substrate preference for unusual fatty acids and are predominantly involved in the incorporation of these fatty acids into seed oils (20, 25). Also, a ricinoleate-specific PDAT from castor bean (RcPDAT) has been reported (24). Co-expression of RcPDAT1 with castor fatty acid hydroxylase in Arabidopsis resulted in a relative increase of 58% hydroxy fatty acids in seeds. Considering the natural occurrence of evolved forms of acyltransferases that are selective for unusual fatty acids, we hypothesized that similar mechanisms take place in flax. Despite the wide range of applications for flax oil, many components involved in TAG biosynthesis have not been characterized at the molecular genetic level. Here we provide this characterization and demonstrate that two pairs of PDAT genes encoding enzymes utilize preferentially substrates containing ALA, and more importantly, one of the pairs (LuPDAT1 and LuPDAT5) has an embryo-preferred expression pattern and appears to contribute mainly to the synthesis of trilinolenin in flax seeds.
EXPERIMENTAL PROCEDURES
Plant Material and Growth Conditions
Flax (L. usitatissimum L.) was grown in the growth chamber with 16 h light at 21 °C and 8 h dark at 18 °C. Seeds were planted in 1 gallon pots containing Metro Mix soil (Greenleaf Products Inc., CA). Plants were fertilized with 17-5-19 (125 g/100 liters) and 12-2-14 (83 g/100 liters) once a week before flowering. From the flowering stage, plants were fertilized weekly with 17-5-19 (280 g/100 liters) until harvest. Individual flowers were tagged at anthesis, and embryos at different stages of development were collected in liquid N2 and stored at −80 °C. Arabidopsis wild-type Columbia and mutant line AS11 were obtained from the Salk Institute via the ABRC (Ohio State University, Columbus, OH). Arabidopsis seeds in plots or plates were cold-treated at 4 °C in the dark for 3 days and then placed into a controlled growth chamber with a constant temperature of 20 °C and a 16-h photoperiod.
Identification and Isolation of Candidate Genes
In general, all primers are summarized in supplemental Table S1. Cloning integrity was confirmed by sequencing at each step.
A BLAST analysis (26) was conducted against the flax genomic database (27) by using AtDGAT1, AtPDAT1, AtDCR, and tung tree VfDGAT2 as the protein query. The theoretical molecular weight and isoelectric point values were calculated using the Compute pI/Mw tool provided in the ExPASy server. To isolate the target genes, total RNA was extracted from the embryo of flax (cultivar AC Emerson) 12 days post-anthesis (DPA) using Plant RNeasy plant mini kit (Qiagen) as described by the manufacturer. The first stand of the cDNA was synthesized using SuperScript III reverse transcriptase (Invitrogen) according to the protocol provided by the supplier. The target genes were amplified from the resulting cDNA as the template for 30 cycles of PCR amplification using Platinum Taq DNA Polymerase High Fidelity (Invitrogen). PCR was performed under the following temperature cycle program: 95 °C for 2 min; 30 cycles of denaturation (95 °C, 20 s), annealing (55 °C, 15 s), and extension (68 °C, 2.5 min); and a final extension at 68 °C for 2 min. To amplify LuDGAT2–1, LuDGAT2–3, LuPDAT2, and LuPDAT6, the forward primers contained a specific restriction site (underlined) and a Kozak translation initiation sequence (italic) to improve the translation of the protein (supplemental Table S1). A specific restriction site was also introduced in the reverse primer (underlined). The PCR products were cloned into the pYES vector collinear to the GAL1 promoter inducible by galactose. The pYES is a modified pYES2.1/V5-HIS vector (Invitrogen) constructed in our laboratory, which contains more restriction sites in its multiple cloning site. For LuDCR1 and LuPDAT1 that could not be amplified by these specially designed primers, the internal primers were used for amplification. For LuDGAT2–2 and LuPDAT5, an internal forward primer and a reverse primer spanning the 3′-untranslated region (3′-UTR) were used for amplification. The PCR products were subcloned into pYES2.1/V5-HIS vector using the pYES2.1 TOPO kit (Invitrogen) as demonstrated by the supplier. To construct the co-expression vectors, LuFAD2-1 (28) and LuFAD3B (4) genes were first amplified using PCR with appropriate primers that allowed specific restriction sites to be added (underlined, supplemental Table S1) to the ends of amplified products and then inserted into multiple cloning site 1 and multiple cloning site 2 of the pESC-URA expression vector (Agilent Technologies), yielding LuFAD2–1-FAD3B/pESC plasmid. The ADH1 terminator: LuFAD2–1:ProGAL10:ProGAL1:LuFAD3B:CYC1 terminator expression cassette of LuFAD2–1-FAD3B/pESC was then excised and subcloned into the recombinant pYES plasmids containing LuPDAT1, LuPDAT2, or LuDGAT1-1 through a one-step, isothermal assembly method described by Gibson (29). The resulting plasmids were referred to as LuFAD2-1-FAD3B-PDAT1/pYES, LuFAD2-1-FAD3B-PDAT2/pYES, and LuFAD2-1-FAD3B-DGAT1-1/pYES.
Real-time PCR Quantification
Total RNA was isolated from vegetative tissues (stems, leaves, apexes, and roots), reproductive tissues (flowers), developing embryos (8, 11, 14, 16, and 20 DPA), and seeds (4, 25, and 40 DPA) of flax cultivar CDC Bethune using RNeasy plant mini kits (Qiagen). Due to technical difficulties in separating embryos from other seed components at 4, 25, and 40 DPA, the whole seeds at these three stages were used for gene expression analysis. The cDNA was synthesized using SuperScript III reverse transcriptase (Invitrogen). Gene-specific primers and probes were designed using Primer Express software (Applied Biosystems) and the sequences are listed in supplemental Table S1. 5′-FAM/3′-quencher-labeled probes were used to assay the genes involved in TAG synthesis, and probes for reference genes were labeled with 5′-VIC/3′-quencher. TaqMan-based quantitative RT-PCR assays were performed using Fast Advance Master mix (Invitrogen) on the ABI PRISM 7900HT Real-time PCR system (Applied Biosystems). PCR efficiency for each amplicon was calculated using a dilution series of a single cDNA sample over several log concentrations. According to the recommendation of Huis et al. (30), two internal reference genes, encoding glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and ubiquitin extension protein (UBI2), were used. The relative expression level was calculated using the comparative Ct method after normalizing to controls using the reference genes.
Heterologous Expression in Yeast Mutant H1246
In general, the constructed recombinant plasmids were transformed into the quadruple mutant strain Saccharomyces cerevisiae H1246 using the LiAc/SS carrier DNA/PEG method (31) and transformants were selected on minimal medium plates lacking uracil. The recombinant yeast cells were first grown in liquid minimal medium with 2% (w/v) raffinose overnight and then inoculated at a starting OD of 0.1 in minimum medium containing 2% (w/v) galactose and 1% (w/v) raffinose (referred to as induction medium) to induce gene expression.
For heterologous expression of single flax genes, yeast cells were grown at 30 °C and yeast transformed with pYESLacZ was used as a control. For the feeding experiments, free fatty acids, including oleic (OA; 18:1cisΔ9), linoleic acid (LA; 18:2cisΔ9,12), ALA, stearidonic acid (SDA; 18:4cisΔ6,9,12,15), dihomo-γ-linolenic acid (DGLA; 20:3cisΔ8,11,14), γ-linolenic acid (GLA; 18:3cisΔ6,9,12), arachidonic acid (AA; 20:4cisΔ5,8,11,14), eicosatrienoic acid (ETA; 20:3cisΔ11,14,17), eicosapentaenoic acid (EPA; 20:5cisΔ5,8,11,14,17), and docosahexaenoic acid (DHA; 22:6cisΔ4,7,10,13,16,19) were dissolved in 0.5 m ethanol. The fatty acid solutions were first dissolved in 0.06% (v/v) tyloxapol (Sigma) and then mixed with induction medium. Tyloxapol is a non-ionic surfactant that was used to disperse the fatty acids into the medium. Yeast was induced in induction medium with 100 μm fatty acid supplementation.
To evaluate the concentration effect of exogenously provided ALA on total TAG content and composition in yeast expressing LuPDAT1 and LuPDAT2, the recombinant yeast cells were cultivated in induction medium supplemented with different concentration of ALA (0 to 300 μm) and harvested at the same growth stage (A600 nm = 6.5 ± 0.05). Approximately 30 mg of dry yeast were weighted out and used for lipid analysis after 16 h freeze drying. One hundred μg of the TAG internal standard, triheptadecanoin (C17:0 TAG), were added to each sample before lipid extraction.
For growth curve construction, yeast cells were grown first in minimum medium containing 2% (w/v) raffinose, and then in induction medium supplemented with three different ALA concentrations (0, 100 μm, and 1 mm). Aliquots (100 μl) were withdrawn from the culture at intervals of 6 or 12 h and diluted 1:10 with sterile water. One hundred microliters of the diluted cells were transferred to a 96-well clear, flat-bottom, polystyrene microplate (UNIPLATE, Whatman). The yeast growth (A600 nm) was measured using the Synergy H4 Hydrid multimode microplate reader (Biotek Instrument, Inc.).
Growth phenotypes were further analyzed by first culturing recombinant yeast cells in minimum medium containing 2% (w/v) raffinose, and then spotting 2.5 μl of cell suspension serially diluted to A600 nm values of 1, 10−1, 10−2, 10−3, and 10−4, onto agar plates containing induction medium supplemented with different concentration of ALA (0, 100 μm, and 1 mm). ALA was first dissolved in 0.5 m ethanol, and then diluted in a warm medium containing 0.01% (v/v) tyloxapol immediately before plating. The same amount of ethanol and typloxapol was added to the plates without fatty acid supplementation. Yeast strains BY4742 (wild-type) and H1246 transformed with empty pYES vector were used as positive and negative controls, respectively. Plates were incubated at 30 °C for 2 days.
For the co-expression study, yeast cells transformed with LuFAD2-1-FAD3B-PDAT1/pYES, LuFAD2-1-FAD3B-PDAT2/pYES, or LuFAD2-1-FAD3B-DGAT1-1/pYES plasmid were inoculated in the same induction medium for 3 days at 20 °C before cell harvest. Yeast cells transformed with LuFAD2-1-FAD3B/pYES were used as a control.
Western Blot Analysis
To produce N-terminal-tagged constructs, the complete ORFs of three LuPDATs (LuPDAT1, LuPDAT2, and LuPDAT6) were first amplified by the primers listed in supplemental Table S1 and then subcloned into the pYES2/NT-C expression vector (Invitrogen). For the generation of C-terminal-tagged constructs, the stop codons of the LuPDATs in the recombinant pYES plasmids were mutated by using the QuikChange mutagenesis kit (Stratagene). Yeast microsomes were prepared as previously described (32). Briefly, the recombinant yeast cells were first cultivated in liquid minimal medium with 2% (w/v) raffinose and then inoculated at a starting OD of 0.4 for 12 h. The cells were harvested, washed, and then resuspended in the homogenization buffer (20 mm Tris-HCl, pH 7.9, 10 mm MgCl2, 1 mm EDTA, 5% (v/v) glycerol, 300 mm ammonium sulfate, 2 mm dithiothreitol). In the same buffer, the cells were broken mechanically with ⅓ volume of 0.5-mm glass beads by a bead beater (Biospec Bartlesville). To remove the cell debris and glass beads, the homogenate was centrifuged at 10,000 × g at 4 °C for 20 min. The recovery supernatant was further centrifuged at 48,000 × g at 4 °C for 1 h. The resulting microsomal pellets were dissolved in 3 mm imidazole buffer (pH 7.4) containing 125 mm sucrose. Equivalent amounts of microsomal proteins (15 μg, as determined by Bio-Rad Bradford assay) were separated by 4–12% gradient Tris-glycine gels and then blotted onto nitrocellulose membranes. The target N-terminal-tagged proteins were detected using anti-HisG-HRP antibody (Invitrogen). The bounded antibodies were detected using SuperSignal Fermo Chemiluminescent Substrate (Pierce). Equal protein loading was ensured by determining the level of constitutively expressed chaperone Kar2p using a rabbit polyclonal anti-Kar2p (Santa Cruz Biotechnology) as the primary antibody followed by incubation with HRP goat anti-rabbit IgG(H+L) secondary antibody (Invitrogen).
Nile Red Fluorescence Detection of Neutral Lipids in Yeast
The Nile red fluorescence assay was conducted as described (32, 33). Briefly, the recombinant yeast cells were cultivated in induction medium with or without ALA supplementation and harvested at the stationary growth stage. Cells with ALA feeding were washed three times with deionized water before Nile red measurement. One hundred-μl aliquots of induced yeast cell culture were transferred to a 96-well dark flat-bottom plate (UNIPLATE, Whatman). The background fluorescence was measured using the Synergy H4 Hydrid multimode microplate reader (Biotek Instrument, Inc.) with emission and excitation filter settings at 485 and 538 nm, respectively. Five microliters of freshly prepared methanolic Nile red solution (0.1 mg/ml) was added first and mixed with yeast culture. The second fluorescence intensity value was measured under the same conditions. The final Nile red value was calculated by subtracting the first measurement from the second measurement (ΔF), and then dividing by the corresponding A600 nm value.
Yeast Lipid Extraction and Analysis
Yeast lipid extraction was performed using the method described previously (32, 34). Briefly, induced yeast cultures at the stationary growth stage were harvested by centrifugation, washed, and resuspended in 1 ml of 0.9% (w/v) sodium chloride (NaCl). Cells were homogenized with an equal volume of glass beads (0.5 mm) by vigorously vortexing for 2 min. Lipids were extracted by the chloroform, methanol, 0.9% aqueous NaCl (2:1:0.9, v/v/v) method. The chloroform phase (lower phase) was collected, dried under nitrogen, and resuspended in 30 μl of chloroform. The extracted lipids were resolved on the thin layer chromatography (TLC) plates (SIL G25, 0.25 mm, Macherey-Nagel, Germany) with the solvent system hexane/diethyl ether/acetic acid (80:20:1). The developed plate was visualized with 3% cupric acetate (w/v) and 8% phosphoric acid (v/v) followed by charring at 280 °C for 20 min. The TAG bands were identified according to triolein and trilinolenin standards.
Fatty acid analysis was performed by gas chromatography (GC)/mass spectrometry (MS) as described previously (35). Briefly, the extracted lipids were developed on a TLC plate using the same solvent system but visualized under UV light after spraying with 0.05% primuline solution. The bands corresponding to TAG were scraped and transmethylated with 5% (w/v) sodium methoxide (NaOMe) at room temperature for 30 min. The resulting fatty acid methyl esters (FAMEs) were extracted with two portions (2 ml each) of hexane. The hexane phases were pooled together and dried under a stream of nitrogen and immediately resuspended in 250 μl of iso-octane with 0.1 mg/ml of C21:0 methyl ester standard. FAMEs were subjected to an Agilent 6890N GC equipped with DB-23 capillary column (30 m × 0.25 mm × 0.25 μm) and a 5975 inert XL Mass Selective Detector. The following temperature program was applied: 165 °C, hold for 4 min, 10 °C/min to 180 °C, hold 5 min, and 10 °C/min to 230 °C, hold 5 min.
Overexpression of LuDGATs and LuPDATs in Arabidopsis
Agrobacterium tumefaciens strain GV3101 and pGreen/pSoup-based dual binary vectors (36) were used for Arabidopsis transformation. The coding regions of target genes were amplified using recombinant pYES plasmids as template with specially designed primers for each gene (see supplemental Table S1). The amplicons were cloned into the pGreen vector under control of the seed-specific napin promoter. The resulting construct and the helper plasmid pSoup were co-transformed into A. tumefaciens GV3101 by electroporation (37). Agrobacterium strains containing the pGreen/pSoup dual binary vectors were used to transform the Arabidopsis wild-type (Columbia) and mutant line AS11 by the floral dipping method (37). Plants transformed with an empty vector pGreen were used as controls. T1 seeds of transgenic plants were selected on half-strength Murashige and Skoog (MS) agar plates supplemented with 80 μm of the herbicide phosphoinothricin. Transformants were then transferred to soil and grown to maturity to produce T2 seeds. The presence of the target genes was confirmed by gene-specific PCR analysis using DNA extracted from T2 young leaf tissue as template. T2 seeds were collected and used for total lipid and fatty acid analysis.
Analysis of Arabidopsis Seed Oil
Total lipid content and the fatty acid composition of T2 seeds were determined by GC. Approximately 10 mg of seeds per replicate were weighted out after stabilizing the seed moisture content in desiccators for 72 h. One hundred μg of triheptadecanoin (C17:0 TAG) were used as a TAG internal standard. Seeds were subjected to treatment with 2 ml of 3 n methanolic HCl and heated at 80 °C for 16 h. The extracted FAMEs were suspended in 1.5 ml of iso-octane with 0.1 mg/ml of C21:0 methyl ester standard and analyzed by GC-MS using the same column and temperature gradient. Total lipid content was determined by multiplying the peak-area ratio of the total fatty acid and the internal standard by the initial internal standard amount.
Flax Seed Oil and Fatty Acid Composition Analysis
Flax embryos of cultivar CDC Bethune at different developmental stages (8, 11, 14, 16, and 20 DPA) and seeds (25, 30, and 40 DPA) were collected, measured for fresh weight, immersed in liquid nitrogen, and then placed at −80 °C for storage. Frozen embryos and seeds (∼15 to 75 mg of fresh weight per biological replicate) were freeze-dried for 4 days and then homogenized in a mortar and pestle in the presence of liquid nitrogen. Homogenates were preceded for lipid extraction and fatty acid analysis as described in Arabidopsis seed oil analysis. The oil and ALA content were calculated on a fresh weight basis.
Phylogenetic Analysis
The multiple sequence alignments of PDAT, DCR, DGAT1, and DGAT2-like family proteins were generated using the ClustalW module (38) within MEGA5 (39) with the default parameters (gap penalty, 10.0; gap length penalty, 0.2; Gonnet matrix). The phylogenetic trees were constructed using the same software with the following parameters: neighbor-joining method, Poisson model, complete deletion, and bootstrap (1000 replicates). Numbers above the branches indicate the percentage of bootstrap values.
Accession Number
Accession numbers of protein sequences from the Arabidopsis Genome Initiative or EMBL/GenBank database or flax genome database (www.phytozome.net/flax) used in the current study are given in supplemental Table S2.
RESULTS
Identification and Isolation of Genes Encoding TAG-synthesizing Enzymes from Flax
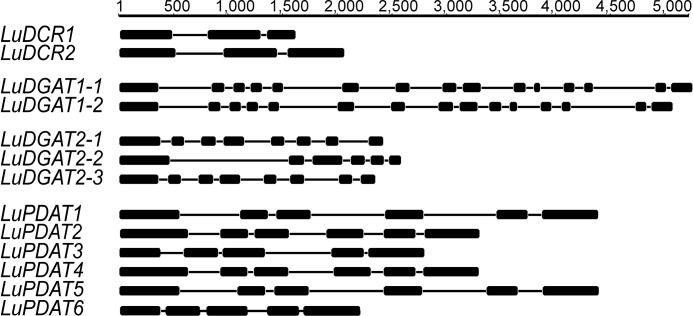
The recently completed flax genome sequence database (27) provided the starting point for identifying flax genes homologous to known genes involved in the final step of TAG synthesis. Using basic local alignment search tool (BLAST), we identified three DGAT2s (LuDGAT2-1, LuDGAT2-2, and LuDGAT2-3), two DCRs (LuDCR1 and LuDCR2), and six PDATs (LuPDAT1, LuPDAT2, LuPDAT3, LuPDAT4, LuPDAT5, and LuPDAT6) in the flax genome. In addition, we had previously reported a DGAT1 (here referred to as LuDGAT1-1) (32) and now identified a second gene (LuDGAT1-2) in the flax genome. General information about the identified genes is listed in Table 1, whereas the gene structures were represented in Fig. 1. Both LuDGAT1 genes contain 15 exons and as with DGAT1 from other plant species, the first exon is the longest and encodes a hydrophilic domain (40). LuDGAT2-1 and LuDGAT2-3 are composed of 8 exons each and share a more similar gene structure than with LuDGAT2-2, which contains only 6 exons. Interestingly, LuDGAT2-3 is located adjacent to LuDGAT2-2 in the genome (supplemental Fig. S1). Six LuPDATs can be divided into three groups based on polypeptide sequences (supplemental Fig. S2A). The genes within each group have a similar gene structure, where LuPDAT3 and LuPDAT6 genes contain 5 exons, one less than in LuPDATs of the other two groups. Phylogenetic analyses of the predicted polypeptides for each class indicate a close relationship with known TAG-synthesizing enzymes from other species (supplemental Fig. S2, A–D). Therefore, we conclude that the identified genes comprise the most probable candidates for encoding TAG-synthesizing enzymes from flax. In addition, the identification of gene pairs for all genes, except to LuDGAT2-2, is consistent with the findings that suggest whole-genome duplication in flax (27). The two genes in a gene pair have a very high degree of sequence identity at both nucleotide and amino acid levels (Table 2). Further genomic analysis revealed that LuDGAT1-1 and LuPDAT5 are located close together on the same chromosome and the other member of the gene pair (LuDGAT1-2 and LuPDAT1) are similarly arranged on a different chromosome (supplemental Fig. S3).
TABLE 1.
General information on individual candidate genes and the encoded polypeptides (deduced from the DNA sequence information)
| Genes | Gene length | Protein length | Molecular mass | Isoelectric point |
|---|---|---|---|---|
| bp | daltons | |||
| DGAT1-1 | 1524 | 507 | 58038.19 | 8.98 |
| DGAT1-2 | 1542 | 513 | 58793.08 | 8.92 |
| DGAT2-1 | 1065 | 354 | 39405.12 | 9.49 |
| DGAT2-2 | 1059 | 352 | 39004.68 | 9.19 |
| DGAT2-3 | 1050 | 349 | 38951.64 | 9.42 |
| DCR1 | 1137 | 378 | 41128.85 | 5.49 |
| DCR2 | 1401 | 466 | 50887.96 | 5.10 |
| PDAT1 | 2088 | 695 | 76891.89 | 8.23 |
| PDAT2 | 2145 | 714 | 79072.61 | 6.4 |
| PDAT3 | 1728 | 575 | 63093.05 | 6.19 |
| PDAT4 | 2148 | 715 | 78923.51 | 6.72 |
| PDAT5 | 2088 | 695 | 76817.64 | 8.28 |
| PDAT6 | 1719 | 572 | 62792.54 | 6.19 |
FIGURE 1.
Genomic DNA structure of homologous genes encoding putative TAG-synthesizing enzymes from flax. The thick lines indicate exons and the thin lines indicate introns. The numbers on top of each group denote the scale in base pairs.
TABLE 2.
Sequence identity between the gene pairs and gene products
| Gene pair | Nucleotide | Amino acid |
|---|---|---|
| % | ||
| DGAT1-1 and DGAT1-2 | 85.9 | 97.7 |
| DGAT2-1 and DGAT2-3 | 91.2 | 93.3 |
| DCR1 and DCR2 | 90.2 | 89.3 |
| PDAT1 and PDAT5 | 97 | 97.1 |
| PDAT2 and PDAT4 | 95.6 | 97.1 |
| PDAT3 and PDAT6 | 95.4 | 96 |
Several DGAT1, DGAT2, and PDAT Genes Are Expressed Preferentially in Seeds and Correlate with Oil Accumulation
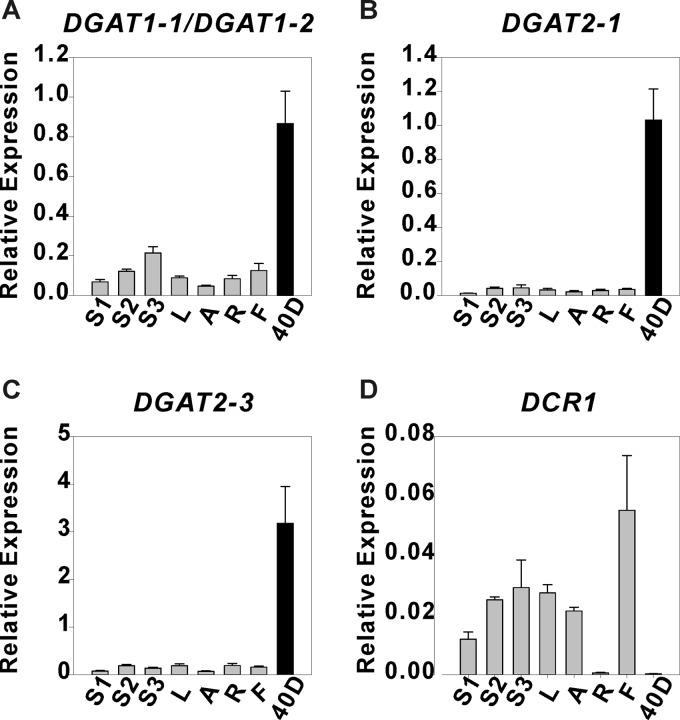
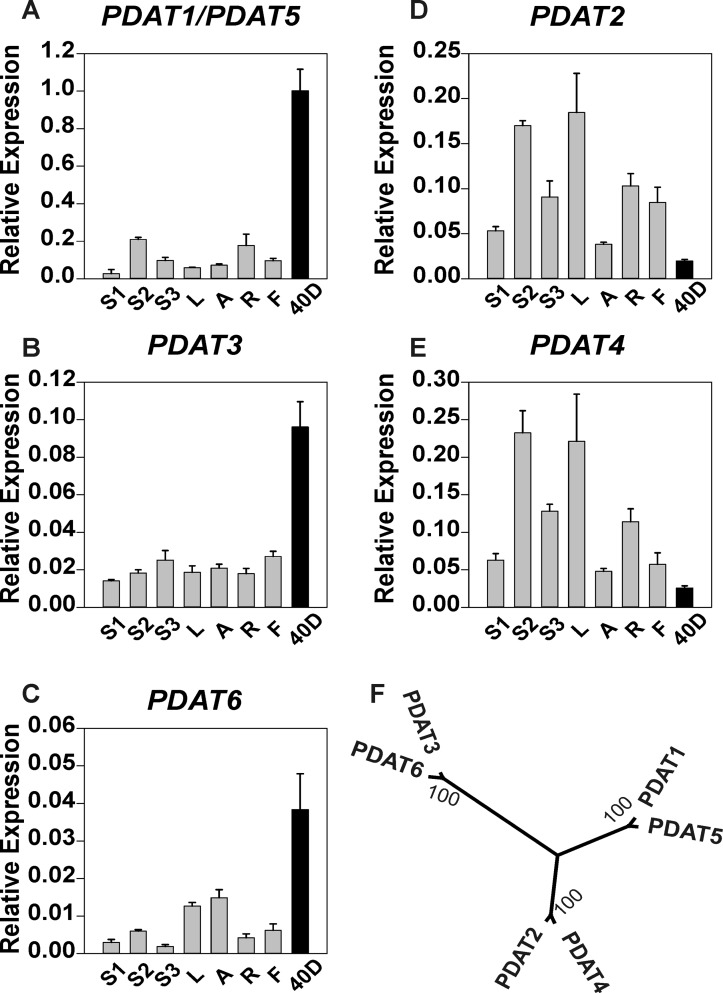
To investigate the potential physiological roles of all candidate genes, we studied the expression patterns of LuDGAT1, LuDGAT2, LuDCR, and LuPDAT in a range of organs including vegetative tissues, reproductive tissues, and mature seeds (40 DPA). Genes encoding LuDGAT1 or LuDGAT2 had significantly higher expression in seeds than in other vegetative tissues (Fig. 2, A–C). In the case of LuDCR, the expression level was significantly higher in vegetative tissues than that in seeds, suggesting that this gene might play a minor role in TAG biosynthesis (Fig. 2D). LuPDAT genes displayed two distinct expression profiles. Transcripts of LuPDAT1/LuPDAT5, LuPDAT3, and LuPDAT6 (Fig. 3, A–C) were detected preferentially in seeds and to a lesser extent in other tissues, and the opposite was observed for LuPDAT2 and LuPDAT4 (Fig. 3, D and E). This result suggests that two groups of PDATs might have unrelated physiological functional roles in flax. An attempt to individually detect the transcript of LuPDAT5 failed, but we were able to isolate the cDNA of LuPDAT5 using the reverse primer targeting 3′-UTR. Due to the very high level of sequence identity between LuPDAT1 and LuPDAT5 (97%), the transcripts of LuPDAT1 and LuPDAT5 were detected together. The transcripts for LuDGAT2-2 could not be detected in any of the tested plant tissues. Attempts to isolate the corresponding cDNAs were also unsuccessful, suggesting that LuDGAT2-2 may not be expressed in the tested tissues.
FIGURE 2.
Quantitative real-time PCR analysis of relative expression of flax DGAT genes in different tissues. The transcript levels of LuDGAT1 (A) and LuDGAT2 (B and C) genes were higher in seeds (40 DPA, black bar) than in other tissues (gray bar) and the opposite was observed for LuDCR1 (D). Data are given as mean ± S.E. (n = 3). S1, immature stem; S2, developing stem; S3, mature stem; L, leaves; A, apexes; R, roots; F, flowers; 40D, mature seeds.
FIGURE 3.
Quantitative real-time PCR analysis of relative expression of flax PDAT genes in different tissues. The LuPDAT1/LuPDAT5 (A), LuPDAT3 (B), and LuPDAT6 (C) genes exhibited higher expression levels in seeds (40 DPA, black bar) than in other tissues (gray bar) and the opposite was observed for LuPDAT2 (D) and LuPDAT4 (E). F, phylogenetic analysis of six flax PDAT proteins. Genes within the same branch of the phylogenetic tree have the similar expression pattern. Data are given as mean ± S.E. (n = 3). S1, immature stem; S2, developing stem; S3, mature stem; L, leaves; A, apexes; R, roots; F, flowers; 40D, mature seeds.
These experiments indicated that LuDGAT1, LuDGAT2-1, LuDGAT2-3, LuPDAT1/PDAT5, LuPDAT3, and LuPDAT6 are preferentially expressed in seeds instead of other tissues. To further decipher the role of these seed-preferred genes in oil synthesis, we analyzed the relationship between gene expression and seed oil and ALA accumulation patterns throughout seed development (Fig. 4, A–I). During seed development, the oil content on a fresh weight basis fit a sigmoidal curve (R2 = 0.969) with the rapid phase of oil accumulation occurring between 8 and 20 DPA (Fig. 4B). The ALA content on a fresh weight basis increased steadily until about 16 DPA (Fig. 4C). The expression of DGATs and PDATs displayed dissimilar temporal regulation. The level of DGAT transcripts (Fig. 4, D–F) peaked during the late stages of seed development, when the rate of oil and ALA accumulation already reached a plateau. However, the highest expression of PDATs (Fig. 4, G–I) was found in the early stages of seed development, during the period of active seed oil and ALA accumulation. This result indicated that DGATs and PDATs might contribute differently to TAG synthesis during flax seed development.
FIGURE 4.
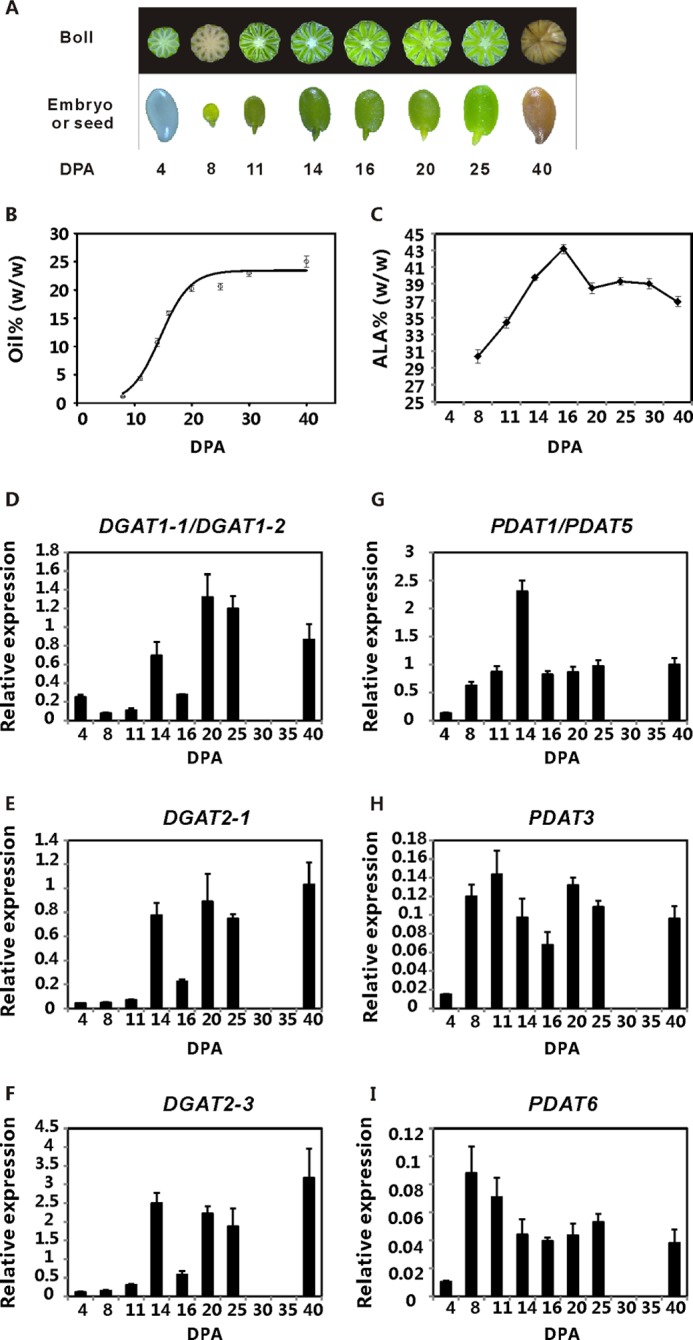
Oil and ALA accumulation correlate better with the expression of LuPDATs than with LuDGATs. A, flax embryos or seeds used for quantitative real-time PCR analysis. The top panel shows the cross-sections of the developing bolls at 4 to 25 DPA and the whole mature boll at 40 DPA from left to right. The bottom panel shows the cleared seed at 4 DPA, developing embryos at 8 to 25 DPA, and the mature seed at 40 DPA. B, oil deposition in seed on a fresh weight basis (% of fresh weight). The mean values of oil content (n = 4) were fitted to a sigmoidal curve (R2 = 0.969) using Sigmaplot 12.3. C, ALA accumulation pattern during seed development. The data are presented on a fresh weight basis (% of fresh weight) and shown as mean ± S.E. (n = 4). D–I, analysis of gene expression patterns of LuDGATs and LuPDATs during seed development. Data are shown as mean ± S.E. (n = 3).
DGATs and PDATs from Flax Are Functional TAG-synthesizing Enzymes
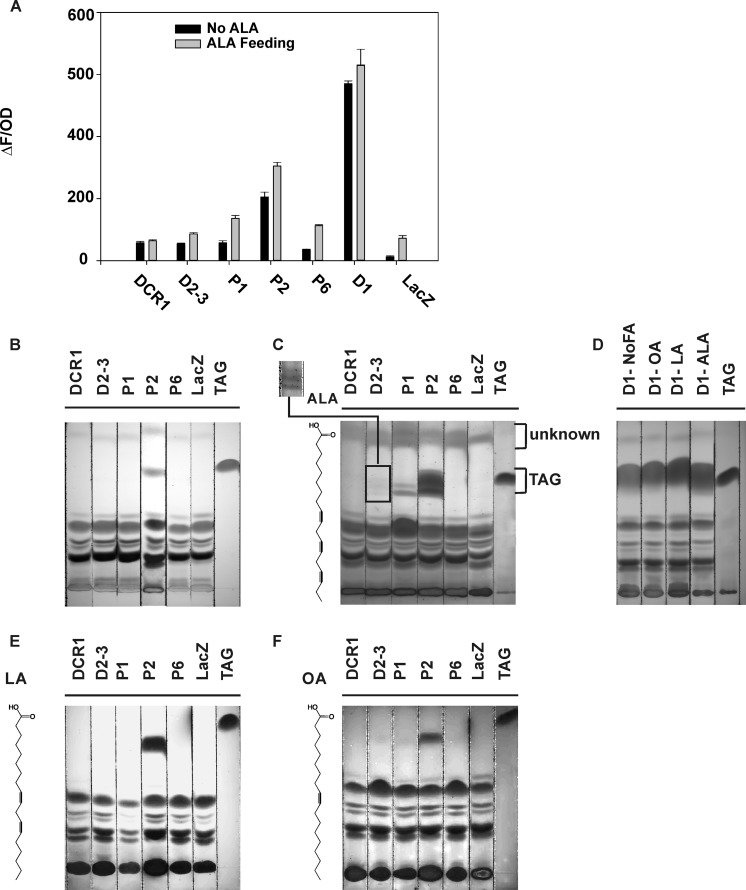
The ability of the polypeptides encoded by LuDCR1, LuDGAT, and LuPDAT genes to catalyze the synthesis of TAG was investigated through a functional assay in yeast. Because genes in a gene pair show high sequence similarity, one gene of each pair, including LuDGAT1-1, LuDGAT2-3, LuDCR1, LuPDAT1, LuPDAT2, and LuPDAT6, were chosen for this assay. The cDNAs of selected genes were isolated from 12 DPA flax developing embryos. The respective open reading frames (ORFs) were expressed in the quadruple mutant strain S. cerevisiae H1246, which lacks four genes DGA1, LRO1, ARE1, and ARE2 encoding DGAT1, PDAT1, ASAT1 (acyl-CoA:sterol acyltransferase 1), and ASAT2, respectively. With the disruption of these four genes, S. cerevisiae H1246 is unable to synthesize TAG and sterol esters (41), thus highlighting the production of TAG that results from the activity of recombinant flax enzymes. It has been previously demonstrated by our group that yeast H1246 expressing LuDGAT1-1 has the ability to synthesize TAG (32), thus it was used as a positive control. Yeast transformed with the pYES vector coding for the bacterial protein LacZ was used as a negative control. Production of TAG in recombinant yeast was detected by the Nile red fluorescent assay (32) as well as by TLC. The results of the Nile red assay (Fig. 5A) showed that besides LuDGAT1-1, LuPDAT2 was the only enzyme that had significant activity in yeast. Because flax oil contains a substantial amount of ALA, which is absent in yeast, we hypothesized that some of these enzymes might be selective for substrates containing ALA. Yeast is capable of importing fatty acids from the environment and converting them to their respective acyl-CoA derivatives (42). Therefore, the expression of the genes was induced in the presence of exogenously added ALA. Nile red results indicated that all enzymes displayed an enhanced activity in the presence of ALA with the exception of LuDCR1 (Fig. 5A). The Nile red assay is a high throughput technique for quantitative measurement of neutral lipids; however, it is unable to distinguish different neutral lipid classes. Therefore, TLC was used to further confirm the production of TAG in recombinant H1246 yeast cells. There was a good correlation between the results from Nile red and TLC analysis (Fig. 5, A–D). Two recombinant PDATs (LuPDAT1 and LuPDAT2) restored TAG synthesis in H1246 when culturing yeast in the presence of ALA (Fig. 5C). The recombinant LuDGAT2-3 also produced TAG, but at significantly lower levels than those found in LuPDAT1, LuPDAT2, and LuDGAT1-1(Fig. 5, C and D). This analysis confirmed that most of the flax genes can be functionally expressed in yeast and their encoded enzymes are able to complement the TAG synthesis mutations in H1246.
FIGURE 5.
Functional complementation assay in yeast strain H1246. Yeast cells expressing several flax genes show enhanced synthesis of TAG in the presence of ALA. A, Nile red fluorescence assay was performed on the recombinant yeast mutant H1246 cultured in medium with ALA (gray bar) or without ALA supplementation (black bar). The value represents the levels of neutral lipids accumulated in the recombinant yeast, which is calculated by dividing Nile red fluorescence (ΔF) by the optical density (OD) at 600 nm. Data are presented as mean ± S.E. (n = 3). Total lipid extracts were prepared from these cultures and the individual lipid classes were separated by thin layer chromatography (TLC) (B and C). B, TLC analysis of yeast lipid in the absence of ALA. C, TLC analysis of yeast lipid in the presence of ALA. The square bracket indicates the position of TAG produced by yeast cells. The inset displays a high contrast image of TAG produced in yeast expressing LuDGAT2-3 in the present of ALA. D, TLC analysis of yeast cells expressing LuDGAT1-1 in the absence or presence of different exogenously provided fatty acids. E and F, TLC analysis of yeast lipid in the presence of LA and OA. Yeast cells transformed with pYESLacZ were used as the negative control. Although yeast cells transformed with pYESLacZ and pYESLuPDAT6 also showed the higher Nile red value in the presence of ALA (A), the TLC plate indicated that the increased value was very likely due to the production of the unknown compound (square bracket indicated) (C). The corresponding fatty acid used for feeding is shown on the left of the figure with the chemical structure. D1, LuDGAT1-1; DCR1, LuDCR1; D2-3, LuDGAT2-3; P1, LuPDAT1; P2, LuPDAT2; P6, LuPDAT6; TAG, triolein TAG standard in B, E, and F, trilinolenin TAG standard in C and D.
LuPDAT1 and LuPDAT2 Have Unique ALA Selectivity
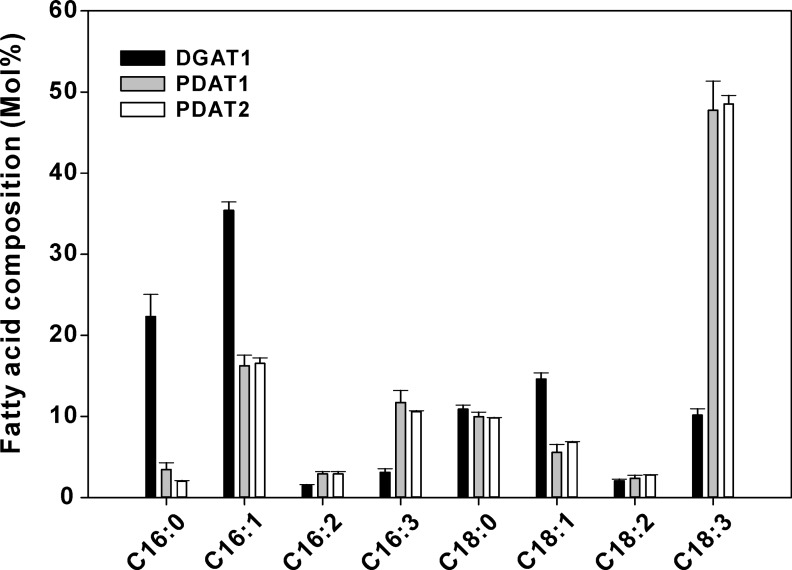
When ALA was exogenously added to induction medium, yeast cells expressing LuPDAT1 and LuPDAT2 produced multiple TAG bands on the TLC plate (Fig. 5C). This was not observed in yeast expressing LuDGAT1-1 (Fig. 5D). Comparison of the migration rates of trilinolenin and triolein standards under our TLC conditions (supplemental Fig. S4) suggested that the observed multiple TAG bands could be explained by the different composition of fatty acids in these TAGs. Therefore, it is plausible to assume that in the presence of ALA, LuPDAT1 and LuPDAT2 produce TAG with a specific fatty acid composition. Indeed, GC analysis of TAG bands with different migration rates indicated that the TAG band with the lower migration rate was composed uniquely of ALA, whereas the band with a higher migration rate contained 65 and 49 mol % ALA for LuPDAT1 and LuPDAT2, respectively (Fig. 6). This result indicated that both LuPDAT1 and LuPDAT2 have the ability to synthesize trilinolenin, which is the major molecular species of TAG in flax oil (43). This experiment also suggested that these two PDATs might be selective for substrates containing ALA, or have a preference for utilizing exogenously imported acyl moieties into yeast. To investigate the origin of this effect, the recombinant yeast strains were cultivated in the presence of OA or LA. As shown in Fig. 5, yeast cells expressing LuPDAT1 and LuPDAT2 produced much weaker TAG bands in these medium conditions (Fig. 5, E and F) than in medium supplemented with ALA (Fig. 5C). It is also worth noting that a substantial amount of TAG was produced by LuDGAT1-1 under all conditions (Fig. 5D). Overall, these results suggest that both LuPDAT1 and LuPDAT2 have a high preference for ALA-containing substrates. Fatty acids commonly found in yeast, including stearic, palmitic, palmitoleic acids, and OA are inefficient substrates for these PDATs.
FIGURE 6.
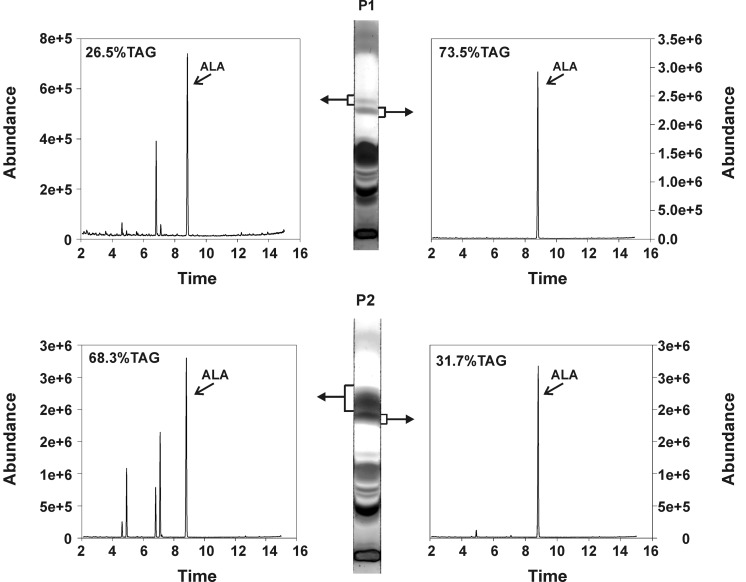
Gas chromatography-mass spectrometry chromatograms of yeast strain H1246 expressing LuPDAT1 and LuPDAT2 in the presence of ALA. The recombinant yeast cells were cultivated in the presence of ALA. The yeast lipids were extracted and separated by a thin layer chromatography (TLC) plate. The compound corresponding to the upper and lower TAG bands (marked by square brackets) was scrapped separately from the TLC plate, transmethylated, and analyzed through GC/MS. The ratio of TAG within each separated band to the total amount of TAG was calculated and the values are shown as percentage on the upper left corner of each chromatograph. GC profiles showed that yeast strain H1246 expressing LuPDAT1 or LuPDAT2 has the ability to produce TAG containing only ALA (trilinolenin), when ALA is added exogenously to the cell. P1, LuPDAT1; P2, LuPDAT2.
LuPDAT1 and LuPDAT2 Have the Similar Preference for ALA-containing Substrates
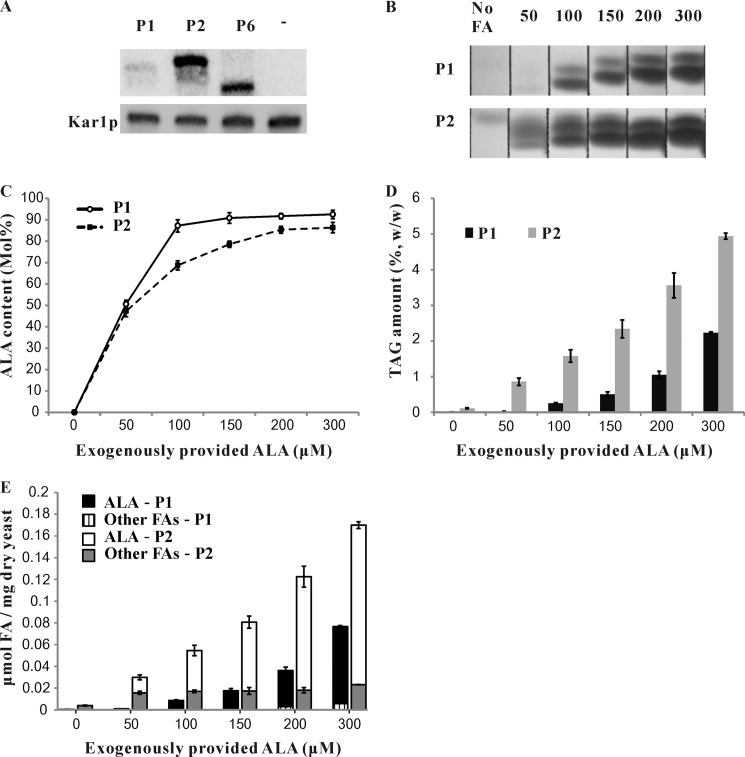
The free fatty acid feeding study revealed that both LuPDAT1 and LuPDAT2 are uniquely ALA-selective. We are also intrigued by the finding that, when ALA was provided exogenously, LuPDAT1 catalyzed the synthesis of a higher amount of trilinolenin with lower total TAG as compared with LuPDAT2 (Fig. 6). To further compare these two enzymes, we first used Western blot to assess the protein expression levels of these PDATs in H1246. Both enzymes with C-terminal fusion tag had reduced TAG-forming ability. Therefore, N-terminal-tagged proteins were used for detection. Western blot analysis revealed that the band corresponding to PDAT2 was markedly stronger than that corresponding to PDAT1 (Fig. 7A), suggesting that H1246 was able to accumulate much higher levels of PDAT2 than PDAT1. It should also be noted that PDAT6 was expressed at a similar protein level to PDAT2, but it failed to complement TAG synthesis in H1246 under all conditions.
FIGURE 7.
Yeast cell expressing LuPDAT1 and LuPDAT2 have similar responses to an increase of exogenously provided ALA. A, representative Western blot analysis showing HisG-LuPDAT protein levels. Microsomes were prepared from yeast expressing HisG-PDATs (see “Experimental Procedures”). Cells transformed with empty pYES2/NT-C vector were used as a negative control, which was annotated as “−” in the image. Equal protein loading was ensured by measuring the constitutively expressed Kar2p in yeast. B, thin layer chromatography (TLC) analysis showing the TAG forming ability of recombinant yeast in the presence of different concentrations of ALA. The yeast cells expressing LuPDAT1 and LuPDAT2 were cultivated in the absence or presence of different ALA concentrations and harvested at the same growth stage (A600 nm = 6.5 ± 0.05). The yeast lipids were extracted and separated by TLC and the corresponding TAG spots are shown in B. C-E, the ALA concentration effect on overall percentage of ALA in TAG (C), total TAG content (D), and amount of fatty acids in TAG (E) of recombinant yeasts. Total TAG content is presented on the basis of yeast dry weight. The amount of ALA and other fatty acids is presented as micromole of fatty acids per mg of yeast dry weight. Data are presented as mean ± S.E. (n = 4). P1, LuPDAT1; P2, LuPDAT2; FA, fatty acid.
It is likely that the higher level of PDAT2 produced in yeast may enhance the rate of channeling ALA into TAG, thus depriving the exogenously introduced ALA in the DAG/PC pool and leading to the formation of TAG with lower content of ALA as compared with PDAT1. To address this possibility, we further investigated the concentration effect of exogenously provided ALA on total TAG content and composition in yeast expressing LuPDAT1 and LuPDAT2 by culturing the recombinant cells in medium supplemented with 0 to 300 μm ALA (Fig. 7, B–E). As shown in Fig. 7C, both PDAT1 and PDAT2 were able to catalyze the production of TAG with up to 90% ALA, but PDAT2 reached the plateau at a much higher concentration of ALA, suggesting that the increased abundance of recombinant PDAT2 would require more ALA-containing substrates to obtain TAG with the same level of ALA as produced by PDAT1. In addition, an increase in ALA concentration from 0 to 300 μm led to ∼168- and 44-fold increases in total TAG content for yeast expressing LuPDAT1 and LuPDAT2, respectively (Fig. 7D). Further quantifying the fatty acid species in TAG revealed that elevated TAG content in these cells was mainly because of the increased amount of ALA (Fig. 7E), suggesting that both enzymes may be more active in the presence of ALA. Taken together, we observed a similar response of LuPDAT1 and LuPDAT2 to an increase of ALA concentration, indicating that LuPDAT2 has similar ALA selectivity as compared with LuPDAT1. The differential levels of protein accumulation, if not entirely, at least partially account for the observed differences in the TAG-forming ability between PDAT1 and PDAT2.
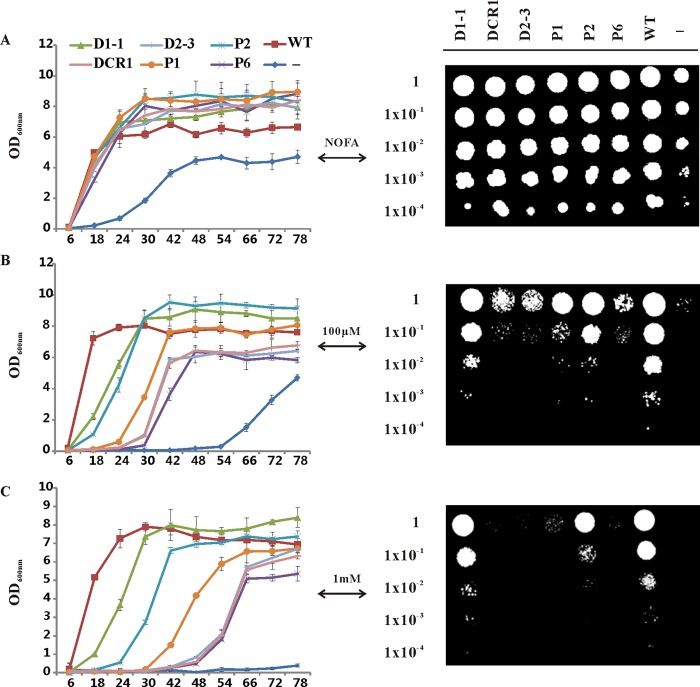
Exogenously Provided Free Fatty Acids Inhibit Growth of the Recombinant Yeast Mutants and This Inhibitory Effect Can Be Rescued in a TAG Accumulation-dependent Manner
We observed that the recombinant yeast mutant displayed a different growth rate upon exposure to free fatty acids. To assess the effect of exogenously provided ALA on recombinant yeast cell growth, we evaluated the cell growth in induction medium supplemented with 0, 100 μm, and 1 mm ALA by both growth curves analysis and the serial dilution plate assay. BY4742 (wild-type) and H1246 harboring the empty vector pYES were used as positive and negative controls, respectively. As shown in Fig. 8, the presence of ALA produced a concentration-dependent inhibitory effect on recombinant yeast growth. Cells with higher TAG-synthesizing ability showed an earlier log phase and earlier appearance on plates, suggesting that the inhibitory effect of ALA was rescued in a TAG accumulation-dependent manner. This result is in agreement with early findings, which reported that TAG biosynthesis plays a crucial role in detoxifying the excess imported unsaturated fatty acids (32, 44). Together, the results implied that TAG-synthesizing enzymatic activity can be physiologically assessed by analyzing the growth of recombinant H1246 in the presence of ALA.
FIGURE 8.
Rescue of lipotoxicity phenotype in H1246. Exogenously supplied ALA inhibits the growth of yeast H1246. This inhibition can be rescued by expressing genes encoding TAG-synthesizing enzymes from flax and the rescuing effect is correlated with the ability to synthesize TAG as demonstrated with Nile red assay and TLC analysis. Growth curves of recombinant yeast H1246 cultured in induction medium with 0 (A), 100 μm (B), and 1 mm (C) ALA, were constructed by measuring the optical density (OD) of the cultures at 600 nm. Data are presented as mean ± S.E. (n = 3 or 6). BY4742 (wild-type) and H1246 harboring the empty vector pYES were used as positive and negative controls, respectively. For negative controls, six independent colonies were analyzed and consistently showed delayed growth and low final density even in the absence of free fatty acid. The panels on the right side, from top to bottom, show the growth of 2.5 μl of each 10-fold dilution (1 to 10−4) of recombinant yeast H1246 spotted onto induction medium plates containing 0, 100 μm, and 1 mm ALA. The negative controls were annotated as “−” in the image. WT, wild-type; D1-1, LuDGAT1-1; DCR1, LuDCR1; D2-3, LuDGAT2-3; P1, LuPDAT1; P2, LuPDAT2; P6, LuPDAT6.
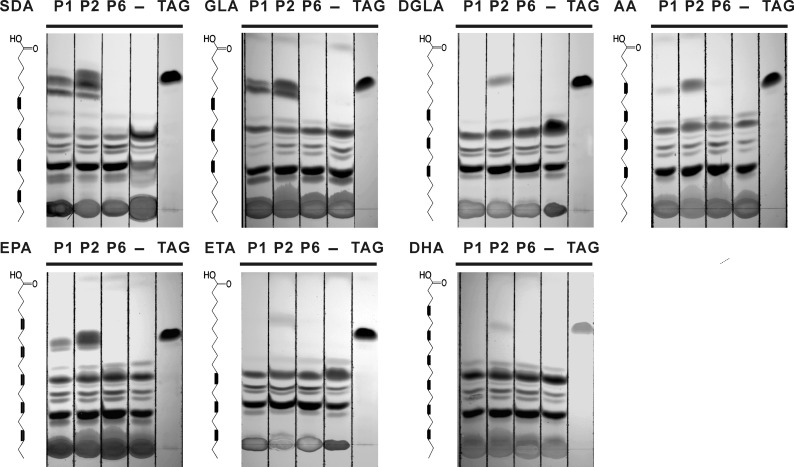
LuPDAT1 and LuPDAT2 Can Also Preferably Utilize Substrates Containing Certain PUFAs
The previous results showed the unique selectivity properties of LuPDAT1 and LuPDAT2 for substrates containing ALA. To further examine the substrate preferences of flax PDATs, we conducted a similar experiment using other PUFAs that are not commonly found in plants, including SDA, DGLA, GLA, AA, ETA, EPA, and DHA. LuPDAT1 and LuPDAT2 appeared to accept substrates containing SDA, GLA, and EPA (Fig. 9), whereas DGLA, AA, ETA, and DHA were markedly poorer substrates for LuPDAT1 and LuPDAT2. Together, the data presented in Figs. 5 and 9 indicated that LuDGAT1 are capable of utilizing a broad range of substrates containing different fatty acids. However, LuPDAT1 and LuPDAT2 have clear preference for certain PUFAs, particularly those with at least three double bonds, such as ALA, GLA, SDA, and EPA.
FIGURE 9.
PUFA-specific activity of LuPDAT1 and LuPDAT2 in yeast strain H1246. Yeast mutant H1246 transformed with LuPDAT genes was cultivated in the presence of different PUFAs fed individually to a final concentration of 100 μm. Yeast transformed with pYESLacZ was used as the negative control, which was annotated as “−” on the TLC plate. The corresponding fatty acid used for feeding is shown on the left of the figure with the chemical structure. P1, LuPDAT1; P2, LuPDAT2; P6, LuPDAT6; TAG, trilinolenin TAG standards; SDA, stearidonic acid; GLA, γ-linolenic acid; DGLA, dihomo-γ-linolenic acid; AA, arachidonic acid; EPA, eicosapentaenoic acid; ETA, eicosatrienoic acid; DHA, docosahexaenoic acid.
Feeding fatty acids to yeast provides an easy and flexible way to introduce novel acyl moieties in yeast endogenous lipids. This method is, however, limited as it does not allow a controlled distribution of exogenous fatty acids to specific glycerolipids as it occurs naturally. To circumvent this problem, LuPDAT1, LuPDAT2, and LuDGAT1-1 were individually co-expressed with LuFAD2-1 and LuFAD3B, thus simulating a natural production of ALA in PC. In this experiment yeast cells expressing LuPDAT1 and LuPDAT2 produced TAG predominantly with ALA, whereas cells expressing LuDGAT1-1 produced TAG mainly with palmitoleic acid (16:1cisΔ9) (Fig. 10). LuPDAT1 and LuPDAT2 were able to produce TAG with an ALA:LA ratio of 10:1, whereas LuDGAT1-1 produced TAG with an ALA:LA ratio of ∼2:1. These results further confirmed that LuPDAT1 and LuPDAT2 have similar preference for ALA-containing substrate.
FIGURE 10.
Comparison of the FAME profile of yeast strain H1246 co-expressing LuPDAT1, LuPDAT2, and LuDGAT1-1 individually with desaturases. When compared with the yeast cell co-expressing LuDGAT1-1 with LuFAD2-1 and LuFAD3B (black bar), co-expression of either LuPDAT1 (gray bar) or LuPDAT2 (white bar) with LuFAD2-1 and LuFAD3B in yeast produces TAG with ALA (C18:3) as the predominant fatty acid. Yeast cultures were induced at 20 °C for 3 days before harvested. Data are presented as mean ± S.E. (n = 3).
Seed-specific Expression of LuDGAT1-1, LuPDAT1, and LuPDAT2 Affects the Fatty Acid Profile and Oil Content in Arabidopsis
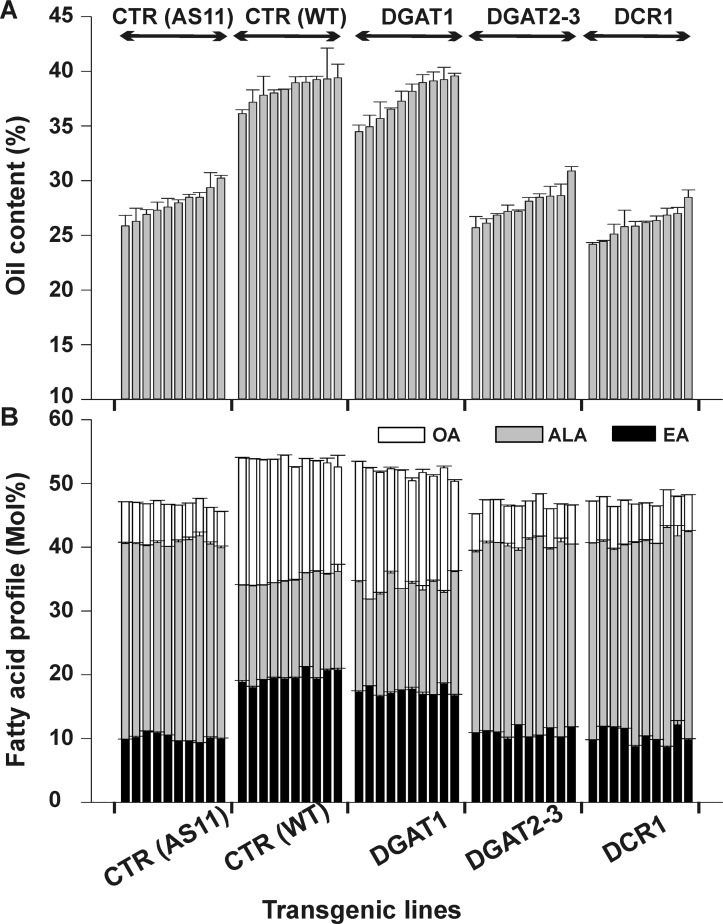
Complementation assays in yeast indicated that LuDGAT1-1, LuDGAT2-3, LuPDAT1, and LuPDAT2 are active TAG-synthesizing enzymes from flax. Although informative, this expression system is limited as yeast lacks the cellular and developmental complexity of multicellular higher plants. To investigate the functionality of flax TAG-synthesizing enzymes in plants, we expressed the ORFs of LuDGAT1-1, LuDGAT2-3, and LuDCR1 in the Arabidopsis dgat1 mutant AS11 under the regulation of the seed-specific napin promoter. AS11 has a reduced amount of TAG and altered fatty acid profile (low OA and eicosenoic acid (20:1cisΔ11) and high ALA) (18). To minimize variation of the genome position and copy number-dependent transgene expression (45, 46), 10 individual transgenic lines were analyzed for each construct. This strategy also helped to compensate for environmental effects that can significantly influence the accumulation of oil in the seeds of each plant even though they were cultivated side-by-side. Analysis of T2 transgenic seed lines indicated that overexpression of LuDGAT1-1 restored seed oil content of AS11 to levels comparable with the empty vector-transformed control wild-type plants (Fig. 11A). LuDCR1 and LuDGAT2 did not complement the reduced TAG phenotype of the AS11 mutant (Fig. 11A). GC analysis of the total lipid extract-FAMEs showed that LuDGAT1-1 could also alter the seed fatty acid profile of AS11 making it similar to wild-type controls. None of the LuDGAT2-3 and LuDCR1 transgenic lines could, however, complement this phenotype (Fig. 11B).
FIGURE 11.
Lipid phenotype of LuDGAT-overexpressing seeds. Overexpressing LuDGAT1-1 in Arabidopsis AS11 background restores the wild-type lipid phenotype. Ten individual transgenic lines were analyzed for each construct. Arabidopsis AS11 mutant and wild-type transformed with empty pGreen plasmid were used as controls (CTR). Data are presented as mean ± S.E. (n = 3). EA, eicosenoic acid. A, seed oil content. Oil content is expressed as percentage of seed dry weight. B, fatty acid composition. Values were obtained from FAME analysis of dry seeds.
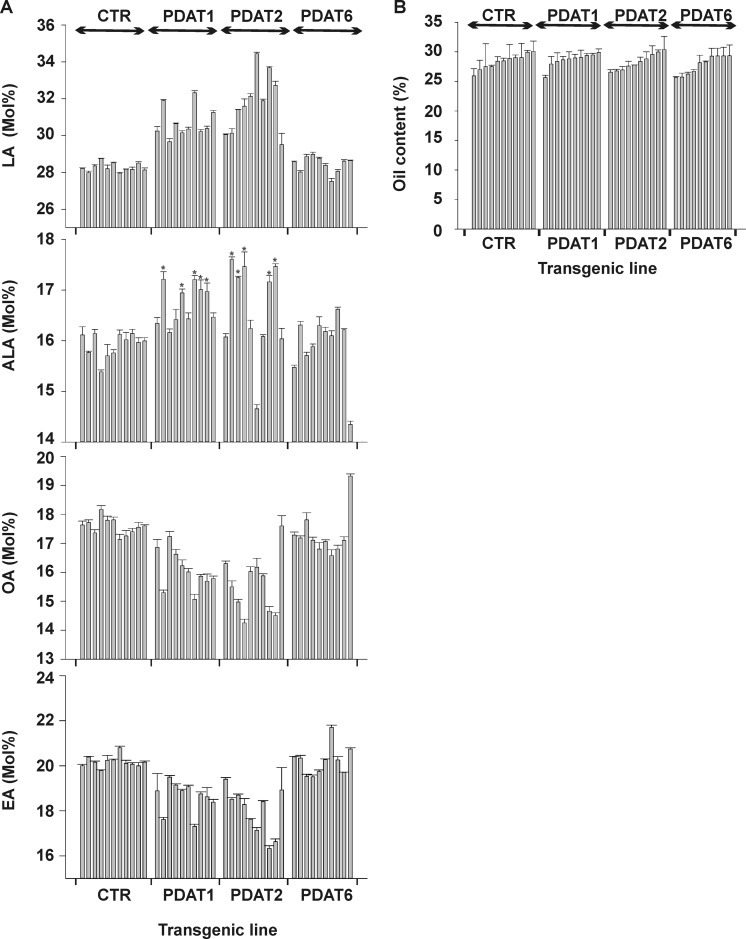
It has been shown that the Arabidopsis PDAT1 knock-out mutant did not have any significant changes in either fatty acid composition or oil content compared with wild-type (47), whereas the attempt to obtain the Arabidopsis dgat1 pdat1 double mutant failed due to the lethal effect of a dgat1pdat1 genotype on pollen development (17). To study the biochemical properties of LuPDATs in plant oil biosynthesis, the constructs carrying the coding region of LuPDATs (LuPDAT1, LuPDAT2, or LuPDAT6) were transformed into Arabidopsis wild-type Columbia. As compared with the empty vector-transformed control wild-type plants, overexpression of the LuPDAT1 and LuPDAT2 increased the PUFA (LA and ALA) content, at the expense of mostly OA and eicosenoic acid (Fig. 12A). This altered fatty acid composition was not found in Arabidopsis lines overexpressing LuPDAT6. As shown in Fig. 12A, the LuPDAT1 lines exhibited a relative increase of LA in the 4.9 to 14.3% range compared with the average exhibited by empty vector transformed wild-type controls. In addition, the ALA content was significantly increased in many lines, with the highest relative increase of 8.1%. The LuPDAT2 transgenic T2 seed lines showed a relative increase of LA by 4.4 to 21.8%. ALA content was generally increased, with the highest relative increase value observed being 10.5%. The oil content of LuPDAT1, LuPDAT2, and LuPDAT6 Arabidopsis transgenic lines were not significantly altered compared with controls (Fig. 12B).
FIGURE 12.
Lipid phenotype of LuPDAT-overexpressing seeds. Ten individual transgenic lines were analyzed for each construct. Wild-type Arabidopsis transformed with empty pGreen plasmid were used as controls (CTR). A, fatty acid composition of LuPDAT-overexpressing seeds. Overexpression of LuPDAT1 and LuPDAT2 in Arabidopsis seeds results in an increased level of LA and ALA. Data are presented as mean ± S.E. (n = 3), with asterisks indicating p < 0.05 (ANOVA, Duncan's multiple range test). EA, eicosenoic acid. B, oil content of LuPDAT-overexpressing seeds. Data are presented as mean ± S.E. (n = 3).
DISCUSSION
Flax seed is a prominent oilseed crop and one of the most important plant-based sources of vegetable oils rich in ALA. Understanding the metabolic pathway of oil synthesis is pivotal for a continuous improvement of flax as an oilseed crop. The goal of this study was to identify flax genes involved in TAG synthesis and functionally characterize the encoded enzymes.
In mainstream oilseed crops such as Brassica napus and Glycine max, DGAT1 has been proposed to be the dominant enzyme involved in the final step of TAG biosynthesis (48, 49). But in plant species that accumulate exotic fatty acids such as ricinoleic, vernolic, and α-eleostearic acid, DGAT2 and PDAT appear to have a more predominant role in TAG synthesis although DGAT1 is also expressed during seed development in these species (20, 49, 50). In the case of flax, we found gene homologues encoding all major TAG-synthesizing enzymes with the exception of DGAT3.
We have previously cloned a DGAT1 from flax (here refereed to LuDGAT1-1) and have demonstrated that it can complement TAG synthesis in the yeast strain H1246 (32). Although this enzyme was considerably active when produced recombinantly in yeast and plants (Figs. 5, A and D, 10, and 11), it did not appear to show a preference for substrates containing ALA (Figs. 5D and 10). Like in other oilseeds, the expression of LuDGAT1 was considerably up-regulated in seeds (Fig. 2A), but a comprehensive analysis throughout seed development indicated that the transcript levels peaked at 20 DPA when the rate of oil accumulation reached a plateau and most ALA is already incorporated in TAG (Fig. 4, B–D). Together, these results suggested that flax DGAT1 appears to play a role in the nonspecific transfer of acyl moieties to TAG during the final stages of seed development. During the time this manuscript was being prepared, we detected an additional gene encoding DGAT1 (here referred to as LuDGAT1-2) in the final released version of the flax genome sequence (27). LuDGAT1-1 and LuDGAT1-2 share 97.7% identity and due to this high degree of homology, their expression was detected together (Figs. 2A and 4D).
In contrast to DGAT1, flax DGAT2-3 failed to complement TAG synthesis in Arabidopsis AS11 mutant (Fig. 11). Production of LuDGAT2-3 recombinant in yeast H1246 resulted in the production of small amounts of neutral lipids in yeast (Fig. 5C) and ineffectively rescued the fatty acid lipotoxicity phenotype (Fig. 8). The transcript of a third DGAT2 homologous gene (LuDGAT2-2) could not be detected or isolated. These results suggested that in flax DGAT2 might contribute minimally to TAG synthesis despite being expressed specifically in seeds. Similarly, LuDCR1 did not show a significant effect on TAG biosynthesis in both yeast and Arabidopsis expression system (Figs. 5 and 11).
In the case of flax PDATs, we analyzed one representative gene from each of the three groups of homologous genes (LuPDAT1, LuPDAT2, and LuPDAT6). Heterologous expression in yeast indicated that both LuPDAT1 and LuPDAT2 were active and able to produce TAG composed predominantly of ALA when ALA was supplemented as a free fatty acid to the medium or endogenously produced through the action of desaturases LuFAD2-1 and LuFAD3B (Figs. 5C and 10). Interestingly, LuPDAT2 has a higher level of protein accumulation than LuPDAT1 in yeast (Fig. 7A), which may account partially for its higher TAG-forming ability. The reason for poor expression levels of LuPDAT1 is currently unknown but may be due to the low mRNA stability or inefficient codon usage. Overexpression of LuPDAT1 or LuPDAT2 in Arabidopsis also resulted in an enhanced content of PUFAs (Fig. 12A). Analysis of gene expression revealed that LuPDAT1/LuPDAT5 transcripts accumulated substantially more in seeds than in other vegetative tissues (Fig. 3A). In fact, expression of LuPDAT1/LuPDAT5 was closely correlated with oil and ALA accumulation in developing embryos, presenting a peak during the rapid phase of lipid accumulation at 14 DPA (Fig. 4, B–G). LuPDAT2 was found to be mostly expressed in vegetative tissues (Fig. 3D), indicating that TAG synthesized by the catalytic action of LuPDAT2 might have physiological functions in vegetative tissues of flax. LuPDAT6 was also highly expressed during the initial stages of embryo development (Fig. 4I) but the encoded enzyme had significantly lower activity in all conditions tested (Figs. 5, 8, 9, and 12). Based on sequence homology (Table 2) and similarities in expression profile (Fig. 3), we suggest that the pairs LuPDAT1/LuPDAT5, LuPDAT2/LuPDAT4, and LuPDAT3/LuPDAT6 may have similar functions in flax. The fatty acid feeding experiment confirmed that LuPDAT5, similar to LuPDAT1, also has the higher preference for ALA-containing substrate (supplemental Fig. S5). On the basis of these results, we propose that LuPDAT1/LuPDAT5 may play a more important role than LuDGAT1 in the production of ALA-containing TAG in developing flax seed. This hypothesis is in agreement with metabolic analyses of the acyl-CoA profile of developing flax seed, which detected only small amounts of ALA-CoA in flax embryo (51).
The identified ALA-selective flax PDATs in this study could be considered as another example of a plant TAG-synthesizing enzyme that displays preference for certain substrates. Our data indicates, however, that these PDATs not only favor substrates containing ALA, but also extend to other PUFAs that are not naturally formed in flax, including SDA, GLA, and EPA (Fig. 9). It appears that the activity of these PDATs is highly dependent on ALA availability. Increasing the concentration of exogenously provided ALA resulted in an enhanced amount of ALA-containing TAG and total TAG contents in yeast expressing LuPDAT1 or LuPDAT2 (Fig. 7, B–E). When ALA was added to the medium, both LuPDAT1 and LuPDAT2 were able to produce TAG with up to 90% ALA (Fig. 7C). In contrast, when ALA was endogenously produced by the co-expression of LuFAD2-1 and LuFAD3B, the total amount of ALA in TAG was only about 50% for LuPDAT1 or LuPDAT2 (Fig. 10). This difference can be explained by two possible reasons. One possible explanation is that the ALA availability may limit the activity of these two PDATs. Inefficient desaturase activity in the heterologous yeast expression system has been recently reported by Dahmen et al. (52). They found that yeast cytochrome b5, which transfers the electrons to the desaturase, poorly interacts with foreign desaturases, thus limiting desaturase enzymatic activity and resulting in the low production of unsaturated fatty acids. Future studies of the overexpression of flax cytochrome b5 in the LuFAD2-1-LuFAD3b-LuPDAT-expressing yeast poses an opportunity to further enhance the production of ALA-containing TAG in yeast by metabolic engineering. The other possible reason is that to produce trilinolenin the flax PDATs require sn-2 ALA-PC and sn-1,2 ALA-DAG. Considering that the excess ALA-CoA derived from the exogenously supplied ALA can be transferred indiscriminately into different glycerolipids, there is a better chance of obtaining sn-1,2 ALA-DAG. In contrast, ALA produced by a FAD3 is restricted to sn-2 PC, which can be utilized by PDAT thus limiting its redistribution to other lipid classes such as DAG. Therefore, the limited amount of sn-1,2 ALA-DAG may restrict the activity of LuPDAT1 and LuPDAT2. The same theory can be used to explain the small but significant increase of ALA content in the seed oil of LuPDAT1- and LuPDAT2-overexpressing Arabidopsis lines. It is possible that the activity of FAD3 in Arabidopsis might not be sufficiently high to supply the substrates required for LuPDAT1 and LuPDAT2. For instance, expression of FAD3 in wild-type Arabidopsis is significantly lower compared with the AS11 mutant (53). Furthermore, labeling experiments indicated that Arabidopsis utilize the PC-derived DAG pool as the major source for TAG biosynthesis (54). PC-derived DAG synthesis, however, may provide mostly sn-2-ALA-DAG. It is possible that when produced in Arabidopsis, LuPDAT1 and LuPDAT2 may rapidly transfer ALA from the sn-2 position of PC to the sn-3 position of the PC-derived DAG. This rapid conversion could limit the chance of rechanneling ALA from PC to the acyl-CoA pool and subsequently incorporating it to the sn-1 position of DAG by the endogenous Arabidopsis enzymes, thus imposing a bottleneck for accumulating ALA in Arabidopsis seed oil.
A phylogenetic analysis of PDATs including the functionally tested enzymes from Arabidopsis (17) and castor bean (24, 50) indicate that LuPDAT1 and RcPDAT1-2 are closely related (supplemental Fig. 2A). Interestingly, both polypeptides display remarkable substrate selectivity and the corresponding genes are preferentially expressed in seeds. The branch containing LuPDAT1 and RcPDAT1-2 lacks a corresponding polypeptide from Arabidopsis. LuPDAT2 is, however, more closely associated with AtPDAT1 and RcPDAT1-1. It is interesting that in Arabidopsis upon AtDGAT1 disruption, AtPDAT1 is responsible for synthesizing the remaining 65–70% of TAG (53) despite of the inability of AtPDAT1 or AtPDAT2 to complement TAG synthesis in H1246 (17). In addition, overexpression of AtPDAT1 in wild-type Arabidopsis background did not produce a significant effect on oil phenotype (23). Similarly, expression of RcPDAT1-1 in Arabidopsis CL37 lines did not result in any substantial increase in hydroxy fatty acids (50). Overexpression of LuPDAT2 in wild-type Arabidopsis, however, led to a significantly increased PUFA level. This difference might be explained by the higher substrate selectivity of LuPDAT2. LuPDAT6 belongs to the same branch of AtPDAT2 and RcPDAT2. Although PDATs in this branch are preferentially expressed in developing seeds, they do not have a significant effect on TAG accumulation. Overall, these findings suggest the existence of a class of plant PDATs that displays substrate selectivity properties.
One of the most remarkable advances in oilseed biotechnology is the successful reconstitution of VLC-PUFA synthesis in transgenic plants (reviewed by Ruiz-López et al. (55)). Flax would become an obvious candidate for this technology because it naturally contains substantial amounts of ALA that is the precursor for VLC-PUFAs. A previous report demonstrated that the introduction of a VLC-PUFA biosynthetic pathway in flax led to a high proportion of Δ6-desaturated C-18 fatty acids (GLA and SDA totaling up to 33% in some plants). The pathway, however, did not efficiently proceed further after the first desaturation step, resulting in a very limited amount of AA and EPA (<5%) in flax oil (56). As demonstrated before, the front-end desaturases and elongases involved in VLC-PUFA have different acyl carrier specificity (57). Desaturases act on PC, whereas elongases occur mainly within the acyl-CoA pool. According to the results presented in Fig. 9, it is likely that LuPDAT1 contributes to a premature channeling of GLA and SDA to TAG, resulting in an insufficient supply of acyl-CoA substrates for elongation. The observation that GLA and SDA were nearly absent from the acyl-CoA pool of the transgenic flax lines (56) further supports this hypothesis. To circumvent this problem, the metabolic pathway for VLC-PUFAs would have to be strategically modified to bypass other acyl moieties that could be utilized by LuPDAT1 until it reaches EPA. For example, the pathway involving utilization of the Δ8-desaturation pathway to convert LA to AA (58) and then further desaturating AA to EPA by Δ17 desaturases (59), would contain intermediates (LA, DGLA, and AA), which are poorly utilized by LuPDAT1 (Figs. 5E and 9).
In conclusion, this study provided new insights into the biosynthesis of TAG in flax. Our results demonstrated the existence of two pairs of flax PDATs that are highly selective for substrates containing ALA. Among them, one pair of PDATs (LuPDAT1/LuPDAT5) is expressed predominately in seeds. In addition, the oil and ALA accumulation during seed development closely correspond to the expression pattern of this seed-preferred PDAT gene pair, suggesting the critical role of these PDATs in seed oil biosynthesis in flax. The identified PDATs help in the understanding of the mechanisms involved in producing TAG with high PUFA content in plants. Our characterization of TAG-synthesizing enzymes in flax will benefit future projects aimed at enriching PUFAs in plants and other organisms.
Supplementary Material
Acknowledgments
We thank Dr. Gordon Rowland for providing flax seed and Dr. Jitao Zou for the use of laboratory facilities at the National Research Council of Canada, Saskatoon.
This work was supported in part by grants from Genome Alberta/Genome Canada, the Canada Research Chairs Program, the Canada Foundation for Innovation, the Research Capacity Program of Alberta Enterprise and Advanced Education, AVAC Ltd., Alberta Innovates Bio Solutions, and the Natural Sciences and Engineering Research Council of Canada (to R. J. W.)

This article contains supplemental Tables S1 and S2 and Figs. S1–S5.
- ALA
- α-linolenic acid
- DGAT
- acyl-CoA:diacylglycerol acyltransferase
- PDATs
- phospholipid:diacylglycerol acyltransferase
- TAG
- triacylglycerol
- VLC-ω-3-PUFA
- very long chain omega-3 polyunsaturated fatty acids
- FAD
- fatty acid desaturases
- PC
- phosphatidylcholine
- LPCAT
- acyl-CoA:lysophosphatidylcholine acyltransferase
- DAG
- diacylglycerol
- DCR
- defective in cuticular ridge
- DPA
- days post-anthesis
- OA
- oleic acid
- LA
- linoleic acid
- SDA
- stearidonic acid
- DGLA
- dihomo-γ-linolenic acid
- GLA
- γ-linolenic acid
- AA
- arachidonic acid
- ETA
- eicosatrienoic acid
- EPA
- eicosapentaenoic acid
- DHA
- docosahexaenoic acid
- FAME
- fatty acid methyl esters.
REFERENCES
- 1. Jhala A. J., Hall L. M. (2010) Flax (Linum usitatissimum L.): current uses and future applications. Aust. J. Basic Appl. Sci. 4, 4304–4312 [Google Scholar]
- 2. Sinclair A. J., Attar-Bashi N. M., Li D. (2002) What is the role of α-linolenic acid for mammals? Lipids 37, 1113–1123 [DOI] [PubMed] [Google Scholar]
- 3. Das U. N. (2006) Essential fatty acids - a review. Curr. Pharm. Biotechnol. 7, 467–482 [DOI] [PubMed] [Google Scholar]
- 4. Vrinten P., Hu Z., Munchinsky M. A., Rowland G., Qiu X. (2005) Two FAD3 desaturase genes control the level of linolenic acid in flax seed. Plant Physiol. 139, 79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Green A. G. (1986) A mutant genotype of flax (Linum usitatissimum L.) containing very low levels of linolenic acid in its seed oil. Can. J. Plant Sci. 66, 499–503 [Google Scholar]
- 6. Rowland G. G. (1991) An EMS-induced low-linolenic-acid mutant in McGregor flax (Linum usitatissimum L.). Can. J. Plant Sci. 71, 393–396 [Google Scholar]
- 7. Rao S., Abdel-Reheem M., Bhella R., McCracken C., Hildebrand D. (2008) Characteristics of high α-linolenic acid accumulation in seed oils. Lipids 43, 749–755 [DOI] [PubMed] [Google Scholar]
- 8. Stymne S., Stobart A. K. (1984) Evidence for the reversibility of the acyl-CoA:lysophosphatidylcholine acyltransferase in microsomal preparations from developing safflower (Carthamus tinctorius L.) cotyledons and rat liver. Biochem. J. 223, 305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee J., Welti R., Schapaugh W. T., Trick H. N. (2011) Phospholipid and triacylglycerol profiles modified by PLD suppression in soybean seed. Plant Biotechnol. J. 9, 359–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu C., Xin Z., Ren Z., Miquel M., Browse J. (2009) An enzyme regulating triacylglycerol composition is encoded by the ROD1 gene of Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 106, 18837–18842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weiss S. B., Kennedy E. P., Kiyasu J. Y. (1960) The enzymatic synthesis of triglycerides. J. Biol. Chem. 235, 40–44 [PubMed] [Google Scholar]
- 12. Hobbs D. H., Lu C., Hills M. J. (1999) Cloning of a cDNA encoding diacylglycerol acyltransferase from Arabidopsis thaliana and its functional expression. FEBS Lett. 452, 145–149 [DOI] [PubMed] [Google Scholar]
- 13. Saha S., Enugutti B., Rajakumari S., Rajasekharan R. (2006) Cytosolic triacylglycerol biosynthetic pathway in oilseeds. Molecular cloning and expression of peanut cytosolic diacylglycerol acyltransferase. Plant Physiol. 141, 1533–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kalscheuer R., Steinbüchel A. (2003) A novel bifunctional wax ester synthase/acyl-CoA:diacylglycerol acyltransferase mediates wax ester and triacylglycerol biosynthesis in Acinetobacter calcoaceticus ADP1. J. Biol. Chem. 278, 8075–8082 [DOI] [PubMed] [Google Scholar]
- 15. Lardizabal K. D., Mai J. T., Wagner N. W., Wyrick A., Voelker T., Hawkins D. J. (2001) DGAT2 is a new diacylglycerol acyltransferase gene family. Purification, cloning, and expression in insect cells of two polypeptides from Mortierella ramanniana with diacylglycerol acyltransferase activity. J. Biol. Chem. 276, 38862–38869 [DOI] [PubMed] [Google Scholar]
- 16. Lung S. C., Weselake R. J. (2006) Diacylglycerol acyltransferase: a key mediator of plant triacylglycerol synthesis. Lipids 41, 1073–1088 [DOI] [PubMed] [Google Scholar]
- 17. Zhang M., Fan J., Taylor D. C., Ohlrogge J. B. (2009) DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell 21, 3885–3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katavic V., Reed D. W., Taylor D. C., Giblin E. M., Barton D. L., Zou J., Mackenzie S. L., Covello P. S., Kunst L. (1995) Alteration of seed fatty acid composition by an ethyl methanesulfonate-induced mutation in Arabidopsis thaliana affecting diacylglycerol acyltransferase activity. Plant Physiol. 108, 399–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng P., Allen W. B., Roesler K., Williams M. E., Zhang S., Li J., Glassman K., Ranch J., Nubel D., Solawetz W., Bhattramakki D., Llaca V., Deschamps S., Zhong G. Y., Tarczynski M. C., Shen B. (2008) A phenylalanine in DGAT is a key determinant of oil content and composition in maize. Nat. Genet. 40, 367–372 [DOI] [PubMed] [Google Scholar]
- 20. Shockey J. M., Gidda S. K., Chapital D. C., Kuan J. C., Dhanoa P. K., Bland J. M., Rothstein S. J., Mullen R. T., Dyer J. M. (2006) Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell 18, 2294–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rani S. H., Krishna T. H., Saha S., Negi A. S., Rajasekharan R. (2010) Defective in Cuticular Ridges (DCR) of Arabidopsis thaliana, a gene associated with surface cutin formation, encodes a soluble diacylglycerol acyltransferase. J. Biol. Chem. 285, 38337–38347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dahlqvist A., Stahl U., Lenman M., Banas A., Lee M., Sandager L., Ronne H., Stymne S. (2000) Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc. Natl. Acad. Sci. U.S.A. 97, 6487–6492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ståhl U., Carlsson A. S., Lenman M., Dahlqvist A., Huang B., Banas W., Banas A., Stymne S. (2004) Cloning and functional characterization of a phospholipid:diacylglycerol acyltransferase from Arabidopsis. Plant Physiol. 135, 1324–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Erp H., Bates P. D., Burgal J., Shockey J., Browse J. (2011) Castor phospholipid:diacylglycerol acyltransferase facilitates efficient metabolism of hydroxy fatty acids in transgenic Arabidopsis. Plant Physiol. 155, 683–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kroon J. T., Wei W., Simon W. J., Slabas A. R. (2006) Identification and functional expression of a type 2 acyl-CoA:diacylglycerol acyltransferase (DGAT2) in developing castor bean seeds which has high homology to the major triglyceride biosynthetic enzyme of fungi and animals. Phytochemistry 67, 2541–2549 [DOI] [PubMed] [Google Scholar]
- 26. Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. (1990) Basic local alignment search tool. J. Mol. Biol. 215, 403–410 [DOI] [PubMed] [Google Scholar]
- 27. Wang Z., Hobson N., Galindo L., Zhu S., Shi D., McDill J., Yang L., Hawkins S., Neutelings G., Datla R., Lambert G., Galbraith D. W., Grassa C. J., Geraldes A., Cronk Q. C., Cullis C., Dash P. K., Kumar P. A., Cloutier S., Sharpe A. G., Wong G. K., Wang J., Deyholos M. K. (2012) The genome of flax (Linum usitatissimum) assembled de novo from short shotgun sequence reads. Plant J. 72, 461–473 [DOI] [PubMed] [Google Scholar]
- 28. Krasowska A., Dziadkowiec D., Polinceusz A., Plonka A., Łukaszewicz M. (2007) Cloning of flax oleic fatty acid desaturase and its expression in yeast. J. Am. Oil Chem. Soc. 84, 809–816 [Google Scholar]
- 29. Gibson D. G. (2011) Enzymatic assembly of overlapping DNA fragments. Methods Enzymol. 498, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huis R., Hawkins S., Neutelings G. (2010) Selection of reference genes for quantitative gene expression normalization in flax (Linum usitatissimum L.). BMC Plant Biol. 10, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gietz R. D., Schiestl R. H. (2007) High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2, 31–34 [DOI] [PubMed] [Google Scholar]
- 32. Siloto R. M., Truksa M., He X., McKeon T., Weselake R. J. (2009) Simple methods to detect triacylglycerol biosynthesis in a yeast-based recombinant system. Lipids. 44, 963–973 [DOI] [PubMed] [Google Scholar]
- 33. Siloto R. M., Truksa M., Brownfield D., Good A. G., Weselake R. J. (2009) Directed evolution of acyl-CoA:diacylglycerol acyltransferase: development and characterization of Brassica napus DGAT1 mutagenized libraries. Plant Physiol. Biochem. 47, 456–461 [DOI] [PubMed] [Google Scholar]
- 34. Bligh E. G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 35. Mietkiewska E., Siloto R. M., Dewald J., Shah S., Brindley D. N., Weselake R. J. (2011) Lipins from plants are phosphatidate phosphatases that restore lipid synthesis in a pah1Δ mutant strain of Saccharomyces cerevisiae. FEBS J. 278, 764–775 [DOI] [PubMed] [Google Scholar]
- 36. Hellens R. P., Edwards E. A., Leyland N. R., Bean S., Mullineaux P. M. (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 42, 819–832 [DOI] [PubMed] [Google Scholar]
- 37. Weigel D., Glazebrook J. (2002) Arabidopsis: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 38. Thompson J. D., Higgins D. G., Gibson T. J. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu Q., Siloto R. M., Lehner R., Stone S. J., Weselake R. J. (2012) Acyl-CoA:diacylglycerol acyltransferase: molecular biology, biochemistry and biotechnology. Prog. Lipid Res. 51, 350–377 [DOI] [PubMed] [Google Scholar]
- 41. Sandager L., Gustavsson M. H., Ståhl U., Dahlqvist A., Wiberg E., Banas A., Lenman M., Ronne H., Stymne S. (2002) Storage lipid synthesis is non-essential in yeast. J. Biol. Chem. 277, 6478–6482 [DOI] [PubMed] [Google Scholar]
- 42. Faergeman N. J., Black P. N., Zhao X. D., Knudsen J., DiRusso C. C. (2001) The acyl-CoA synthetases encoded within FAA1 and FAA4 in Saccharomyces cerevisiae function as components of the fatty acid transport system linking import, activation, and intracellular utilization. J. Biol. Chem. 276, 37051–37059 [DOI] [PubMed] [Google Scholar]
- 43. Ciftci O. N., Przybylski R., Rudzińska M. (2012) Lipid components of flax, perilla, and chia seeds. Eur. J. Lipid Sci. Technol. 114, 794–800 [Google Scholar]
- 44. Petschnigg J., Wolinski H., Kolb D., Zellnig G., Kurat C. F., Natter K., Kohlwein S. D. (2009) Good fat, essential cellular requirements for triacylglycerol synthesis to maintain membrane homeostasis in yeast. J. Biol. Chem. 284, 30981–30993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tang J., Scarth R., Fristensky B. (2003) Effects of genomic position and copy number of Acyl-ACP thioesterase transgenes on the level of the target fatty acids in Brassica napus L. Mol. Breed. 12, 71–81 [Google Scholar]
- 46. Ahmad A., Zhang Y., Cao X. F. (2010) Decoding the epigenetic language of plant development. Mol. Plant. 3, 719–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mhaske V., Beldjilali K., Ohlrogge J., Pollard M. (2005) Isolation and characterization of an Arabidopsis thaliana knockout line for phospholipid:diacylglycerol transacylase gene (At5g13640). Plant Physiol. Biochem. 43, 413–417 [DOI] [PubMed] [Google Scholar]
- 48. Lock Y. Y., Snyder C. L., Zhu W., Siloto R. M., Weselake R. J., Shah S. (2009) Antisense suppression of type 1 diacylglycerol acyltransferase adversely affects plant development in Brassica napus. Physiol. Plant 137, 61–71 [DOI] [PubMed] [Google Scholar]
- 49. Li R., Yu K., Hildebrand D. F. (2010) DGAT1, DGAT2 and PDAT expression in seeds and other tissues of epoxy and hydroxy fatty acid accumulating plants. Lipids 45, 145–157 [DOI] [PubMed] [Google Scholar]
- 50. Kim H. U., Lee K. R., Go Y. S., Jung J. H., Suh M. C., Kim J. B. (2011) Endoplasmic reticulum-located PDAT1-2 from castor bean enhances hydroxy fatty acid accumulation in transgenic plants. Plant Cell Physiol. 52, 983–993 [DOI] [PubMed] [Google Scholar]
- 51. Ruiz-López N., Haslam R. P., Venegas-Calerón M., Larson T. R., Graham I. A., Napier J. A., Sayanova O. (2009) The synthesis and accumulation of stearidonic acid in transgenic plants: a novel source of “heart-healthy” omega-3 fatty acids. Plant Biotechnol. J. 7, 704–716 [DOI] [PubMed] [Google Scholar]
- 52. Dahmen J. L., Olsen R., Fahy D., Wallis J. G., Browse J. (2013) Cytochrome b5 coexpression increases Tetrahymena thermophila Δ6 fatty acid desaturase activity in Saccharomyces cerevisiae. Eukaryot. Cell 12, 923–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xu J., Carlsson A. S., Francis T., Zhang M., Hoffman T., Giblin M. E., Taylor D. C. (2012) Triacylglycerol synthesis by PDAT1 in the absence of DGAT1 activity is dependent on re-acylation of LPC by LPCAT2. BMC Plant Biol. 12, 4–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bates P. D., Browse J. (2011) The pathway of triacylglycerol synthesis through phosphatidylcholine in Arabidopsis produces a bottleneck for the accumulation of unusual fatty acids in transgenic seeds. Plant J. 68, 387–399 [DOI] [PubMed] [Google Scholar]
- 55. Ruiz-López N., Sayanova O., Napier J. A., Haslam R. P. (2012) Metabolic engineering of the omega-3 long chain polyunsaturated fatty acid biosynthetic pathway into transgenic plants. J. Exp. Bot. 63, 2397–2410 [DOI] [PubMed] [Google Scholar]
- 56. Abbadi A., Domergue F., Bauer J., Napier J. A., Welti R., Zähringer U., Cirpus P., Heinz E. (2004) Biosynthesis of very-long-chain polyunsaturated fatty acids in transgenic oilseeds: constraints on their accumulation. Plant Cell 16, 2734–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Domergue F., Abbadi A., Ott C., Zank T. K., Zähringer U., Heinz E. (2003) Acyl carriers used as substrates by the desaturases and elongases involved in very long-chain polyunsaturated fatty acids biosynthesis reconstituted in yeast. J. Biol. Chem. 278, 35115–35126 [DOI] [PubMed] [Google Scholar]
- 58. Qi B., Fraser T., Mugford S., Dobson G., Sayanova O., Butler J., Napier J. A., Stobart A. K., Lazarus C. M. (2004) Production of very long chain polyunsaturated omega-3 and omega-6 fatty acids in plants. Nat. Biotechnol. 22, 739–745 [DOI] [PubMed] [Google Scholar]
- 59. Pereira S. L., Huang Y. S., Bobik E. G., Kinney A. J., Stecca K. L., Packer J. C., Mukerji P. (2004) A novel ω3-fatty acid desaturase involved in the biosynthesis of eicosapentaenoic acid. Biochem. J. 378, 665–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.