Background: ppGalNAc-T13 is up-regulated in high metastatic murine lung cancer cell lines.
Results: Trimeric Tn on Syndecan 1 forms a complex with integrin α5β1 and MMP-9, leading to cell adhesion and cancer metastasis.
Conclusion: Reduction of GM1 induces ppGalNAc-T13 expression, and its product trimeric Tn enhances metastasis via integrins.
Significance: A novel key molecule in cancer metastasis is identified.
Keywords: Glycolipids, Glycosylation, Integrins, Lung Cancer, Metastasis, O-glycans, Tn Antigen
Abstract
We demonstrated previously that ppGalNAc-T13 (T13), identified as an up-regulated gene with increased metastasis in a DNA microarray, generated trimeric Tn (tTn) antigen (GalNAcα1-Ser/Thr)3 on Syndecan 1 in highly metastatic sublines of Lewis lung cancer. However, it is not known how tTn antigen regulates cancer metastasis. Here, we analyzed the roles of tTn antigen in cancer properties. tTn antigen on Syndecan 1 increased cell adhesion to fibronectin in an integrin-dependent manner. Furthermore, cell adhesion to fibronectin induced phosphorylation of focal adhesion kinase and paxillin in T13-transfectant cells. In the search of Syndecan 1-interacting molecules, it was demonstrated that tTn antigen-carrying Syndecan 1 interacted with integrin α5β1 and matrix metalloproteinase 9 and that these molecules shifted to a glycolipid-enriched microdomain/rafts along with increased metastatic potential in T13-transfectant cells. We also identified a tTn substitution site on Syndecan 1, demonstrating that tTn on Syndecan 1 is essential for the interaction with integrin α5β1 as well as for the reaction with mAb MLS128. These data suggest that high expression of the ppGalNAc-T13 gene generates tTn antigen on Syndecan 1 under reduced expression of GM1, leading to enhanced invasion and metastasis via the formation of a molecular complex consisting of integrin α5β1, Syndecan 1, and MMP-9 in the glycolipid-enriched microdomain/rafts.
Introduction
Metastasis is a major cause of death in human cancers and contains multiple processes, from the detachment from the primary tumor sites to the formation of metastatic foci (1). Among the numerous factors involved in these processes, several types of carbohydrates on the cell surface, particularly O-glycans, play important roles in the attachment and invasion of cancer cells and in their survival in the blood stream (2). O-glycan chains in glycoproteins undergo qualitative and quantitative alteration and are often truncated and/or highly sialylated in cancer cells (2–4). Among them, expression levels of Tn antigen (GalNAcα1-Ser/Thr), which has been considered as a tumor-associated antigen, are increased in many types of human cancers (5). However, it is not well known how O-glycan structures are involved in the acquisition of metastatic potential.
In the analysis of mechanisms for cancer metastasis, we demonstrated previously that MMP-92 was recruited to the membrane microdomains named GEM/rafts by reduced levels of ganglioside GM1, resulting in its efficient secretion and activation and, eventually, in increased invasion and metastatic potentials (6). We also found that ppGalNAc-T13, which is a family member of ppGalNAc transferases, was up-regulated as a result of reduced GM1, leading to enhanced metastasis by formation of trimeric Tn (tTn) antigen (three consecutive GalNAc-substituted Ser/Thr) on Syndecan 1 (Sdc1) (7). However, the roles of trimeric Tn antigen on Sdc1 and mechanisms for tTn antigen in the enhancement of cancer metastasis remain to be investigated.
Here, we demonstrate novel functions of tTn antigen on Sdc1: enhancement of cell adhesion to fibronectin through complex formation with integrin α5β1, enhancement of invasion and metastatic activity by recruitment of MMP-9 to GEM/rafts, and increase of phosphorylation levels of FAK and paxillin. These results might provide a persuasive basis to explain the mechanistic roles of ppGalNAc-T13 in cancer metastasis. Furthermore, these results should propose not only potential biomarkers for prediction of metastasis but also a molecular target for antimetastatic therapy.
MATERIALS AND METHODS
Antibodies
Mouse anti-tTn antibody (MLS128) was generated by Numata et al. (8). Goat anti-ppGalNAc-T13 antibody (T-18), rabbit anti-Sdc1 antibody (H-174), rabbit anti-integrin α5 antibody (H-104) and anti-integrin β1 antibody (M-106), hamster anti-integrin β1 antibody (Hmβ1-1), rabbit anti-FAK antibody (C-20), rabbit anti-phospho-FAK antibodies (Tyr-576, Tyr-577, Tyr-861, and Tyr-925), anti-phospho-paxillin antibodies (Tyr-31 and Tyr-181), and normal Syrian hamster IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rat anti-Syndencan-1 antibody (281-2) and rabbit anti-phospho-FAK antibody (Tyr-397) were from BD Transduction Laboratories (San Jose, CA). Mouse anti-phospho-paxillin (Tyr-118) and anti-rabbit IgG antibody conjugated with HRP were from Cell Signaling Technology (Beverly, MA). Anti-mouse IgG antibody conjugated with HRP was purchased from Amersham Bioscience. Anti-rabbit IgG antibody conjugated with HRP was from Cell Signaling Technology. FITC-labeled anti-mouse IgG antibody was purchased from ICN/Cappel (Durham, NC). FITC-labeled streptavidin was from EY Laboratories, Inc. (San Mateo, CA). Anti-mouse IgG conjugated with HRP (Mouse TrueBlotTM ULTRA) was from Bay Bioscience (Kobe, Japan). Anti-rat IgG antibody conjugated with Alexa Fluor 405, anti-rabbit IgG antibody conjugated with Alexa Fluor 488, and anti-mouse IgG antibody conjugated with Alexa Fluor 564 were purchased from Invitrogen.
Cell Lines and Culture
Establishment of high metastatic sublines (C4-ly, lymph node; C4-sc, lung) from Lewis lung cancer cell lines (sublines) H7 and C4 was as described (6). These sublines were maintained in DMEM supplemented with 7.5% FBS at 37 °C in a humidified atmosphere containing 5% CO2. Establishment of stable transfectant cells of ppGalNAc-T13 cDNA (T13-TF) and control cells was as described (7). These clones were cultured in DMEM supplemented with 7.5% FBS and G418 (350 μg/ml). Establishment of stable knockdown cells of ppGalNAc-T13 gene (T13-KD) and control cells was as described (7). These clones were cultured in DMEM supplemented with 7.5% FBS and blasticidin (2 μg/ml). GM1 synthase RNAi transfectant (GM1-Si) cells and control cells were cultured in DMEM supplemented with 7.5% FBS and puromycin (6 μg/ml) (Calbiochem).
Preparation of siRNAs
StealthTM siRNAs against mouse Sdc1 were designed by Invitrogen. GL2, siRNA for a firefly luciferase (B-Bridge International, Inc., CA), was used as a negative control. They were transfected into a T13-TF line (TF-1) and a vector control line (Vc-1) or T13-KD line (KD-1) and a vector control line (C-1) with Lipofectamin 2000TM (Invitrogen) according to the protocol of the manufacturer. Selected target sequences for knockdown of Sdc1 are shown in supplemental Table S1.
Matrigel Invasion Assay
Procedures for invasion assay were as described (7).
Cell Adhesion Assays Using a Real-time Cell Electronic Sensing (RT-CES) System
ACEA e-platesTM (ACEA Biosciences, San Diego, CA) were coated with fibronectin (FN) or poly-L-lysine in PBS (5 μg/ml) for 1 h at 37 °C. The plates were washed with PBS and coated with 0.5% BSA in PBS for 1 h at 37 °C. The wells were washed with PBS before the addition of culture medium without serum, and cells (2.5 × 104) were added on ACEA e-plates coated with extracellular matrix proteins. The adhesion of cells was monitored continuously using the RT-CES system (Wako Pure Chemical, Osaka, Japan) for 24 h. To block the cell adhesion mediated by integrins, cells were pretreated with 200 μg/ml of GRGDSP peptide or its control peptide, GRADSP in serum-free medium for 30 min. To block the cell adhesion in which tTn antigen is involved, cells were pretreated with anti-tTn mAb (10 μg/ml) or control IgG in serum-free medium for 30 min.
Immunofluorescence
Cells were plated on precoated plates with FN after being treated and rotated under serum-free conditions. After incubation for 0, 5, 15, and 30 min, cells were fixed in paraformaldehyde (4% in PBS for 10 min) and treated with 0.1% Triton X-100 in PBS for 10 min at room temperature. Nonspecific binding was blocked with 2.5% BSA in PBS for 60 min at room temperature. Cells were stained with the first antibodies (rat anti-Sdc1 pAb, rabbit anti-integrin β1 pAb, or mouse anti-tTn mAb) in PBS containing 0.5% BSA for 60 min at room temperature and then with anti-rat IgG conjugated with Alexa Fluor 405, anti-rabbit IgG conjugated with Alexa Fluor 488, and anti-mouse IgG conjugated with Alexa Fluor 564 in PBS containing 0.5% BSA for 45 min at room temperature. The resulting staining patterns were imaged using a confocal microscope (Fluoview FV10i, Olympus, Tokyo, Japan). It was confirmed that there is no cross-reaction between the authentic antigens and non-relevant secondary reagents. DIC indicates images of differential interference contrast microscopy.
Cell Lysis and Western Immunoblotting
Cell lysis and Western immunoblotting were performed as described (7).
Phosphorylation Levels of FAK and Paxillin during Adhesion to FN
Cells were detached after culturing in serum-free medium for 16 h, and the cell suspension was added to precoated plates with FN and followed by harvesting at the indicated time points (0, 5, 15, or 30 min). Immunoblotting was performed as described (7).
Immunoprecipitation
Immunoprecipitation was performed as described (7).
Isolation of Raft Fraction
GEM/rafts were isolated using a detergent extraction method essentially as described by Mitsuda et al. (9). Cells (1.0 × 107) were plated in 15-cm culture dishes, cultured up to 90% confluency, and then three dishes of cells were used for each preparation. After washing twice with ice-cold PBS, the cells were lysed in 1 ml of MNE/Triton X-100 buffer (1% Triton X-100, 25 mm MES-NaOH (pH 6.5), 150 mm NaCl, 5 mm EGTA, 1 mm Na3VO4, 1 mm PMSF, 1 μg/ml aprotinin) and then Dounce-homogenized 15 times. Samples were placed on the bottom of Ultra-ClearTM centrifuge tubes (Beckman Instruments) and mixed with an equal volume of 80% (w/v) sucrose in MNE buffer without Triton X-100. Then, 2 ml of 30% sucrose (w/v) in MNE buffer without Triton X-100 was overlaid, and 1 ml of 5% (w/v) sucrose in MNE buffer without Triton X-100 was layered on top. The samples were centrifuged at 100,000 × g in an SW50.1 rotor for 16 h at 4 °C. The entire procedure was performed at 4 °C. From the top of the gradient, 0.5 ml of each fraction was collected to yield 10 fractions. GEM/rafts were isolated using a detergent extraction method essentially as described by Mitsuda et al. (9).
Metastasis Assay
The spontaneous metastasis assay was performed as described (6). In brief, cells (2 × 106/mouse) were inoculated subcutaneously into the thigh of age-matched female C57BL/6 mice (Nippon SLC, Hamamatsu, Japan). Mice were sacrificed 4 weeks after injection, and metastatic nodules on the surface of lungs were counted by naked eye. Microscopic examination of tissues was performed to confirm the identity of metastatic foci. All mouse experiments were performed following the guideline of the Nagoya University Committee on Animal Research. When these guidelines were constructed, the Principles of Laboratory Animal Care (National Institutes of Health Publication 86-23, revised 1985) were followed, as well as the guideline from the Ministry of Education, Culture, Sports, and Technology of Japan.
Immunohistochemical Staining
Immunohistochemical staining was performed using the standard streptavidin-biotin-peroxidase complex (SAB) method. Deparaffinized sections (4 μm thick) were treated with 0.3% hydrogen peroxide (H2O2) in absolute methanol for 20 min to block endogenous peroxidase activity. After blocking nonspecific antibody binding by incubation with Protein Block Serum-FreeTM (Dako, Carpinteria, CA) for 30 min at room temperature, the sections were incubated with mouse anti-tTn mAb (5 μg/ml) for 16 h at 4 °C. Biotinylated secondary antibody (Vector) was then applied for 45 min. The sections were incubated with peroxidase-conjugated streptavidin (Vector) for 30 min, and the reaction products were visualized using 3,3′-diaminobenzidine (Dako). Nuclear counterstaining was performed using hematoxylin. Negative control staining was performed without primary antibodies.
Construction and Transfection of Expression Vectors with Point Mutations
Mouse Sdc1 cDNA was cloned as described (7). Point mutants of the putative modification site of tTn antigen were generated by PCR for site-directed mutagenesis using a KOD-Plus mutagenesis kitTM (TOYOBO, Ohtsu, Japan). Mutant construction of the putative glycosylation site corresponding to nucleotides 238–246 (relative to the start codon) of Sdc1 was designed by changing ACCAGCAGC to gCCgcCgcC to obtain the mutation plasmid from pEE14/Sdc1. For transient transfection, 8 μg of pEE14/Sdc1 or its mutant plasmids were transfected into TF-1 clones (50–60% confluent) in 10-cm culture dishes using Lipofectamine 2000TM (Invitrogen) according to the instructions of the manufacturer. After 48 h, collection of secretory proteins was performed as described below.
Preparation of Secretory Proteins
Preparation of secretory proteins was performed as described (7), and the procedures are described in the supplemental Materials and Methods.
Purification of Secretory Proteins
A series of secretory proteins of Sdc1Fc was purified by using a protein A-Sepharose column. After binding to secretory proteins, elution buffer (0.1 m citric acid buffer (pH 3.5) and 150 mm NaCl) was added to the column, and then eluted proteins were mixed with neutralizing buffer (1 m Tris-HCl (pH 8.2) and 150 mm NaCl). The buffer containing purified secretory proteins was changed to Tris-based buffer (50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1 mm CaCl2, and 1 mm MgCl2).
Pull-down Assay
A series of purified secretory proteins were pulled down by using 30 μl of protein A-Sepharose at 4 °C. Then, lysates were incubated at 4 °C. For the antibody blocking assay, anti-tTn antibody was incubated with protein A-Sepharose conjugated with a series of secretory proteins prior to the addition to lysates.
Statistical Analysis
The statistical significance of data was determined by using Student's t test.
RESULTS
Knockdown of Sdc1 Resulted in the Suppression of Invasion Activity
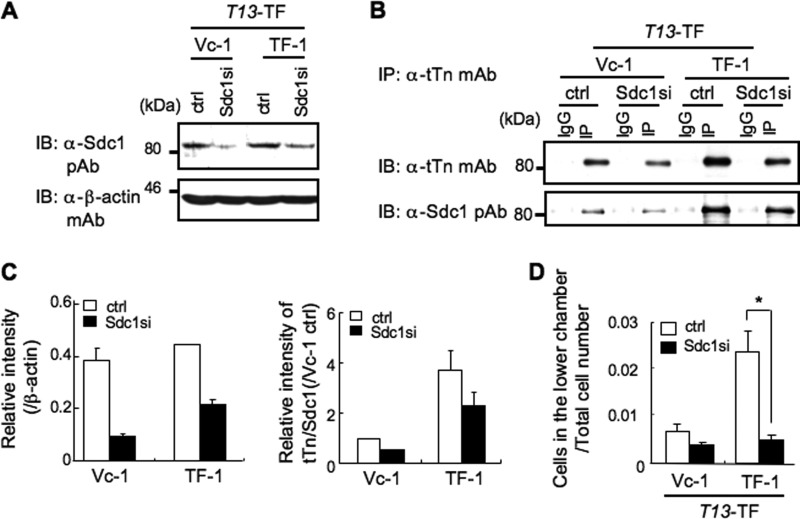
In a previous study, we revealed that the ppGalNAc-T13 gene was identified as a highly expressed gene in the high metastatic sublines (C4-sc) derived from a Lewis lung cancer clone (C4). Therefore, we established stable transfectant lines of ppGalNAc-T13 (T13-TF, TF-1, and TF-2) from C4 or stable silenced lines of ppGalNAc-T13 (T13-KD, KD-1, and KD-2) from C4-sc, resulting in the increased or reduced expression levels of tTn antigen on cell surface and invasion activity, respectively (supplemental Fig. S1A). It was also demonstrated that tTn antigen was formed on Sdc1 molecules in the transfectant cells (supplemental Fig. S1B). Then, we performed knockdown of Sdc1 with siRNA using T13-TF (Fig. 1, A and C, left panel). Immunoprecipitation/immunoblotting revealed that tTn antigen on Sdc1 in T13-TF clones decreased significantly compared with vector controls (Fig. 1, B and C, right panel). When treated with anti-Sdc1 siRNA, TF-1 showed much stronger suppression of invasion activity compared with Vc-1 (Fig. 1D). These data suggest that tTn antigen on Sdc1 is indeed involved in the invasion activity. Similar results were obtained in T13-KD clones.
FIGURE 1.
The tTn antigen on Sdc1 modulates invasion activity of Lewis lung cancer cells as shown by Sdc1 silencing. A, a knockdown line of Sdc1 with shRNA into T13-TF was established. Immunoblotting (IB) was performed with anti-Sdc1 pAb by using whole cell lysate in T13-TF. β-actin was used as a control (Ctrl). B, after immunoprecipitation with an anti-tTn mAb in siRNA-treated T13-TF clones (Vc-1 and TF-1), immunoblotting was performed with each antibody. IgG, normal mouse IgG; IP, immunoprecipitation with anti-tTn mAb. C, results of densitometry scans of the bands in A (left panel) and B (right panel). D, invasion assay in which 5 × 105 cells were seeded in the upper chamber in the absence of serum. After 24 h, invaded cell numbers were counted. The columns represent mean ± S.D. (n = 3). A representative of three independent experiments is shown. *, p < 0.01.
tTn Antigen-expressing Cells Showed Increased Cell Adhesion to FN in an Integrin-dependent Manner
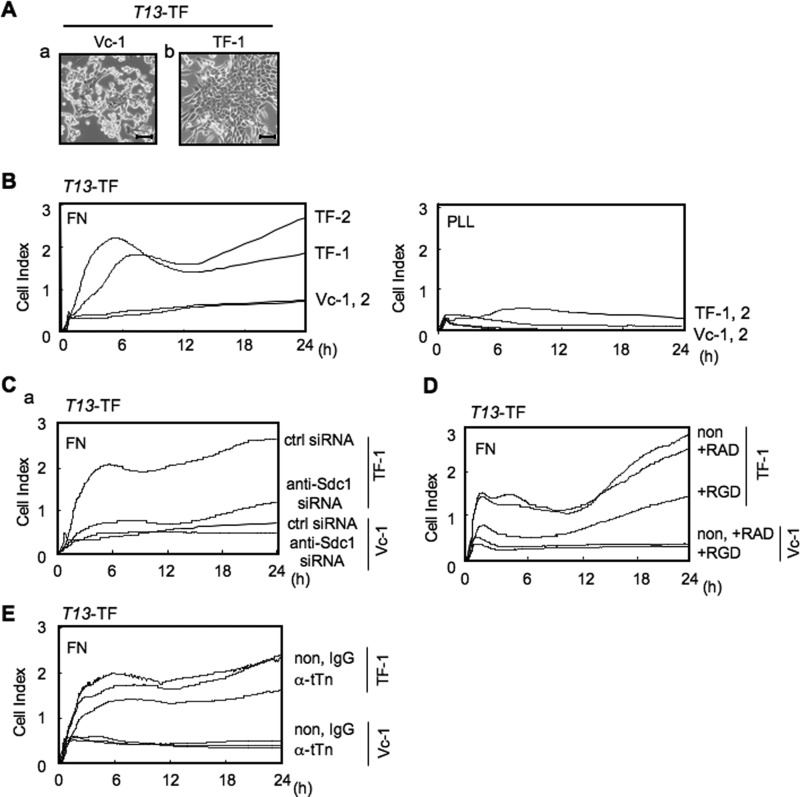
It has been reported recently that the Sdc1 molecule acts as a cell and matrix adhesion receptor (10, 11). Therefore, intensity of adhesion and spreading of T13-TF clones and their control cells was analyzed by the RT-CES system (Fig. 2A). ACEA e-plates were coated with laminin (LN), collagen type I (CL), FN, or poly-L-lysine (PLL) as a control (supplemental Fig. S2). Consequently, cell adhesion dramatically increased in T13-TF clones compared with controls for fibronectin-coated plates under FCS-free conditions (Fig. 2B). For poly-L-lysine, the adhesion intensities were very low and did not increase, even in the later phase, in either T13-TF or control cells. Moreover, knockdown of Sdc1 with siRNA in T13-TF clones showed much stronger suppression of adhesion activity compared with a control clone (Fig. 2C). Because cell adhesion to FN is well known to be integrin-dependent (11, 12), we performed an RT-CES analysis in the presence of RGD peptide to examine whether cell adhesion of T13-TF clones to FN occurred in an integrin-dependent manner. As expected, the RGD peptide efficiently suppressed more than 75% of the adhesion to FN in T13-TF (Fig. 2D). To investigate whether tTn antigen is involved in the cell adhesion to FN, cells were pretreated with anti-tTn antibody prior to the adhesion assay, resulting in the suppression of cell adhesion to FN in T13-TF (Fig. 2E). Similar results were obtained in T13-KD clones (supplemental Fig. S3).
FIGURE 2.
Expression of tTn antigen on Sdc1 increased cell adhesion to FN in an integrin-dependent manner. Cells were seeded in the wells of 96-well e-plates at 2.5 × 104 cells/well, and cell attachment and spreading were monitored by the RT-CES system. A, morphology of the T13-TF lines. B, the e-plates were precoated with FN or poly-L-lysine (PLL) as described under “Experimental Procedures.” C, knockdown with siRNA against Sdc1 in T13-TF clones was also analyzed by using FN-coated plates. Ctrl, control. D, cell adhesion to FN was analyzed to examine the effects of a blocking peptide. Cells were pretreated with GRGDSP (+RGD) peptide or its control peptide GRADSP (+RAD) in serum-free medium for 30 min before the adhesion assay. E, cell adhesion to FN was analyzed to examine its dependence on tTn by using anti-tTn mAb. Cells were pretreated with anti-tTn mAb (α-tTn) in serum-free medium for 30 min before the adhesion assay. A representative of three independent experiments is shown.
Costaining of Sdc1, Integrin β1, and tTn Antigen during Adhesion to FN
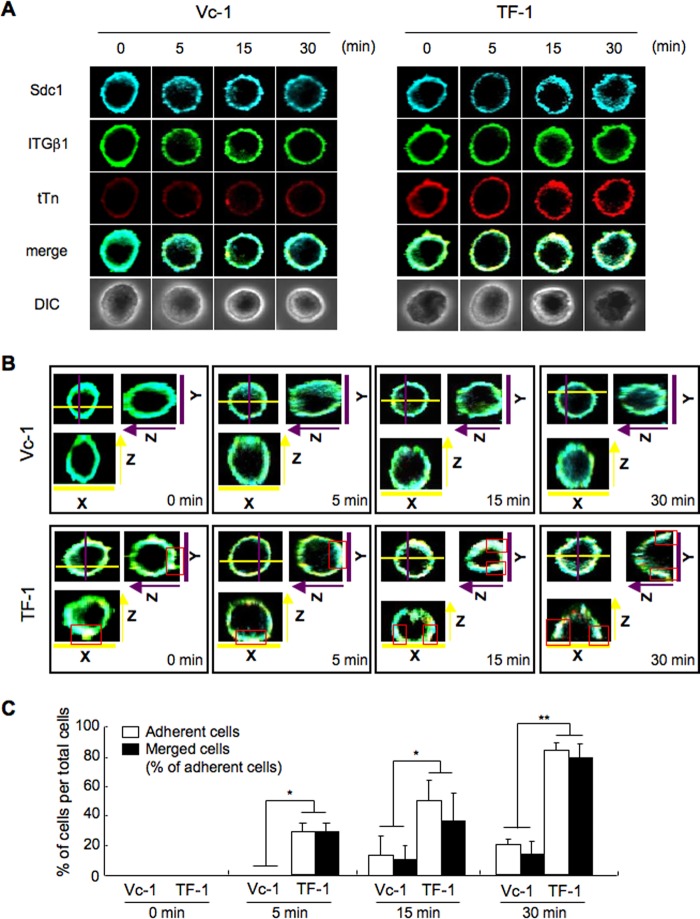
We analyzed the distribution patterns of Syndecan 1, integrin β1, and trimeric Tn antigen during the cell adhesion by immunocytochemical staining. Cells (Vc-1 and TF-1) were plated on precoated plates with FN and fixed after incubation for 0, 5, 15, and 30 min. The localization of Syndecan 1, integrin β1, and trimeric Tn antigen at various stages of adhesion is shown in Fig. 3A. Syndecan 1/integrin β1/trimeric Tn antigen were uniformly stained at the cell adhesion sites at 0 or 5 min in TF-1, and these three molecules were costained (white) at or around the adhesion sites at 15 or 30 min when cell adhesion to FN was completed in TF-1 (Fig. 3B). On the other hand, Syndecan 1 and integrin β1 partially costained in Vc-1 during slower adhesion to FN than TF-1. In addition, adherent cell numbers were counted in several areas and plotted in Fig. 3C, showing that most of the adherent cells were costained with these molecules in both of T13-TF clones (TF-1 and Vc-1). These results suggest the possibility that Syndecan 1/integrin β1/trimeric Tn antigen cooperatively exert their functions in the cell adhesion to FN, particularly in TF-1 at the early stage of adhesion events. Similar results were obtained in T13-KD clones (supplemental Fig. S4).
FIGURE 3.
Costaining of Sdc1, integrin β1, and trimeric Tn antigen during adhesion to FN. A, cells were plated on precoated plates with FN and rotated under serum-free conditions. After incubation for 0, 5, 15, and 30 min, cells were fixed in paraformaldehyde (4% in PBS for 10 min) and treated with 0.1% Triton X-100 in PBS for 10 min at room temperature. Then, cells were stained for Sdc1 (blue), integrin β1 (ITGβ1, green), and trimeric Tn antigen (tTn, red), and their images were observed using a confocal microscope. The resulting staining patterns were imaged using a confocal microscope (Fluoview FV10i, Olympus). It was confirmed that there is no cross-reaction between the individual antigens and non-relevant second reagents. DIC, images of differential interference contrast microscope. B, similar images as shown in A. Images of the y-z axis in Y (purple line). The right side in the image is the adhesion site. Images of the x-z axis in X (yellow line). The bottom in the image is the adhesion site. Red boxes indicate costaining sites. C, the numbers of adherent cells and merged cells in all cells detected in the images in B were counted, and a percentage was obtained. The columns represent mean ± S.D. (n = 20). *, p < 0.01; **, p < 0.001.
Trimeric Tn Antigen on Sdc1 Enhanced Tyrosine Phosphorylation in FAK and Paxillin during Cell Adhesion to FN
In a past study, an integrin β1 and Sdc1-mediated pathway via FN has been reported (11, 13, 14). Therefore, we further analyzed signaling molecules related to cell adhesion or spreading to clarify whether integrin- and Sdc1-mediated cell adhesion to FN promoted a downstream signaling pathway, including FAK and paxillin, on the basis of tTn antigen. Enhanced phosphorylation of FAK (Tyr-397, Tyr-576, Tyr-577, and Tyr-925) was exhibited in T13-TF clones during cell attachment to an FN-coated plate (Fig. 4A, upper panel). Phosphorylation levels of paxillin (Tyr-31, Tyr-118, and Tyr-181) were also enhanced in T13-TF clones compared with the controls (Fig. 4A, lower panel). When cell attachment to FN was blocked by an RGD peptide or anti-tTn mAb, phosphorylation levels of FAK and paxillin were reduced markedly. Band intensities were plotted in Fig. 4B. These results suggested that the increased invasion and metastatic activity were induced via the FAK and paxillin signaling pathway in the cell adhesion to FN in T13-TF clones.
FIGURE 4.
The tTn antigen on Sdc1 enhanced FAK and paxillin signals during cell adhesion to FN. A, T13-TF clones were detached with 0. 02% EDTA in PBS after culture in serum-free conditions at 37 °C for 16 h, and the cell suspension was added to precoated plates with FN at the indicated time points (0, 5, 15, or 30 min) as described under “Experimental Procedures.” Immunoblotting (IB) was performed with antibodies specifically reactive to the individual phosphorylation sites of FAK (Tyr-397, Tyr-576, Tyr-577, Tyr-861, and Tyr-925) or paxillin (Tyr-31, Tyr-118, and Tyr-181). B, the bands in A were scanned, and band intensities were plotted after correction by those of individual total proteins. A representative of one independent experiment is shown.
tTn Antigen on Sdc1 Is Involved in the Complex Formation Consisting of Sdc1, Integrin α5β1, and MMP-9
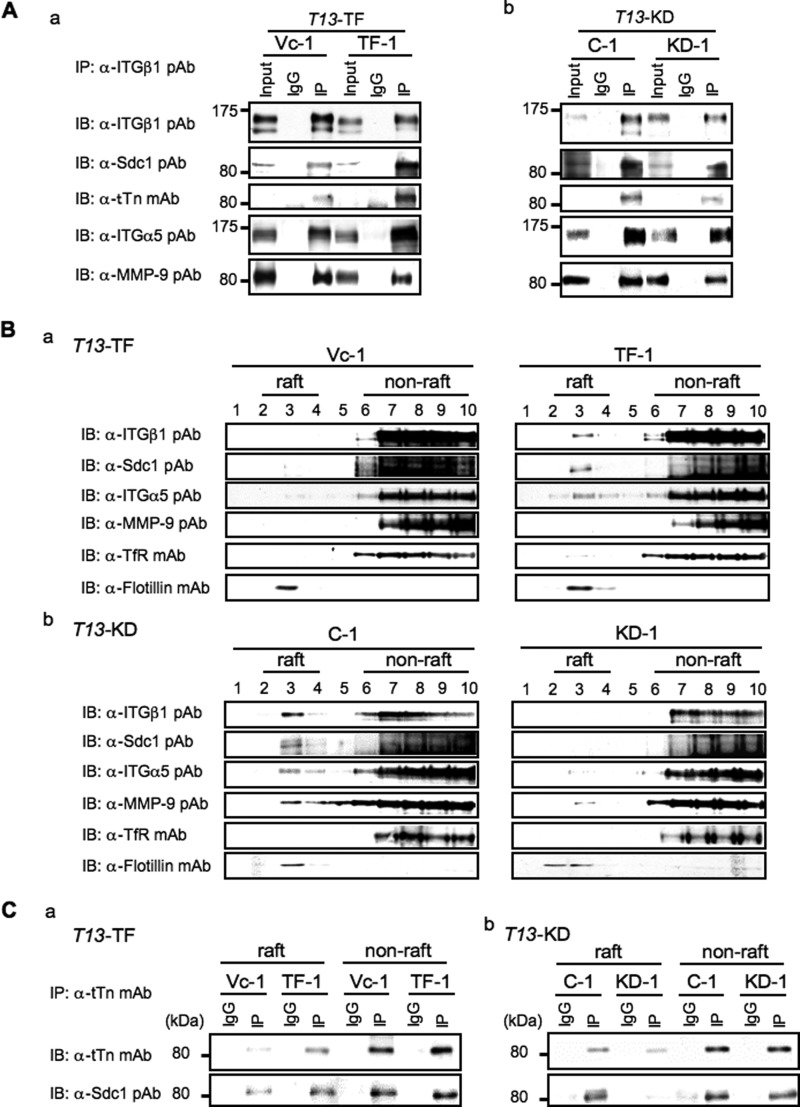
To clarify how tTn antigen on Sdc1 regulate the cell adhesion to FN that seemed to be mediated by integrins, immunoprecipitates with anti-integrin β1 pAb were subjected to immunoblotting using anti-Sdc1 pAb. Consequently, a strong Sdc1 band was detected, suggesting definite interaction between integrins and tTn antigen on Sdc1 in T13-TF clones (Fig. 5Aa). Furthermore, integrin α5 was also contained in this complex in T13-TF clones. T13-KD clones showed the complex consisting of integrin α5β1 and tTn antigen on Sdc1 with less intensity of bands (Fig. 5Ab). Other integrin α series were simultaneously examined for coprecipitation, but none of these were found in the immunoprecipitates, suggesting that the main partner of β1 is α5. In addition, MMP-9 was detected in the immunoblotting of the immunoprecipitates from both of these cells (Fig. 5A, bottom panel), suggesting possible complex formation of these molecules and binding of MMP-9 to integrins irrespective of tTn.
FIGURE 5.
The tTn antigen on Sdc1 is involved in complex formation with integrin α5β1 and shifts of the components of the complex to GEM/rafts in highly metastatic lines. A, cell lysates from T13-TF (a) and T13-KD (b) clones were immunoprecipitated with 2 μg of anti-integrin β1 (ITGβ1) pAb or normal hamster IgG at 4 °C. After SDS-PAGE of the immunoprecipitates, immunoblotting was performed as described under “Experimental Procedures.” Input, total cell lysates; IgG, normal hamster IgG; IP, immunoprecipitates with anti-ITGβ1 pAb. B, shifts of tTn antigen on Sdc1, integrins, and MMP-9 to the GEM/rafts in highly metastatic lines. Cells were lysed using Triton X-100, and the extracts were fractionated with discontinuous sucrose density gradient centrifugation. Fractions from T13-TF (a) and T13-KD (b) were subjected to immunoblotting using the antibodies against the proteins indicated. Transferrin receptor (TfR) was used as a non-raft marker, and flotillin was used as a raft marker. C, the raft fraction (2–4) and non-raft fraction (7–9) were combined and immunoprecipitated with 2 μg of anti-tTn mAb or normal mouse IgG at 4 °C. After SDS-PAGE of the immunoprecipitates, immunoblotting was performed with each Ab (upper panel, anti-tTn mAb; lower panel, anti-Sdc1 pAb). IgG, normal mouse IgG; IP, immunoprecipitates with anti-tTn mAb. A representative result of three independent experiments is shown.
Shift of tTn Antigen-Carrying Sdc1 to GEM/rafts in High Metastatic Lines
Because we found that integrin β1 and MMP-9 localized to GEM/rafts in highly metastatic lines in a previous study (6), we examined the intracellular localization of tTn antigen on Sdc1 and its interaction with integrin α5β1 by isolating the GEM/rafts fraction from the Triton X-100 extracts of cells. Ten fractions from the discontinuous sucrose density gradient were prepared and analyzed for distribution of these molecular complexes as well as a raft marker, flotillin 1, and a non-raft marker, transferrin receptor (Fig. 5B). Immunoblot analysis showed that most of flotillin 1 was found in fraction 3, indicating that it contained the GEM/rafts fraction. Sdc1 and integrin α5β1 also appeared in the raft fraction of TF-1 in T13-TF lines but not in controls (Fig. 5Ba). In contrast, all of these molecules disappeared in the raft fraction of T13-KD line, whereas they were detected in the GEM/rafts of the control (C-1) (Fig. 5Bb). Similar results were obtained in the analysis of the GM1-Si line and its control (Vc-1) (supplemental Fig. S5). To examine whether tTn antigen on Sdc1 localized in GEM/rafts, we performed immunoprecipitation/immunoblotting by using a raft fraction (2–4) and non-raft fraction (7–9) in the T13-TF and T13-KD lines (Fig. 5C). Interestingly, we found that more tTn-carrying Sdc1 was detected in GEM/rafts of T13 transfectants than in control cells. These data suggest that tTn antigen on Sdc1 interacts with integrin α5β1 and MMP-9 in GEM/rafts, leading to enhanced invasion and metastasis.
tTn Antigen on Sdc1 Plays an Essential Role in Cancer Metastasis
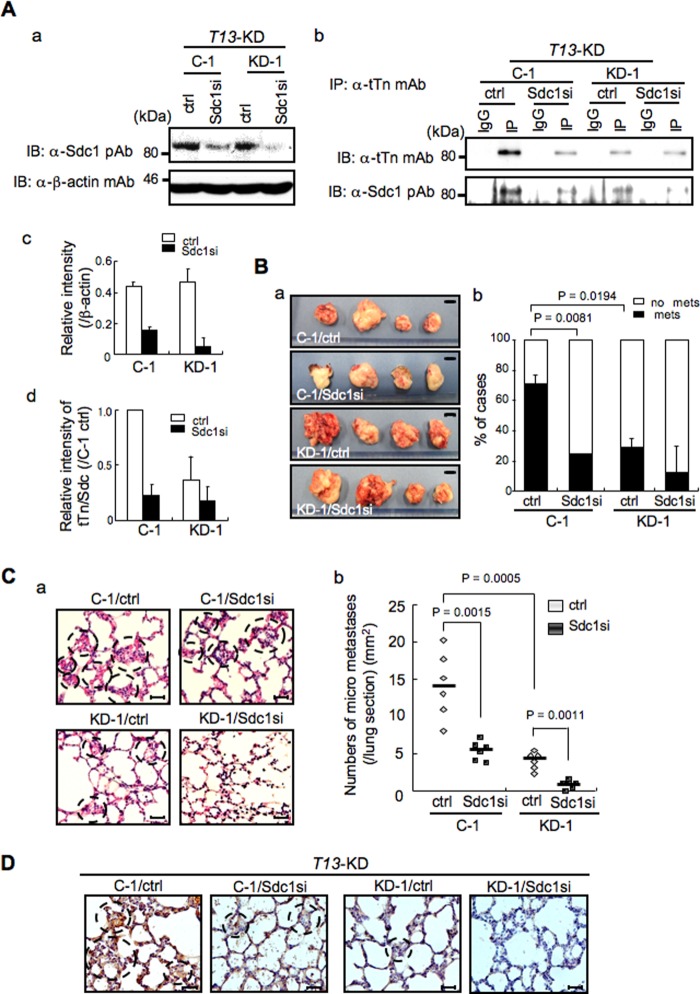
Previously, we performed preliminary metastasis experiments by generating T13-KD clones, showing significantly reduced lung metastasis in T13-KD clones in vivo (supplemental Fig. S6, A and B). In immunohistochemical staining, lung metastatic foci derived from control cells were strongly stained with anti-tTn antibody compared with T13-KD clones (supplemental Fig. S6C). These data suggest that tTn antigen-positive cells cause enhanced lung metastasis. To further clarify whether tTn antigen on Sdc1 is involved in enhanced lung metastasis, we analyzed metastasis experiments by knockdown of Sdc1 using the C4-sc control and T13-KD lines. Knockdown lines of Sdc1 with shRNA were established using T13-KD clones, and immunoblotting with anti-Sdc1 pAb revealed sufficient suppression of Sdc1 (Fig. 6A, a and c). Immunoblotting with anti-tTn mAb and anti-Sdc1 pAb using immunoprecipitates with anti-tTn mAb also showed a marked reduction on tTn/Sdc1 in C-1 (Fig. 6A, b and d). C-1 in T13-KD control clones treated with anti-Sdc1 siRNA (C-1/Sdc1si) showed a markedly decreased incidence of lung metastasis compared with KD-1/Sdc1si (Sdc1-silenced clone) in T13-KD clones (Fig. 6B, b), whereas tumor sizes at injected sites varied with no significant differences (a). In microscopy analysis, C-1/Sdc1si showed much decreased micrometastasis foci in lung sections compared with KD-1/Sdc1si in T13-KD clones (Fig. 6C, a and b). Furthermore, immunohistochemical staining revealed that C-1/Sdc1si showed decreased expression of tTn antigen in lung metastatic foci (Fig. 6D). These data indicate that tTn antigen on Sdc1 definitely regulates metastatic potential.
FIGURE 6.
The tTn antigen on Sdc1 plays an essential role in cancer metastasis. Metastasis experiments were performed by Sdc1-silenced T13-KD (C-1/Sdc1si and KD-1/Sdc1si) and their control lines (C-1/ctrl and KD-1/ctrl). A, knockdown lines of Sdc1 with shRNA in T13-KD were established. Immunoblotting (IB) was performed with anti-Sdc1 pAb using whole cell lysate of T13-KD lines. β-actin was used as a control (a). After immunoprecipitation with an anti-tTn mAb from lysates of Sdc1si and control (ctrl) lines of T13-KD clones, immunoblotting was performed with each antibody. IgG, normal mouse IgG; IP, immunoprecipitation with anti-tTn mAb. A representative of three independent experiments is shown. The results of densitometry scans of the bands in a and b are presented in c and d, respectively. B, examples of primary tumors with injection (subcutaneously) of Sdc1-silenced T13-KD lines (a) and a histogram depicting the percentage of mice that developed lung metastasis after injection of each cell line (b). Scale bar = 1 cm. Bars represent mean ± S.D. (C-1, KD-1 (control): n = 7; C-1, KD-1 (Sdc1si): n = 8). C, micrometastasis foci in lungs were observed using microscopy in Sdc1-silenced T13-KD lines (a). Whisker plots show the numbers of the metastatic foci (b). Scale bar = 30 μm. Bars represent the median value (n = 6). D, immunohistochemical staining by anti-tTn antibody was performed using lung sections in Sydecan 1-silenced T13-KD lines.
Identification of Modification Sites of tTn Antigen on Sdc1
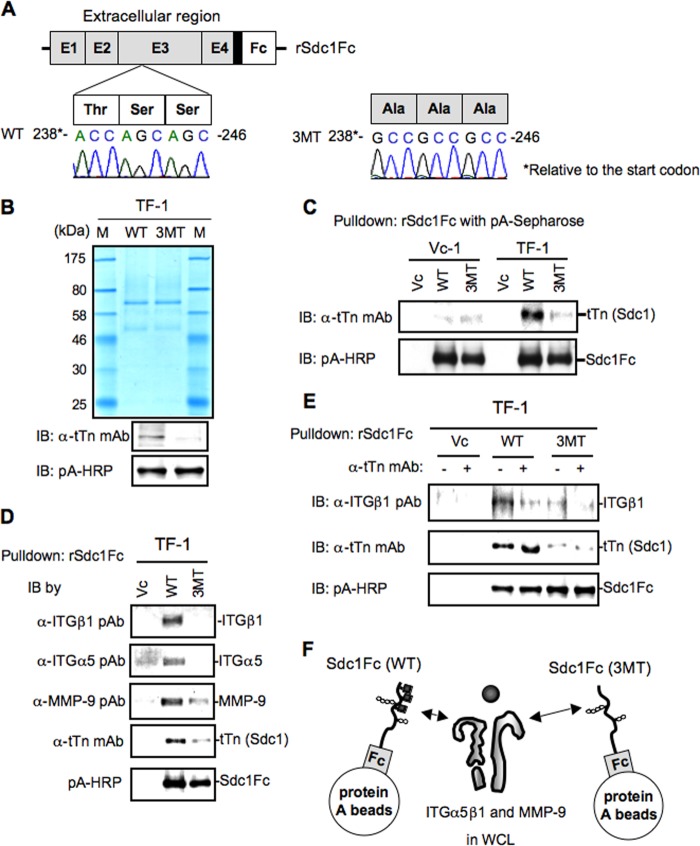
To examine modification sites of tTn antigen on Sdc1, we prepared expression vectors for point mutants at the putative modification sites. As three consecutive amino acids of serine and threonine, there was only one site (from the N terminus, amino acids 80–82) in Sdc1 in which point mutations were generated (Fig. 7A). Transient transfection of the mutant expression vectors into a T13-TF line resulted in the generation of almost single components in the culture supernatants in both WT and mutant (3MT) vectors (Fig. 7B). Using these recombinant proteins, immunoblotting was performed with anti-tTn antibody, showing a band only in the WT sample from TF-1 (Fig. 7C). These results indicate that the correct substitution site for tTn antigen on Sdc1 is at amino acids 80–82.
FIGURE 7.
Identification of the substitution site with tTn antigen on Sdc1. A, design of soluble Sdc1 expression vectors (3MT, mutant). The DNA sequence shows the point mutations in Sdc1 exon 4. Transversion 238-ACCAGCAGC-246 → 238-GCCGCCGCC-246 is predicted to convert residues from TSS to AAA. B, a series of supernatants in TF-1 after transfection was purified by using a protein A column. After SDS-PAGE of 1 μg of purified samples, Coomassie Brilliant Blue staining and immunoblotting (IB) were performed as described under “Experimental Procedures.” WT, wild type of Sdc1; 3MT, mutant of Sdc1. C, a series of secretory proteins was pulled down using protein A-Sepharose at 4 °C. After SDS-PAGE of the pull-down samples, immunoblotting was performed as described under “Experimental Procedures.” Vc, vector control. D, cell lysates of Vc-1 (T13 transfectant control) were mixed with rSdc1Fc-pA-Sepharose purified from TF-1 with WT or 3MT of the rSdc1Fc expression vector, and bound proteins were pulled down. The pull-down samples were applied for immunoblotting using the indicated antibodies. Immunoblotting was performed with individual antibodies. Note that only WT rSdc1Fc bound with integrins. E, a pull-down with rSdc1Fc-pA-Sepharose was performed as in A, except that anti-tTn mAb was added to rSdc1Fc-pA-Sepharose before the reaction with cell lysates. Note that the pull-down efficiency of integrins was reduced markedly by pretreatment with anti-tTn mAb. F, schematic illustrating that integrin α5β1 and MMP-9 in whole cell lysates (WCL) were pulled down via tTn with rSdc1Fc-pA-Sepharose.
To analyze whether tTn antigen on Sdc1 directly binds integrin β1, we performed a pull-down assay using recombinant Sdc1Fc with wild-type or mutated substitution sites (80–82) (WT or 3MT) with integrin α5β1 and MMP-9 in whole cell lysates. Interestingly, tTn antigen on Sdc1Fc (WT) brought about high levels of pull-down of integrin β1 as well as α5 compared with the mutant Sdc1Fc (3MT) (Fig. 7D), indicating ternary complex formation of these molecules, as suggested in Fig. 5A. The fact that pretreatment with anti-tTn antibody resulted in the reduced pull-down of Sdc-Fc (WT) (Fig. 7E) also suggests that tTn antigen of Sdc1 directly bound to integrin α5β1 (F).
tTn Antigen Generated by ppGalNAc-T13 on Sdc1 Leads to Cancer Metastasis
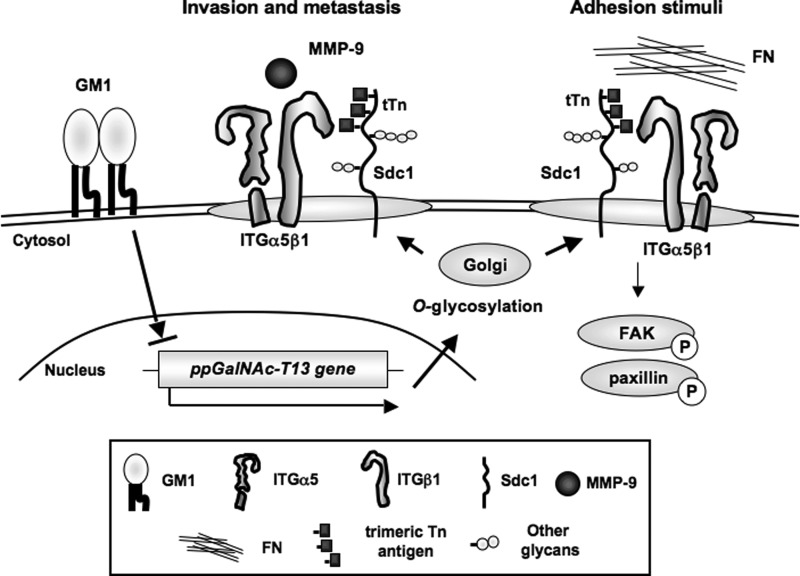
All results described above are summarized in Fig. 8. GM1-Si lines showed a high expression of the ppGalNAc-T13 gene, and highly metastatic cell lines, in turn, showed reduced GM1 expression on the cell surface (7). To examine whether low expression levels of GM1 resulted in the formation of tTn antigen on Sdc1 by ppGalNAc-T13, we performed immunoprecipitation/immunoblotting, showing that tTn antigen on Sdc1 was expressed on GM1-Si clones by the ppGalNAc- T13 gene (supplemental Fig. S5). The schematic illustrates that expression levels of GM1 decreased along with up-regulation of the ppGalNAc-T13 gene and resultant generation of tTn antigen on Sdc1, leading to high metastasis. tTn antigen on Sdc1 directly binds to integrin α5β1, enhances the phosphorylation levels of FAK and paxillin during cell adhesion to fibronectin, and recruits MMP-9 to GEM/rafts, leading to enhanced cell invasion and cancer metastasis.
FIGURE 8.
High expression of the ppGalNAc-T13 gene enhances cancer metastasis by formation of trimeric Tn antigen. The schematic illustrates that expression levels of GM1 decrease and that the ppGalNAc-T13 gene is up-regulated along with high metastasis, leading to the generation of trimeric Tn antigen on Sdc1 molecules. The trimeric Tn antigen on Sdc1 might enhance integrin α5β1 functions, resulting in increased adhesion to fibronectin via the FAK/paxillin pathway. Moreover, the trimeric Tn antigen on Sdc1 recruits MMP-9 to GEM/rafts, resulting in enhancement of cell invasion and metastatic potential. The structure of the trimeric Tn antigen on Sdc1 may provide a potential biomarker for the prediction of high metastasis and also a molecular target for the therapies of highly metastatic cancers.
DISCUSSION
There has been an increasing number of reports on the important roles of O-glycans in tumor progression and phenotypes in various types of cancers (15, 16). Although a few core proteins modified by the ppGalNAc-Ts family have been identified in many types of cancer so far (17–19), the mechanisms by which these glycosylations regulate functions of the carrier proteins and, thereby, cancer phenotypes, such as invasion and metastasis, have not been demonstrated clearly. In particular, the Tn antigen has been considered to be a representative cancer-associated O-type carbohydrate antigen. However, mechanisms for its expression and roles in cancer properties have not been well demonstrated. On the other hand, it has been reported that tTn enhanced tumor properties and that anti-tTn mAb (MLS128) treatment inhibited cell growth in colon carcinoma (20), whereas tTn antigen-carrying molecules have not yet been identified.
To date, 16 distinct ppGalNAc-T members have been identified in mammals (21, 22). The ppGalNAc-T13 gene was highly and mainly expressed in the brain and at very low or undetectable levels in other tissues. ppGalNAc-T13 was able to form a tTn epitope, three consecutive GalNAc-Ser/Thr structures (23). There have been no reports on the role of ppGalNAc-T13 in cancers except for high mRNA expression levels in bone marrow of stage 4 neuroblastoma patients (24). Therefore, our study is the first to identify ppGalNAc-T13 as a responsible molecule for cancer metastasis on the basis of solid experimental results.
In a previous report, we first identified Sdc1 as a tTn antigen-carrying molecule that undergoes modification by ppGalNAc-T13, leading to enhanced metastasis (7). In this study, we demonstrated that tTn antigen on Sdc1 binds to the integrin α5β1 complex, resulting in enhanced cell adhesion to fibronectin with increased phosphorylation of FAK and paxillin and increased invasion and motility by modulating integrin α5β1 and MMP-9 localization.
Integrins are a major family of cell surface receptors that mediate attachment to the extracellular matrix, and integrin-mediated adhesive interactions are intimately involved in the regulation of many cellular functions, such as tumor cell growth, metastasis, leukocyte homing, and activation (25, 26). Syndecans are classical coreceptors for growth factors, angiogenic factors, and chemokines, act as cell and matrix adhesion receptors (27), and influence cell spreading and motility (28, 29). In concert with integrins, Sdc1 regulates the activation of integrin αVβ3 and αVβ5 during cell adhesion to vitronectin on mammary carcinoma cells, fibroblasts, and endothelial cells (10, 30).
One of the most intriguing results in the function study of ppGalNAc-T13 is that T13-TF clones showed increased cell adhesion, specifically to fibronectin (Fig. 2B). Accordingly, the RGD peptide, a motif in the fibronectin for integrin binding, blocked cell adhesion to fibronectin, and anti-tTn mAb also blocked cell adhesion to fibronectin (Fig. 2, D and E). Furthermore, knockdown of Sdc1 strongly suppressed cell adhesion to fibronectin (Fig. 2C). These data indicate that trimeric Tn antigen on Sdc1 itself might regulate integrin functions, i.e. adhesion to fibronectin and cell migration. Immunoprecipitation/immunoblotting revealed interaction between integrins and Sdc1 and suggested that integrin α5β1 could strongly bind to trimeric Tn antigen on Sdc1 in T13-TF and C4-sc control (in T13-KD control) (Fig. 5A).
The most crucial issue here is how trimeric Tn modulate structures and functions of integrins and whether the binding of these molecules (or epitope) is direct or indirect. The results shown in Fig. 7 suggest that trimeric Tn binds directly to the integrin α5β1 complex or its associating molecules, if present.
The expression of trimeric Tn antigen caused enhancement of phosphorylation levels of FAK and paxillin during cell attachment to fibronectin (Fig. 4). Phosphorylation of FAK at Tyr-576 and Tyr-577 is required for maximal FAK catalytic activity for phosphorylation of paxillin at Tyr-31 and Tyr-181, which have been reported to be important in cell migration and focal adhesion turnover (31). Accordingly, the RGD peptide and anti-tTn antibody definitely suppressed site-specific phosphorylation levels of FAK and paxillin. These data strongly suggest that FAK/paxillin-mediated signaling transduced via integrin α5β1 at the early stage of adhesion to fibronectin causes increased invasion activity.
As for roles of Sdcs in tumor phenotypes, Sdc1 was reported to be a hybrid type proteoglycan containing heparan sulfate and chondroitin sulfate with the capability of growth factor binding (32). For cancer metastasis, Sdc-2 suppressed MMP-2 activation and metastasis of Lewis lung cancer (33). It was reported recently that two major types of proteoglycan, together with their receptor RAGE (receptor for advanced glycation end products) are involved in the metastasis of Lewis lung cancer cells (34). These results are very intriguing but hard to be correlated with our results reported here. The Sdc1 bands appeared as a single band at near 80 kDa and corresponded with those (85 kDa) treated with chondroitinase ABC and heparitinase 1 using Lewis lung cancer (33), suggesting that the cell lines used in this study did not contain proteoglycans. These findings suggest that the observations reported here are not a result of the lack of glycosaminoglycan chains.
There have been a number of studies that indicate that glycosphingolipids are located mainly at GEM/rafts and modulate cell signaling. Consequently, GEM/rafts are involved in the regulation of a wide variety of cell events and associated with various pathological situations and diseases (35, 36). In fibroblasts, overexpressed GM1 altered GEM organization, resulting in the dispersion of platelet-derived growth factor receptor to the non-GEM fraction (9). In melanomas, overexpression of GM1 synthase resulted in decreased cell proliferation and invasion activity because of the altered profiles of ganglioside species in GEM/rafts (37). In this study, the dramatic recruitment of integrin α5β1 and MMP-9 to GEM/rafts was induced on the basis of the formation of trimeric Tn antigen on Sdc1 by down-regulation of GM1 (Fig. 5). Accordingly, increased tTn antigen on Sdc1 in GM1 synthase-silenced clones induced changes in the localization of integrins and MMP-9 to GEM/rafts (supplemental Figs. S4 and S5). These data suggest that the absence of GM1 might be a trigger to bring about the increase of malignant properties in cancer cells by formation of tTn antigen on Sdc1 and formation of a molecular complex with integrin α5β1 and/or MMP-9 by being recruited to GEM/rafts.
In our previous paper, metastasis analysis showed that a poor coalescent to the facia and peritoneum was formed and that the number of lung metastasis foci was reduced significantly in T13-KD lines (7). In this study, the knockdown of Sdc1 in T13-KD control clones exhibited a significant suppression of lung metastasis compared with non-treated cells (Fig. 6). Furthermore, in lung sections, metastatic tumor regions were stained by anti-tTn antibody, suggesting that tTn antigen-positive cells have an advantage to form lung metastasis foci.
Given the broad spectrum of glycans that affect tumor progression, a range of glycan-specific targeting approaches might be considered for novel drug development, i.e. glycopeptides-based vaccination for targeting mucins (38, 39). To avoid crucial side effects, it would be desirable to identify extremely specific molecules to elicit invasive or metastatic properties in cancer cells. The tTn antigen on Sdc1 might be a very specific and promising tumor marker and also a candidate for molecular targets of cancer therapeutics to eradicate highly metastatic cells.
Supplementary Material
Acknowledgments
We thank T. Mizuno and Y. Nakayasu for technical assistance and M. Asai and K. Ushida for technical teaching of histochemical analyses.
This study was supported by Ministry of Education, Culture, Sports, and Technology of Japan Grants-in-Aid for Scientific Research 21390076, 24390078, and 23590371 and by Ministry of Education, Culture, Sports, and Technology of Japan Grant-in-Aid for Scientific Research on Innovative Areas 23110008.

This article contains supplemental Figs. S1–S6, Table S1, and Materials and Methods.
- MMP-9
- matrix metalloproteinase 9
- GEM
- glycolipid-enriched microdomain
- FAK
- focal adhesion kinase
- RT-CES
- real-time cell electronic sensing
- FN
- fibronectin
- pAb
- polyclonal antibody.
REFERENCES
- 1. Meng X., Zhong J., Liu S., Murray M., Gonzalez-Angulo A. M. (2012) A new hypothesis for the cancer mechanism. Cancer Metastasis Rev. 31, 247–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fuster M. M., Esko J. D. (2005) The sweet and sour of cancer. Glycans as novel therapeutic targets. Nat. Rev. Cancer 5, 526–542 [DOI] [PubMed] [Google Scholar]
- 3. Kannagi R. (2004) Molecular mechanism for cancer-associated induction of sialyl Lewis X and sialyl Lewis A expression. The Warburg effect revisited. Glycoconj. J. 20, 353–364 [DOI] [PubMed] [Google Scholar]
- 4. Li M., Song L., Qin X. (2010) Glycan changes. Cancer metastasis and anti-cancer vaccines. J. Biosci. 35, 665–673 [DOI] [PubMed] [Google Scholar]
- 5. Ju T., Otto V. I., Cummings R. D. (2011) The Tn antigen-structural simplicity and biological complexity. Angew. Chem. Int. Ed. Engl. 50, 1770–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Q., Furukawa K., Chen H. H., Sakakibara T., Urano T., Furukawa K. (2006) Metastatic potential of mouse Lewis lung cancer cells is regulated via ganglioside GM1 by modulating the matrix metalloprotease-9 localization in lipid rafts. J. Biol. Chem. 281, 18145–18155 [DOI] [PubMed] [Google Scholar]
- 7. Matsumoto Y., Zhang Q., Akita K., Nakada H., Hamamura K., Tokuda N., Tsuchida A., Matsubara T., Hori T., Okajima T., Furukawa K., Urano T., Furukawa K. (2012) pp-GalNAc-T13 induces high metastatic potential of murine Lewis lung cancer by generating trimeric Tn antigen. Biochem. Biophys. Res. Commun. 419, 7–13 [DOI] [PubMed] [Google Scholar]
- 8. Numata Y., Nakada H., Fukui S., Kitagawa H., Ozaki K., Inoue M., Kawasaki T., Funakoshi I., Yamashina I. (1990) A monoclonal antibody directed to Tn antigen. Biochem. Biophys. Res. Commun. 170, 981–985 [DOI] [PubMed] [Google Scholar]
- 9. Mitsuda T., Furukawa K., Fukumoto S., Miyazaki H., Urano T., Furukawa K. (2002) Overexpression of ganglioside GM1 results in the dispersion of platelet-derived growth factor receptor from glycolipid-enriched microdomains and in the suppression of cell growth signals. J. Biol. Chem. 277, 11239–11246 [DOI] [PubMed] [Google Scholar]
- 10. Beauvais D. M., Rapraeger A. C. (2010) Syndecan 1 couples the insulin-like growth factor-1 receptor to inside-out integrin activation. J. Cell Sci. 123, 3796–3807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morgan M. R., Humphries M. J., Bass M. D. (2007) Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 8, 957–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hynes R. O. (2002) Integrins. Bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 13. Mitra S. K., Hanson D. A., Schlaepfer D. D. (2005) Focal adhesion kinase. In command and control of cell motility. Nat. Rev. Mol. Cell Biol. 6, 56–68 [DOI] [PubMed] [Google Scholar]
- 14. Clark E. A., Brugge J. S. (1995) Integrins and signal transduction pathways. The road taken. Science 268, 233–239 [DOI] [PubMed] [Google Scholar]
- 15. Tarp M. A., Clausen H. (2008) Mucin-type O-glycosylation and its potential use in drug and vaccine development. Biochim. Biophys. Acta 1780, 546–563 [DOI] [PubMed] [Google Scholar]
- 16. Brockhausen I. (1999) Pathways of O-glycan biosynthesis in cancer cells. Biochim. Biophys. Acta 1473, 67–95 [DOI] [PubMed] [Google Scholar]
- 17. Wu Y. M., Liu C. H., Hu R. H., Huang M. J., Lee J. J., Chen C. H., Huang J., Lai H. S., Lee P. H., Hsu W. M., Huang H. C., Huang M. C. (2011) Mucin glycosylating enzyme GALNT2 regulates the malignant character of hepatocellular carcinoma by modifying the EGF receptor. Cancer Res. 71, 7270–7279 [DOI] [PubMed] [Google Scholar]
- 18. Taniuchi K., Cerny R. L., Tanouchi A., Kohno K., Kotani N., Honke K., Saibara T., Hollingsworth M. A. (2011) Overexpression of GalNAc-transferase GalNAc-T3 promotes pancreatic cancer cell growth. Oncogene 30, 4843–4854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park J. H., Nishidate T., Kijima K., Ohashi T., Takegawa K., Fujikane T., Hirata K., Nakamura Y., Katagiri T. (2010) Critical roles of mucin 1 glycosylation by transactivated polypeptide N-acetylgalactosaminyltransferase 6 in mammary carcinogenesis. Cancer Res. 70, 2759–2769 [DOI] [PubMed] [Google Scholar]
- 20. Morita N., Yajima Y., Asanuma H., Nakada H., Fujita-Yamaguchi Y. (2009) Inhibition of cancer cell growth by anti-Tn monoclonal antibody MLS128. Biosci. Trends 3, 32–37 [PubMed] [Google Scholar]
- 21. Tian E., Ten Hagen K. G. (2009) Recent insights into the biological roles of mucin-type O-glycosylation. Glycoconj. J. 26, 325–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bennett E. P., Mandel U., Clausen H., Gerken T. A., Fritz T. A., Tabak L. A. (2012) Control of mucin-type O-glycosylation. A classification of the polypeptide GalNAc-transferase gene family. Glycobiology 22, 736–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y., Iwasaki H., Wang H., Kudo T., Kalka T. B., Hennet T., Kubota T., Cheng L., Inaba N., Gotoh M., Togayachi A., Guo J., Hisatomi H., Nakajima K., Nishihara S., Nakamura M., Marth J. D., Narimatsu H. (2003) Cloning and characterization of a new human UDP-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase, designated pp-GalNAc-T13, that is specifically expressed in neurons and synthesizes GalNAc α-serine/threonine antigen. J. Biol. Chem. 278, 573–584 [DOI] [PubMed] [Google Scholar]
- 24. Berois N., Blanc E., Ripoche H., Mergui X., Trajtenberg F., Cantais S., Barrois M., Dessen P., Kågedal B., Bénard J., Osinaga E., Raguénez G. (2006) ppGalNAc-T13. A new molecular marker of bone marrow involvement in neuroblastoma. Clin. Chem. 52, 1701–1712 [DOI] [PubMed] [Google Scholar]
- 25. Giancotti F. G., Mainiero F. (1994) Integrin-mediated adhesion and signaling in tumorigenesis. Biochim. Biophys. Acta 1198, 47–64 [DOI] [PubMed] [Google Scholar]
- 26. Chen Q., Kinch M. S., Lin T. H., Burridge K., Juliano R. L. (1994) Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J. Biol. Chem. 269, 26602–26605 [PubMed] [Google Scholar]
- 27. Xian X., Gopal S., Couchman J. R. (2010) Syndecans as receptors and organizers of the extracellular matrix. Cell Tissue Res. 339, 31–46 [DOI] [PubMed] [Google Scholar]
- 28. McQuade K. J., Rapraeger A. C. (2003) Syndecan 1 transmembrane and extracellular domains have unique and distinct roles in cell spreading. J. Biol. Chem. 278, 46607–46615 [DOI] [PubMed] [Google Scholar]
- 29. Langford J. K., Yang Y., Kieber-Emmons T., Sanderson R. D. (2005) Identification of an invasion regulatory domain within the core protein of syndecan-1. J. Biol. Chem. 280, 3467–3473 [DOI] [PubMed] [Google Scholar]
- 30. Beauvais D. M., Ell B. J., McWhorter A. R., Rapraeger A. C. (2009) Syndecan 1 regulates αvβ3 and αvβ5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J. Exp. Med. 206, 691–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brown M. C., Turner C. E. (2004) Paxillin. Adapting to change. Physiol. Rev. 84, 1315–1339 [DOI] [PubMed] [Google Scholar]
- 32. Deepa S. S., Yamada S., Zako M., Goldberger O., Sugahara K. (2004) Chondroitin sulfate chains on syndecan-1 and syndecan-4 from normal murine mammary gland epithelial cells are structurally and functionally distinct and cooperate with heparan sulfate chains to bind growth factors. A novel function to control binding of midkine, pleiotrophin, and basic fibroblast growth factor. J. Biol. Chem. 279, 37368–37376 [DOI] [PubMed] [Google Scholar]
- 33. Munesue S., Yoshitomi Y., Kusano Y., Koyama Y., Nishiyama A., Nakanishi H., Miyazaki K., Ishimaru T., Miyaura S., Okayama M., Oguri K. (2007) A novel function of syndecan-2, suppression of matrix metalloproteinase-2 activation, which causes suppression of metastasis. J. Biol. Chem. 282, 28164–28174 [DOI] [PubMed] [Google Scholar]
- 34. Mizumoto S., Takahashi J., Sugahara K. (2012) Receptor for advanced glycation end products (RAGE) functions as receptor for specific sulfated glycosaminoglycans, and anti-RAGE antibody or sulfated glycosaminoglycans delivered in vivo inhibit pulmonary metastasis of tumor cells. J. Biol. Chem. 287, 18985–18994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hakomori S. I. (2010) Glycosynaptic microdomains controlling tumor cell phenotype through alteration of cell growth, adhesion, and motility. FEBS Lett. 584, 1901–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Inokuchi J. (2010) Membrane microdomains and insulin resistance. FEBS Lett. 584, 1864–1871 [DOI] [PubMed] [Google Scholar]
- 37. Dong Y., Ikeda K., Hamamura K., Zhang Q., Kondo Y., Matsumoto Y., Ohmi Y., Yamauchi Y., Furukawa K., Taguchi R., Furukawa K. (2010) GM1 / GD1b / GA1 synthase expression results in the reduced cancer phenotypes with modulation of composition and raft-localization of gangliosides in a melanoma cell line. Cancer Sci. 101, 2039–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Musselli C., Ragupathi G., Gilewski T., Panageas K. S., Spinat Y., Livingston P. O. (2002) Reevaluation of the cellular immune response in breast cancer patients vaccinated with MUC1. Int. J. Cancer 97, 660–667 [DOI] [PubMed] [Google Scholar]
- 39. Noujaim A. A., Schultes B. C., Baum R. P., Madiyalakan R. (2001) Induction of CA125-specific B and T cell responses in patients injected with MAb-B43.13. Evidence for antibody-mediated antigen-processing and presentation of CA125 in vivo. Cancer Biother. Radiopharm. 16, 187–203 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.