Abstract
Cytotoxic T lymphocyte (CTL) response is a critical component of the immune response to tumors, therefore optimal induction of CTL responses to tumor antigens is highly desired for developing efficient cancer immunotherapy. IL-27 is a member of the IL-12 family of cytokines that is comprised of an IL-12 p40-related protein subunit, EBV-induced gene 3 (EBI3), and a p35-related subunit, p28. IL-27 functions through IL-27R and has been shown to have potent anti-tumor activity via activation of a variety of immune components, including anti-tumor CD8+ T cell responses. However, the exact mechanisms of how IL-27 enhances anti-tumor CD8+ T cell responses are not fully understood. In this paper we mainly discuss the evidences that suggest novel mechanisms by which IL-27 enhances anti-tumor CTL responses, including IL-27 inhibition of activation-induced cell death; the phenotypes of IL-27-stimulated CTLs; IL-27-induced CTL IL-10/IL-21 production and IL-27-mediated suppression of regulatory T cell responses. These evidences suggest that IL-27 may have a great potential to be utilized in boosting anti-tumor CTL responses in human cancer patients.
Keywords: IL-27, anti-tumor cytotoxic T lymphocyte (CTLs), cancer, p28, p35, p53, IL-21
Introduction
Cytotoxic T lymphocyte (CTL) response is a critical component of the immune response to tumors, therefore optimal induction of CTL responses to tumor antigen is highly desired for developing efficient cancer immunotherapy. Previous studies have revealed that some cytokines, such as interleukin-12 (IL-12) and a variety of other cytokines [1-12], are potent stimulators of anti-tumor CTL responses. IL-12 is recognized as a master regulator of Th1/Tc1 responses [13,14]. However, in contrast to its significant anti-tumor and anti-metastatic activities documented in preclinical studies, clinical trials with IL-12 used as a single agent, or as a vaccine adjuvant, have shown limited efficacy in most cases [14,15]. It has recently been found that IL-12 up-regulates Tim-3 expression in T cells and induces T cell exhaustion, and thus IL-12-induced T cell responses could not be sustained to resulting in tumor rejection [16]. Thus, more effective application of IL-12, and of newly identified IL-12 family members such as IL-27, should be evaluated as therapeutic agents, as it may has more potential in the treatment cancer patients.
Since IL-27 was first reported having anti-tumor activity in 2004 [8,9], the potent antitumor activity of IL-27 has been verified in various tumor models, and many studies have shown CD8+ T cell-dependent tumor rejection [8-10,12,17]. The enhancing roles of endogenous IL-27 in the generation of anti-tumor CTL responses were also demonstrated using IL-27Rα-deficient mice [18-20]. Although these studies provide a strong case for the role of IL-27 in inducing anti-tumor CTL responses, it is not fully understood how IL-27 enhances anti-tumor CTL responses. In this paper we review the evidences that suggest novel mechanisms by which IL-27 enhances anti-tumor CTL responses.
IL-27 biology
IL-27 is a heterodimeric cytokine composed of two subunits: EBI3 and p28 [21]. Since it is structurally and functionally similar to IL-12, it was classified into the IL-12 cytokine family. Co-expression of EBI3 and p28 within the same cell is required for IL-27 to employ its biological functions [21]. Early studies demonstrated that activated antigen-presenting cells are the main source of both human and murine IL-27. The expression of human IL-27 has been detected in monocytes, dendritic cells, endothelial cells, and trophoblast cells [21,22], while the expression of murine IL-27 has been detected in activated macrophages and microglia cells [21,23]. IL-27 receptor (IL-27R) is also a heterodimeric molecule that consists of WSX-1 (IL-27Rα) and gp130. They have been shown to coexpress on a variety of immune cell types, including CD4+ T cells, CD8+ T cells, NK cells, monocytes, mast cells, neutrophils, and B cells [24,25]. Therefore, IL-27 has pleiotropic functions that depend on the cell type on which IL-27R is expressed. Although WSX-1 has high affinity to IL-27 in the absence of gp130 [21], it is insufficient to mediate IL-27 signal transduction [24]. In contrast, IL-27 does not bind to gp130 in the absence of WSX-1 [26]. Thus, coexpression of WSX-1 and gp130 in the same cell is pivotal for IL-27 to execute its biological activities.
The interaction of IL-27 with IL-27R has been shown to activate Janus Kinase (JAK) 1-2, Tyrosine kinase (TYK) 2, STAT 1-5 in naïve CD4+ T cells [27]. Co-immunoprecipitation studies and pull-down assays show that the intracellular domain of WSX-1 is constitutively associated with JAK1 and contributes to tyrosine phosphorylation of STAT1 [28], while gp130 constitutively interacts with JAK1/TYK2 and elicits a strong activation of STAT3 [24,29]. By studying STAT1-/- and STAT3-/- murine T cells, STAT3 was shown to be indispensable for IL-27-mediated proliferative effect on naïve CD4+ T cells, while STAT1 was found to be dispensable [29]. In contrast, Yoshimura et al. reported that IL-27 preferentially induces STAT3 in fully activated CD4+ T cells, while both STAT1 and STAT3 are activated in early activated T cells upon IL-27 stimulation [30]. Since IFN-α/β/γ utilize JAK1/2, TYK1/2, and STAT1 [31,32], IL-6 has the ability to activate JAK1/2, TYK2, STAT1/3 [33,34], IL-27 was considered to have similar biological actions to IFN-α/β/γ and IL-6. IL-27 is a pleiotropic cytokine capable of regulating Th1, Th2, Th17, and FoxP3+ Treg responses [35]. IL-27 was initially found to induce T-bet expression and promote Th1 responses [36]; later studies using IL-27Rα-/- mice and pathogen/autoimmune disease models have revealed that IL-27 actually inhibits Th1 responses [37,38]. However, the molecular mechanisms of IL-27-mediated Th1 suppression remain unclear. It is well established that IL-27 inhibits Th2 and Th17 responses via blocking the expression of transcription factors GATA-3 (Th2) and RoRγt (Th17) [27,39]. IL-27 also appears to inhibit CD4+ CD25+ Foxp3+ regulatory T cell (Treg) responses [40,41]. Another notable function of IL-27 is its ability in inducing IL-10 production in essentially all subtypes of T lymphocytes [42-46]. A variety of mechanisms, including activation of STAT1/STAT3 [42], induction of ICOS, c-Maf and IL-21 [47-49] have been proposed. IL-27 induces IL-21 production mainly by CD4+ T cells [47,50,51].
The multi-faced anti-tumor activities of IL-27
In the past decade, IL-27 has attracted considerable interest as a potent antitumor cytokine due to its similarities to IL-12. IL-12 has been proven to be effective in controlling tumor growth and metastases. The mechanisms responsible for the anti-tumor effects of IL-12 include: promoting IFN-γ production, anti-angiogenesis, triggering programmatic changes in suppressive cellular components within tumors, and enhancing lytic abilities of CTLs, NK cells and NKT cells to eradicate tumors [52,53]. However, IL-12 treatment of human cancer patients is associated with severe side effects, which may be due to its excessive pro-inflammatory effects. Since IL-27 was discovered in 2002, the potent anti-tumor effect of IL-27 has come into agreement in almost all literatures with multiple mechanisms involved [19,54]. However, the mechanisms by which IL-27 exerts its anti-tumor activity vary among different types of tumors. The potent antitumor activity of IL-27 has been verified in various immunogenic tumor models, and many studies have shown CD8+ T cell-dependent tumor rejection [8-10,12,17]. While in other tumor models, IL-27 also exerted its antitumor activity through various other mechanisms. For instance, in poorly immunogenic tumors such as B16F10 melanoma, IL-27 can enhance NK cell response [55], inhibit angiogenesis [56], and directly suppress tumor cell proliferation [57]. IL-27 can also suppress NK-resistant head and neck squamous cell carcinoma through inducing antibody-dependent cellular cytotoxicity [58]. In lung cancer model, over-expression of IL-27 or treatment with recombinant IL-27 can decrease expressions of vimentin, COX-2, and its metabolite (PGE2) in lung cancer cells [59], which results in the reduction of cancer cell migration and invasion. IL-27 is also capable of inhibiting human multiple myeloma cell growth as well as osteoclast activity [60]. Collectively, these studies suggest that IL-27 can exert its anti-tumor activity through direct inhibition cancer cell growth, proliferation and migration; inhibiting tumor angiogenesis, enhancing NK activity and more importantly, enhancing tumor-specific CTL responses.
The roles of IL-27 in CTL differentiation and survival
Although previous studies [19,54] provide a strong case for the role of IL-27 in inducing anti-tumor CTL responses, it is not fully understood how IL-27 enhances anti-tumor CTL responses. In the literature, IL-27 has been shown to be able to directly stimulate CD8+ T cells. IL-27 was shown to activate STAT1 and subsequently augmented the expression of two related T-box transcriptional factors: T-bet and Eomes [19,61], enhance proliferation and IFN-γ production by CD8+ T cells [19], and promote CD8+ T cells to express IL-12Rβ2 and granzyme B [61,62]. IL-27-induced IL-21 was shown to be pivotal for CD8+ T cells to produce granzyme B [63]. We recently [64] analyzed the direct effects of IL-27 on tumor antigen specific CD8+ T cells using the tumor P1CTL T cell receptor (TCR) transgenic model [65]. As outlined in Figure 1, we have found that IL-27 exerts three STAT1/STAT3 activation-mediated effects on tumor antigen specific CD8+ T cells, which provides new explanations for why IL-27 enhances anti-tumor CTL responses. First, IL-27 up-regulates anti-apoptotic molecules such as Bcl-2 and inhibits activation of Caspase 3 in activated CD8+ T cells, which leads to improved survival of activated CD8+ T cells. The survival enhancing effect of IL-27 on CD8+ T cells is consistent with a recent study showing that IL-27 can inhibit activation-induced cell death and enhance the survival of CD4+ T cells [66]. The strong anti-apoptosis effect of IL-27 could explain why, in tumor models, more CTLs accumulate in the IL-27-positive tumor microenvironment. Second, IL-27 induces a unique memory precursor cell (MPC)/effector phenotype in activated CD8+ T cells, characterized by up-regulation of SOCS3, Bcl-6, Sca-1, T-bet, Blimp1 and Perforin, and down-regulation of Eomes and IFN-γ. SOCS3 and Bcl-6 have recently been shown to be critical in establishing CD8+ T cell memory [67]. Sca-1 is a cell membrane molecule usually expressed on self-renewing stem cells and is a marker of central memory CD8+ T cells [68,69]. Up-regulation of T-bet and down-regulation of Eomes have been demonstrated in IL-12-stimulated CD8+ T cells previously [52], and are associated with CD8+ T cell differentiation into effector but not memory T cells [52,67]. The controversy of up-regulation of T-bet with down-regulation of IFN-γ in P1CTL can be explained by IL-27 induction of Bcl-6, which has been shown to inhibit T-bet induced IFN-γ production [70]. Unlike previous studies, we actually observed deceases of Granzyme B and IFN-γ by CD8+ T cells after IL-27 stimulation. Collectively, these results suggest that IL-27 programs tumor antigen specific CD8+ T cells into MPC-like effector cells. This phenotype can potentially increase CTL “stemness”, without affecting their effector functions such as cytotoxicity [64]. Consistent with the anti-tumor effects of IL-27, recent other studies [71,72] have revealed that T cells with concomitant memory and terminally differentiated phenotype are more efficient in rejecting tumors than T cells that are extreme in Th1/Tc1 differentiation. Third, IL-27 induces high amount of IL-10 production by CTL and our study suggests that IL-27-induced CTL IL-10 production contributes to MPC phenotype, T cell memory and tumor rejection [64].
Figure 1.
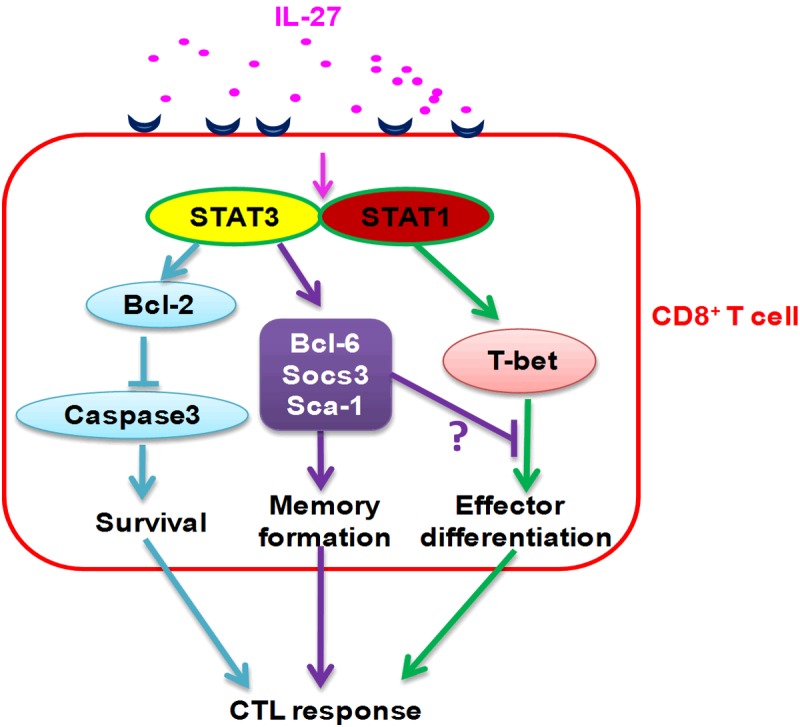
IL-27 directly stimulates CD8+ T cells and programs them into memory effector CTL cells.
The roles of IL-27/IL-21/IL-10 axis in anti-tumor CTL responses and CTL memory
A notable function of IL-27 is its ability in inducing IL-10 production in essentially all subtypes of T lymphocytes [42-46]. Although the role of IL-10 in tumor immunity is often controversial, increasing evidence suggests a positive role of IL-10 in the induction of anti-tumor CTL responses. For instance, in IL-10-deficient mice, anti-tumor CTL responses were weakened [3] and increased numbers of FoxP3+ Treg cells were found [73]; whereas in IL-10 transgenic mice, anti-tumor CTL responses were primed and shown to be responsible for tumor rejection [3,74]. In our recent study, we have shown that IL-27-induced CTL IL-10 production contributes to CTL-mediated tumor rejection [64]. This conclusion is supported by three lines of evidence. First, adoptive transfer of IL-27-stimulated P1CTL cells, which produce high amounts of IL-10, had better therapeutic efficacy than IL-27-stimulated IL-10-deficient P1CTL. Second, adoptive transfer of P1CTL or IL-10-/- P1CTL cells into Rag2-/- or Rag2-/- IL-10-/- mice with established J558-IL-27 tumors shows that P1CTL cells can reject tumors better than IL-10-/- P1CTL cells. Third, J558-IL-27 tumor cells, which failed to grow into tumors in BALB/c mice due to a potent anti-tumor CTL response, could grow into tumors in IL-10-/- BALB/c mice. IL-10 producing CD8+ T cells are usually considered as suppressor cells that down-regulate T cell responses [75]. However, some studies showed that IL-10 producing CTLs were more highly activated and cytolytic than IL-10-deficient CTLs [3,76]. In our recent study, we found that IL-27-stimulated IL-10-deficient CTLs expressed similar levels of IFN-γ, Granzyme B and perforin, and exhibited similar levels of cytotoxicity to target cells compared to their WT counterparts. However, IL-10-deficiency significantly reduced the expression of IL-27-induced survival molecules, such as SOCS3, Bcl-2, and Bcl-6 in CTLs. Thus, IL-27 induced CTL IL-10 production increases their survival potential, which can lead to stronger CTL responses. Our finding that IL-27 induced CTL IL-10 production contributes to their efficacy in tumor rejection suggests that IL-10 producing CTLs are better effectors rather than suppressor cells.
IL-27 also induces IL-21 production by CD4+ [47,50,51] and CD8+ T cells [63]. IL-21 is a member of the IL-2 family of cytokines. It is mainly produced by CD4+ T cells and acts on T cells, B cells, NK cells and dendritic cells [77,78]. IL-21 signals through IL-21R and activates Jak1 and Jak3, leading to activation of STAT1, 3 and 5; however, the activation of STAT3 appears to be the most important STAT protein for IL-21 signaling [79]. IL-21 induces Granzyme B expression in CD8+ T cells [63] and enhances anti-viral CTL responses [80-83] and CTL-mediated tumor rejection [4-7,84]. Recently, IL-21-induced STAT3 activation has been shown to play a critical role in the survival and memory responses of CD8+ T cells [67,85]. Given that IL-21 has similar effects of IL-10 in anti-tumor immunity, we expect that IL-27-induced CTL IL-21 production by T cells also contribute to the induction of anti-tumor CTL responses. However, this hypothesis remains to be tested. The roles of IL-27-induced IL-10 and IL-21 production by CD4+ T cells also remain to be determined.
IL-10 has also been shown to play a vital role in CD8+ T cell memory [86], presumably via induction of SOCS3 [67]. In our recent study we find that in the absence of IL-10, the capacity for IL-27 induction of MPC phenotype in CD8+ T cells is greatly diminished, which is mainly reflected by reduced expression of SOCS3, Bcl-2, and Bcl-6. Adoptive transfer experiments suggest that IL-10-deficient CD8+ T cells do not give rise to good memory response compared to IL-27-stimulated WT P1CTL cells. Thus, IL-27 induced CTL IL-10 production contributes to CTL memory response.
IL-27 suppresses treg responses in the tumor microenvironment
A few lines of evidence suggest that IL-27 has a profound impact on the homeostasis of Foxp3+ regulatory T cells. IL-27 has been shown to inhibit the conversion of inducible T regulatory cells (iTreg) by inhibiting the expression of Foxp3, CD25 and CTLA4 [40,41]. IL-27Rα-deficient (WSX-1-/-) [87] and IL-27 EBI3-/- mice [88] exhibit increased Treg conversion and expansion during autoimmune responses. IL-27 transgenic mice are deficient for Treg cells and develop systemic inflammation at 8-11 week of age [89]. However, recent evidences also suggest that IL-27 may be needed for Treg survival [66,90]. These features of IL-27 suggest that Treg responses, and subsequently CTL responses in the tumor microenvironment may be affected by IL-27. Indeed, studies using tumor models have revealed reduced numbers of Foxp3+ Treg cells and increased CTL responses in the tumor microenvironment of IL-27-positive tumors [12,20,59]. Our recent study (Liu et al, submitted) shows that the frequencies of Foxp3+ CD4+ Treg cells in tumors are significantly decreased in IL-27-overexpressed tumors, while Treg numbers increased in tumors from EBI3-/- mice. While these results suggest negative correlation of Treg response and IL-27, it remains unclear if IL-27 directly mediate Treg suppression in the tumor microenvironment or alternatively via suppression of IL-2 production [89]. Regardless of the mechanisms, it is likely that IL-27 can enhance anti-tumor CTL responses via inhibition of Treg responses.
Concluding remarks and future directions
Accumulating evidences suggest that IL-27 promotes anti-tumor CTL responses in general [19,54]. As demonstrated in Figure 1, IL-27 can enhance anti-tumor CTL responses via directly stimulating CD8+ T cells, inhibiting activation induced cell death and programming CTLs into memory effector cells with unique features [64]. Some of the features, such as enhancing CTL survival without reducing cytolytic activity, can clearly benefit anti-tumor CTL response; whereas other features, such as inhibition of CTL IFN-γ production, may affect CTL-mediate tumor rejection. IL-27-induced CTL IL-10 production actually benefits CTL survival and CTL-mediated tumor rejection. IL-27 is also likely to influence CTL responses via affecting Treg, Th17, Tr1 (producing IL-10 and IL-21) and myeloid cells [46,54] (Figure 2). Recently, IL-27 is shown to induce the expression of PD-L1 in CD4+ T cells [91], a major mediator of T cell exhaustion [92,93]. It remains unclear if IL-27 induces PD-L1 expression in CTL cells; if so, it may represents another negative factor for the generation of CTL responses. Selective induction positive features and blocking negative molecules such as PD-L1 will further enhance IL-27-mediated anti-tumor CTL response.
Figure 2.
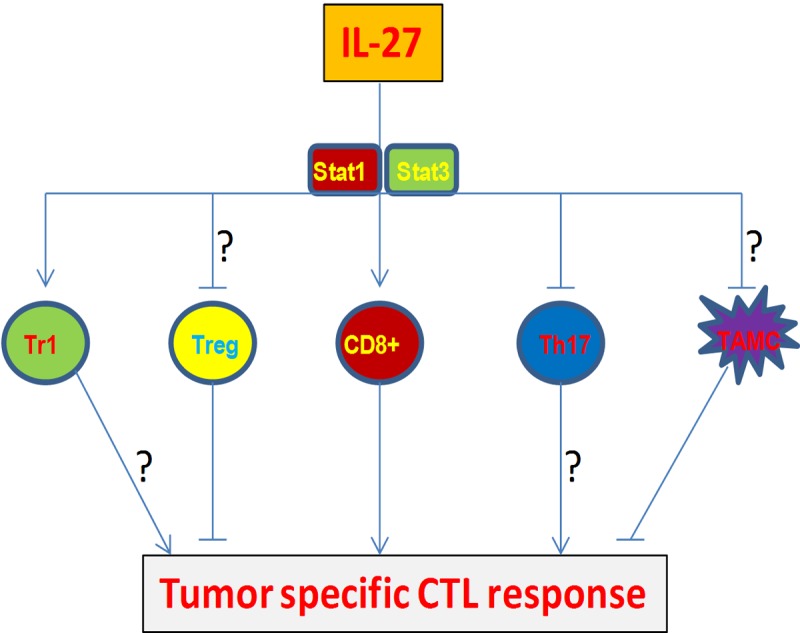
IL-27 affects tumor specific CTL responses via a variety of mechanisms. TAMC: tumor associated myeloid cells.
Currently, cytokine based cancer therapy are mainly used in the following ways, namely, systemic administration; local delivery; cytokine-secreting tumor cell vaccination; and the use of cytokines as adjuvant for cancer vaccine and adoptive cell therapy. A recent study [94] suggests that irradiated, IL-27-positive cancer cells induced protective T cell responses against subsequent tumor cell challenge. However, our recent experiments using irradiated, IL-27-secreting B16F10 cells failed to generate protective CTL responses (Liu et al, unpublished). This finding is supported by another report that using IL-12 and IL-27 combined gene therapy. It was found that potent CTL responses can only be induced by delivery of IL-12 first, followed by IL-27 delivery [17]. Thus, IL-27 does not induce, but amplify existing CTL responses. As such, IL-27 should be used with other vaccines to amplify CTL responses. It is also expected that IL-27 can enhance cancer vaccine induced CTL memory. Future research should be focused on identification of the most appropriate ways to use IL-27 as a therapeutic for the generation of anti-cancer CTL responses.
Acknowledgements
This study is supported in part by grants R01CA138427 (to XFB) and R01CA155521 (to JY) from National Cancer Institute.
Disclosure of conflict of interest
None.
References
- 1.Giovarelli M, Musiani P, Modesti A, Dellabona P, Casorati G, Allione A, Consalvo M, Cavallo F, di Pierro F, De Giovanni C, et al. Local release of IL-10 by transfected mouse mammary adenocarcinoma cells does not suppress but enhances antitumor reaction and elicits a strong cytotoxic lymphocyte and antibody-dependent immune memory. J Immunol. 1995;155:3112–3123. [PubMed] [Google Scholar]
- 2.Berman RM, Suzuki T, Tahara H, Robbins PD, Narula SK, Lotze MT. Systemic administration of cellular IL-10 induces an effective, specific, and long-lived immune response against established tumors in mice. J Immunol. 1996;157:231–238. [PubMed] [Google Scholar]
- 3.Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, Blaisdell S, Basham B, Dai J, Grein J, Sheppard C, Hong K, Cutler C, Turner S, Laface D, Kleinschek M, Judo M, Ayanoglu G, Langowski J, Gu D, Paporello B, Murphy E, Sriram V, Naravula S, Desai B, Medicherla S, Seghezzi W, McClanahan T, Cannon-Carlson S, Beebe AM, Oft M. IL-10 Elicits IFNgamma-Dependent Tumor Immune Surveillance. Cancer Cell. 2011;20:781–796. doi: 10.1016/j.ccr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Ma HL, Whitters MJ, Konz RF, Senices M, Young DA, Grusby MJ, Collins M, Dunussi-Joannopoulos K. IL-21 activates both innate and adaptive immunity to generate potent antitumor responses that require perforin but are independent of IFN-gamma. J Immunol. 2003;171:608–615. doi: 10.4049/jimmunol.171.2.608. [DOI] [PubMed] [Google Scholar]
- 5.Kishida T, Asada H, Itokawa Y, Cui FD, Shin-Ya M, Gojo S, Yasutomi K, Ueda Y, Yamagishi H, Imanishi J, Mazda O. Interleukin (IL)-21 and IL-15 genetic transfer synergistically augments therapeutic antitumor immunity and promotes regression of metastatic lymphoma. Mol Ther. 2003;8:552–558. doi: 10.1016/s1525-0016(03)00222-3. [DOI] [PubMed] [Google Scholar]
- 6.Di Carlo E, Comes A, Orengo AM, Rosso O, Meazza R, Musiani P, Colombo MP, Ferrini S. IL-21 induces tumor rejection by specific CTL and IFN-gamma-dependent CXC chemokines in syngeneic mice. J Immunol. 2004;172:1540–1547. doi: 10.4049/jimmunol.172.3.1540. [DOI] [PubMed] [Google Scholar]
- 7.Smyth MJ, Hayakawa Y, Cretney E, Zerafa N, Sivakumar P, Yagita H, Takeda K. IL-21 enhances tumor-specific CTL induction by anti-DR5 antibody therapy. J Immunol. 2006;176:6347–6355. doi: 10.4049/jimmunol.176.10.6347. [DOI] [PubMed] [Google Scholar]
- 8.Salcedo R, Stauffer JK, Lincoln E, Back TC, Hixon JA, Hahn C, Shafer-Weaver K, Malyguine A, Kastelein R, Wigginton JM. IL-27 mediates complete regression of orthotopic primary and metastatic murine neuroblastoma tumors: role for CD8+ T cells. J Immunol. 2004;173:7170–7182. doi: 10.4049/jimmunol.173.12.7170. [DOI] [PubMed] [Google Scholar]
- 9.Hisada M, Kamiya S, Fujita K, Belladonna ML, Aoki T, Koyanagi Y, Mizuguchi J, Yoshimoto T. Potent antitumor activity of interleukin-27. Cancer Res. 2004;64:1152–1156. doi: 10.1158/0008-5472.can-03-2084. [DOI] [PubMed] [Google Scholar]
- 10.Chiyo M, Shimozato O, Iizasa T, Fujisawa T, Tagawa M. Antitumor effects produced by transduction of dendritic cells-derived heterodimeric cytokine genes in murine colon carcinoma cells. Anticancer Res. 2004;24:3763–3767. [PubMed] [Google Scholar]
- 11.Chiyo M, Shimozato O, Yu L, Kawamura K, Iizasa T, Fujisawa T, Tagawa M. Expression of IL-27 in murine carcinoma cells produces antitumor effects and induces protective immunity in inoculated host animals. Int J Cancer. 2005;115:437–442. doi: 10.1002/ijc.20848. [DOI] [PubMed] [Google Scholar]
- 12.Salcedo R, Hixon JA, Stauffer JK, Jalah R, Brooks AD, Khan T, Dai RM, Scheetz L, Lincoln E, Back TC, Powell D, Hurwitz AA, Sayers TJ, Kastelein R, Pavlakis GN, Felber BK, Trinchieri G, Wigginton JM. Immunologic and therapeutic synergy of IL-27 and IL-2: enhancement of T cell sensitization, tumor-specific CTL reactivity and complete regression of disseminated neuroblastoma metastases in the liver and bone marrow. J Immunol. 2009;182:4328–4338. doi: 10.4049/jimmunol.0800471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 14.Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, Anichini A. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang ZZ, Grote DM, Ziesmer SC, Niki T, Hirashima M, Novak AJ, Witzig TE, Ansell SM. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J Clin Invest. 2012;122:1271–1282. doi: 10.1172/JCI59806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu S, Lee DA, Li S. IL-12 and IL-27 sequential gene therapy via intramuscular electroporation delivery for eliminating distal aggressive tumors. J Immunol. 2010;184:2348–2354. doi: 10.4049/jimmunol.0902371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinozaki Y, Wang S, Miyazaki Y, Miyazaki K, Yamada H, Yoshikai Y, Hara H, Yoshida H. Tumor-specific cytotoxic T cell generation and dendritic cell function are differentially regulated by interleukin 27 during development of anti-tumor immunity. Int J Cancer. 2009;124:1372–1378. doi: 10.1002/ijc.24107. [DOI] [PubMed] [Google Scholar]
- 19.Morishima N, Mizoguchi I, Okumura M, Chiba Y, Xu M, Shimizu M, Matsui M, Mizuguchi J, Yoshimoto T. A pivotal role for interleukin-27 in CD8+ T cell functions and generation of cytotoxic T lymphocytes. J Biomed Biotechnol. 2010;2010:605483. doi: 10.1155/2010/605483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Natividad KD, Junankar SR, Mohd Redzwan N, Nair R, Wirasinha RC, King C, Brink R, Swarbrick A, Batten M. Interleukin-27 Signaling Promotes Immunity against Endogenously Arising Murine Tumors. PLoS One. 2013;8:e57469. doi: 10.1371/journal.pone.0057469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 22.Larousserie F, Pflanz S, Coulomb-L’Hermine A, Brousse N, Kastelein R, Devergne O. Expression of IL-27 in human Th1-associated granulomatous diseases. J Pathol. 2004;202:164–171. doi: 10.1002/path.1508. [DOI] [PubMed] [Google Scholar]
- 23.Sonobe Y, Yawata I, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. Production of IL-27 and other IL-12 family cytokines by microglia and their subpopulations. Brain Res. 2005;1040:202–207. doi: 10.1016/j.brainres.2005.01.100. [DOI] [PubMed] [Google Scholar]
- 24.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 25.Batten M, Ghilardi N. The biology and therapeutic potential of interleukin 27. J Mol Med (Berl) 2007;85:661–672. doi: 10.1007/s00109-007-0164-7. [DOI] [PubMed] [Google Scholar]
- 26.Scheller J, Schuster B, Holscher C, Yoshimoto T, Rose-John S. No inhibition of IL-27 signaling by soluble gp130. Biochem Biophys Res Commun. 2005;326:724–728. doi: 10.1016/j.bbrc.2004.11.098. [DOI] [PubMed] [Google Scholar]
- 27.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 29.Kamiya S, Owaki T, Morishima N, Fukai F, Mizuguchi J, Yoshimoto T. An indispensable role for STAT1 in IL-27-induced T-bet expression but not proliferation of naive CD4+ T cells. J Immunol. 2004;173:3871–3877. doi: 10.4049/jimmunol.173.6.3871. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimura T, Takeda A, Hamano S, Miyazaki Y, Kinjyo I, Ishibashi T, Yoshimura A, Yoshida H. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J Immunol. 2006;177:5377–5385. doi: 10.4049/jimmunol.177.8.5377. [DOI] [PubMed] [Google Scholar]
- 31.Schindler C, Shuai K, Prezioso VR, Darnell JE Jr. Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992;257:809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- 32.Shuai K, Schindler C, Prezioso VR, Darnell JE Jr. Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992;258:1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- 33.Stahl N, Boulton TG, Farruggella T, Ip NY, Davis S, Witthuhn BA, Quelle FW, Silvennoinen O, Barbieri G, Pellegrini S, et al. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994;263:92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- 34.Heinrich PC, Horn F, Graeve L, Dittrich E, Kerr I, Muller-Newen G, Grotzinger J, Wollmer A. Interleukin-6 and related cytokines: effect on the acute phase reaction. Z Ernahrungswiss. 1998;37(Suppl 1):43–49. [PubMed] [Google Scholar]
- 35.Hall AO, Silver JS, Hunter CA. The immunobiology of IL-27. Adv Immunol. 2012;115:1–44. doi: 10.1016/B978-0-12-394299-9.00001-1. [DOI] [PubMed] [Google Scholar]
- 36.Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, Grewal IS, de Sauvage FJ. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 37.Hamano S, Himeno K, Miyazaki Y, Ishii K, Yamanaka A, Takeda A, Zhang M, Hisaeda H, Mak TW, Yoshimura A, Yoshida H. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19:657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 38.Holscher C, Holscher A, Ruckerl D, Yoshimoto T, Yoshida H, Mak T, Saris C, Ehlers S. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol. 2005;174:3534–3544. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 39.Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, Hunter CA, Cua DJ, Kastelein RA. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 40.Neufert C, Becker C, Wirtz S, Fantini MC, Weigmann B, Galle PR, Neurath MF. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37:1809–1816. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- 41.Huber M, Steinwald V, Guralnik A, Brustle A, Kleemann P, Rosenplanter C, Decker T, Lohoff M. IL-27 inhibits the development of regulatory T cells via STAT3. Int Immunol. 2008;20:223–234. doi: 10.1093/intimm/dxm139. [DOI] [PubMed] [Google Scholar]
- 42.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 43.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 44.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 45.Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol. 2008;180:2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 46.Hunter CA, Kastelein R. Interleukin-27: balancing protective and pathological immunity. Immunity. 2012;37:960–969. doi: 10.1016/j.immuni.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, Sharpe AH, Karp CL, Miaw SC, Ho IC, Kuchroo VK. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu J, Yang Y, Qiu G, Lal G, Wu Z, Levy DE, Ochando JC, Bromberg JS, Ding Y. c-Maf regulates IL-10 expression during Th17 polarization. J Immunol. 2009;182:6226–6236. doi: 10.4049/jimmunol.0900123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Batten M, Ramamoorthi N, Kljavin NM, Ma CS, Cox JH, Dengler HS, Danilenko DM, Caplazi P, Wong M, Fulcher DA, Cook MC, King C, Tangye SG, de Sauvage FJ, Ghilardi N. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. J Exp Med. 2010;207:2895–2906. doi: 10.1084/jem.20100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei L, Laurence A, Elias KM, O’Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 53.Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM, Trinchieri G, Rosenberg SA, Restifo NP. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011;121:4746–4757. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murugaiyan G, Saha B. IL-27 in tumor immunity and immunotherapy. Trends Mol Med. 2013;19:108–116. doi: 10.1016/j.molmed.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Oniki S, Nagai H, Horikawa T, Furukawa J, Belladonna ML, Yoshimoto T, Hara I, Nishigori C. Interleukin-23 and interleukin-27 exert quite different antitumor and vaccine effects on poorly immunogenic melanoma. Cancer Res. 2006;66:6395–6404. doi: 10.1158/0008-5472.CAN-05-4087. [DOI] [PubMed] [Google Scholar]
- 56.Shimizu M, Shimamura M, Owaki T, Asakawa M, Fujita K, Kudo M, Iwakura Y, Takeda Y, Luster AD, Mizuguchi J, Yoshimoto T. Antiangiogenic and antitumor activities of IL-27. J Immunol. 2006;176:7317–7324. doi: 10.4049/jimmunol.176.12.7317. [DOI] [PubMed] [Google Scholar]
- 57.Yoshimoto T, Morishima N, Mizoguchi I, Shimizu M, Nagai H, Oniki S, Oka M, Nishigori C, Mizuguchi J. Antiproliferative activity of IL-27 on melanoma. J Immunol. 2008;180:6527–6535. doi: 10.4049/jimmunol.180.10.6527. [DOI] [PubMed] [Google Scholar]
- 58.Matsui M, Kishida T, Nakano H, Yoshimoto K, Shin-Ya M, Shimada T, Nakai S, Imanishi J, Yoshimoto T, Hisa Y, Mazda O. Interleukin-27 activates natural killer cells and suppresses NK-resistant head and neck squamous cell carcinoma through inducing antibody-dependent cellular cytotoxicity. Cancer Res. 2009;69:2523–2530. doi: 10.1158/0008-5472.CAN-08-2793. [DOI] [PubMed] [Google Scholar]
- 59.Ho MY, Leu SJ, Sun GH, Tao MH, Tang SJ, Sun KH. IL-27 directly restrains lung tumorigenicity by suppressing cyclooxygenase-2-mediated activities. J Immunol. 2009;183:6217–6226. doi: 10.4049/jimmunol.0901272. [DOI] [PubMed] [Google Scholar]
- 60.Cocco C, Giuliani N, Di Carlo E, Ognio E, Storti P, Abeltino M, Sorrentino C, Ponzoni M, Ribatti D, Airoldi I. Interleukin-27 acts as multifunctional antitumor agent in multiple myeloma. Clin Cancer Res. 2010;16:4188–4197. doi: 10.1158/1078-0432.CCR-10-0173. [DOI] [PubMed] [Google Scholar]
- 61.Schneider R, Yaneva T, Beauseigle D, El-Khoury L, Arbour N. IL-27 increases the proliferation and effector functions of human naive CD8+ T lymphocytes and promotes their development into Tc1 cells. Eur J Immunol. 2011;41:47–59. doi: 10.1002/eji.201040804. [DOI] [PubMed] [Google Scholar]
- 62.Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J, Yoshimoto T. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J Immunol. 2005;175:1686–1693. doi: 10.4049/jimmunol.175.3.1686. [DOI] [PubMed] [Google Scholar]
- 63.Mittal A, Murugaiyan G, Beynon V, Hu D, Weiner HL. IL-27 induction of IL-21 from human CD8+ T cells induces granzyme B in an autocrine manner. Immunol Cell Biol. 2012;90:831–835. doi: 10.1038/icb.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Z, Liu JQ, Talebian F, Wu LC, Li S, Bai XF. IL-27 enhances the survival of tumor antigen-specific CD8(+) T cells and programs them into IL-10-producing, memory precursor-like effector cells. Eur J Immunol. 2013;43:468–479. doi: 10.1002/eji.201242930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu JQ, Bai XF. Overcoming immune evasion in T cell therapy of cancer: lessons from animal models. Curr Mol Med. 2008;8:68–75. doi: 10.2174/156652408783565531. [DOI] [PubMed] [Google Scholar]
- 66.Kim G, Shinnakasu R, Saris CJ, Cheroutre H, Kronenberg M. A novel role for IL-27 in mediating the survival of activated mouse CD4 T lymphocytes. J Immunol. 2013;190:1510–1518. doi: 10.4049/jimmunol.1201017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity. 2011;35:792–805. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med. 2005;11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 69.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, Paulos CM, Muranski P, Restifo NP. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oestreich KJ, Huang AC, Weinmann AS. The lineage-defining factors T-bet and Bcl-6 collaborate to regulate Th1 gene expression patterns. J Exp Med. 2011;208:1001–1013. doi: 10.1084/jem.20102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirschhorn-Cymerman D, Budhu S, Kitano S, Liu C, Zhao F, Zhong H, Lesokhin AM, Avogadri-Connors F, Yuan J, Li Y, Houghton AN, Merghoub T, Wolchok JD. Induction of tumoricidal function in CD4+ T cells is associated with concomitant memory and terminally differentiated phenotype. J Exp Med. 2012;209:2113–2126. doi: 10.1084/jem.20120532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Curran MA, Geiger TL, Montalvo W, Kim M, Reiner SL, Al-Shamkhani A, Sun JC, Allison JP. Systemic 4-1BB activation induces a novel T cell phenotype driven by high expression of Eomesodermin. J Exp Med. 2013;210:743–755. doi: 10.1084/jem.20121190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tanikawa T, Wilke CM, Kryczek I, Chen GY, Kao J, Nunez G, Zou W. Interleukin-10 ablation promotes tumor development, growth, and metastasis. Cancer Res. 2012;72:420–429. doi: 10.1158/0008-5472.CAN-10-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Groux H, Cottrez F, Rouleau M, Mauze S, Antonenko S, Hurst S, McNeil T, Bigler M, Roncarolo MG, Coffman RL. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J Immunol. 1999;162:1723–1729. [PubMed] [Google Scholar]
- 75.Noble A, Giorgini A, Leggat JA. Cytokine-induced IL-10-secreting CD8 T cells represent a phenotypically distinct suppressor T-cell lineage. Blood. 2006;107:4475–4483. doi: 10.1182/blood-2005-10-3994. [DOI] [PubMed] [Google Scholar]
- 76.Trandem K, Zhao J, Fleming E, Perlman S. Highly activated cytotoxic CD8 T cells express protective IL-10 at the peak of coronavirus-induced encephalitis. J Immunol. 2011;186:3642–3652. doi: 10.4049/jimmunol.1003292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5:688–698. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- 78.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 79.Zeng R, Spolski R, Casas E, Zhu W, Levy DE, Leonard WJ. The molecular basis of IL-21-mediated proliferation. Blood. 2007;109:4135–4142. doi: 10.1182/blood-2006-10-054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yi JS, Ingram JT, Zajac AJ. IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection. J Immunol. 2010;185:4835–4845. doi: 10.4049/jimmunol.1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 84.Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siegel AM, Heimall J, Freeman AF, Hsu AP, Brittain E, Brenchley JM, Douek DC, Fahle GH, Cohen JI, Holland SM, Milner JD. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;35:806–818. doi: 10.1016/j.immuni.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Foulds KE, Rotte MJ, Seder RA. IL-10 is required for optimal CD8 T cell memory following Listeria monocytogenes infection. J Immunol. 2006;177:2565–2574. doi: 10.4049/jimmunol.177.4.2565. [DOI] [PubMed] [Google Scholar]
- 87.Cox JH, Kljavin NM, Ramamoorthi N, Diehl L, Batten M, Ghilardi N. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J Exp Med. 2011;208:115–123. doi: 10.1084/jem.20100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu JQ, Liu Z, Zhang X, Shi Y, Talebian F, Carl JW Jr, Yu C, Shi FD, Whitacre CC, Trgovcich J, Bai XF. Increased Th17 and regulatory T cell responses in EBV-induced gene 3-deficient mice lead to marginally enhanced development of autoimmune encephalomyelitis. J Immunol. 2012;188:3099–3106. doi: 10.4049/jimmunol.1100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wojno ED, Hosken N, Stumhofer JS, O’Hara AC, Mauldin E, Fang Q, Turka LA, Levin SD, Hunter CA. A role for IL-27 in limiting T regulatory cell populations. J Immunol. 2011;187:266–273. doi: 10.4049/jimmunol.1004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hall AO, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, Pritchard GH, Silver JS, Bouladoux N, Stumhofer JS, Harris TH, Grainger J, Wojno ED, Wagage S, Roos DS, Scott P, Turka LA, Cherry S, Reiner SL, Cua D, Belkaid Y, Elloso MM, Hunter CA. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hirahara K, Ghoreschi K, Yang XP, Takahashi H, Laurence A, Vahedi G, Sciume G, Hall AO, Dupont CD, Francisco LM, Chen Q, Tanaka M, Kanno Y, Sun HW, Sharpe AH, Hunter CA, O’Shea JJ. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity. 2012;36:1017–1030. doi: 10.1016/j.immuni.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 93.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1 (PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang J, Tian H, Li C, Cheng L, Zhang S, Zhang X, Wang R, Xu F, Dai L, Shi G, Chen X, Li Y, Du T, Deng J, Liu Y, Yang Y, Wei Y, Deng H. Antitumor effects obtained by autologous Lewis lung cancer cell vaccine engineered to secrete mouse Interleukin 27 by means of cationic liposome. Mol Immunol. 2013;55:264–74. doi: 10.1016/j.molimm.2013.02.006. [DOI] [PubMed] [Google Scholar]


