Abstract
Cigarette smoking (CS) is the primary cause of preventable morbidity and mortality. Abundant clinical evidence suggests that CS is more harmful to women; however, the mechanisms responsible for these differences are not yet known. CS alters endothelial function, the redox state, inflammation, and global DNA methylation, which is associated with one-carbon metabolism and the transsulfuration pathway. However, it is not known whether the previously identified alterations are sex-gender related. Healthy adult men and oral contraceptive-free women with regular menstrual cycles were enrolled; women were examined during the follicular phase. Men had higher plasma levels of uric acid, total bilirubin, homocysteine, glutamylcysteine, total glutathione, cysteinylglycine; had more monocytes and released more TNF-alpha from human monocytes derived macrophages (hMDMs), but they had fewer platelets and lower levels of DNA methylation, and their hMDMs released less TNF-alpha after LPS stimulation. MDA, taurine, cysteine, arginine, ADMA, and SDMA were not different. CS decreased global DNA methylation more in women and increased the platelet, monocyte, and lymphocyte counts and the homocysteine, arginine, and ADMA levels only in women, whereas increased the neutrophil and eosinophil counts only in men. Additionally, CS reduced the sex-gender differences in total bilirubin, basal and LPS-induced TNF-alpha release, total glutathione, and glutamylcysteine, leaving unchanged cysteinylglycine, taurine, SDMA, MDA, and cysteine. These data suggest that cardiovascular risk factors seem to come earlier in young healthy female smokers than in young healthy male smokers, supporting the greater alarmism regarding the effects of CS in women and providing a basis for understanding the sex-gender differences. These results also suggest that cessation programs targeting women are needed.
Keywords: Cigarette smoking, sex-gender differences, ADMA, transsulfuration pathway, global DNA methylation
Introduction
The effects of cigarette smoking (CS) on health are well known, with CS being the main cause of preventable morbidity and mortality [1]. In the US population, CS-related death is the leading cause of preventable death in women [2]. CS greatly increases a woman’s relatively risk of myocardial infarction [3] and cardiovascular outcomes [2], especially in women who use oral contraceptives (OC) [4]. In addition, it has been calculated that the risk of myocardial infarction due to CS is greater in women than in men [5]. Despite the substantial declines in the prevalence of smoking, at least among adults in the Western world, the prevalence of smoking is increasing among girls [6], and smoking cessation is more frequent among men than women [7].
The underlying mechanisms by which CS might be more harmful to smoker women are still unclear. CS can affect many functions that can have prognostic implications for subsequent cardiovascular diseases (CVDs), such as the production of reactive oxygen species [8], the induction of inflammatory processes [9], and alterations in endothelial function [10] and global DNA methylation [11,12], which is associated with the one-carbon metabolism pathway. Homocysteine plays a central role in this pathway, in which it is converted into methionine or cleared by the transsulfuration pathway [13]. However, it is scanty known whether the previously identified mechanisms are influenced by sex-gender.
The term “sex-gender” reflects both physiological distinctions between each sex, dictated by genetics and biology, and environmental and social influences (e.g., diet, lifestyle, social status, and cultural influences) [14].
Therefore, this study aimed to investigate the previously identified mechanisms in young healthy adult smokers and non-smokers. We examined the endothelial function by evaluating an endogenous inhibitor of NO synthesis, asymmetric dimethylarginine (ADMA) [10]; the precursor of NO synthesis L-arginine; and symmetric dimethyl arginine (SDMA), which seems to favour inflammation [15]. We also measured the total glutathione, glutamylcysteine, cysteinylglycine, and cysteine levels together with the level of homocysteine, which has a role in the transsulfuration pathway [13].
The levels of lipid peroxidation, which is an oxidative stress indicator; the hydrophilic antioxidant uric acid [16]; and total bilirubin [17] were evaluated because they have different correlations with CVD; the first is inversely correlated with CVD, and the second is directly associated with the risk of CVD [18,19]. The platelets, white blood cell (WBC) and subtype counts are considered markers of inflammation [8], as is TNF-alpha release from human monocytes-derived macrophages (hMDMs). Cytokine release was evaluated under basal conditions and after stimulation with lipopolysaccharide (LPS), an endotoxin produced by gram-negative bacteria that binds Toll-like receptor 4 [20] and is also present in tobacco [21].
Materials and methods
Ethics statement
The study was approved by the local ethical committee of Azienda Ospedaliero-Universitaria of Sassari. Informed consent was obtained, and blood sampling was performed during a voluntary blood donation. Women were asked to donate blood during the follicular phase, and all participants were informed that an aliquot of blood would be kept for the study. Blood chemistry analyses were performed as a scheduled service for blood donors.
Population
A total of 83 healthy adult men and 85 healthy adult women with regular menstrual cycles (28 days) aged between 18 and 40 years were enrolled during the period from July 2007 to November 2010 at the Servizio di Diagnosi e Cura di Endocrinologia, Azienda Ospedaliero-Universitaria, Sassari, and at Diabetologia Aziendale ASL 2 Olbia, San Giovanni di Dio Hospital. The subjects were free of kidney, liver, heart, and endocrine diseases and had not had an infectious disease for at least two months prior to the study. None of the subjects were on long-term medications, including OC for women. All women were analysed during the follicular phase (1-10 days) and had not used OC for at least three months to ensure an appropriate wash-out period. A regular smoker was defined as a subject who smoked at least one cigarette per day at baseline.
Biochemical tests and blood cell counts
Fasting venous blood samples (8 and 10 am) were obtained and used for laboratory assessments of the selected biomarkers. Blood was collected using the appropriate anticoagulant, and the plasma was aliquoted and used within 1 month after storage at –80 °C to measure the cysteine, homocysteine, total glutathione, cysteinylglycine, glutamylcysteine, arginine, ADMA and SDMA levels and to measure the MDA level as described in Campesi et al. [22]. The platelet, WBC and WBC subset counts were performed using whole blood aliquots. WBCs were also used to determine the degree of global DNA methylation as described in Sotgia et al [23].
Isolation of human monocytes and differentiation into hMDMs
Monocytes were isolated from aliquots of blood (30 ml) withdrawn from healthy volunteers according to Campesi et al. [22]. Purified monocytes were obtained by adhesion; non-adherent cells (mainly lymphocytes) were removed by gentle washing with PBS. hMDMs were differentiated by culturing the isolated monocytes for 8-10 days in a 5% CO2 incubator at 37 °C in RPMI 1640 supplemented with 20% foetal bovine serum, 1% antibiotic-antimycotic, and 10 mM HEPES. hMDMs were prepared and characterised as described in Campesi et al [22].
Quantification of tumour necrosis factor-α release from hMDMs
hMDMs (4 x 104/cm2) were incubated in a 96-well plate for 24 h in the absence or presence of 100 ng/ml LPS (Sigma Aldrich, Milano, Italia). The supernatants were then collected and stored at -80 °C until analysis of the tumour necrosis factor-α release using a commercial ELISA kit (human TNF-α/TNFSF1A DuoSet ELISA kit, R & D Systems, Milano, Italy) following the manufacturer’s instructions. All samples were assayed in duplicate.
Statistical analysis
Statistical analysis was performed by comparing men with women as a function of smoking status. This analysis was performed using the Family-Wise Error Rate (FWER) approach, and the values were corrected for multiple-hypothesis testing using Bonferroni’s correction (if the probability of type I error is set at α and m tests are performed, each test is controlled at the level α* = α/m; Bonferroni, 1936). This correction guarantees that the probability of a false positive is at most equal to α [24].
The distribution of the samples was assessed using the Kolmogorov-Smirnov and Shapiro tests. The strength of the association between pairs of variables was measured using the Pearson Product Moment correlation coefficient when the data were normally distributed and with the Spearman Product Moment correlation coefficient when the data had a non-Gaussian distribution. These analyses were performed using SigmaStat software.
Results
Age, body weight, and height
A total of 83 men and 85 women were enrolled and stratified based on smoking status; 28 males and 32 females smoked, and 55 males and 53 females did not smoke. As shown in Table 1, all groups were well matched for age, but as expected, men were heavier and taller than women independent of CS.
Table 1.
Age, body weight and height of the enrolled subjects
| M (N = 55) | W (N = 53) | SM (N = 28) | SW (N = 32) | |
|---|---|---|---|---|
| Age (years) | 28 (18-39) | 27 (20-40) | 25.5 (19-37) | 26 (18-40) |
| Weight (kg) | 73 (55-105) | 53 (38-70)* | 66.5 (51-95) | 55 (44-78)† |
| Height (m) | 1.74 (1.62-1.90) | 1.60 (1.50-1.74)* | 1.73 (1.63-1.85) | 1.62 (1.50-1.80)† |
The data are expressed as medians and ranges. All women were analysed during the follicular phase. M = male non-smokers; W = female non-smokers; SM = male smokers; SW = female smokers.
P < 0.001 versus M;
P < 0.001 versus SM; N = sample size.
Evaluation of endothelial function
Notably, in healthy young adults, CS affected the levels of ADMA (an endogenous inhibitor of NOS) and L-arginine (the precursor of NO) in a sex-gender specific manner. Specifically, ADMA was not different between non-smokers but it was more elevated (approximately 20%) in female smokers than in males smokers, and consequently, the ADMA/SDMA ratio differed only between men and women who smoke (Figure 1). The L-arginine level did not differ between non-smokers, but it was more elevated in female smokers than in female non-smokers. Finally, the SDMA level and the ADMA/arginine ratio did not sexually diverge either in smokers or non-smokers (Figure 1).
Figure 1.
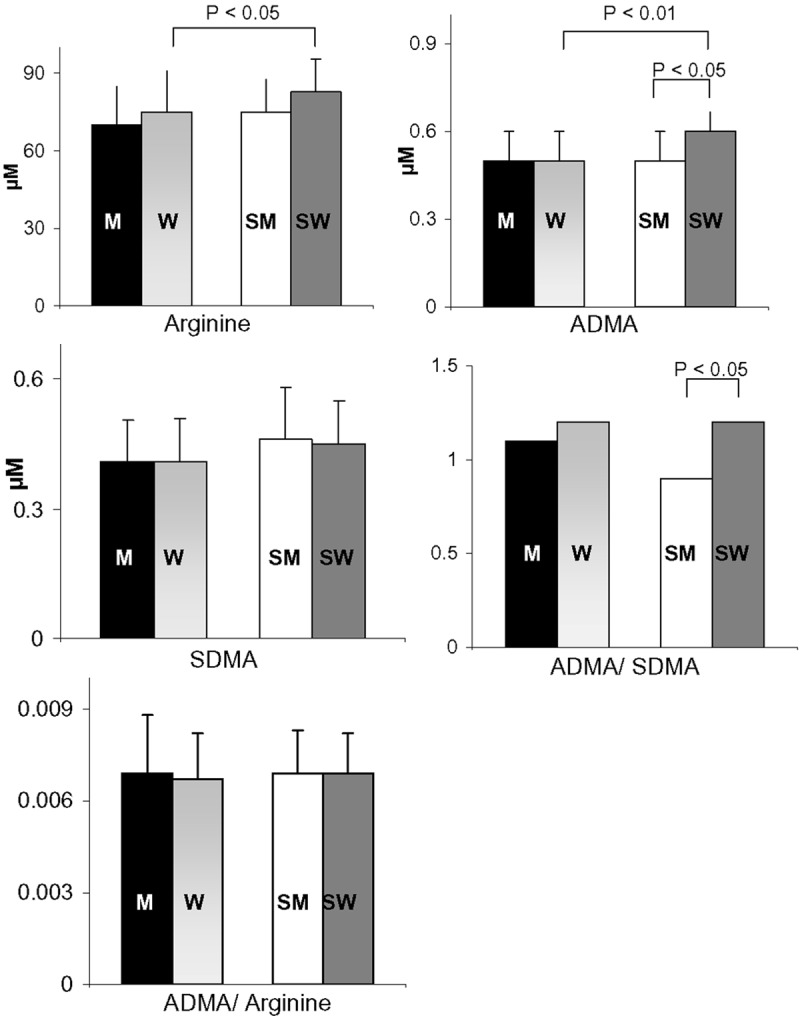
Arginine, ADMA, SDMA, ADMA/SDMA and ADMA/arginine levels in male non-smokers (M; N = 36), female non-smokers (W; N = 44), male smokers (SM; N = 21), and female smokers (SW; N = 26). All women were analysed during the follicular phase. The data are expressed as the means ± std. dev. or as median values. Bars represent the statistical differences.
Plasma thiols involved in the methylation and transsulfuration processes.
The homocysteine level was affected by sex-gender, being lower in female non-smokers than in male non-smokers, and by CS, being elevated only in female smokers (Figure 2). The plasma levels of total glutathione, its immediate precursor glutamylcysteine, and cysteinylglycine (the degradation product of glutathione) diverged in non-smokers, being significantly lower in women than in men (-13.5%, -15%, and -22%, respectively). Notably, these differences, with the exception of that for cysteinylglycine, were not observed in smokers (Figure 2). Finally, the levels of cysteine and taurine, one of the end products of cysteine metabolism and an endogenous scavenger of hypochlorous acid [25], did not diverge between men and women and were not affected by CS (Figure 2).
Figure 2.
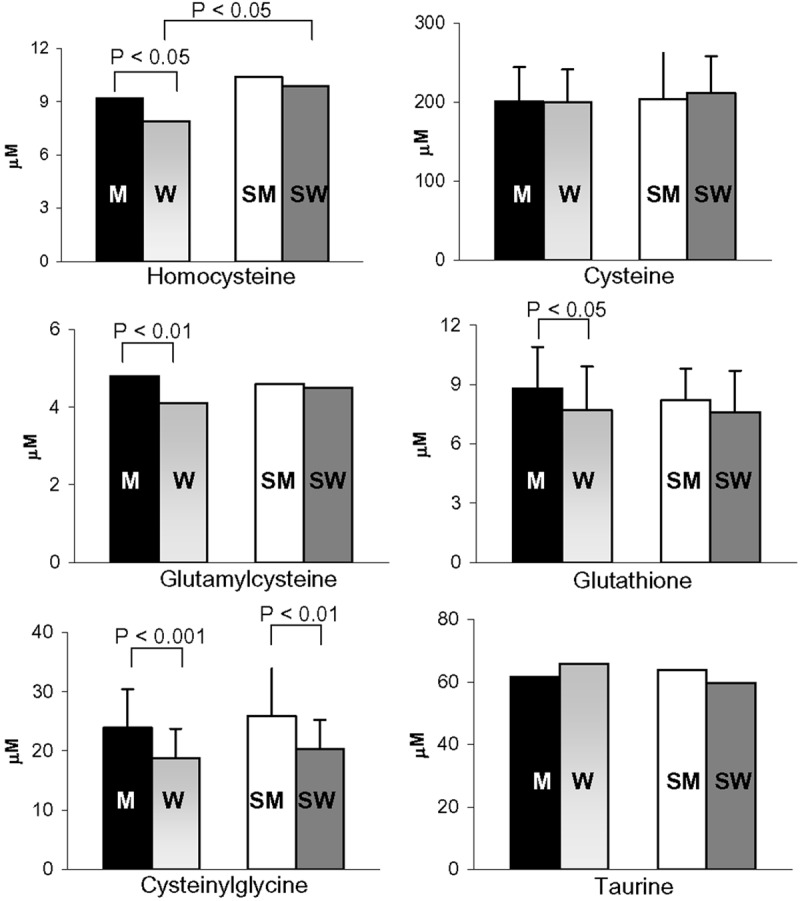
Effect of regular CS on the plasma anti-oxidant and pro-oxidant levels in male non-smokers (M; N = 36), female non-smokers (W; N = 44), male smokers (SM; N = 21), and female smokers (SW; N = 26). All women were analysed during the follicular phase. The data are expressed as the means ± std. dev. or as median values. Bars represent the statistical differences.
Global DNA methylation
DNA methylation alters the gene expression pattern in cells and plays a crucial role in the development of many diseases, including cardiovascular diseases [12,26]. In non-smokers, the level of global DNA methylation was significantly higher in women than in men (Figure 3). CS reduced global DNA methylation in both men and women; however, the reduction was greater in women than in men (Figure 3).
Figure 3.
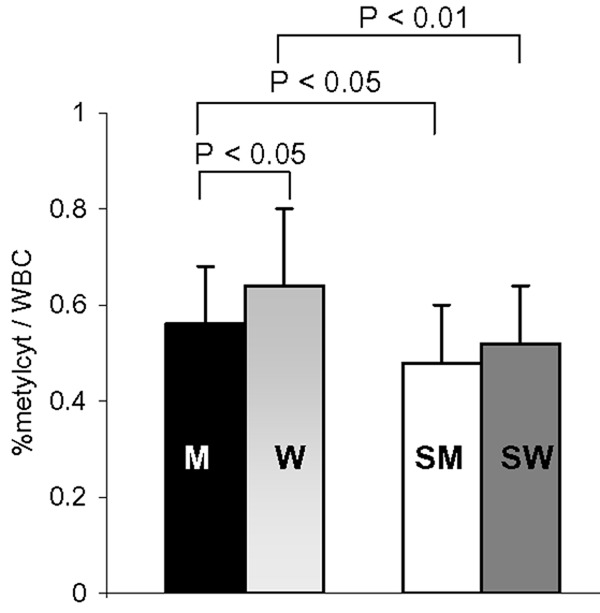
Effect of smoking on global DNA methylation in WBCs from male non-smokers (M; N = 37), female non-smokers (W; N = 45), male smokers (SM; N = 21), and female smokers (SW; N = 26). All women were analysed during the follicular phase. The data are expressed as the mean ± std. dev. Bars represent the statistical differences.
Effect of regular CS and sex-gender on the redox state and inflammatory parameters
Plasma lipid peroxidation, uric acid, and total bilirubin levels
The MDA level did not differ between men and women and was not affected by CS (data not shown), whereas in non-smokers, the total bilirubin and uric acid levels were significantly lower in women than in men. CS reduced the magnitude of the sex-gender difference observed for the total bilirubin level, but it did not significantly affect the uric acid level (Figure 4).
Figure 4.
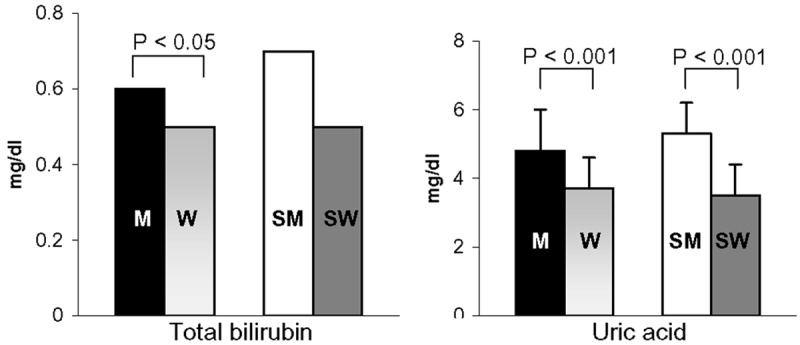
Effect of regular CS on the plasma bilirubin and uric acid levels in male non-smokers (M; N = 55), female non-smokers (W; N = 53), male smokers (SM; N = 28), and female smokers (SW; N = 32). All women were analysed during the follicular phase. The data are expressed as the means ± std. dev. or as median values. Bars represent the statistical differences.
Platelet, WBC and WBC subset counts
As shown in Figure 5, all values were within the normal range for non-smokers and smokers. As expected, the platelet count and monocyte counts were higher in women and in men (Figure 5), whereas the WBC, neutrophil, eosinophil, basophil, and lymphocyte counts did not differ between men and women. Notably, CS significantly increased the platelet count only in women and affected the WBC count and the counts of all WBC subtypes (Figure 5) except basophils. CS elevated the WBC count in both men and women. However, neutrophils and eosinophils were significantly increased only in men, whereas lymphocytes and monocytes were elevated only in women.
Figure 5.
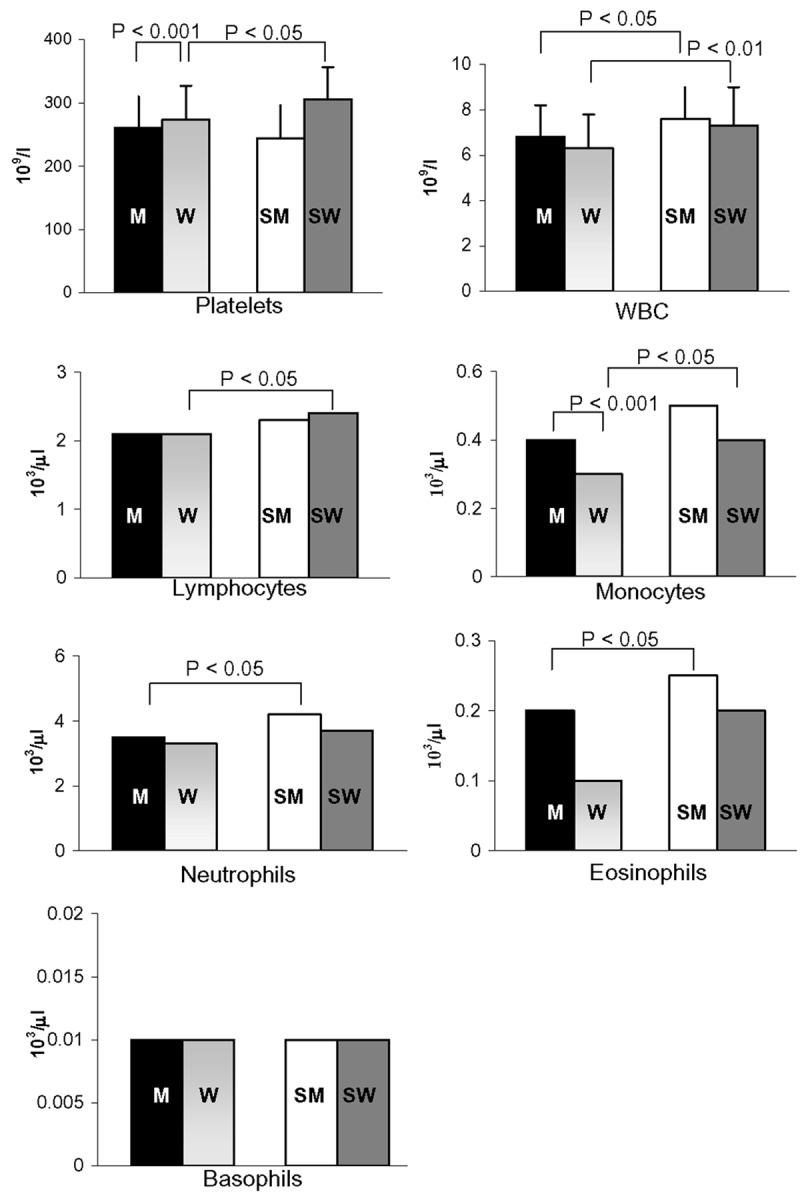
Effects of regular CS and sex-gender on blood cell counts in male non-smokers (M; N = 55), female non-smokers (W; N = 53), male smokers (SM; N = 28), and female smokers (SW; N = 32). All women were analysed during the follicular phase. The data are expressed as the means ± std. dev. or as median values. Bars represent the statistical differences.
Basal and LPS-induced release of TNF-alpha from hMDMs
The basal level of TNF-alpha released from hMDMs was significantly higher for male non-smokers than female non-smokers (Figure 6). This difference was not observed for smokers. LPS dramatically increased the release of TNF-alpha from hMDMs obtained from all cohorts (Figure 6). Notably, the level of LPS-induced TNF-alpha release was higher in female non-smokers than male non-smokers, whereas there was no statistically significant difference observed between men and women who smoke.
Figure 6.
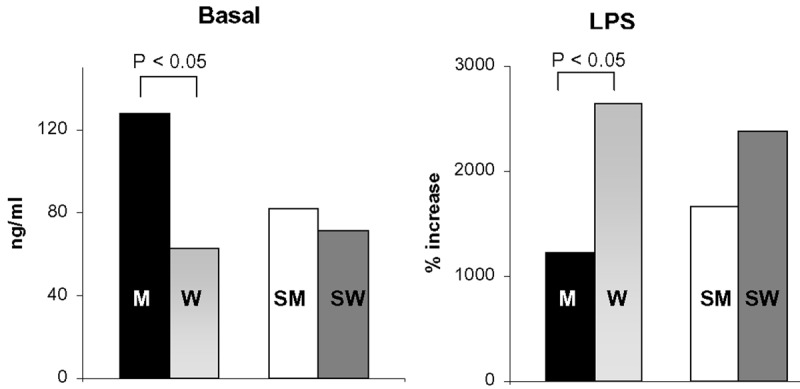
Basal and LPS-induced TNF-a release by hMDMs obtained from male non-smokers (M; N = 25), female non-smokers (W; N = 46), male smokers (SM; N = 24), and female smokers (SW; N = 25). All women were analysed during the follicular phase. The data are expressed as medians. Bars represent the statistical differences.
Analysis of correlations
In female non-smokers, age did not correlate with any of the studied parameters, whereas in female smokers, age was negatively associated with the lymphocyte (r = -0.47; P = 0.007) and platelet counts (r = -0.34; P = 0.04) and the cysteinylglycine (r = -0.53; P = 0.006), cysteine (r = -0.50; P = 0.01) and arginine levels (r = -0.41; P = 0.03). Age was also positively associated with the levels of taurine (r = 0.46; P = 0.04) and DNA methylation (r = 0.40; P = 0.04) in female smokers. Even fewer correlations were found for men. Age was positively associated with the basophil count (r = 0.27; P = 0.04) and the MDA level (r = 0.46; P = 0.04) in non-smokers and in smokers, respectively.
In addition, body weight was related with few parameters. In female non-smokers, weight was positively correlated with the WBC count (r = 0.28; P = 0.04) and negatively correlated with the total bilirubin level (r = -0.36; P = 0.008), whereas in female smokers, was correlated only with the ADMA/arginine ratio (r = 0.40; P = 0.04). Finally, in male non-smokers, weight was negatively correlated with the arginine (r = -0.35; P = 0.04) and cysteinylglycine levels (r = -0.37; P = 0.03), whereas in male smokers, weight was correlated only with the uric acid level (r = 0.45; P = 0.01).
In male and female non-smokers, the ADMA level was positively correlated with the arginine level (r = 0.55; P < 0.001 and r = 0.42; P = 0.004 respectively, the SDMA level (r = 0.60; P < 0.001 in men and r = 0.49; P < 0.001 in women), the ADMA/SDMA ratio (r = 0.55; P < 0.001 in men and r = 0.41; P = 0.006 in women), and the ADMA/arginine ratio (r = 0.60; P < 0.001 in men and r = 0.53; P < 0.001 in women) and negatively associated with the taurine (r = -0.48; P = 0.003 in men and r = -0.30; P = 0.05 in women) and MDA levels (r = -0.52; P = 0.002 in men and r = -0.65; P < 0.001 in women). In addition, in male non-smokers, the ADMA level was positively correlated with the cysteinylglycine level (r = 0.38; P = 0.02) and negatively associated with the monocyte (r = -0.34; P = 0.04), whereas in female non-smokers, ADMA was inversely correlated with the neutrophil count (r = -0.31; P = 0.04).
In female smokers, the ADMA level was positively correlated with the arginine level (r = 0.57; P = 0.002), the ADMA/SDMA ratio (r = 0.63; P < 0.001), the ADMA/arginine ratio (r = 0.78; P < 0.001), and the homocysteine level (r = 0.45; P = 0.02), whereas in male smokers, the ADMA level was positively correlated with the SDMA level (r = 0.54; P = 0.04) and negatively correlated with the monocyte count (r = -0.45; P = 0.03) and the levels of homocysteine (r = -0.61; P = 0.003), cysteine (r = -0.44; P = 0.02), glutamylcysteine (r = -0.65; P = 0.001), and MDA (r = -0.50; P = 0.02).
In non-smokers of both sexes, the SDMA level was positively associated with the arginine level (r = 0.35; P = 0.04 in men and r = 0.39; P = 0.009 in women). In addition, the SDMA level was negatively associated with the ADMA/SDMA ratio in female non-smokers (r = -0.56; P < 0.001) and with the taurine level in male non-smokers (r = -0.36; P = 0.03). In male and female smokers, SDMA was negatively associated with the ADMA/SDMA level (r = -0.48; P = 0.03 and r = -0.58; P = 0.002 respectively). The SDMA level was also negatively associated with the cysteinylglycine and cysteine levels in female smokers (r = -0.52; P = 0.007 and r = -0.44; P = 0.02, respectively) and with the basophil count in male smokers (r = -0.74; P < 0.001).
In male non-smokers, the total glutathione level was directly associated with the levels of cysteinylglycine and glutamylcysteine (r = 0.48; P = 0.003 and r = 0.40; P = 0.02, respectively), whereas in male smokers, the total glutathione level was directly associated with the glutamylcysteine level (r = 0.73; P < 0.001) and inversely associated with the arginine level (r = -0.52; P = 0.02). In female non-smokers, the total glutathione level was only positively associated with the glutamylcysteine level (r = 0.40; P = 0.008), whereas in female smokers, it was positively correlated with the levels of cysteine (r = 0.55; P = 0.004), homocysteine (r = 0.63; P < 0.001), and cysteinylglycine (r = 0.48; P = 0.01) and inversely correlated with the taurine level (r = -0.52; P = 0.01).
In all studied groups, global DNA methylation was negatively correlated with the WBC count (r = -0.93; P < 0.001 in male non-smokers, r = -0.94; P < 0.001 in female non-smokers, r = -0.93; P < 0.001 in male smokers and r = -0.92; P < 0.001 in female smokers), neutrophil count (r = -0.79; P < 0.001 in male non-smokers, r = -0.85; P < 0.001 in female non-smokers, r = -0.68; P < 0.001 in male smokers and r = -0.72; P < 0.001 in female smokers), and lymphocyte count (r = -0.52; P < 0.001 in male non-smokers, r = -0.65; P < 0.001 in female non-smokers, r = -0.52; P = 0.02 in male smokers and r = -0.56; P = 0.003 in female smokers). In male and female non-smokers, global DNA methylation was inversely correlated with the monocyte count (r = -0.43; P = 0.008 for men and r = -0.56; P < 0.001 for women), In female smokers, DNA methylation was negatively correlated with the taurine level (r = -0.44; P = 0.03) and the platelet count (r = -0.49; P = 0.01), whereas DNA methylation was inversely associated with the monocyte count only in male smokers (r = -0.50; P = 0.02).
Discussion
The major goal of this study was to determine whether regular CS affects inflammation and endothelial markers, global DNA methylation and the transsulfuration pathway to better understand why smoking is more harmful to women than men. Female non-smokers had lower plasma levels of glutamylcysteine, cysteinylglycine, and total glutathione than male non-smokers, whereas the level of cysteine, the limiting amino acid in glutathione synthesis, was not sexually divergent. The previous results suggest that there are sexual dimorphisms in transsulfuration, which provides a catabolic route leading to sulphate [27]. It is important to note that, at least in rat liver, the key enzyme in glutathione synthesis is present at a lower level in females [22]. Notably, the levels of the sulphur-containing amino acids homocysteine, glutamylcysteine, total glutathione and cysteinylglycine were strongly influenced by CS, and these effects were sex-gender specific. In particular, the slowdown in the transsulfuration pathway was not observed in female smokers and became comparable to that in men, with the exception of the cysteinylglycine level, which remained reduced by approximately 22%. Further confirmation of the sex-gender differences in transsulfuration comes from the sex-gender differences observed in the correlations between sulphur-containing compounds. Specifically, the total glutathione level was positively correlated with the levels of both its precursor and its metabolite only in male non-smokers; the total glutathione level was correlated with only the level of its precursor in female smokers.
Notably, the levels of two molecules that affect endothelial function [28], homocysteine and ADMA, were increased by CS in women, whereas in men, CS elevated only the homocysteine level. Furthermore, this effect was weaker in men than in women. The increase in homocysteine was directly linked to the increase in ADMA, which occurred only in women, suggesting that CS also affects the demethylation process in a sex-gender-specific manner. In addition, these findings suggest that the complex interplay between homocysteine and methylated arginines [28] is disrupted by CS in a sex-gender-specific manner. Homocysteine and ADMA are risk factors for cardiovascular diseases and thus, CS is more dangerous for women than men considering that sex-gender differences in endothelial function may underlie sex-gender differences in CVD [29].
The published data on the effects of CS on plasma antioxidants are not consistent with studies indicating that the levels of these antioxidants are unchanged, decreased, or increased [30-35]. The observed variability might be the result of differences in the study populations, e.g., differences in age, hormonal status, and OC use, and these differences make it impossible to obtain a perfect overview of the effects of CS on the antioxidant status. In our study, the plasma uric acid levels were not affected by CS in either men or women. Notably, CS reduced the magnitude of the sex-gender disparity observed between male and female non-smokers with respect to the levels of total bilirubin, total glutathione, and glutamylcysteine.
Given the pro-inflammatory properties of CS, it is not surprising that it can stimulate the bone marrow, leading to increases in platelets and WBCs in the bloodstream. Because the platelet count is associated with vascular and non-vascular death [36] the observed increase in the platelet count in female smokers could be clinically relevant and could partially explain the more deleterious effect of smoking in women.
CS also had a sex-gender-divergent effect on the WBC and WBC subset counts. As previously shown [37], these counts were increased by CS with the exception of the lymphocyte count, and this increase is considered a predictor of CVD [38]. Notably, the counts of cells that are present during every phase of atherogenesis, such as monocytes [39], were elevated by CS only in women. It is important to note that an increase in the monocyte count is associated with an increase of 15% in the risk for heart disease [40]. Monocytes differentiate into macrophages in the vessels and can be a source of cytokines such as TNF-alpha [41], which promotes endothelial dysfunction [42]. Here, the basal level of TNF-alpha release in men was more than twice that in women, whereas the level of TNF-alpha release induced by LPS was typically greater in women than in men. Collectively, these results reveal that hMDMs orchestrate TNF-alpha release in a sex-gender-dependent manner. Once again, the literature data on the effects of CS on cytokine release are conflicting. Most previous studies used alveolar macrophages [43,44] and did not report the donor’s sex, with some exceptions [45,46]. Previously, we demonstrated that TNF-alpha release, at least in women, depends on the hormonal status [22], with basal cytokine release being significantly greater from hMDMs derived from women that use OC than from hMDMs derived from non-users [22]. In contrast, LPS-induced release is more elevated for hMDMs obtained from women who did not use OC [22].
The global DNA methylation was lower in young men than in young women, suggesting that sex-gender needs to be taken into consideration in studies that assess global DNA methylation as an epigenetic marker for the risk of diseases. Some authors have reported that the level of global DNA methylation is higher in men than in women [47,48], but these studies included elderly subjects and did not consider the menstrual cycle phase or the use of OC or hormone replacement therapy. These factors could explain the divergence because total DNA methylation is influenced by the use of OC [22], being inversely associated with the concentrations of sex hormones (estradiol, testosterone) in postmenopausal women with low serum folate levels [49]. In addition, global DNA methylation could be modified by numerous lifestyle-related factors, such as the consumption of compounds with estrogenic activity [50]. In this study, CS reduced the level of global DNA methylation, especially in women. Some previous studies did not found an association between CS and global DNA methylation [51,52], but the hormonal status was not reported even though these studies analysed women aged from 35 to 75 years [53] or elderly men [54]. We observed that global DNA methylation was positively associated with age only in female smokers. The sex-gender differences in global DNA methylation could have remarkable consequences because global DNA methylation correlates with atherosclerosis [26]. Indeed, it is known that CS is a risk factor for breast cancer [55] and that leukocyte DNA hypomethylation is independently associated with breast cancer development [53]. The described sex-gender differences suggest that sex-gender and smoking need to be taken into consideration when studying global DNA methylation and when the relationships between DNA methylation and health outcomes are being assessed.
In conclusion, the present study provides convincing evidence that regular CS induces significant alterations in DNA methylation, the platelet count, the WBC count, the WBC subset count, endothelial function, transsulfuration, and hMDM function. Notably, the majority of variations were observed in young adult women, providing a basis for understanding the greater alarm for women than men [56] and suggesting that cessation programs targeting young women are urgently needed. Among individuals 18-40 years of age, CS did not substantially affect risk factors in men. Instead, we observed significant changes in women at an age at which women are normally free from risk factors, and these changes could contribute to the onset of CVD in women. Finally, the results suggest that the normal range for healthy subjects should be determined according to sex-gender and smoking status.
Acknowledgements
This study was supported by a grant from Legge Regionale n. 7: “Promozione della ricerca scientifica e dell’innovazione tecnologica in Sardegna” and by a grant from Regione Sardegna “Progetti di farmacovigilanza attiva, finanziabili attraverso i fondi fv 2008/09”. We are grateful to the mayor of Osilo and all Municipal Hall staff for the laboratory space that they dedicated to the National Sex–Gender Laboratory.
Disclosure of conflict of interest
None.
References
- 1.WHO Media centre. Tobacco. Fact sheet N°339. 2013. Updated May 2013.
- 2.Institute of Medicine. Women’s Health Research: Progress, Pitfalls, and Promise. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 3.Mahonen MS, McElduff P, Dobson AJ, Kuulasmaa KA, Evans AE. Current smoking and the risk of non-fatal myocardial infarction in the WHO MONICA Project populations. Tob Control. 2004;13:244–250. doi: 10.1136/tc.2003.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190:S5–22. doi: 10.1016/j.ajog.2004.01.061. [DOI] [PubMed] [Google Scholar]
- 5.Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378:1297–1305. doi: 10.1016/S0140-6736(11)60781-2. [DOI] [PubMed] [Google Scholar]
- 6.Center for Disease Control Prevention. Cigarette smoking among adults USA 2007. MMWR. 2008;57:1221–1226. [Google Scholar]
- 7.Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 8.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007;131:1557–1566. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- 9.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34:J258–265. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Edirisinghe I, Rahman I. Cigarette smoke-mediated oxidative stress, shear stress, and endothelial dysfunction: role of VEGFR2. Ann N Y Acad Sci. 2010;1203:66–72. doi: 10.1111/j.1749-6632.2010.05601.x. [DOI] [PubMed] [Google Scholar]
- 11.Philibert RA, Beach SR, Brody GH. Demethylation of the aryl hydrocarbon receptor repressor as a biomarker for nascent smokers. Epigenetics. 2012;7:1331–1338. doi: 10.4161/epi.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baccarelli A, Wright R, Bollati V, Litonjua A, Zanobetti A, Tarantini L, Sparrow D, Vokonas P, Schwartz J. Ischemic heart disease and stroke in relation to blood DNA methylation. Epidemiology. 2010;21:819–828. doi: 10.1097/EDE.0b013e3181f20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wierzbicki AS. Homocysteine and cardiovascular disease: a review of the evidence. Diab Vasc Dis Res. 2007;4:143–150. doi: 10.3132/dvdr.2007.033. [DOI] [PubMed] [Google Scholar]
- 14.Franconi F, Carru C, Spoletini I, Malorni W, Vella S, Mercuro G, Deidda M, Rosano G. A GENS-based approach to cardiovascular pharmacology: impact on metabolism, pharmacokinetics and pharmacodynamics. Ther Deliv. 2011;2:1437–1453. doi: 10.4155/tde.11.117. [DOI] [PubMed] [Google Scholar]
- 15.Schepers E, Barreto DV, Liabeuf S, Glorieux G, Eloot S, Barreto FC, Massy Z, Vanholder R. Symmetric dimethylarginine as a proinflammatory agent in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:2374–2383. doi: 10.2215/CJN.01720211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeum KJ, Russell RM, Krinsky NI, Aldini G. Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch Biochem Biophys. 2004;430:97–103. doi: 10.1016/j.abb.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Jansen T, Daiber A. Direct Antioxidant Properties of Bilirubin and Biliverdin. Is there a Role for Biliverdin Reductase? Front Pharmacol. 2012;3:30. doi: 10.3389/fphar.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimm H, Yun JE, Jo J, Jee SH. Low serum bilirubin level as an independent predictor of stroke incidence: a prospective study in Korean men and women. Stroke. 2009;40:3422–3427. doi: 10.1161/STROKEAHA.109.560649. [DOI] [PubMed] [Google Scholar]
- 19.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 21.Sebastian A, Pehrson C, Larsson L. Elevated concentrations of endotoxin in indoor air due to cigarette smoking. J Environ Monit. 2006;8:519–522. doi: 10.1039/b600706f. [DOI] [PubMed] [Google Scholar]
- 22.Campesi I, Sanna M, Zinellu A, Carru C, Rubattu L, Bulzomi P, Seghieri G, Tonolo G, Palermo M, Rosano G, Marino M, Franconi F. Oral contraceptives modify DNA methylation and monocyte-derived macrophage function. Biol Sex Differ. 2012;3:4. doi: 10.1186/2042-6410-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sotgia S, Carru C, Franconi F, Fiori PB, Manca S, Pettinato S, Magliona S, Ginanneschi R, Deiana L, Zinellu A. Rapid quantification of total genomic DNA methylation degree by short-end injection capillary zone electrophoresis. J Chromatogr A. 2008;1185:145–150. doi: 10.1016/j.chroma.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 24.Wang K, Li M, Bucan M. Pathway-based approaches for analysis of genomewide association studies. Am J Hum Genet. 2007;81:1278–1283. doi: 10.1086/522374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franconi F, Di Leo MA, Bennardini F, Ghirlanda G. Is taurine beneficial in reducing risk factors for diabetes mellitus? Neurochem Res. 2004;29:143–150. doi: 10.1023/b:nere.0000010443.05899.2f. [DOI] [PubMed] [Google Scholar]
- 26.Hiltunen MO, Yla-Herttuala S. DNA methylation, smooth muscle cells, and atherogenesis. Arterioscler Thromb Vasc Biol. 2003;23:1750–1753. doi: 10.1161/01.ATV.0000092871.30563.41. [DOI] [PubMed] [Google Scholar]
- 27.Rao AM, Drake MR, Stipanuk MH. Role of the transsulfuration pathway and of gamma-cystathionase activity in the formation of cysteine and sulfate from methionine in rat hepatocytes. J Nutr. 1990;120:837–845. doi: 10.1093/jn/120.8.837. [DOI] [PubMed] [Google Scholar]
- 28.Dayal S, Lentz SR. ADMA and hyperhomocysteinemia. Vasc Med. 2005;10(Suppl 1):S27–33. doi: 10.1191/1358863x05vm599oa. [DOI] [PubMed] [Google Scholar]
- 29.Bacon SL, Lavoie KL, Arsenault A, Dupuis J, Pilote L, Laurin C, Gordon J, Gautrin D, Vadeboncoeur A. The research on endothelial function in women and men at risk for cardiovascular disease (REWARD) study: methodology. BMC Cardiovasc Disord. 2011;11:50. doi: 10.1186/1471-2261-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puertas MC, Martinez-Martos JM, Cobo MP, Carrera MP, Mayas MD, Ramirez-Exposito MJ. Plasma oxidative stress parameters in men and women with early stage Alzheimer type dementia. Exp Gerontol. 2012;47:625–630. doi: 10.1016/j.exger.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Michelet F, Gueguen R, Leroy P, Wellman M, Nicolas A, Siest G. Blood and plasma glutathione measured in healthy subjects by HPLC: relation to sex, aging, biological variables, and life habits. Clin Chem. 1995;41:1509–1517. [PubMed] [Google Scholar]
- 32.Moriarty SE, Shah JH, Lynn M, Jiang S, Openo K, Jones DP, Sternberg P. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic Biol Med. 2003;35:1582–1588. doi: 10.1016/j.freeradbiomed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Mathru M, Dries DJ, Barnes L, Tonino P, Sukhani R, Rooney MW. Tourniquet-induced exsanguination in patients requiring lower limb surgery. An ischemia-reperfusion model of oxidant and antioxidant metabolism. Anesthesiology. 1996;84:14–22. doi: 10.1097/00000542-199601000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Haj Mouhamed D, Ezzaher A, Neffati F, Douki W, Gaha L, Najjar MF. Effect of cigarette smoking on plasma uric acid concentrations. Environ Health Prev Med. 2011;16:307–312. doi: 10.1007/s12199-010-0198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Endler G, Hamwi A, Sunder-Plassmann R, Exner M, Vukovich T, Mannhalter C, Wojta J, Huber K, Wagner O. Is low serum bilirubin an independent risk factor for coronary artery disease in men but not in women? Clin Chem. 2003;49:1201–1204. doi: 10.1373/49.7.1201. [DOI] [PubMed] [Google Scholar]
- 36.van der Bom JG, Heckbert SR, Lumley T, Holmes CE, Cushman M, Folsom AR, Rosendaal FR, Psaty BM. Platelet count and the risk for thrombosis and death in the elderly. J Thromb Haemost. 2009;7:399–405. doi: 10.1111/j.1538-7836.2008.03267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rana JS, Boekholdt SM, Ridker PM, Jukema JW, Luben R, Bingham SA, Day NE, Wareham NJ, Kastelein JJ, Khaw KT. Differential leucocyte count and the risk of future coronary artery disease in healthy men and women: the EPIC-Norfolk Prospective Population Study. J Intern Med. 2007;262:678–689. doi: 10.1111/j.1365-2796.2007.01864.x. [DOI] [PubMed] [Google Scholar]
- 38.Margolis KL, Manson JE, Greenland P, Rodabough RJ, Bray PF, Safford M, Grimm RH Jr, Howard BV, Assaf AR, Prentice R. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women’s Health Initiative Observational Study. Arch Intern Med. 2005;165:500–508. doi: 10.1001/archinte.165.5.500. [DOI] [PubMed] [Google Scholar]
- 39.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 40.Olivares R, Ducimetiere P, Claude JR. Monocyte count: a risk factor for coronary heart disease? Am J Epidemiol. 1993;137:49–53. doi: 10.1093/oxfordjournals.aje.a116601. [DOI] [PubMed] [Google Scholar]
- 41.Zimmermann HW, Trautwein C, Tacke F. Functional role of monocytes and macrophages for the inflammatory response in acute liver injury. Front Physiol. 2012;3:56. doi: 10.3389/fphys.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valente AJ, Rozek MM, Sprague EA, Schwartz CJ. Mechanisms in intimal monocyte-macrophage recruitment. A special role for monocyte chemotactic protein-1. Circulation. 1992;86:III20–5. [PubMed] [Google Scholar]
- 43.Yamaguchi E, Itoh A, Furuya K, Miyamoto H, Abe S, Kawakami Y. Release of tumor necrosis factor-alpha from human alveolar macrophages is decreased in smokers. Chest. 1993;103:479–483. doi: 10.1378/chest.103.2.479. [DOI] [PubMed] [Google Scholar]
- 44.Xu J, Xu F, Lin Y. Cigarette smoke synergizes lipopolysaccharide-induced interleukin-1beta and tumor necrosis factor-alpha secretion from macrophages via substance P-mediated nuclear factor-kappaB activation. Am J Respir Cell Mol Biol. 2011;44:302–308. doi: 10.1165/rcmb.2009-0288OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ward AJN, Chana KK, Thomas CM, Fenwich PS, Donnelly LE. P121Differential responses of M1 and M2 monocyte-derived macrophage phenotypes in COPD. Thorax. 2011;66:A116. [Google Scholar]
- 46.Amoruso A, Gunella G, Rondano E, Bardelli C, Fresu LG, Ferrero V, Ribichini F, Vassanelli C, Brunelleschi S. Tobacco smoke affects expression of peroxisome proliferator-activated receptor-gamma in monocyte/macrophages of patients with coronary heart disease. Br J Pharmacol. 2009;158:1276–1284. doi: 10.1111/j.1476-5381.2009.00442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang FF, Cardarelli R, Carroll J, Fulda KG, Kaur M, Gonzalez K, Vishwanatha JK, Santella RM, Morabia A. Significant differences in global genomic DNA methylation by gender and race/ethnicity in peripheral blood. Epigenetics. 2011;6:623–629. doi: 10.4161/epi.6.5.15335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Maarri O, Becker T, Junen J, Manzoor SS, Diaz-Lacava A, Schwaab R, Wienker T, Oldenburg J. Gender specific differences in levels of DNA methylation at selected loci from human total blood: a tendency toward higher methylation levels in males. Hum Genet. 2007;122:505–514. doi: 10.1007/s00439-007-0430-3. [DOI] [PubMed] [Google Scholar]
- 49.Ulrich CM, Toriola AT, Koepl LM, Sandifer T, Poole EM, Duggan C, McTiernan A, Issa JP. Metabolic, hormonal and immunological associations with global DNA methylation among postmenopausal women. Epigenetics. 2012;7:1020–1028. doi: 10.4161/epi.21464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kundakovic M, Champagne FA. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav Immun. 2011;25:1084–1093. doi: 10.1016/j.bbi.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hillemacher T, Frieling H, Moskau S, Muschler MA, Semmler A, Kornhuber J, Klockgether T, Bleich S, Linnebank M. Global DNA methylation is influenced by smoking behaviour. Eur Neuropsychopharmacol. 2008;18:295–298. doi: 10.1016/j.euroneuro.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Terry MB, Delgado-Cruzata L, Vin-Raviv N, Wu HC, Santella RM. DNA methylation in white blood cells: association with risk factors in epidemiologic studies. Epigenetics. 2011;6:828–837. doi: 10.4161/epi.6.7.16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi JY, James SR, Link PA, McCann SE, Hong CC, Davis W, Nesline MK, Ambrosone CB, Karpf AR. Association between global DNA hypomethylation in leukocytes and risk of breast cancer. Carcinogenesis. 2009;30:1889–1897. doi: 10.1093/carcin/bgp143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moore LE, Pfeiffer RM, Poscablo C, Real FX, Kogevinas M, Silverman D, Garcia-Closas R, Chanock S, Tardon A, Serra C, Carrato A, Dosemeci M, Garcia-Closas M, Esteller M, Fraga M, Rothman N, Malats N. Genomic DNA hypomethylation as a biomarker for bladder cancer susceptibility in the Spanish Bladder Cancer Study: a case-control study. Lancet Oncol. 2008;9:359–366. doi: 10.1016/S1470-2045(08)70038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson KC, Miller AB, Collishaw NE, Palmer JR, Hammond SK, Salmon AG, Cantor KP, Miller MD, Boyd NF, Millar J, Turcotte F. Active smoking and secondhand smoke increase breast cancer risk: the report of the Canadian Expert Panel on Tobacco Smoke and Breast Cancer Risk (2009) Tob Control. 2011;20:e2. doi: 10.1136/tc.2010.035931. [DOI] [PubMed] [Google Scholar]
- 56.Tan YY, Gast GC, van der Schouw YT. Gender differences in risk factors for coronary heart disease. Maturitas. 2010;65:149–160. doi: 10.1016/j.maturitas.2009.09.023. [DOI] [PubMed] [Google Scholar]


