Abstract
Although cervical cardiac transplantation is a well recognized useful model in diverse experimental settings, its widespread use, however, has been significantly hampered by the technical challenges relevant to small vessel anastomosis. We herein introduced a simplified two-stitch sleeve technique into arterial anastomosis during the course of cervical cardiac transplantation in mice. Cervical transplantation of allogenic and syngeneic cardiac grafts was conducted to assess the feasibility of this two-stitch sleeve technique in arterial anastomosis. Venous anastomosis was completed by the one-suture end-to-end microsuture technique, while arterial anastomosis was conducted by invaginating the recipient right common carotid artery into the graft left common carotid artery along with two guiding stitches. The two-stitch sleeve technique significantly simplified the procedures for arterial anastomosis as compared with that of the traditional microsuture technique (5.5 ± 1.8 min vs. 15.7 ± 3.0 min). However, the survival time for allografts (8.0 ± 0.2 day vs. 8.0 ± 0.4 day) and the long-term patency for syngeneic grafts (> 120 days) were the same as the grafts implanted by the traditional microsuture technique. This simplified sleeve technique is easy to learn, particularly for beginners without microsuture experience, and therefore, it has the great potential for widespread use in transplant immunology.
Keywords: Anastomosis, heart transplantation, microsuture, two-stitch sleeve technique
Introduction
Upon the introduction of murine abdominal heterotopic cardiac transplantation by Corry and colleagues in 1973 [1], this model has been widely employed as a useful tool for the study of allograft rejection and dissection of other immunological questions. In 1991, Chen further introduced the microsuture technique into murine cervical cardiac transplantation [2], in which two sequential cardiac transplantations could be conducted in the same recipient mouse using the abdominal and cervical site to test donor-type organ-specific tolerance. More importantly, the introduction of cervical heart transplantation significantly reduced trauma and circulation disturbance to the recipient mice. However, the original approach described for cervical cardiac transplantation requires 5 to 6 interrupted stitches for anastomosis of the right common carotid artery at the recipient side with the innominate artery at the graft side [2], which is time consuming and technically challenging. Particularly, diameter for the mouse common carotid artery (200~300 μm) is about 2 times smaller than that of the innominate artery, and this discrepancy demands extensive experience for microsurgery. As a result, technical challenges associated with the anastomosis of small vessels significantly hampered the widespread use of this model.
To circumvent the above mentioned difficulties, we herein introduce a simplified two-stitch sleeve technique for arterial anastomosis in cervical cardiac transplantation. Specifically, only two guiding stitches are needed for arterial anastomosis, which greatly simplifies the surgical procedures for anastomosis and remarkably reduces the ischemic time for grafts. We further demonstrated evidence that this sleeve technique does not affect the survival time for allografts and the long-term patency for syngeneic grafts as compared with that of the traditional microsuture technique.
Materials and methods
Animals
Wild-type C57BL/6J (H-2b) and BALB/c (H-2d) mice were purchased from the Animal Experimental Center of Wuhan University, and male mice with 8-12 wk old (22-26 g) were used for the study. The mice were housed in a SPF facility at the Tongji Medical College. All surgical procedures were carried out by one specific surgeon. All studies were conducted in accordance with the NIH guidelines and were approved by the Animal Care and Use Committee (ACUC) in Tongji Hospital.
Surgical instruments and supplies
The SZM45 operating microscope with magnification ranging from 7X to 45X for providing a clear view of small vessels was from Sunny Optical Technology (Yuyao, China). The micro-monopole electro-coagulator for cauterizing small vessel branches was originated from Evergreen (Wuhan, China). Microsurgical scissors, forceps and so on were purchased from Fine Science Tools (Foster City, CA, USA). Silk suture (6-0), 4-0 nylon on 13 mm (3/8 4×10) needles, and 11-0 nylon on 5 mm (3/8) needles were derived from Jinhuan Medical Products (Shanghai, China). The temperature controller for resuming body temperature after operation was a product from RWD Life Science (Shenzhen, China).
Surgical approaches
Anesthesia
All mice were anesthetized with a single intraperitoneal (i.p.) injection of pentobarbital sodium (Merck Chemicals, Shanghai, China) dissolved in normal saline at the dose of 100 mg/kg.
Harvest of cardiac graft (8~10 min)
The donor mouse was fixed in supine position. A U-sharp incision was made on the abdominal wall to expose the inferior vena cava, and through which 0.5 ml heparin solution (125 U/ml) was then administered. Both inferior vena cava and abdominal aorta were transected 1 min after heparin injection. The anterior chest wall was flipped over to expose inner content. After removing the thymus as a whole, the soft tissues between the ascending aorta and the pulmonary artery were bluntly dissected as much as possible (Figure 1A). Upon the ligation of inferior vena cava, the right superior vena cava was ligated with the homolateral auricular appendix and dissected distally. It would be better to ligate and dissect the left auricular appendix as well. The innominate artery and the aortic arch between the left common carotid artery and left subclavian artery were next ligated with 6-0 silk suture and divided distally. The left common carotid artery was sequentially separated from the surrounding fat tissues and transected as distally as possible in anticipation of anastomosis (Figure 1B). After transecting the pulmonary artery at its first bifurcation, the pulmonary veins and left superior vena cava were ligated en masse with 6-0 silk suture and were cut from the ligature. The cardiac graft was then preserved in 4°C normal saline (Figure 1C).
Figure 1.
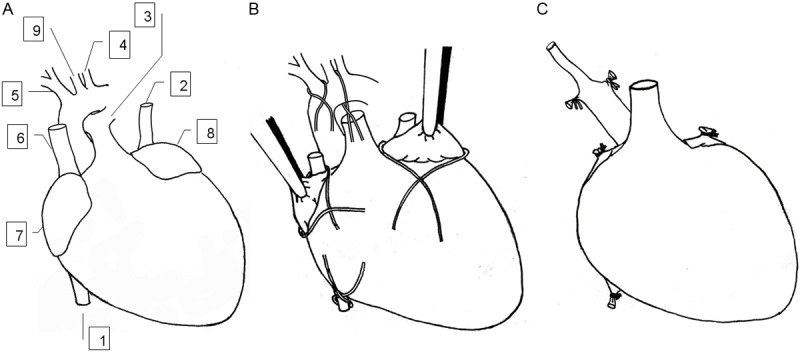
Paradigm for harvesting cardiac graft. A: In situ location of blood vessels in the donor heart. B: Ligation placed on the donor heart. The inferior vena cava was ligated, the right superior vena cava was ligated with homolateral auricular appendix and dissected distally. The left auricular appendix was ligated and dissected distally. The innominate artery and the aortic arch between the left common carotid artery and left subclavian artery were ligated and divided distally. The left common carotid artery was isolated from surrounding fat tissues and transected as distally as possible. The pulmonary artery was transected at its first bifurcation. C: The prepared cardiac graft ready for implantation. 1, inferior vene cava; 2, left superior vena cava; 3, pulmonary artery; 4, left subclavian artery; 5, innominate artery; 6, right superior vena cava; 7, right auricular appendix; 8, left auricular appendix; and 9, left common carotid artery.
Recipient preparation (10~12 min)
The recipient mouse was fixed in supine position, and its head was immobilized by hooking the upper incisor teeth. After the right neck was disinfected and shaved with 70% alcohol, an incision was made from the midpoint of the right clavicle to the lower mandible, and the incised skin was stretched by retractors in a proper strength. A segment of external vein was bluntly mobilized from the ambient connective tissues with small branches been cauterized. The caudal end was firstly clamped to let the back coming blood distend the external vein. The cranial end was then ligated and transected. To expose the right common carotid artery, the right salivary gland and the sternomastoid muscle were ligated and excised. A segment of common carotid artery was next dissociated from surrounding tissues, and the proximal end was clamped, while the distal end was ligated and transected.
Anastomosis of graft pulmonary artery with recipient external vein (7~9 min)
For implantation, the cardiac graft was placed in the fossa formed by the stretched skin in a position for the left common carotid artery and pulmonary artery in graft facing the recipient right common carotid artery and external vein, respectively. A one-suture technique was utilized for end-to-end anastomosis of the graft pulmonary artery and the recipient external vein [3]. Briefly, one stay stitch was anchored at the left side of the vessels, while the posterior and anterior wall were closed with running sutures by in-out/out-in and out-in/in-out sequence, respectively. After the last stitch was applied, the free end of the thread was cut with 1 mm left in a knotless fashion adopted from Mao et. al [4]. The first and last stitch should be applied closely to the stay stitch with short bites to prevent bleeding [5]. Ten stitches in total would be sufficient for the anastomosis.
Two-stitch sleeve technique for anastomosis of the common arteries (4~6 min)
A forceps with 0.1 mm tip width was employed to mechanically dilate the donor left common carotid artery with appropriate strength. The right common carotid artery in the recipient was served as the feeding artery, in which the adventitial tags were gently stripped. The stumps of the vessels were flushed with heparin solution before anastomosis. The first guiding suture was next placed 0.2~0.3 mm (the same length as the diameter of the common carotid artery) beyond the stump of the graft common carotid artery by piercing from outside to inside with a 11-0 nylon thread, and then out through the open stump to pick up the free edge of the recipient common carotid artery from inside (Figure 2A). The same thread was then pierced out from the inside of the receiving artery near the primary piercing point (Figure 2B). A second guiding suture was next placed in a similar manner 180 degrees apart from the first guiding suture. The first guiding suture was left untied until the second one was finished. Both guiding sutures were then gently stretched to introduce the feeding artery into the receiving artery and knotted with themselves for fixation (Figure 2C). The receiving artery was gently stretched to avoid crumpling. For controls, the grafts were subjected to arterial anastomosis using the traditional technique [2]. Abdominal heart transplantation was conducted as previously described [6].
Figure 2.
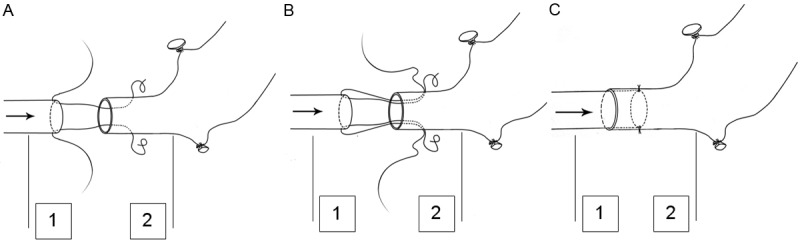
Illustration of the two-stitch sleeve technique. A: Guiding sutures are begun with piercing the donor common carotid artery from outside, and picked up at the free edge of the recipient common carotid artery from inside. The first guiding suture is placed 0.2~0.3 mm (the same length as the diameter of the common carotid artery) beyond the vessel stump. B: The same thread is then pierced out from the inside of the receiving artery in close proximity of the primary piercing point. C: The guiding sutures are gently stretched to introduce the feeding artery into the receiving artery and knotted with itself for fixation. The arrow shows the direction of the blood flow. 1, the feeding artery (recipient common carotid artery); 2, the receiving artery (donor common carotid artery).
Revascularization and completion of the operation (10~15 min)
Upon the completion of anastomosis, the venous clamp was firstly released, and the arterial clamp was next interruptedly released until the absence of obvious bleeding (Figure 3). In general, the graft would return to sinus rhythm within a minute but in a slower tempo for contraction. After the position of the graft was adjusted without affecting the patency of the vessels, skin was closed with 4-0 nylon in one layer. The animal was then placed on the heated cushion of the temperature controller to maintain its anal temperature at 37°C until its full resuscitation.
Figure 3.
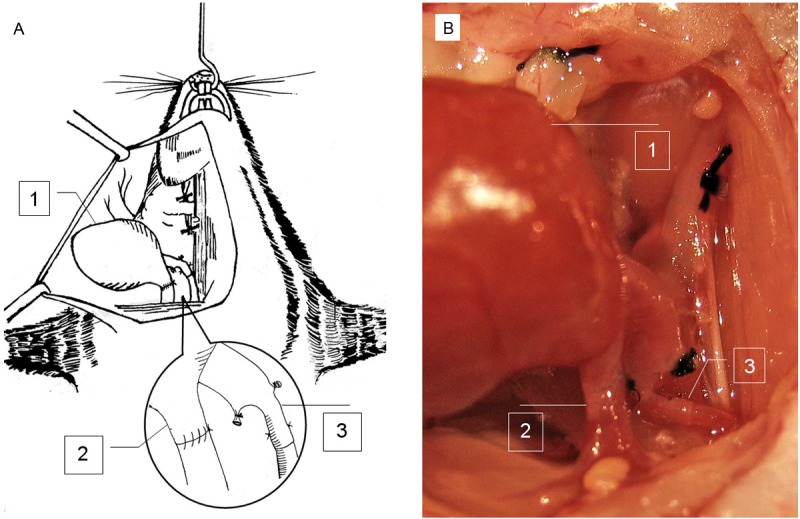
Schematic view of cervical cardiac implantation. A: A graphic picture for clear illustration of cervical cardiac transplantation. B: An image of transplanted cardiac graft. The submandibular gland is ligated and removed. Venous anastomosis is completed by one-suture end-to-end microsuture technique. Arterial anastomosis is finished by the two-stitch sleeve technique. The inset shows the arterial and venous anastomosis. 1, cardiac graft; 2, the donor pulmonary artery is anastomosed to the recipient right external jugular vein in an end-to-end manner; and 3, the donor right common carotid artery is anastomosed to the recipient right common carotid artery by the end-in-end sleeve technique.
Assessment of allograft rejection
Allograft rejection was blindly determined by palpation of the grafts and scored by a semi quantitative scale twice daily as previously reported [7,8], in which 0 stands for a fully rejected graft (no contraction), while 4 represents a well functioning graft. Cessation of graft heart beat was further validated by direct visualization.
Histology
Arterial junction with the sleeve technique was harvested at indicated time, and then subjected to hematoxylin and eosin (HE) staining using the established techniques [9].
Ultrasonic Doppler analysis
Blood flow velocity was measured by Ultrasonic Doppler analysis in a blinded fashion by an experienced doctor [10]. Two-D short-axis view of blood flow Images at the site of right common carotid artery was obtained using the Ultrasonic Doppler System (MyLab Twice, ESAOTE, Italy) equipped with a high frequency linear-array transducer (LA435, 6~18 MHz).
Statistical analysis
All data are expressed as mean ± SD. Comparisons between two groups were analyzed by the unpaired Student’s t test. Allograft survival curves were generated by the Kaplan and Meier method [7]. Allograft survival differences between groups were determined using the log-rank (Mantel-Cox) test. In all cases, p < 0.05 was considered with statistical significance.
Results
Survival time for the cardiac allografts
We first sought to examine the differences for allograft survival time between the traditional microsuture technique and our two-stitch sleeve technique. For this purpose, we conducted cervical heterotopic transplantation of BALB/c-derived cardiac grafts into C57BL/6J recipient mice by employing the traditional microsuture technique and the two-stitch sleeve technique, respectively. Ten mice were included in each study group, and the graft was defined as operative failure for exclusion if its survival time is < 3 days. Of interestingly note, we failed to observe a perceptible difference for the graft survival time between mice in the traditional group and the sleeve group as manifested by the similar median survival time (MST) (8.0 ± 0.4 day vs. 8.0 ± 0.2 day, Figure 4A). However, our sleeve technique has greatly reduced the time for arterial anastomosis as compared with that of the traditional microsuture technique (5.5 ± 1.8 min vs. 15.7 ± 3.0 min, p < 0.01, Figure 4B). We also conducted cervical transplantation of a donor-strain derived second cardiac allograft in recipients 3 weeks after abdominal cardiac transplantation. In line with our expectation, the second graft was rejected much faster than that of the first graft (MST) (3.0 ± 0.0 day vs. 8.0 ± 0.4 day, p < 0.01, Figure 4A).
Figure 4.
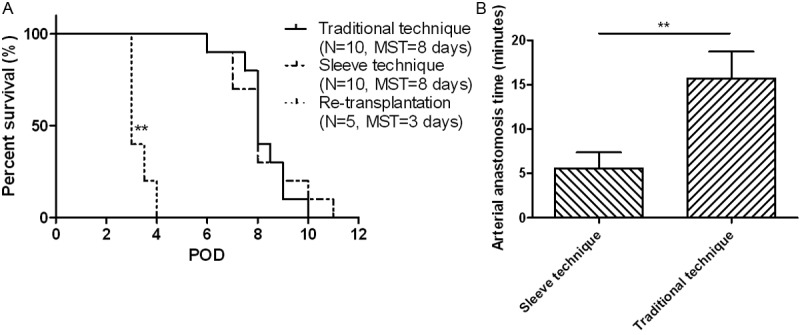
A: Survival time for the cardiac allografts. BALB/c derived cardiac allografts were implanted into C57BL/6J mice at the cervical site using the traditional microsuture technique and the two-stitch sleeve technique, respectively. For re-transplantation, the recipient mice were first transplanted with a cardiac allograft at the abdominal site, and 3 weeks later a second cardiac allograft was implanted at the cervical site using the two-stitch sleeve technique. B: Comparison for the time of arterial anastomosis between the sleeve technique and traditional technique. POD, post operative day; MST, median survival time. **, p < 0.01.
Long-term patency for syngeneic cardiac grafts
The next important question is whether our two-stitch sleeve technique affects the long-term graft patency rate. To address this question, we conducted syngeneic cardiac transplantation in C57BL/6J mice. In consistent with our expectation, syngeneic grafts resulted from our sleeve technique displayed similar long-term patency as that of grafts conducted by the traditional technique. Specifically, all 12 syngeneic grafts carried out by our sleeve technique and traditional technique still scored 3 at the end of experimentation (120 days after transplantation, N=6 in each group).
To further confirm the above results, we performed Ultrasonic Doppler analysis of blood flow velocity in the carotid arteries. Due to the small size and complexity of the vessels at the site of graft, the measurements were conducted at the point where bidirectional blood flow existed. As shown in Figure 5A and 5B, the irrigation of the right (graft) side common carotid artery (sleeve anastomosis) displayed a comparable blood flow velocity as that of control common carotid artery in the left side on day 50 after transplantation. Importantly, a similar pattern of blood flow velocity was also noted in the recipient mice with the traditional technique at day 50 (Figure 5C and 5D) of transplantation. Together, these data support that our two-stitch sleeve technique does not have a discernable impact on long-term graft patency as compared with that of the traditional microsuture technique.
Figure 5.
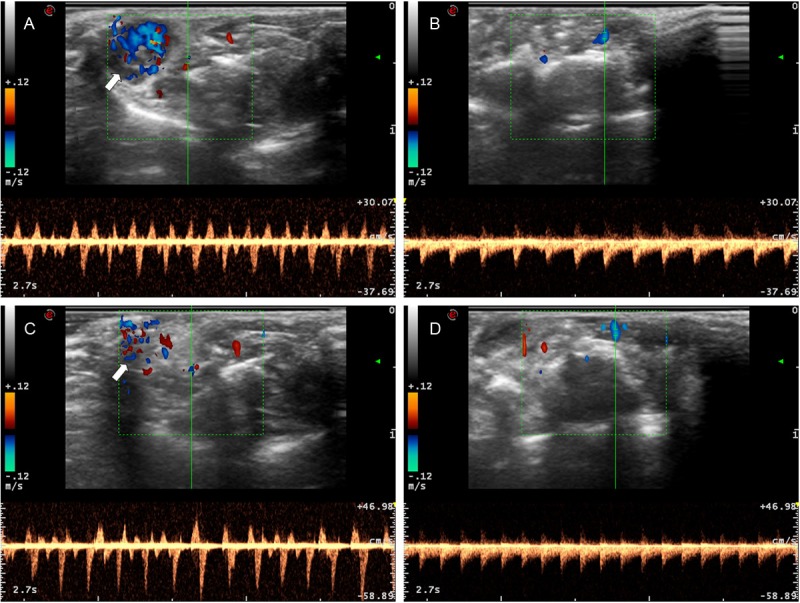
Ultrasonic Doppler analysis of blood flow in syngeneic grafts 50 days after transplantation. Bidirectional blood flow velocity with the two-stitch sleeve technique (A) and its control left common carotid artery (B). Bidirectional blood flow velocity with the traditional microsuture technique (C) and its left control common carotid artery (D). Green lines represent the location of blood flow measured, while white arrows are pointed to the cardiac grafts.
Results for histological analysis
We further conducted HE staining of sections of arterial conjunctions to examine the histological changes in grafts with our two-stitch sleeve technique and the traditional technique at indicated time points. It was noted that both receiving and feeding artery were intact in syngeneic grafts 24 h after transplantation (Figure 6A). Importantly, in consistent with the Ultrasonic Doppler results, the feeding arteries were remained untouched overtime and still patent after day 50 (Figure 6C) of transplantation. However, the receiving artery was found to be fused with the feeding artery and lost its initial morphology overtime (Figure 6C). Also, a similar arterial morphology could be noted in the proximal irrigating common carotid artery with the traditional microsuture technique (Figure 6B and 6D).
Figure 6.
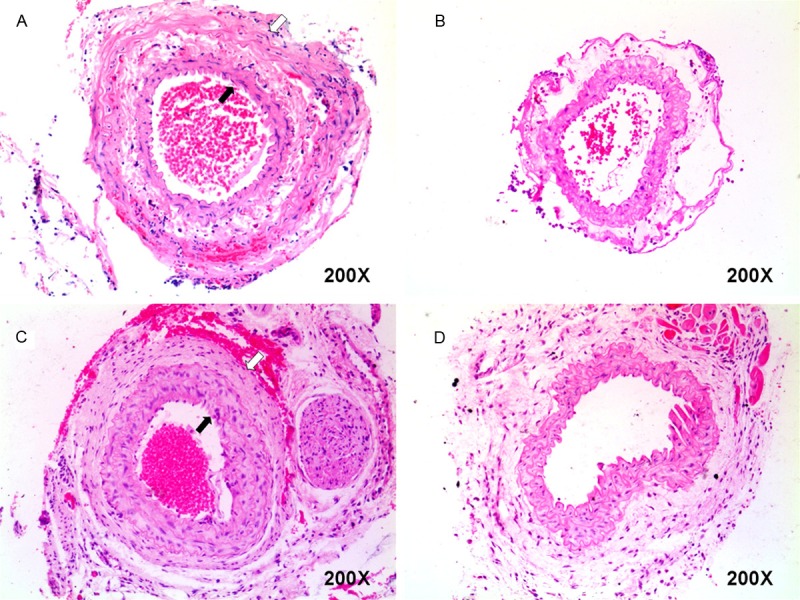
Histological analysis of the arterial conjunctions. A: A representative histological result for the arterial conjunction 24 h after the two-stitch sleeve anastomosis. B: A representative histological result for the proximal irrigating common carotid artery 24 h after the traditional microsuture anastomosis. C: Result for the arterial conjunction of a syngenic graft 50 days after the sleeve anastomosis. D: Result for the proximal irrigating common carotid artery 50 days after the traditional microsuture technique in a syngeneic graft. The open arrows indicate the outer receiving artery, while the filled arrows indicate the inner feeding artery (Original magnification, ×200).
Discussion
In the present report, we introduced a simplified two-stitch sleeve technique for murine cervical heterotopic cardiac transplantation. We also presented evidence supporting that this technique possesses a significant improvement as compared with that of the traditional microsuture technique by simplifying the procedures for vessel anastomosis and reducing the ischemic time for the grafts. Given that the donor pulmonary artery wall and the recipient external vein are too thin and fragile to be identified and anastomosed, it would be better to immerse the vessels in normal saline to let them to be floated and visualized. A knotless technique introduced by Mao and colleagues can be also employed for venous anastomosis as illustrated previously [4], which would allow the operator to adjust the size of anastomosis after completion of all sutures and to save the time for knotting.
The left common carotid artery is slightly larger than the right common carotid artery, which renders it to be the best candidate as the receiving artery. Unlike other reported techniques [11-14], our sleeve technique does not require extra-vascular stitches or holding stitches except for the two guiding stitches. For the traditional microsuture technique, at least 6 stitches and 14 min are needed to complete the arterial anastomosis. In sharp contrast, our sleeve technique only requires 2 guiding stitches along with 5 min to finish the anastomosis. The significant reduction of ischemic time and intraluminal sutures for the graft manifested by our sleeve technique would assure a higher successful rate for the cervical transplantation. Moreover, our histological and Ultrasonic Doppler studies revealed that cardiac grafts implanted by our sleeve technique showed similar patency as that of grafts with the traditional microsuture technique.
The sleeve technique has also been previously employed for the study of allograft arteriosclerosis by Dambrin and colleagues [15], in which they demonstrated that both bleeding and thrombosis were rare cases. Similarly, bleeding and thrombosis are very unusual in our cervical cardiac transplantation model as well, and the overall successful rate was about 90~95% in our one year experience. However, bleeding and thrombosis were the leading complications in our early training process. To our experience, the invaginating depth of the feeding artery could be a key factor relevant to this complication, and the most optimal depth is about the same length as the diameter of the receiving stump. A deeper invagination is likely to result in arterial crumpling, while a shorter one would be difficult to reach homeostasis. Also, the symmetry of the two guiding stitches, the bite length apart from the stump and degrees apart from the cross-section could be factors affecting the final outcome. Of note, temperature recovery is another important factor to prevent thrombosis formation after skin closure. Particularly, it is important to interruptedly release the arterial clamp for prevention of bleeding and prolapse of the feeding artery. Furthermore, the integrity of the feeding arterial intima and heparin flushing are also factors to prevent thrombosis.
In summary, the sleeve technique was originally introduced by Murphy in 1897 [16], and it was then revisited by Lauritzen in 1978 [17]. During the past 3 decades, a great deal of effort has been devoted to improve this technique with applications both in experimental and clinical settings [12,13,18-22]. We herein in this report introduced our two-stitch sleeve technique into the murine cervical cardiac transplantation model. To our knowledge, this is the smallest vessel anastomosis accomplished by the sleeve technique thus far. Some investigators once questioned its application in small vessels [18], while our data in this report demonstrated evidence supporting that our two-stitch sleeve technique is highly feasible in small vessels. This simplified sleeve technique is easy to learn, particularly for beginners without microsuture experience, and therefore, it has the great potential for widespread use in the settings with anastomosis of small vessels.
Acknowledgements
This work was supported by grants to CYW from the National Natural Science Foundation of China (81130014), the Chinese Ministry of Science & Technology (2012BAI39B05), and the European Foundation for the Study of Diabetes (EFSD)/Chinese Diabetes Society (CDS)/Lilly Program for Collaborative Diabetes Research between China and Europe.
Disclosure of conflict of interest
The manuscript has neither been submitted nor published elsewhere. All authors have read and agreed to the content within the manuscript. All authors declare no financial or other conflict of interest relevant to the subject of this article.
References
- 1.Corry RJ, Winn HJ, Russell PS. Heart transplantation in congenic strains of mice. Transplant Proc. 1973;5:733–735. [PubMed] [Google Scholar]
- 2.Chen ZH. A technique of cervical heterotopic heart transplantation in mice. Transplantation. 1991;52:1099–1101. doi: 10.1097/00007890-199112000-00035. [DOI] [PubMed] [Google Scholar]
- 3.Qian SG, Fung JJ, Demetris AV, Ildstad ST, Starzl TE. Orthotopic liver transplantation in the mouse. Transplantation. 1991;52:562–564. doi: 10.1097/00007890-199109000-00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao M, Liu X, Tian J, Yan S, Lu X, Gueler F, Haller H, Rong S. A novel and knotless technique for heterotopic cardiac transplantation in mice. J Heart Lung Transplant. 2009;28:1102–1106. doi: 10.1016/j.healun.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 5.Niimi M. The technique for heterotopic cardiac transplantation in mice: experience of 3000 operations by one surgeon. J Heart Lung Transplant. 2001;20:1123–1128. doi: 10.1016/s1053-2498(01)00309-6. [DOI] [PubMed] [Google Scholar]
- 6.Liu F, Kang SM. Heterotopic heart transplantation in mice. J Vis Exp. 2007;6:238. doi: 10.3791/238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y, Yin H, Han J, Huang B, Xu J, Zheng F, Tan Z, Fang M, Rui L, Chen D, Wang S, Zheng X, Wang CY, Gong F. Extracellular hmgb1 functions as an innate immune-mediator implicated in murine cardiac allograft acute rejection. Am J Transplant. 2007;7:799–808. doi: 10.1111/j.1600-6143.2007.01734.x. [DOI] [PubMed] [Google Scholar]
- 8.Martins PN. Assessment of graft function in rodent models of heart transplantation. Microsurgery. 2008;28:565–570. doi: 10.1002/micr.20544. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S, Lv JW, Yang P, Yu Q, Pang J, Wang Z, Guo H, Liu S, Hu J, Li J, Leng J, Huang Y, Ye Z, Wang CY. Loss of dicer exacerbates cyclophosphamide-induced bladder overactivity by enhancing purinergic signaling. Am J Pathol. 2012;181:937–946. doi: 10.1016/j.ajpath.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 10.Rao X, Zhong J, Zhang S, Zhang Y, Yu Q, Yang P, Wang MH, Fulton DJ, Shi H, Dong Z, Wang D, Wang CY. Loss of methyl-CpG-binding domain protein 2 enhances endothelial angiogenesis and protects mice against hind-limb ischemic injury. Circulation. 2011;123:2964–2974. doi: 10.1161/CIRCULATIONAHA.110.966408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duminy FJ. A new microvascular “sleeve” anastomosis. J Surg Res. 1989;46:189–194. doi: 10.1016/0022-4804(89)90054-1. [DOI] [PubMed] [Google Scholar]
- 12.Gahankari DR, Lalwani NR, Phatak AM. Classification and comparison of five techniques of end-to-end microarterial anastomoses in rats: a new proposed technique. Microsurgery. 1995;16:793–802. doi: 10.1002/micr.1920161206. [DOI] [PubMed] [Google Scholar]
- 13.Guneren E, Odaci E, Yildiz L, Akbas H, Eroglu L, Kaplan S. Use of longitudinal invaginating matrix sutures in microarterial sleeve anastomoses. Scand J Plast Reconstr Surg Hand Surg. 2004;38:1–4. doi: 10.1080/02844310310010571. [DOI] [PubMed] [Google Scholar]
- 14.Zhang XY, Wheatley AM. Application of a new sleeve anastomosis technique to graft rearterialization in rat liver transplantation. Microsurgery. 1996;17:472–476. doi: 10.1002/(SICI)1098-2752(1996)17:8<472::AID-MICR11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 15.Dambrin C, Calise D, Pieraggi MT, Thiers JC, Thomsen M. Orthotopic aortic transplantation in mice: a new model of allograft arteriosclerosis. J Heart Lung Transplant. 1999;18:946–951. doi: 10.1016/s1053-2498(99)00051-0. [DOI] [PubMed] [Google Scholar]
- 16.Cuadros CL. History of sleeve anastomosis. Plast Reconstr Surg. 1988;82:1102–1103. doi: 10.1097/00006534-198812000-00044. [DOI] [PubMed] [Google Scholar]
- 17.Lauritzen C. A new and easier way to anastomose microvessels. An experimental study in rats. Scand J Plast Reconstr Surg. 1978;12:291–294. doi: 10.3109/02844317809013008. [DOI] [PubMed] [Google Scholar]
- 18.Kanaujia RR, Hoi KI, Miyamoto Y, Ikuta Y, Tsuge K. Further technical considerations of the sleeve microanastomosis. Plast Reconstr Surg. 1988;81:725–734. doi: 10.1097/00006534-198805000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama Y, Soeda S, Iino T, Uchida A. Is the sleeve anastomosis a risky technique? Br J Plast Surg. 1987;40:288–294. doi: 10.1016/0007-1226(87)90125-1. [DOI] [PubMed] [Google Scholar]
- 20.Siemionow M. Histopathology of microarterial anastomoses: end-to-end versus end-in-end (sleeve) technique. J Hand Surg Am. 1990;15:619–625. doi: 10.1016/s0363-5023(09)90025-9. [DOI] [PubMed] [Google Scholar]
- 21.Sully L, Nightingale MG, O'Brien BM, Hurley JV. An experimental study of the sleeve technique in microarterial anastomoses. Plast Reconstr Surg. 1982;70:186–192. doi: 10.1097/00006534-198208000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Moskowitz M, Ostad D, Kessler K, Siebert JW. New four-stitch sleeve anastomosis: an experimental study in rats with reports of clinical use. Microsurgery. 1996;17:291–294. doi: 10.1002/(SICI)1098-2752(1996)17:6<291::AID-MICR1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]


