Abstract
The purpose of this study was to examine whether low frequency (<100 kHz), low intensity (<100 mW/cm2, spatial peak temporal peak) ultrasound can be an effective treatment of venous stasis ulcers, which affect 500 000 patients annually costing over $1 billion per year. Twenty subjects were treated with either 20 or 100 kHz ultrasound for between 15 and 45 min per session for a maximum of four treatments. Healing was monitored by changes in wound area. Additionally, two in vitro studies were conducted using fibroblasts exposed to 20 kHz ultrasound to confirm the ultrasound's effects on proliferation and cellular metabolism. Subjects receiving 20 kHz ultrasound for 15 min showed statistically faster (p < 0.03) rate of wound closure. All five of these subjects fully healed by the fourth treatment session. The in vitro results indicated that 20 kHz ultrasound at 100 mW/cm2 caused an average of 32% increased metabolism (p < 0.05) and 40% increased cell proliferation (p < 0.01) after 24 h when compared to the control, non-treated cells. Although statistically limited, this work supports the notion that low-intensity, low-frequency ultrasound is beneficial for treating venous ulcers.
INTRODUCTION
This work describes the encouraging results of a pilot human study (20 subjects) indicating that low frequency (<100 kHz), low intensity (<100 mW/cm2, spatial peak temporal peak) ultrasound can be used as an effective tool in chronic wound management, specifically treatment of venous stasis ulcers. Although statistically limited due to the small (n = 20) subject population, this study is of importance as no satisfactory in vivo or animal model, which incorporates all the complexities of chronic wounds, exists (Lindblad, 2000).
The treatment of chronic wounds, including diabetic, venous, and decubitus ulcers has an annual cost of over $25 billion on the American health system (Sen et al., 2009), of which venous leg ulcers contributed nearly $1 billion in 1991 (Phillips and Dover, 1991). Over 500 000 patients are treated for venous ulcers annually (Margolis and Cohen, 1994) and this treatment constitutes approximately 1% of total health care costs in the western world (Nelzen, 2000). Multiple factors contribute to the delayed healing of venous ulcers including ambulatory venous hypertension often associated with venous reflux, poor nutrition, advancing age, smoking, and medical compliance (i.e., following physician's guidelines and recommendations) (Gohel et al., 2005). The direct wound care cost associated with venous ulcers exceeds $2400 per month, but also is associated with indirect costs due to limited productivity and quality of life issues related to pain and depression (Olin et al., 1999). New therapies are needed to both directly help patients and to decrease the costs related to these wounds.
In terms of using ultrasound to treat venous ulcers, the common knowledge appears to be that reported in the review (Uhlemann et al., 2003), where it was concluded that “the quality of studies supporting the effectiveness of ultrasound as a therapeutic modality in wound healing is rather poor” and hence “the scientific basis for the use of ultrasound in wound healing is not well established.” The outcome of the research presented herein not only expands the knowledge base related to interaction of ultrasound and biological tissue but also supports the notion that the use of low-intensity, low-frequency ultrasound is beneficial for the treatment of chronic venous ulcers.
The primary decisions for ultrasound parameters involved choice of frequency range and intensity level used in insonification. To the best of the authors' knowledge, Dyson and her associates should be credited for their pioneering efforts of promoting in vivo therapeutic ultrasound treatment of venous ulcers (Dyson et al., 1976), performed at 1 W/cm2 and a frequency of 3 MHz, with treatment times ranging from 5–10 min, depending on the size. In a later publication, Young and Dyson (1990) suggested that 1 MHz ultrasound at an intensity (SATA) of 100 mW/cm2 could be useful in accelerating the inflammatory and early proliferative stages of repair in full-thickness acute wounds in a rat model. The intensity level not exceeding 100 mW/cm2 (spatial peak temporal peak) was selected intentionally partly because it follows Dyson’ and her team's findings and also because it is considered safe even at prolonged (>250 min) exposure time, according to the 2008 statement on mammalian in vivo ultrasonic biological effects from the American Institute of Ultrasound in Medicine (AIUM, 2008). In the same Young study (Young and Dyson, 1990), it was reported that the application of somewhat lower frequency (750 kHz vs 1 MHz) resulted in faster healing, when compared the treatment outcome obtained at the higher frequency (3 MHz). They ascribed this effect to non-thermal ultrasound mechanisms, such as stable cavitation and cavitation-related acoustic microstreaming, which are more likely to be present at the lower frequency. From the perspective of this work, it should be reiterated that the outcomes of Young and Dyson (1990) were observed in an acute wound model, as opposed to the chronic wound environment found in venous ulcers and considered here. In another relevant study (Peschen et al., 1997), 24 chronic venous ulcer patients were exposed to 30 kHz continuous wave ultrasound at 100 mW/cm2 intensity for 10 min, three times a week. This treatment resulted in enhanced rate of wound healing (55.4% reduction in the treatment group vs 16.5% reduction in the control group). In an in vitro study (Doan et al., 1999) of 1 MHz (25% duty cycle at intensities ranging from 0.1 to 1 W/cm2) and 45 kHz (continuous wave at intensities 5–50 mW/cm2) ultrasound on fibroblasts, osteoblasts, and monocytes, it was determined that the 45 kHz treatment resulted in increased (compared with 1 MHz treatment) production of collagen and non-collagenous proteins. Based on the above described works and additional comprehensive literature search, including a review of the results of Sundaram et al. (2003), the ultrasound field exposure parameters presented in this study [100 mW/cm2ISPTP with a pulse repetition frequency (PRF) of 1 Hz and duty cycle of 50%] were eventually chosen.
As already noted, this paper describes a 20 patient pilot study, during which patients suffering from venous ulcers were exposed to ultrasound with the goal of determining the effects of acoustic energy on wound healing. The efficacy of the ultrasound was determined by tracking reduction in wound size on a weekly basis. Following the in vivo study, two additional in vitro experiments were performed using the ultrasound parameters from the best performing group to confirm and examine the anticipated cellular effects of the ultrasound treatment.
More specifically, the auxiliary in vitro studies were designed to provide insight into the elements of ultrasound exposure which produce the largest cellular response as it relates to wound healing. The working hypothesis for this study was that the insonification caused ultrasound cell proliferation (mitosis) and concurrently increased cellular metabolic activity. Fibroblast cells were chosen as they have been previously shown by Doan et al. (1999) to respond to low frequency (45 kHz), low-intensity (30 mW/cm2) ultrasound. Both intensity and PRF [shown to be biologically relevant by Lewin and Chivers (1980)] were anticipated to play a role in ultrasound assisted wound healing, so they were included as two additional, adjustable variables in order to determine their effects on cellular metabolism (effects of the varying of PRF and intensities were not studied in the cellular proliferation experiment).
MATERIALS AND METHODS
Therapeutic tool: Ultrasound applicator
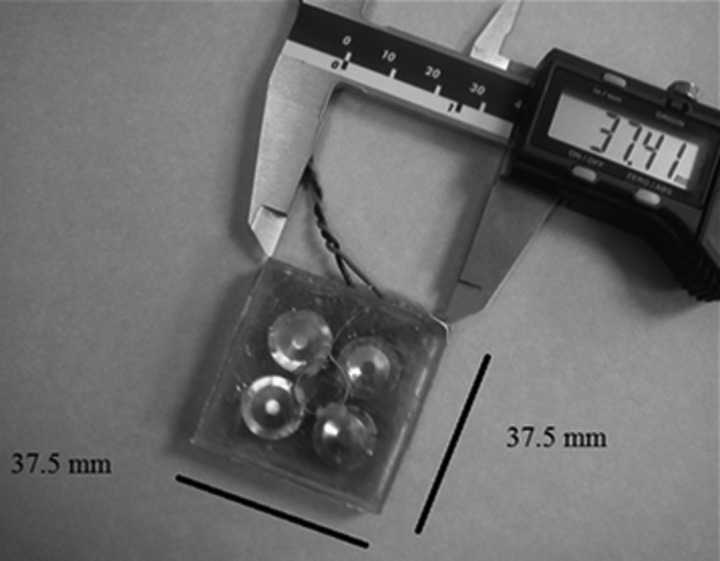
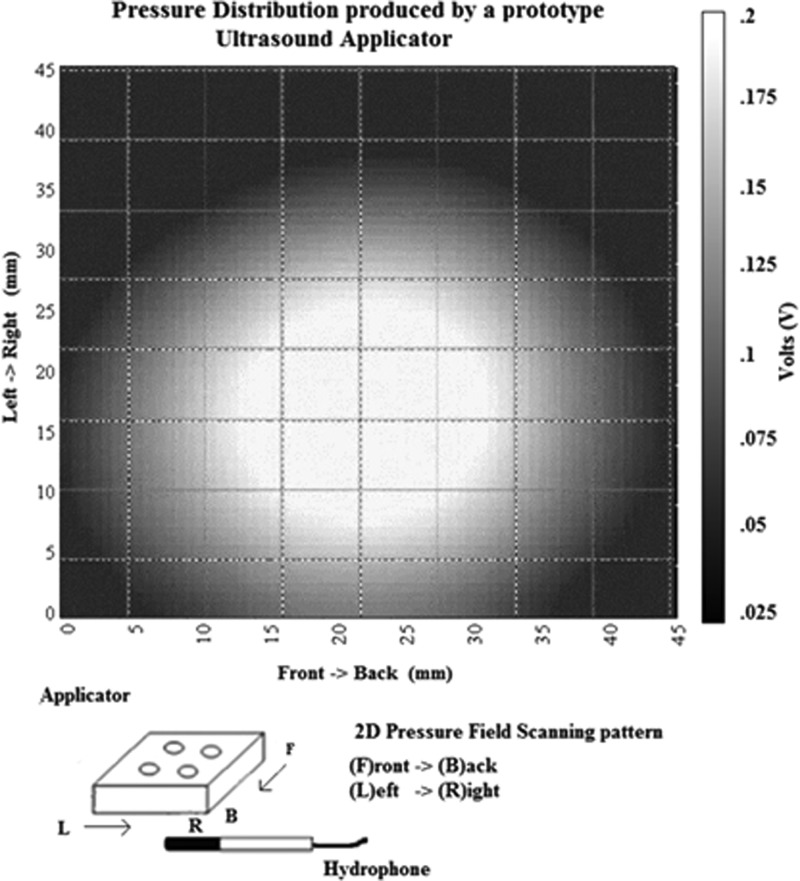
The construction and operation of the flexural transducer used in this study was described in detail in Sunny et al. (2012). Briefly, the applicator, shown in Fig. 1, was driven by a tone-burst with 1 Hz PRF and 500 ms pulse duration, from a custom rechargeable battery-operated driving unit. The light weight (<100 g) small foot print (approximately 35 mm × 35 mm × 10 mm) applicator was designed to be suitable for tether-free, fully portable wound healing applications. The acoustic output of the applicator was determined in 22 °C de-ionized water, using a calibrated hollow cylinder hydrophone, with a diameter of 2 mm (Lewin and Chivers, 1981). The pressure amplitude map of the applicator's acoustic output distribution is shown in Fig. 2. The pressure measurements were performed at a distance of 2.5 mm away from the transmitting surface of the transducer (to mimic a clinical application), and the applicator pressure amplitude was set to 55 kPa (100 mW/cm2ISPTP) prior to treatment. It has previously been determined that the transducer did not experience any self-heating and resulted in less than a 1 °C increase in skin temperature with a 45 min application.
Figure 1.
Photograph of flexural ultrasound applicator used in both in vivo human study as well as in vitro fibroblast study. Within the transducer, the four piezoelectric elements can be seen. The specific spacing of the elements created a uniform field output, as depicted in Fig. 2.
Figure 2.
Two-dimensional beam plot showing ultrasound field distribution of the flexural ultrasound applicator at the axial distance of 2.5 mm. It can be seen that the individual elements in the applicator work synergistically to create a uniform field distribution.
Wound size determination: Digital camera
At the end of each treatment session, a digital photograph was taken using a Fujifilm Finepixs700 digital camera (Fujifilm Holdings Corp, Tokyo, Japan). Each picture had a 1 in. marker in the frame, which allowed for the determination of the scale of the picture for precise determination of wound size. The wound boundaries were traced by hand and the surface area was calculated using a previously described program (Papazoglou et al., 2010) created using matlab software (MathWorks Inc., Natick, MA).
Patient selection
Twenty patients with chronic venous stasis ulcers were recruited from Drexel University Wound Healing Center. The study protocol was reviewed and approved by the Drexel University College of Medicine Institutional Review Board (IRB). Eligible patients were between the ages of 18 and 65, had a documented venous ulcer for at least 8 weeks with a surface area at least 1 cm2 [as determined by standard length times width ruler measurement (Langemo et al., 2008) by the nursing staff] and had a CEAP (a classification system comprised of clinical severity, etiology, anatomy, and pathophysiology) clinical severity of 6. Subjects with moderate to severe vascular insufficiency (ankle brachial index < 0.75 or toe-brachial index < 0.5) were excluded, as it impairs tissue oxygen perfusion and wound healing (Wallace and Stacey, 1998).
All enrolled subjects continued to have standard wound care treatment as determined by the physician, including weekly or bi-weekly debridement, compression therapy, edema control, and any prescribed topical therapies. Upon enrollment, subjects were randomly assigned to one of four experimental groups: 15 min of 20 kHz ultrasound, 45 min of 20 kHz ultrasound, 15 min of 100 kHz ultrasound, or 15 min of sham (no treatment). All active treatments were at 100 mW/cm2 SPTP. Due to the non-normality of distribution of wound initial sizes and ages of the wounds, the logarithmic transforms of the areas and ages were calculated to facilitate comparisons and later statistical analysis. The transform technique is described by Gelfand et al. (2002). The calculated values for each group are presented in Table TABLE I..
TABLE I.
Population data, where the numbers in parentheses indicate standard deviation.
| Sham, 15 min (n = 5) | 20 kHz,15 min (n = 5) | 20 kHz, 45 min (n = 5) | 100 kHz, 15 min (n = 5) | |
|---|---|---|---|---|
| Mean ulcer duration in log weeks (SD) | 0.67 (1.23) | 0.93 (1.13) | 1.64 (1.04) | 1.54 (1.56) |
| Mean ulcer size in log cm2 (SD) | 2.71 (1.20) | 2.63 (0.85) | 3.04 (2.47) | 4.52 (1.80) |
Patient treatment
During each treatment session, topical anesthetic (typically Lidocaine) was applied to the wounds before ultrasound insonification. The ultrasound applicator, wrapped in an acoustically transparent sterile wound dressing (Tegaderm®, 3 M, St. Paul, MN) and coated with sterile ultrasound gel, was placed directly on the wound and affixed using surgical tape (see Fig. 3). The applicator was then turned on for the amount of time dictated by the patient's experimental group. Following insonification, the applicator was removed and a photograph for wound size determination was taken. There were no adverse events reported throughout the pilot study.
Figure 3.
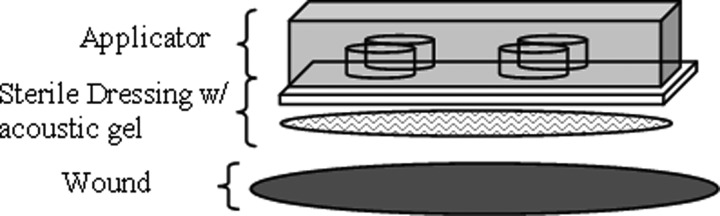
In vivo experimental setup showing ultrasound applicator covered with an acoustically transparent wound dressing with ultrasound gel coupled to the wound bed. The entire setup is attached to the wound with surgical tape.
Fibroblast preparation and ultrasound exposure
Mouse fibroblasts (3T3-swiss albino cells) were purchased from American Type Culture Collection and were all used between passage numbers 7 and 15 (a method of tracking how many times a cell populations has been re-cultured). The fibroblasts, chosen due their documented role in wound healing (Moulin et al., 1996), were seeded in sterile six-well transparent plates at 1 × 104 and 2 × 104 cells/well (two concentrations were used to rule out any possible effects on the assays used; p > 0.4) in 2 mL of Dulbecco's modified Eagle's medium (Life Technologies, Carlsbad, CA) with 10% bovine sera. The six-well plates were then sealed off to maintain the sterile environment. The cells were cultured between 18 and 20 h to allow cells to adhere to the wells and begin cell growth cycle, throughout which cells were maintained in an incubator at 37 °C, with 5% CO2 and 80%–90% humidity. After this initial growth period, cells were removed from incubation for exposure with the ultrasound, which occurred at room temperature.
For the cellular metabolism in vitro experiment, all cells were exposed to 20 kHz ultrasound for 15 min (chosen based on results of the in vivo treatment). To investigate whether the ultrasound parameters could be manipulated to produce the greatest cellular response relative to the control group, two additional pulse repetition frequencies (10 and 20 Hz) and intensities (50 and 200 mW/cm2) were studied in addition to those from the human study. The complete exposure matrix for the cellular metabolism study is shown in Table TABLE II.. The exposure parameters of Table TABLE II. marked in gray match those used in the human study. Each exposure parameter was repeated six times.
TABLE II.
Ultrasound exposure matrix for in vitro fibroblast study. Shaded areas correspond to human subjects' exposure. Intensity presented as spatial peak, temporal peak.
| Intensity (mW/cm2) | 50 | 100 | 200 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pulse repetition frequency (Hz) | 1 | 10 | 20 | 1 | 10 | 20 | 1 | 10 | 20 |
A final in vitro experiment was conducted to investigate cellular proliferation as it relates to ultrasound treated cells. The treated cells for this experiment were exposed to 20 kHz ultrasound, 1 Hz PRF, and 100 mW/cm2 intensity for 15 min (a combination of exposures found to produce the greatest response relative to the control groups from the prior two studies, shown in Sec. 3).
Prior to the complete description of the in vitro experimental setup, it is important to note that the potential of introducing undesirable artifacts during the insonification was recognized and given thoughtful consideration. Specifically, the geometry of the exposure container (see Fig. 4) was intentionally chosen to closely mimic the in vivo exposure conditions. Similarly, the biological assays chosen were carefully selected to, respectively, determine cellular metabolism (alamarBlue) and proliferation (BrdU).
Figure 4.
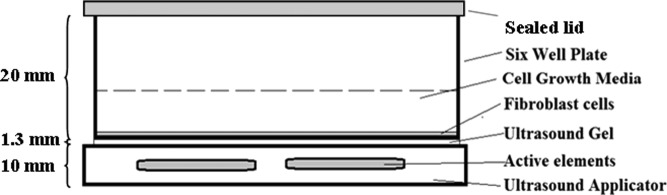
In vitro experimental setup showing ultrasound applicator and sterile plate containing fibroblasts in a growth media.
When the cells from both experiments were ready for insonification, the ultrasound applicator (Fig. 1) was placed below the six-well plate to transfer the ultrasound energy in a non-contact, sterile manner, shown in Fig. 4. The geometry of the experimental setup was chosen to mimic (as closely as possible) the in vivo clinical exposure conditions. The acoustic output of the applicators was adjusted to account for any attenuation experienced due to the relatively thin (1.3 mm, or 0.017 λ) plastic bottom of the well plate. Treatment and control (sham) exposures were performed simultaneously in separate well plates, to minimize any potential variability between samples, while eliminating concerns of sham group exposure to any potential reflected ultrasound. Due to the relatively long (75 mm) wavelengths of 20 kHz ultrasound, the pressure gradient across the sample (∼2 mm) at any time point was negligible. With this, the experimental setup was not subject to nodes or anti-node formation within the medium and therefore was not a concern. Even in the worst case scenario, cells would be exposed to a maximum of 200 mW/cm2ISPTP, which, under the experimental conditions used here, complies with FDA safety guidelines. After exposure, cells were cultured for an additional 24 h prior to the cellular metabolism and proliferation assays.
Cellular metabolism assay
After a 24-h incubation period following insonification, the cell medium was replaced with fresh solution of 90% media with a 10% alamarBlue (Invitrogen Corp., Carlsbad, CA), a reagent used for assessing cellular metabolism in vitro (Voytik-Harbin et al., 1998). This reagent assesses cellular metabolism by showing an increase in the level of fluorescence as nutrients in the media are consumed. This level was then measured using a fluorescence reader (Tecan US Inc., Morrisville, NC) with an excitation wavelength of 560 nm and an emission wavelength of 590 nm. The fluorescence of the treatment cells was compared to the control cell plates to determine percent increase. There were six wells for each of the treatment and control groups.
Cellular proliferation assay
After the same 24-h incubation period following insonification, cell proliferation was measured using a 5-bromo-2′-deoxyuridine (BrdU)assay, a standard test for measuring cellular proliferation (Porstmann et al., 1985). The BrdU calorimetric assay procedure was performed as per the manufacturer's protocol (Roche Scientific, Indianapolis, IN). The cell media was removed and BrdU, diluted 1:1000 in media with 10% sera, was added to the cells for 3 additional hours of incubation prior to adding the fixing and denaturing agents from the kit. The newly synthesized cellular DNA that incorporated the BrdU was then labeled using a BrdU labeling agent which attached to the BrdU. The reaction product was then quantified by measuring the absorbance in a fluorescence reader (Tecan US Inc., Morrisville, NC) at 370 nm (referenced at 492 nm). The absorbance of the treatment cells was then compared to the control cell samples to determine relative changes(%) in proliferation. There were six samples for each of the treatment and control groups.
RESULTS
Clinical, in vivo study
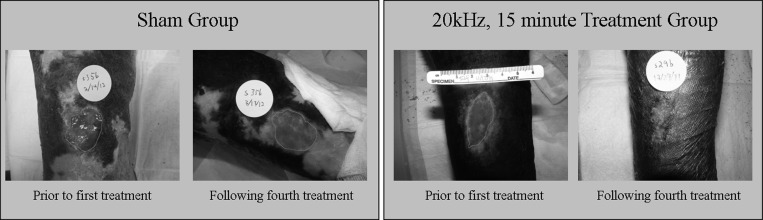
Of the total 20 patients who enrolled in the study, 10 subjects healed and 10 did not heal during the four treatments of the study. Eight of the 15 treated ulcers healed in the four treatment time frame in comparison to two of the five in the sham group. One particular group, namely, those patients receiving 20 kHz for 15 min, experienced complete healing in 100% (n = 5) of subjects by the fourth treatment. A complete list of outcomes is given in Table TABLE III.. Representative photos of a non-healing and healing patient from the sham and 20 kHz, 15 min treatment group are shown in Fig. 5.
TABLE III.
Healing outcomes by experimental groups.
| Healing (number of subjects) | Non-healing (number of subjects) | |
|---|---|---|
| Sham | 2 | 3 |
| 20 kHz, 15 min | 5 | 0 |
| 20 kHz, 45 min | 1 | 4 |
| 100 kHz, 15 min | 2 | 3 |
Figure 5.
(Color online) Representative wound photos from a non-healing ulcer in the sham group (left) and a healing ulcer in the 20 kHz, 15 min treatment group (right). Images feature line tracing the wound perimeter as part of the wound sizing software.
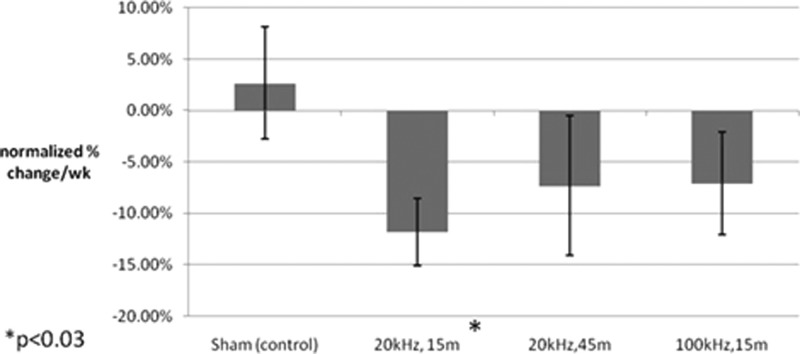
As already noted, the progress of healing was assessed through wound size reduction. All wound areas were normalized to the initial size. This was done to facilitate intra-wound comparisons due to the variance in the initial wound sizes (0.7–22 cm2). Therefore, the percent reductions from the normalized initial wound sizes for each treatment type were plotted in Fig. 6.
Figure 6.
Comparison of percent change in wound size by experimental group as measured using manually traced images which were analyzed using computer software designed by this lab group. Bars indicate standard error.
The results presented in Fig. 6 indicate that the average rate of closure for the 20 kHz, 15 min treatment group was statistically (p < 0.03) improved, resulting in faster reduction in area per week compared to the sham group, as determined by a one-tailed student's t-test. Although the mean rate of change in wound size was negative (indicating healing) in the other two treatment groups (20 kHz, 45 min and 100 kHz, 15 min), the differences were not statistically significant from those obtained with sham, which had an average increase in wound size of 3% per week (n = 5).
In addition, it should be noted that the average initial wound size for the five human subjects in the 20 kHz, 15 min treatment group was nearly identical to the average non-healing ulcer in the sham group (4.8 cm2 vs 4.7 cm2), showing that wounds of the same size, when treated with ultrasound did heal, whereas those not-treated did not heal. Furthermore, the five healing wounds of the 20 kHz, 15 min treatment group had an initial size which was nearly twice as large as the average healing ulcer for the overall study (4.8 cm2 vs 2.7 cm2), highlighting the efficacy of this particular treatment. A post hoc analysis, using g*power software described in (Erdfelder et al., 1996), determined that the effect size for the 20 kHz, 15 in treatment compared to the sham was 0.96 (>0.5 is generally considered to be a large effect size). This small pilot study was not large enough, nor was it intended, for power analysis. A larger follow-up study is being prepared.
In vitro results
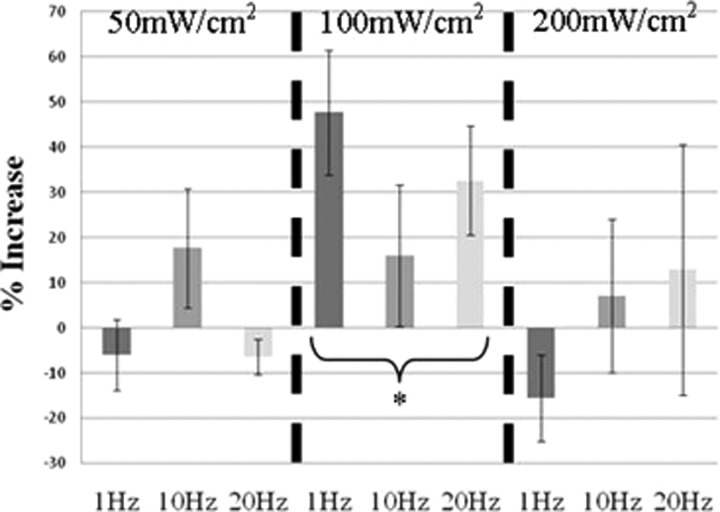
The results of the cellular metabolism assay comparing the various treatment groups to the control are shown below in Fig. 7. A two-factor analysis of variance determined that whereas there was no statistical difference between the applied pulse repetition frequencies (p > 0.05), the intensity was statistically significant (p = 0.021). A post hoc Tukey test (a typically used method to evaluate which means are statistically different from one another in a multiple group analysis) confirmed that the 100 mW/cm2 group statistically outperformed both the 50 mW/cm2 group (p < 0.05) and the 200 mW/cm2 group (p < 0.04).
Figure 7.
Average increase in metabolic activity of fibroblast cells for cells exposed to ultrasound with respect to cells that were not exposed to ultrasound (incubator control/sham). The groups are separated into the three intensity levels (50 100, and 200 mW/cm2 SPTP), and within each level, the dark gray bar represents the 1 Hz PRF, the medium gray represents 10 Hz PRF, and the light gray represents 20 Hz PRF. Bars indicate standard error with six repetitions of each condition. *p < 0.05.
The follow-up in vitro experiment measuring cellular proliferation had similarly encouraging results. The cells receiving ultrasound treatment (20 kHz for 15 min at 100 mW/cm2) had an average of 35% increased cellular proliferation when compared to the control cells, a statistically significant increase (p < 0.01).
DISCUSSION
The outcome of the human study described provides valuable insight into the possibility of using ultrasound energy in chronic wound management. The initial results show that there may be a dose response (here defined as an optimal combination of frequency, PRF, and intensity resulting in various amounts of total energy, in J, or energy density, in J/cm2) which is worth further investigation in future studies. In both the cellular metabolism and human studies, the group which received the 15 min of 20 kHz (633 J or ∼63 J/cm2) ultrasound at 1 Hz PRF outperformed the other treatment doses. Larger doses (i.e., those received by the 45 min at 20 kHz ultrasound or the higher intensity 200 mW/cm2in vitro group) did not show improvements in proliferation or wound healing compared to the lower (633 J) treatment dose. These findings seem to be supported by those reported by Johns (2002), whose review paper explores previously reported efficacy of different frequencies (45 kHz–3 MHz) and doses (2–150 J/cm2) and suggests that different exposure parameters may require unique energy densities or doses to reach therapeutic efficacy.
With respect to reduction in wound size, all three treatment groups showed a net reduction in wound size, whereas the sham had an average increase in wound size. As the goal of this pilot study was to both determine whether ultrasound promoted wound healing is achievable as well as to determine which ultrasound parameters had the greatest effect (in terms of rate of wound closure and total number of healed subjects), the treatment outcome indicates that 20 kHz insonification for 15 min is the most promising to be further examined with the larger cohort of subjects.
It is worthwhile to note that whereas the inclusion criteria dictated that enrolled wounds were at least 1 cm2 in initial size, the initial size determination was completed using the standard ruler method, which measured the longest axis of the wound and multiplied it by the longest perpendicular width (Langemo et al., 2008) and statistically (p < 0.001) overestimated true area by up to 44%. In contrast, post hoc digital image analysis was conducted using the aforementioned tracing and computational calculation of wound area. This digital analysis method, which is accurate to 0.1 cm2 (Keast et al., 2004), revealed that three wounds were less than 1 cm2 in area at the time of enrollment. However, as these wounds were in three separate groups it was deemed that they would not affect statistical analysis and they were included in the 20 subject sample.
The promising in vitro fibroblast study results indicate that low intensity, low frequency ultrasound promotes healing from the cellular level. The safe levels of ultrasound did not cause thermal effects or damage to the fibroblasts during insonification. Increased cellular metabolism and subsequent proliferation play vital roles in the wound healing process. The in vitro study data also indicated that PRF did not play a role in increasing cellular metabolism. To further determine how ultrasound influences the healing process, collagen assays will be performed in future experiments, as collagen production leads to the extracellular matrix comprising granulation tissue, which predominates the proliferative phase of wound healing. Future human studies will follow a larger cohort of subjects for a longer period of time (up to 12 treatments) and will assess the optical properties of edema using an optical spectroscopy system. Additionally, the geometry of the active elements within the applicator will be systematically modified to allow customized and personalized treatment based upon the wound shape and area. In conclusion, the data presented appear to support the notion that low-intensity (100 mW/cm2) low-frequency (20 kHz) ultrasound accelerates the healing process through increased cell proliferation and metabolism determined by in vitro studies and a faster rate of wound closure seen in a preliminary human pilot study.
ACKNOWLEDGMENTS
This work was primarily supported by the NIH Grant No. 5 R01 EB009670. The authors would also like to thank the staff at the Drexel University Wound Healing Center for their assistance and use of their facilities. Three of the authors (J.A.S., C.R.B., and Y.S.) are grateful for their partial support received from the Calhoun Biomedical Engineering Endowment of Drexel University. Finally, the prematurely departed contribution of Dr. Elisabeth Papazoglou, who was instrumental in getting the diagnostic aspects of this research off the ground, is respectfully acknowledged.
References
- AIUM (2008). “ Statement on mammalian in vivo ultrasonic biological effects,” American Institute of Ultrasound in Medicine, Laurel, MD.
- Doan, N., Reher, P., Meghji, S., and Harris, M. (1999). “ In vitro effects of therapeutic ultrasound on cell proliferation, protein synthesis, and cytokine production by human fibroblasts, osteoblasts, and monocytes,” J. Oral Maxillofac. Surg. 57, 409–419. 10.1016/S0278-2391(99)90281-1 [DOI] [PubMed] [Google Scholar]
- Dyson, M., Franks, C., and Suckling, J. (1976). “ Stimulation of healing of varicose ulcers by ultrasound,” Ultrasonics 14, 232–236. 10.1016/0041-624X(76)90024-X [DOI] [PubMed] [Google Scholar]
- Erdfelder, E., Faul, F., and Buchner, A. (1996). “ GPOWER: A general power analysis program,” Behav. Res. Methods Instrum. Comput. 28, 1–11. 10.3758/BF03203630 [DOI] [Google Scholar]
- Gelfand, J. M., Hoffstad, O., and Margolis, D. J. (2002). “ Surrogate endpoints for the treatment of venous leg ulcers,” J. Invest. Dermatol. 119, 1420–1425. 10.1046/j.1523-1747.2002.19629.x [DOI] [PubMed] [Google Scholar]
- Gohel, M. S., Taylor, M., Earnshaw, J. J., Heather, B. P., Poskitt, K. R., and Whyman, M. R. (2005). “ Risk factors for delayed healing and recurrence of chronic venous leg ulcers—An analysis of 1324 legs,” Eur. J. Vasc. Endovasc. Surg. 29, 74–77. 10.1016/j.ejvs.2004.10.002 [DOI] [PubMed] [Google Scholar]
- Johns, L. D. (2002). “ Nonthermal effects of therapeutic ultrasound: the frequency resonance hypothesis,” J. Athletic Training 37, 293. [PMC free article] [PubMed] [Google Scholar]
- Keast, D. H., Bowering, C. K., Evans, A. W., Mackean, G. L., Burrows, C., and D'Souza, L. (2004). “ MEASURE: A proposed assessment framework for developing best practice recommendations for wound assessment,” Wound Repair Regen. 12, s1–s17. [DOI] [PubMed] [Google Scholar]
- Langemo, D., Anderson, J., Hanson, D., Hunter, S., and Thompson, P. (2008). “ Measuring wound length, width, and area: Which technique?,” Adv. Skin Wound Care 21, 42. 10.1097/01.ASW.0000284967.69863.2f [DOI] [PubMed] [Google Scholar]
- Lewin, P., and Chivers, R. (1980). “ On viscoelastic models of the cell membrane,” Acoust. Lett. 4, 85–89. [Google Scholar]
- Lewin, P. A., and Chivers, R. C. (1981). “ Two miniature ceramic ultrasonic probes,” J. Phys. E 14, 1420–1424. 10.1088/0022-3735/14/12/017 [DOI] [Google Scholar]
- Lindblad, W. (2000). “ Animal models in wound healing research: do we need more?,” Wound Repair Regen. 8, 81. [DOI] [PubMed] [Google Scholar]
- Margolis, D. J., and Cohen, J. H. (1994). “ Management of chronic venous leg ulcers: A literature-guided approach,” Clin. Dermatol. 12, 19–26. 10.1016/0738-081X(94)90253-4 [DOI] [PubMed] [Google Scholar]
- Moulin, V., Castilloux, G., Jean, A., Garrel, D., Auger, F., and Germain, L. (1996). “ In vitro models to study wound healing fibroblasts,” Burns 22, 359–362. 10.1016/0305-4179(95)00167-0 [DOI] [PubMed] [Google Scholar]
- Nelzen, O. (2000). “ Leg ulcers: Economic aspects,” Phlebology 15, 110–114. 10.1007/s005230070005 [DOI] [Google Scholar]
- Olin, J. W., Beusterien, K. M., Childs, M. B., Seavey, C., McHugh, L., and Griffiths, R. I. (1999). “ Medical costs of treating venous stasis ulcers: evidence from a retrospective cohort study,” Vasc. Med. 4, 1–7. [DOI] [PubMed] [Google Scholar]
- Papazoglou, E. S., Zubkov, L., Mao, X., Neidrauer, M., Rannou, N., and Weingarten, M. S. (2010). “ Image analysis of chronic wounds for determining the surface area,” Wound Repair Regen. 18, 349–358. 10.1111/j.1524-475X.2010.00594.x [DOI] [PubMed] [Google Scholar]
- Peschen, M., Weichenthal, M., Schopf, E., and Vanscheidt, W. (1997). “ Low-frequency ultrasound treatment of chronic venous leg ulcers in an outpatient therapy,” Acta Derm. Venereol. 77, 311–314. [DOI] [PubMed] [Google Scholar]
- Phillips, T. J., and Dover, J. S. (1991). “ Leg ulcers,” J. Am. Acad. Dermatol. 25, 965–987. 10.1016/0190-9622(91)70295-D [DOI] [PubMed] [Google Scholar]
- Porstmann, T., Ternynck, T., and Avrameas, S. (1985). “ Quantitation of 5-bromo-2-deoxyuridine incorporation into DNA: An enzyme immunoassay for the assessment of the lymphoid cell proliferative response,” J. Immunol. Meth. 82, 169–179. 10.1016/0022-1759(85)90236-4 [DOI] [PubMed] [Google Scholar]
- Sen, C., Gordillo, G., Roy, S., Kirsner, R., Lambert, L., Hunt, T., Gottrup, F., Gurtner, G., and Longaker, M. (2009). “ Human skin wounds: A major and snowballing threat to public health and the economy,” Wound Repair Regen. 17, 763. 10.1111/j.1524-475X.2009.00543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram, J., Mellein, B. R., and Mitragotri, S. (2003). “ An experimental and theoretical analysis of ultrasound-induced permeabilization of cell membranes,” Biophys. J. 84, 3087–3101. 10.1016/S0006-3495(03)70034-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunny, Y., Bawiec, C. R., Nguyen, A. T., Samuels, J. A., Weingarten, M. S., Zubkov, L. A., and Lewin, P. A. (2012). “ Optimization of un-tethered, low voltage, 20–100 kHz flexural transducers for biomedical ultrasonics applications,” Ultrasonics 52, 943–948. 10.1016/j.ultras.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlemann, C., Heinig, B., and Wollina, U. (2003). “ Therapeutic ultrasound in lower extremity wound management,” Int. J. Lower Extremity Wounds 2, 152. 10.1177/1534734603257988 [DOI] [PubMed] [Google Scholar]
- Voytik-Harbin, S. L., Brightman, A. O., Waisner, B., Lamar, C. H., and Badylak, S. F. (1998). “ Application and evaluation of the alamarBlue assay for cell growth and survival of fibroblasts,” In Vitro Cell. Dev. Biol.: Anim. 34, 239–246. 10.1007/s11626-998-0130-x [DOI] [PubMed] [Google Scholar]
- Wallace, H. J., and Stacey, M. C. (1998). “ Levels of tumor necrosis factor-α (TNF-α) and soluble TNF receptors in chronic venous leg ulcers—Correlations to healing status,” J. Invest. Dermatol. 110, 292–296. 10.1046/j.1523-1747.1998.00113.x [DOI] [PubMed] [Google Scholar]
- Young, S. R., and Dyson, M. (1990). “ Effect of therapeutic ultrasound on the healing of full-thickness excised skin lesions,” Ultrasonics 28, 175–180. 10.1016/0041-624X(90)90082-Y [DOI] [PubMed] [Google Scholar]