Abstract
Objective
Developmental changes at the interface of affective and cognitive systems are examined over a three year period in pediatric bipolar disorder (PBD).
Methods
Thirteen participants with PBD and 10 healthy controls (HC) matched on demographics and IQ were scanned at baseline, 16 weeks, and after three years. All patients received pharmacotherapy based on a medication algorithm. A pediatric affective color matching paradigm was used to probe cognitive processing under emotional challenge.
Results
Behavioral data illustrated that patients in PBD group were slower in response time at baseline, but normalized by three years. On fMRI analyses at baseline, in response to emotional vs. neutral words, patients with PBD showed greater activation, relative to HC, in the right dorsal lateral prefrontal cortex (DLPFC) and amygdala, ventral lateral prefrontal cortex (VLPFC), bilateral anterior cingulate cortex (ACC), and ventral striatum. The increased activation in cortical areas in the PBD group normalized with no differences from HC by 16 weeks. By three years, normalization was observed in not only the cortical, but also the subcortical regions such as amygdala and striatum.
Limitations
These preliminary findings need to be replicated with a larger sample, although it can be challenging to obtain large samples for longitudinal studies.
Conclusions
Greater activation in fronto-striatal and fronto-limbic circuits were observed in unmedicated patients with PBD. With systematic pharmacotherapy for patients with PBD, the time course of recovery was characterized by initial prefrontal changes at 16 weeks, which extended to subcortical normalization at three years. These preliminary findings illustrated that, following appropriate treatment coupled with normal brain development, patients with PBD showed normalization in brain function.
Keywords: Pediatric bipolar disorder, Functional magnetic resonance imaging (fMRI), Emotion, treatment effects
1. Introduction
Pediatric bipolar disorder (PBD) is an early onset variant of the bipolar diathesis (Birmaher et al., 2009; Geller et al., 2002; Pavuluri et al., 2005), and patients are often burdened with persistent neurocognitive deficits, despite achieving symptomatic recovery in mood (Pavuluri et al., 2009). The cognitive deficits in the domains of attention, response inhibition and executive function correlate with poor academic performance (Pavuluri et al., 2005). Emerging findings highlight the impairment at the interface of cognitive and affective brain circuitry that is responsible for combined deficits in executive function and mood stability in PBD (Leibenluft et al., 2007; Passarotti et al., 2010a, 2010b; Rich et al., 2005). One important question, however, is whether the neural circuitry abnormalities in PBD normalize over time - either due to normative brain development or treatment. Longitudinal studies of neural circuitry abnormalities in PBD are critical as significant changes occur in the brain throughout childhood and adolescence (Casey et al., 2005) and thus, any examination of brain “normalization” in these patients should take into account normal developmental processes. Additionally, longitudinal studies have the potential in aiding with prognosis as well as estimating the utility of the treatment algorithms.
To date, few longitudinal studies of brain function have been conducted in patients with PBD. Gogtay et al. (2007) examined structural brain changes over time and found that adolescents with bipolar disorder exhibited an increase in grey matter density in the left temporal cortex and a decrease in gray matter density in the anterior cingulate cortex. In another study, Bitter et al. (2011) found that over one year, those with PBD did not exhibit the normative increase in amygdala volume that was found in healthy controls (HC). Despite these earlier clues from structural neuroimaging studies, little is known about the functional neuroanatomical changes that occur over time in PBD.
Normative development in adolescence has illustrated increased top-down regulation with maturation of fronto-limbic activity (Casey and Jones, 2010; Somerville et al., 2011; Somerville et al., 2010). Our recent studies have revealed that resting state limbic hyperconnectivity is associated with better cognitive and affective circuitry function (Wu et al., In press) and that increased amygdala engagement in affective circuitry was associated with response to short-term pharmacotherapy (Wegbreit et al., 2011). Untreated patients with PBD have demonstrated hyperactive amygdala, insufficiently regulated by VLPFC (Pavuluri et al., 2007; Pavuluri et al., 2008; Pavuluri et al., 2010). Furthermore, the DLPFC and striatum serve multiple functions including the cognitive modulation of emotion (Badgaiyan, 2010; Hare et al., 2008), attention (Saint-Cyr, 2003) and response inhibition (Padmanabhan et al., 2011). Untreated patients with PBD showed greater activity in the striatum during response inhibition, which normalized with eight weeks of pharmacotherapy (Passarotti et al., 2011a; Pavuluri et al., 2011). It is not clear if the activity of these affective and cognitive fronto-limbic and fronto-striatal brain regions during the steep adolescent developmental curve will continue to improve, partially recover, or remain abnormal in PBD.
The current study utilized the pediatric color matching task that has previously yielded consistently reliable findings (Passarotti et al., 2010c; Pavuluri et al., 2008; Pavuluri et al., 2011; Pavuluri et al., 2010; Wegbreit et al., 2011) to probe the interface of affective VLPFC-amygdala circuitry and the cognitive DLPFC-striatal circuitry in PBD and HC. We followed participants over a three year developmental period when the patients received systematic pharmacotherapy for optimal recovery. Based on the leads from conventional fMRI (Mayanil et al., 2011; Pavuluri et al., 2011), patients are predicted to show normalization relative to HC in cortico-subcortical fMRI activity. Alternatively, PBD patients might show partial recovery among these circuits with residual impairment.
2. Methods
2.1. Participants
The sample consisted of 13 individuals with PBD (type I and II) and ten IQ and demographically matched HC (Table 1). All patients were unmedicated at baseline and received pharmacotherapy based on a standardized medication algorithm (Pavuluri et al., 2004). Participants were aged 10–18 years and were clinically assessed and scanned at baseline (13.4 ± 2.5 years), at 16 weeks (16 ± 14 weeks), and at three years follow-up (3.2 ± 1.1 years, Table 1). This study was approved by the institutional review board at the University of Illinois at Chicago. Verbal or written assent was obtained from all of the participants in addition to written consent from parents. Inclusion criteria for patients were a diagnosis of BD type I (mixed N = 2, manic N = 6) or type II (N = 5) according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV; American Psychiatric Association 1994), and a baseline score greater than 12 on the Young Mania Rating Scale (YMRS) (Young et al., 1978). Six of the participants in the PBD group were comorbid for attention-deficit /hyperactivity disorder (ADHD). Inclusion criteria for HC were no current and past DSM-IV diagnosis for axis I disorders or a family history of affective illness, and a score less than 12 on the YMRS at baseline. Exclusion criteria for both groups were substance abuse through urine toxicology screen; serious medical problems (e.g., epilepsy); IQ less than 70 as determined by the Wechsler Abbreviated Scale of Intelligence (WASI) (1999); and contraindications to MRI studies including metallic implants, retractors or braces, and claustrophobia.
Table 1.
Demographic and Clinic Characteristics of Participants with PBD and HC
| HC (n=10) | PBD (n=13) | Analyses | |
|---|---|---|---|
| Variables | Mean (SD) | Mean (SD) | T(P) |
| Age (years) at Baseline | 13.61 (2.97) | 13.25 (2.27) | 0.33 (0.75) |
| IQ* | 105.10 (14.39) | 99.92 (17.17) | 0.77 (0.45) |
| YMRS at Baseline | 2.40 (2.59) | 25.62 (6.48) | 10.65 (0.000) |
| YMRS at 16 weeks | 0.78 (1.56) | 9.36 (12.74) | 2.00 (0.06) |
| YMRS at three years | 2.60 (1.58) | 8.54 (4.33) | 4.11 (0.000) |
| CDRS-R at Baseline | 19.20 (1.48) | 42.54 (18.19) | 4.03 (0.001) |
| CDRS-R at 16 weeks | 18.56 (1.01) | 25.00 (7.20) | 2.65 (0.02) |
| CDRS-R at three years | 18.80 (1.14) | 34.08 (12.09) | 3.96 (0.001) |
| N (%) | N (%) | χ2(P) | |
| Sex | |||
| Male | 7 (70) | 9 (69) | 0.002 (0.97) |
| Female | 3 (30) | 4 (31) | |
| Race | |||
| White | 6 (60) | 9 (69) | 0.21 (0.65) |
| Other | 4 (40) | 4 (31) | |
| Handedness | |||
| Right | 9 (90) | 11 (85) | 0.14 (0.70) |
| Left | 1 (10) | 2 (15) | |
| ADHD Comorbid | 0 (0) | 6 (46) | |
IQ was measured using WASI (Wechsler Abbreviated Scale of Intelligence);
PBD = Pediatric Bipolar Disorder; HC = Healthy Controls; YMRS = Young Mania Rating Scale; CDRS-R = Child Depression Rating Scale-Revised.
2.2. Clinical Assessment
Board-certified child psychiatrists completed the Washington University Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) (Geller et al., 1998) supplemented by the episode characterization of bipolar disorder from the KSADS – Present and lifetime version (Kaufman et al., 2000). Diagnostic interviews were also completed by doctoral-level clinicians with established inter-rater reliability (Cohen’s kappa = 0.94). Information from the interview and all other clinical measures were reviewed to make a consensus clinical diagnosis. The primary clinical measures were the YMRS and the Child Depression Rating Scale-Revised (CDRS-R) (Poznanski et al., 1984).
2.3. Pharmacotherapy
All PBD patients were medication free or were washed out of medication at baseline. Medications used in the treatment paradigm are summarized in Table 2. Psychostimulants were used in six of the 13 participants, and non-stimulants were used in 2 participants with PBD with comorbid ADHD, at the end of three years. The average number of medications received by each patient was 2.0 ± 1.1 at 16 weeks and 2.8 ± 1.5 at three years.
Table 2.
Medication Used to Treat Participants with Pediatric Bipolar Disorder
| Type of Medication | Baseline to 16 weeks n (%) |
16 weeks to three years n (%) |
|---|---|---|
| Mood stabilizers (Lithum, divalproex sodium, oxcarbazepine, lamotrigine) | 9 (82) | 11 (85) |
| Second-generation antipsychotics (resperidone, quetiapine, aripiprazole) | 5 (45) | 12 (92) |
| Psychostimulants (methylphenidate, long-acting form, mixed amphetamine salts long-acting form) | 4 (36) | 6 (46) |
| Non-stimulants (Atomoxetine) | - | 2 (15) |
| Serotonin reuptake inhibitors | 2 (18) | - |
| Benztropine | 1 (9) | 1 (8) |
2.4. Pediatric Affective Color Matching Task
The primary task for the study was the Pediatric Affective Color Matching Task. During the task, participants were asked to match the color of a word to one of two colored circles presented on the left and right side of the word on a display screen (Pavuluri et al., 2008). The target words were negative, positive, and neutral valence words. Negative words included themes such as depression, disappointment, and rejection. Positive words reflected feelings of positivity, such as happiness, energy, accomplishment, and success. Words across affect conditions were matched on frequency of use (Bradley, 1999; Gilhooly and Logie, 1980; Klein, 1964; Kucera et al., 1967) and were at an eight-year-old reading level.
In each three second trial, a word was presented for 200 ms, followed by a response period of 2800 ms, during which participants pressed a button (left or right) to match the color of the word to the correct color dot that appeared on either side of the words (Fig. S1). Participants were told to “respond as quickly as possible.” Participants were presented with separate blocks (30 s each) of positive, negative, and neutral word conditions (5 blocks of each condition) in a pseudo-random order, with each block consisting of ten trials. Each block was separated by a ten second fixation period (total of 15 fixation blocks), allowing the hemodynamic responses to return to resting level before the next block of trials. Trials were balanced for the location of correct responses (left or right dots) and the colors used. The total duration of the task was approximately ten min.
As described in previous studies (Pavuluri et al., 2008), the paradigm had the advantage of (1) assessing the impact of an emotional challenge on the cognitive control required to match the color; (2) presenting words briefly (200 ms) to limit prefrontal processing of affective valence and semantic meaning; and (3) requiring a relatively simple cognitive judgment and response. Visual stimuli were projected onto a screen that was viewed via a back projection system with a color high-resolution liquid crystal display projector. A camera monitored participant’s right eye to make sure they focused on the visual stimuli during the fMRI scan. To familiarize participants with the paradigm and the MR scanner environment, a mock scan was performed before imaging studies.
2.5. MRI Protocol
MRI studies were performed using a 3.0 Tesla whole body scanner (Signa, General Electric Medical System, Milwaukee, WI). Functional images were acquired using echo-planar imaging, which is sensitive to regional alterations in blood flow via blood oxygen level-dependent (BOLD) contrast effects. Parameters for functional scans were: TE=25 ms; flip angle=90°; field of view=20×20 cm; acquisition matrix=64×64; TR=2.5 s; 5 mm slice thickness with 1 mm gap. Anatomical images were acquired in the axial plane (three-dimensional spoiled gradient recalled, 1.5 mm thick contiguous axial slices) to coregister and normalize the functional data.
2.6. Clinical and Behavioral Data Analysis
Reaction time and accuracy for words (negative, positive, neutral), time (baseline, 16 weeks, three years), and groups (PBD, HC) were analyzed using repeated measures ANOVAs. Clinical and demographic data were evaluated by t tests and χ2.
2.7. Image Processing and Data Analysis
The fMRI data were analyzed with FEAT (FMRI Expert Analysis Tool) Version 4.1.8, a tool of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). Preprocessing included motion correction, slice-timing correction, spatial smoothing (a 8mm Gaussian filter at full-width-half-maximum), intensity normalization using a grand mean scaling factor, and high-pass temporal filtering (sigma=100.0s) to remove low frequency drift. Brain extraction and registration to high resolution and template (Montreal Neurological Institute, MNI152) images were carried out by BET (Brain Extraction Tool) (Smith, 2002) and FNIRT (FMRIB's Nonlinear Image Registration Tool) (Jenkinson et al., 2002; Jenkinson and Smith, 2001; Klein et al., 2009), respectively. Time-series statistical analysis was performed by FMRIB's Improved Linear Model (Woolrich et al., 2001), in which a general linear model (GLM) framework of the stimulus onset types as explanatory variables, convolved with a hemodynamic response function (HRF) with time derivatives, was used to fit the time series data of each voxel.
During first level analysis, the GLM model was set up with three regressors: negative, positive, and neutral words. First level analyses consisted of contrast images for the negative vs. neutral as well as positive vs. neutral words for each individual. In the higher level analysis, we conducted whole-brain repeated measures ANOVA with two groups (PBD and HC), two word conditions (negative vs. neutral words, positive vs. neutral words), and two time points. Separate analyses evaluated three combinations of two time points (baseline to three years, baseline to 16 weeks, and 16 weeks to three years). Our primary interest was the baseline to three-year follow up, as we were interested in longitudinal change over the course of development. These analyses allowed us to examine activation changes in patients compared to controls, over the course of treatment and development, and whether these activations were moderated by word valence. Z statistical images were thresholded using clusters determined by Z > 2.3 and a corrected cluster significance threshold of p < 0.05 based on Gaussian random field (GRF) theory (Forman et al., 1995; Friston et al., 1993; Worsley et al., 1992). The relationship between brain responses and clinical measurements was assessed voxelwise in the whole brain by using YMRS and CDRS-R scores as covariates in regression analyses.
3. Results
3.1. Clinical and Demographic Characteristics
Demographic and clinical measurements are summarized in Table 1. No significant differences were found between PBD and HC groups for age, IQ, gender, race, and handedness. For the YMRS, there was a significant group by time interaction [F (2, 36) = 14.92, p < 0.01]. Follow-up analyses indicated that the YMRS scores in the PBD group decreased from baseline to three years [t(12) = 8.90, p < 0.01], but they remained higher than the HC’s YMRS scores at both time points [PBD vs. HC at baseline: t(21) = 10.65, p < 0.01; PBC vs. HC at three years: t(21) = 4.11, p < 0.01]. For the CDRS-R, there was also significant group by time interaction [F (2, 36) = 3.40, p <0.05]. Analogous to the YMRS, the CDRS-R scores in the PBD group tended to decrease from baseline to three years, but remained higher than the HC group, both at baseline [t(21) = 4.03, p < 0.01] and at the three year follow-up [t(21) = 3.96, p < 0.01].
3.2. Behavioral Data
Table 3 reports the average response time and accuracy for each group across time points. The ANOVAs revealed a main effect of group, reflecting slower response times for the PBD group compared to HC [F(3, 16) = 4.13, p < 0.05], as well as a main effect of time reflecting faster response times at 3-year follow-up compared to baseline [F(6, 13) = 3.45, p < 0.05]. No significant findings were found for accuracy at any time point (Table 3).
Table 3.
Response Time and Accuracy Measures for Participants with Pediatric Bipolar Disorder (PBD) and Healthy Controls (HC) at Baseline, 16 weeks, and three years
| HC Mean (SD) |
PBD Mean (SD) |
|||||
|---|---|---|---|---|---|---|
| Response time (ms) |
Baseline (n=10) | 16 weeks (n=9) |
Three years (n=10) |
Baseline (n=13) |
16 weeks (n=11) |
Three years (n=13) |
| Negative words | 553 (131) | 538 (101) | 499 (95) | 682 (199) | 685 (206) | 560 (116) |
| Positive words | 536 (109) | 563 (124) | 498 (107) | 749 (162) | 751 (206) | 527 (126) |
| Neutral words | 540 (124) | 558 (113) | 495 (110) | 741 (144) | 796 (222) | 531 (149) |
| Accuracy (%) | ||||||
| Negative words | 92 (16) | 93 (11) | 92 (9) | 81 (19) | 71 (37) | 87 (13) |
| Positive words | 91 (15) | 97 (7) | 97 (5) | 85 (16) | 73 (33) | 88 (14) |
| Neutral words | 90 (15) | 95 (8) | 93 (6) | 84 (18) | 71 (35) | 88 (15) |
PBD = Pediatric Bipolar Disorder; HC = Healthy Controls.
3.3. Imaging Data
Our focus was on the areas of the brain that responded differently between groups (PBD and HC) at two time points (baseline and three years), and whether this pattern was affected by the valence of words (negative, positive, neutral). The group by time by valence ANOVA revealed no three-way interactions, but did reveal a significant group by time (from baseline to three years) interaction in the right DLPFC [Broadmann Area (BA) 46], VLPFC [BA 47, right BA 45], bilateral anterior cingulate cortex (ACC), ventral striatum, and right amygdala (Figure 1A and Table 4). Follow-up analyses suggested that at baseline, the PBD group exhibited higher activation in the right DLPFC [t(21) = 2.34, p = 0.03] and bilateral ventral striatum [t(21) = 2.65, p = 0.02]. Interestingly, at three-year follow-up, the groups did not significantly differ on activation in these regions (Fig. 1D). We followed up above results to evaluate how things changed at 16 weeks. There was also a significant interaction of group by time (from baseline to 16 weeks) in the right DLPFC [Broadmann (BA) 46], and occipital cortex (Figure 1B and Table 4). From 16 weeks to three years, no interactions of group by time were found.
Figure 1.
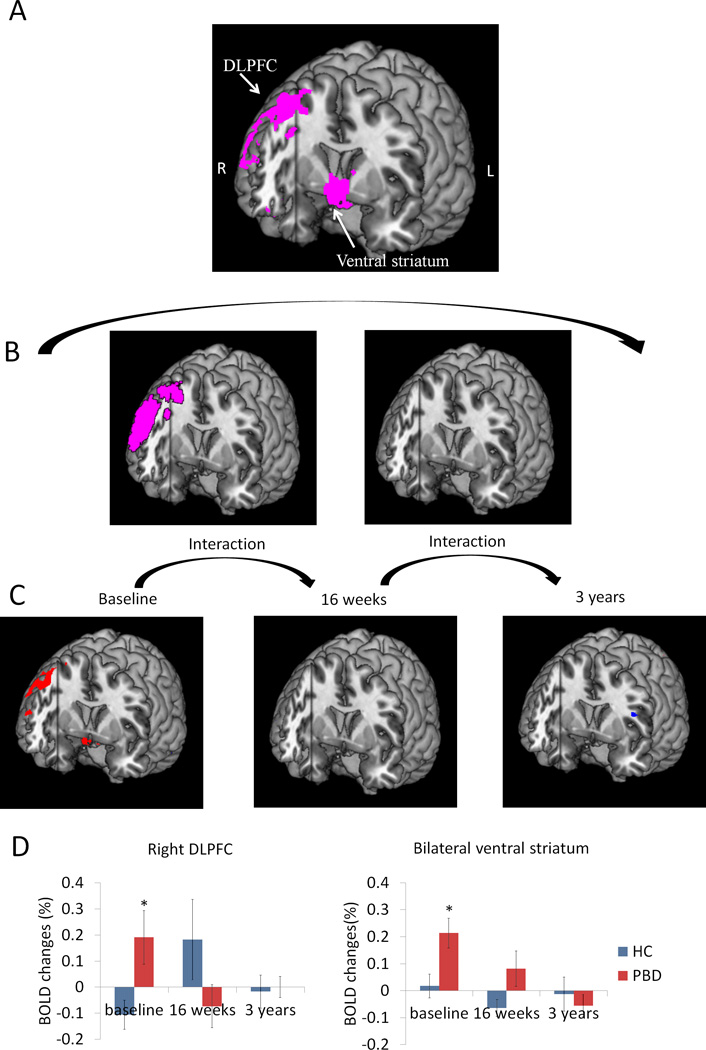
Group differences in activation during affective color matching paradigm were moderated by time. Panel A) PBD differed from HC in activation of right DLPFC and bilateral ventral striatum between baseline and 3-year follow up. B) Interactions between groups and time were also explored for baseline to 16 weeks (left) and for 16 weeks to three years (right). Significant group differences between baseline to 16 weeks were found, but not between 16 weeks to three years. C) Group difference at each time point is shown to inform interactions. In red are regions where PBD > HC. In blue are regions where HC > PBD. D) Blood-oxygenation level dependent (BOLD) signal changes are plotted for the right DLPFC and bilateral ventral striatum to elucidate the nature of group differences across time. The interaction between time and group was significant [F(4, 15) = 3.43, p = 0.04]. The PBD group showed higher levels of activation at baseline relative to HC [t(21) = 2.34, p = 0.03 for right DLPFC, t(21) = 2.65, p = 0.02 for bilateral ventral striatum], and over time, their activation normalized and became similar to that of the HC group.
Table 4.
ANOVA Group by Time Interaction Effect
| ANOVA group by time effect |
Talairach coordinates for peak values |
BA | Volume (mm3) |
Peak F values |
|---|---|---|---|---|
| Baseline - three years | ||||
| R DLPFC | 52, 32, 34 | 46 | 2856 | 19.89 |
| R VLPFC | 58, 26, 4 | 45 | 1952 | 17.82 |
| R VLPFC | 18, 32, −16 | 47 | 2520 | 13.90 |
| R Pregenual ACC | 6, 44, 18 | 32 | 3160 | 16.76 |
| L Pregenual ACC | −2, 40, 8 | 32 | 2352 | 12.55 |
| R Dorsal ACC | 2, 36, 18 | 32 | 2560 | 15.30 |
| L Dorsal ACC | −2, 36, 18 | 32 | 3256 | 13.94 |
| R Amygdala | 14, 6, −24 | 272 | 12.92 | |
| L Striatum | −2, 6, −6 | 984 | 12.92 | |
| R Striatum | 6, 8, −16 | 3216 | 18.07 | |
| Baseline - 16 weeks | ||||
| R DLPFC | 50, 30, 32 | 46 | 5544 | 19.38 |
| R Occipital cortex | 54, −74, 14 | 39 | 22996 | 27.39 |
| 16 weeks - three years | ||||
| None | ||||
Abbreviations: BA, Broadmann areas; ACC, anterior cingulate cortex; DLPFC, dorsal lateral prefrontal cortex; VLPFC, ventral lateral prefrontal cortex.
In summary, the group by time interaction was characterized by higher activation in the PBD group at baseline in the right DLPFC and amygdala, VLPFC, bilateral ACC and ventral striatum. There was a reduction of activity at 16 weeks in the DLPFC regions alone. By three years post treatment, the PBD group showed normalization of activation level in both cortical (DLPFC, VLPFC, ACC) and subcortical regions (amygdala and striatum).
3.4. Correlation between activation and clinical measurements
Within subject activation difference between baseline and 3 years was correlated with treatment response, defined as change in YMRS and CDRS-R scores between these time periods. No significant relationship between neural change and treatment response was found in whole-brain correlational analyses. However, the small number of PBD participants in this study (n=13) likely contributed to a lack of significant correlations, thus the absence of significance may not represent absence of relationship, especially given the significant clinical recovery in a carefully diagnosed sample.
4. Discussion
This is the first fMRI study to examine both the short (16 weeks) and long-term (three years) course of functional activity in PBD. The results of the present study revealed two distinct BOLD response patterns – one associated with short-term pharmacotherapy, and another with long-term treatment. Relative to HC, patients with PBD exhibited greater activation in the emotional and cognitive processing circuitries at baseline, including the right DLPFC, amygdala, bilateral VLPFC, ventral striatum, and ACC. After a 16 week short-term pharmacotherapy, heightened activation in right DLPFC was reduced in the PBD group. After three years of treatment, both cortical and subcortical regions showed normalization in the PBD group. Albeit preliminary, these results suggest that cortical and subcortical areas undergo differential rates of change in PBD, with initial cortical response followed by subcortical changes observed after longer treatment period. With regard to behavioral performance, although not significant, patients demonstrated longer response time relative to HC at all times points, but all participants showed improvement over time.
4.1 Cortical changes after pharmacotherapy in PBD
As a crucial subregion of prefrontal cortex, the DLPFC is involved in several cognitive processes (i.e., shifting attention, working memory, and response inhibition) as well as affect regulation processes along with VLPFC (Chang et al., 2004; Fichtenholtz et al., 2004; Manes et al., 2002; Phillips et al., 2003). We have previously found that PBD patients demonstrated enhanced activation in DLPFC at an untreated baseline state, which was followed by a treatment-related decrease using the same pediatric affective color matching task (Pavuluri et al., 2010). The present results largely replicated these findings. After medication treatment for a short period (16 weeks), there was a decrease in DLPFC activation in PBD, in which the overactivation observed at baseline reduced to a level that was similar to that of HC. Reductions in DLPFC overactivity may be a consistent early marker of normalization in cognitive and affect regulation processes during recovery, and this marker may be especially apparent during tasks that probe the interface of these functions.
We also found that bilateral VLPFC (BA 47 and 45) exhibited higher activity at baseline, but only normalized relative to HC after three years of treatment. It could be that VLPFC is normalized much later, relative to DLPFC changes, as it has the specific role of emotional control, having to modulate amygdala that also was normalized only after three years. The VLPFC is located just inferior to the DLPFC, and the VLPFC/DLPFC junction receives input from both limbic and superior temporal regions, suggesting that this is a region that integrates cognitive and affective functions (Petrides and Pandya, 2002). Functional activation studies support the role of the VLPFC in response inhibition and reward related processes (Casey et al., 1997; Garavan et al., 2002; Passarotti and Pavuluri, 2011b), which suggest, along with connectivity findings, that the VLPFC is crucial for regulating subcortical structures in emotional processing and impulse control (Aron et al., 2003; Botvinick et al., 2001; Menon et al., 2001; Petrides and Pandya, 2002). The elevated activation in untreated PBD at baseline has been reported by many studies that have employed different stimuli, including emotional words (Passarotti et al., 2010c) and neutral faces (Rich et al., 2006) in patients with both bipolar Type I and II. However, some studies also found that VLPFC showed reduced activation to emotional words and faces in untreated PBD Type I relative to HC (Pavuluri et al., 2007; Pavuluri et al., 2008). This discrepancy may be due to the task used or heterogeneity of PBD patients examined in these studies, such as bipolar disorder Type I only or Types I and II. It may be that patients with varying severity of illness would recruit VLPFC differentially to regulate subcortical structures during emotion processing. Further study of bipolar subtypes may clarify the impact of illness type and severity on brain functional changes.
Pregenual and dorsal ACC, regions involved in emotional and cognitive regulation (Bush et al., 2000), showed similarly reduced response to medication after three years of treatment, corresponding with the time frame of changes over the VLPFC. The present observations of increased activation in the ACC in untreated PBD patients (Passarotti et al., 2010c; Pavuluri et al., 2007; Pavuluri et al., 2008) and reduced activation after treatment (Pavuluri et al., 2011) are consistent with previous studies’ findings. Normalized response to medication was observed in the ACC and VLPFC after three years, but not after 16 weeks of treatment, which was similar to the time frame of the subcortical response to treatment (discussed below). This concurrent change between the VLPFC, ACC, and subcortical regions suggest that the normalization of function in these cortical structures may be more closely linked to subcortical alterations. The functional recovery in the DLPFC, VLPFC and ACC observed from baseline to short and longer term treatments suggests a decreased deployment of regulation over subcortical structures in PBD after medication.
4.2. Subcortical changes after pharmacotherapy in PBD
Similar to VLPFC, several subcortical regions (bilateral striatum and right amygdala) exhibited normalized activity after three years of treatment, but not after 16 weeks. These structures have been shown to be involved in emotional and motivational aspects of behavior (Berns et al., 2001; Hare et al., 2005; Jensen et al., 2003; Shohamy, 2011) and have exhibited heightened levels of activity in untreated PBD patients (Malhi et al., 2004; Passarotti et al., 2010b). Previous treatment studies have shown that the amygdala and striatum continued to exhibit relatively greater activation after 8 weeks of treatment (Passarotti et al., 2011a) similar to the short-term outcome in this study. Given that the activation in these subcortical regions normalized only after three years of treatment, it is possible that these subcortical structures may be more treatment-resistant and thus require greater time to recover.
4.3. Potential hierarchical recovery of affective and cognitive interface regions in PBD
Of the interface between affective and cognitive circuits, cortical and subcortical regions may be conceptualized along a tiered hierarchal organization. At the highest regulatory level, both the DLPFC and the VLPFC have extended connections with all other tertiary association regions of the brain and with each other (Petrides, 2005; Petrides and Pandya, 2002). The DLPFC and VLPFC are also well connected with ACC, which has both cognitive and affective components, as supported by both activation and connectivity studies (Bush et al., 2000; Devinsky et al., 1995). These cortical regions are well connected to more primitive subcortical regions that are engaged in emotional processing, such as the ventral striatum and amygdala (Bush et al., 2000; Haber and Knutson, 2010). All these regions showed abnormal response at baseline, but the rate of normalization appears to follow a pattern in which the higher cortical interfacing regions showed normalization shortly after treatment, while the lower subcortical interfacing regions showed normalization by three years of treatment. This pattern suggests the possibility that highest interface centers are most malleable to pharmacological intervention, whereas changes in lower interface centers may be mediated in part by physiological alterations in the higher cortical interfacing regions, in addition to developmental features and longer term systematic treatment for neural circuitry recovery in PBD. This conjuncture can be evaluated with the inclusion of a bipolar group without medication and a healthy control group who are given medication. However, given that ethical considerations prohibit such study design, perhaps animal models can be pursued to address this limitation.
4.4. Limitations
Certain limitations of the present study need to be noted. The sample size was small, so the results should therefore be considered preliminary as non-significant effects may have been due to a Type II error. However, the strengths of the study include HC participants who were matched to PBD participants on age, sex, race, handedness, and IQ. In addition, the PBD patients received multiple psychiatric medications (Mean± SD, 3±1) according to a systematic algorithm during the three years of follow-up. Although it cannot be inferred that medication specifically contributed to the treatment effects observed, this heterogeneity in pharmacotherapy also made the results of this study more generalizable to PBD populations.
5. Conclusions
In sum, the present study demonstrated the long-term course of neural activity and connectivity in individuals being treated for PBD. Whereas changes in the DLPFC were evident after short-term treatment, changes in subcortical activation were not evident until several years after the start of treatment. Although preliminary, these results suggest that the interface between affective and cognitive circuits in PBD is modifiable with development coupled with a long-term and systematic pharmacotherapy algorithm.
Supplementary Material
Figure S1. Pediatric Affective Color Matching Paradigm.
Acknowledgments
We are grateful to the children and families who participated in the study.
Role of funding source
This research was funded by NARSAD Independent Investigator Award, NIH K23 MH 79935-01A2 and K24MH096011.
She is supported by NIMH funding 1R01MH085639, 1R01MH081019 and K24MH096011.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Author Pavuluri designed the study, and Author Yang analyzed the data. Author Yang and Lu wrote the manuscript. Author Pavuluri, Wu, and Stevens helped to analyze the data and give suggestions on the manuscript. Author Wegbreit and Shankman corrected the manuscript. Author Fitzgerald and Levitan finished subject testing and data collection. All authors contributed to and have approved the final manuscript.
Conflict of interest
Dr. Pavuluri has currently no conflicts to declare. In the past three year period, she received honorarium from Bristol-Myers Squibb. Drs. Yang, Lu, Wu, Stevens, Wegbreit, and Shankman disclose no conflicts of interests. Fitzgerald and Levitan disclose no conflicts of interests.
Reference List
- Psychological Corporation, Wechsler Abbreviated Scal of Intelligence (WASI) San Antonio (Texas): Hardourt Brace & Company; 1999. [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD. Dopamine is released in the striatum during human emotional processing. Neuroreport. 2010;21:1172–1176. doi: 10.1097/WNR.0b013e3283410955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS, McClure SM, Pagnoni G, Montague PR. Predictability modulates human brain response to reward. J Neurosci. 2001;21:2793–2798. doi: 10.1523/JNEUROSCI.21-08-02793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Monk K, Kalas C, Goldstein B, Hickey MB, Obreja M, Ehmann M, Iyengar S, Shamseddeen W, Kupfer D, Brent D. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66:287–296. doi: 10.1001/archgenpsychiatry.2008.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitter SM, Mills NP, Adler CM, Strakowski SM, DelBello MP. Progression of amygdala volumetric abnormalities in adolescents after their first manic episode. J Am Acad Child Adolesc Psychiatry. 2011;50:1017–1026. doi: 10.1016/j.jaac.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bradley MM. The Center for Research in Psychophysiology. Gainesville (Florida): University of Florida; 1999. LP: Affective norms for English words (ANEW): Stimuli, instruction manual and affective ratings. [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J Am Acad Child Adolesc Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. quiz 1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. J Cognitive Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder - A functional magnetic resonance imaging investigation. Arch Gen Psychiat. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Fichtenholtz HM, Dean HL, Dillon DG, Yamasaki H, McCarthy G, LaBar KS. Emotion-attention network interactions during a visual oddball task. Brain Res Cogn Brain Res. 2004;20:67–80. doi: 10.1016/j.cogbrainres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved Assessment of Significant Activation in Functional Magnetic-Resonance-Imaging (Fmri) - Use of a Cluster-Size Threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frakowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Human Brain Mapping. 1993;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RAP, Stein EA. Dissociable executive functions in the dynamic control of behavior: Inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Geller B, Warner K, Williams M, Zimerman B. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL and TRF. J Affect Disord. 1998;51:93–100. doi: 10.1016/s0165-0327(98)00176-1. [DOI] [PubMed] [Google Scholar]
- Geller B, Zimerman B, Williams M, Delbello MP, Bolhofner K, Craney JL, Frazier J, Beringer L, Nickelsburg MJ. DSM-IV mania symptoms in a prepubertal and early adolescent bipolar disorder phenotype compared to attention-deficit hyperactive and normal controls. J Child Adolesc Psychopharmacol. 2002;12:11–25. doi: 10.1089/10445460252943533. [DOI] [PubMed] [Google Scholar]
- Gilhooly KJ, Logie RH. Age of acquisition, imagery, consreteness, familiarity and ambiguity measures for 1944 words. Behavior Research Methods & Instrumentation. 1980;12:395–427. [Google Scholar]
- Gogtay N, Ordonez A, Herman DH, Hayashi KM, Greenstein D, Vaituzis C, Lenane M, Clasen L, Sharp W, Giedd JN, Jung D, Nugent TF, 3rd, Toga AW, Leibenluft E, Thompson PM, Rapoport JL. Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. J Child Psychol Psychiatry. 2007;48:852–862. doi: 10.1111/j.1469-7610.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biol Psychiatry. 2005;57:624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-Sads-Pl. J Am Acad Child Adolesc Psychiatry. 2000;39:1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein GS. Semantic Power Measured through the Interference of Words with Color-Naming. Am J Psychol. 1964;77:576–588. [PubMed] [Google Scholar]
- Kucera H, Francis WN, JB C, Twaddell WF. Computational Analysis of Present-Day American English. Providence (Rhode Island): Brown University Press; 1967. [Google Scholar]
- Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, Berghorst LH, Joshi P, Robb A, Schachar RJ, Dickstein DP, McClure EB, Pine DS. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007;164:52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Lagopoulos J, Sachdev P, Mitchell PB, Ivanovski B, Parker GB. Cognitive generation of affect in hypomania: an fMRI study. Bipolar Disord. 2004;6:271–285. doi: 10.1111/j.1399-5618.2004.00123.x. [DOI] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Mayanil T, Wegbreit E, Fitzgerald J, Pavuluri M. Emerging biosignature of brain function and intervention in pediatric bipolar disorder. Minerva Pediatr. 2011;63:183–200. [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, Geier CF, Ordaz SJ, Teslovich T, Luna B. Developmental changes in brain function underlying the influence of reward processing on inhibitory control. Dev Cogn Neurosci. 2011;1:517–529. doi: 10.1016/j.dcn.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Pavuluri MN. Brain functional domains inform therapeutic interventions in attention-deficit/hyperactivity disorder and pediatric bipolar disorder. Expert Rev Neurother. 2011b;11:897–914. doi: 10.1586/ern.11.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of response inhibition in pediatric bipolar disorder and attention deficit hyperactivity disorder. Psychiatry Res. 2010a;181:36–43. doi: 10.1016/j.pscychresns.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010b;49:1064–1080. doi: 10.1016/j.jaac.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Differential engagement of cognitive and affective neural systems in pediatric bipolar disorder and attention deficit hyperactivity disorder. J Int Neuropsychol Soc. 2010c;16:106–117. doi: 10.1017/S1355617709991019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Fronto-limbic dysfunction in mania pre-treatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology (Berl) 2011a;216:485–499. doi: 10.1007/s00213-011-2243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2005;44:846–871. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Henry DB, Devineni B, Carbray JA, Naylor MW, Janicak PG. A pharmacotherapy algorithm for stabilization and maintenance of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:859–867. doi: 10.1097/01.chi.0000128790.87945.2f. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Henry DB, Moss M, Mohammed T, Carbray JA, Sweeney JA. Effectiveness of lamotrigine in maintaining symptom control in pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2009;19:75–82. doi: 10.1089/cap.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, O'Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, O'Connor MM, Harral EM, Sweeney JA. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Res. 2008;162:244–255. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Lu LH, Carbray JA, Sweeney JA. Double-blind randomized trial of risperidone versus divalproex in pediatric bipolar disorder: fMRI outcomes. Psychiatry Res. 2011;193:28–37. doi: 10.1016/j.pscychresns.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Parnes SA, Fitzgerald JM, Sweeney JA. A pharmacological functional magnetic resonance imaging study probing the interface of cognitive and emotional brain systems in pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2010;20:395–406. doi: 10.1089/cap.2009.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children's depression rating scale. J Am Acad Child Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- Rich BA, Schmajuk M, Perez-Edgar KE, Pine DS, Fox NA, Leibenluft E. The impact of reward, punishment, and frustration on attention in pediatric bipolar disorder. Biol Psychiatry. 2005;58:532–539. doi: 10.1016/j.biopsych.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Cyr JA. Frontal-striatal circuit functions: context, sequence, and consequence. J Int Neuropsychol Soc. 2003;9:103–127. doi: 10.1017/s1355617703910125. [DOI] [PubMed] [Google Scholar]
- Shohamy D. Learning and motivation in the human striatum. Curr Opin Neurobiol. 2011;21:408–414. doi: 10.1016/j.conb.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J Cogn Neurosci. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegbreit E, Ellis JA, Nandam A, Fitzgerald JM, Passarotti AM, Pavuluri MN, Stevens MC. Amygdala functional connectivity predicts pharmacotherapy outcome in pediatric bipolar disorder. Brain Connect. 2011;1:411–422. doi: 10.1089/brain.2011.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Wu M, Lu LH, Passarotti AM, Wegbreit E, Fitzgerald J, Pavuluri M. Functional Connectivity Reveals Abnormal Affective, Executive and Sensorimotor Resting State Networks in Psychotropic Na ve Patients with Pediatric Mania. doi: 10.1503/jpn.120073. In press. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Pediatric Affective Color Matching Paradigm.


