Abstract
Renewed interest in using pharmacological ascorbate (AscH−) to treat cancer has prompted interest in leveraging its cytotoxic mechanism of action. A central feature of AscH− action in cancer cells is its ability to act as an electron donor to O2 for generating H2O2. We hypothesized that catalytic manganoporphyrins (MnPs) would increase AscH− oxidation rates, thereby increasing H2O2 fluxes and cytotoxicity. Three different MnPs were tested (MnTBAP, MnT2EPyP, and MnT4MPyP) exhibiting a range of physicochemical and thermodynamic properties. Of the MnPs tested, MnT4MPyP exerted the greatest effect on increasing the rate of AscH− oxidation as determined by the concentration of ascorbate radical [Asc•−] and the rate of oxygen consumption. At concentrations that had minimal effects alone, combining MnPs and AscH− synergized to decrease clonogenic survival in human pancreatic cancer cells. This cytotoxic effect was reversed by catalase, but not superoxide dismutase, consistent with a mechanism mediated by H2O2. MnPs increased steady-state concentrations of Asc•− upon ex vivo addition to whole blood obtained either from mice infused with AscH− or patients treated with pharmacologic AscH−. Lastly, tumor growth in vivo was inhibited more effectively by combining MnT4MPyP with AscH−. We concluded that MnPs increase the rate of oxidation of AscH− to leverage H2O2 flux and ascorbate-induced cytotoxicity.
INTRODUCTION
Adenocarcinoma of the pancreas is the fourth leading cause of cancer death in the United States and is increasing in incidence [1]. Current studies have investigated an entirely new approach, using pharmacological ascorbate as an adjuvant to radiotherapy, to treat pancreatic cancer. Intravenous ascorbate (i.e., ascorbic acid, vitamin C), but not oral ascorbate, produces high plasma concentrations [2], which are in the range cytotoxic to tumor cells [3, 4, 5]. Ascorbate induces oxidative stress and cytotoxicity in pancreatic cancer cells, which appears to be greater in tumor vs. normal cells [6]. We have firmly established that pharmacological ascorbate is a pro-drug for delivery of hydrogen peroxide (H2O2) in vitro and in vivo via its autoxidation [3, 6, 7]. A recent phase I study has demonstrated that pharmacological ascorbate is safe and well-tolerated in oncology patients [8]. In addition, phase I studies specifically in pancreatic cancer treatment have demonstrated that pharmacological ascorbate, combined with standard of care chemotherapy regimens, is safe and well-tolerated and may lead to overall clinical benefit [9, 10].
Ascorbate (AscH−, vitamin C) is a classic donor antioxidant [11]. AscH− scavenges oxidizing free radicals by donating an electron/hydrogen atom forming ascorbate radical (Asc•−), thereby “repairing” the oxidizing radical. In sequential one-electron oxidations, AscH− can donate two electrons to oxygen resulting in formation of dehydroascorbic acid (DHA) and H2O2. The sequential one-electron oxidation of ascorbate can occur via the dianion; Asc2− auto-oxidizes in the presence of dioxygen to produce the Asc•−, dehydroascorbic acid, and H2O2 [12, 13, 14]. Asc•− can dismute, reduce thermodynamically accessible metals, or be reduced by enzymes. At physiological pH (pH 7.4), very little (0.01 %) of the total AscH− is present as the dianion (pKa1 = 4.1 and pKa2 = 11.4 [12]). In the presence of redox active transition metals (e.g. iron, copper or manganese), there is a substantial increase in the level of Asc•− and the associated rate of AscH− oxidation, leading to the production of O2•− and H2O2 [15, 16]. Superoxide can further react with AscH− to form H2O2. Redox active metals can increase the flux of H2O2 by increasing the rate of oxidation of AscH−. The reduced metal can react with O2 to form O2•− and then with the aid of superoxide dismutase (SOD), H2O2 is formed. Although AscH− can act as an electron donor to convert O2•− to H2O2, the efficiency of SOD would make this a minor process in most settings.
Manganoporphyrins (MnPs, manganese porphyrins) are being developed as SOD mimics [17]. The Mn3+ is chelated by a substituted porphyrin ring; substituents on the porphyrin ring system affect the half-cell reduction potential (E1/2) of the central Mn3+ to Mn2+ [18]. The reduction potential correlates with the ability of the Mn3+ to enter into redox reactions with AscH− [19]. Both AscH− and MnPs are well known antioxidants and can protect cells against oxidizing species generated as a result of metabolism, disease, and ionizing stimuli like radiation [20, 21, 22, 23]. However, at pharmacological concentrations, AscH− acts as a pro-oxidant. MnPs increase the flux of AscH−-generated peroxide in vitro [18, 24, 25]. In order to enhance AscH-oxidation, the MnPs must be able to redox cycle with AscH−. Comparing the half-cell reduction potential (E1/2) of MnPs to that of the Asc•−/AscH− couple (neutral pH), one can predict whether a MnP would efficiently redox cycle with AscH− and O2 to produce H2O2.
We hypothesized that MnPs would enhance the rate of AscH− oxidation as a function of their reduction potential, thereby increasing the flux of H2O2. This increased flux of H2O2 will then potentiate AscH−-induced cytotoxicity. Here we investigated the effects of MnPs on the [Asc•−]ss in an AscH− solution and on oxygen consumption rate (OCR), both associated with an increased flux of H2O2, correlating the chemical findings with biological endpoints.
MATERIALS AND METHODS
Cell Culture and Reagents
The human pancreatic cancer cell lines MIAPaCa-2, Panc-1 and AsPC-1 were purchased from ATCC and passaged for fewer than six months after receipt. No additional authentication was performed. Mn(III) tetrakis(N-methylpyridinium-4-yl) porphyrin pentachloride (MnT4MPyP) and Mn(III) tetrakis(4-benzoic acid) porphyrin chloride (MnTBAP) were purchased from Axxora Platform (San Diego, CA, USA). Mn(III) meso-tetrakis(N-ethylpyridinium-2-yl) porphyrin pentachloride (AEOL 10113, MnT2EPyP) were from Dr. James D. Crapo (National Jewish Medical and Research Center, Denver, CO). Because MnPs undergo photooxidation [26], the solid was stored in colored vials at −20 °C, or when dissolved in nanopure water, the solution was stored at 4 °C in colored vials. Clonogenic survival, adenovirus transfections and western blots were performed as previously described [6]
Oxygen consumption via Clark Electrode
The rate of oxygen consumption (OCR, −d[O2]/dt) was determined using a Clark electrode oxygen monitor (YSI Inc., Yellow Springs, OH, USA) connected to an ESA Biostat multi-electrode system (ESA Products, Dionex Corp., Chelmsford, MA, USA) in PBS (GIBCO) or in (10 % FBS) DMEM [27]. The effect of different MnPs on the OCR of AscH− was then determined. Accumulation of H2O2 was determined using catalase (Sigma).
Electron Paramagnetic Resonance
EPR spectra were obtained with a Bruker EMX ESR spectrometer (Bruker BioSpin; Billerica, MA), using an ER 4119HS cavity. To make stable stock solutions of AscH− (100 mM), L-ascorbic acid was dissolved in PBS (50 mM, treated with Chelex resin, pH 7.4–7.6) [28]. Samples were contained in Pyrex capillary tubes (Fisher Scientific, Pittsburg, PA, USA) with 1 mm outer diameter, supported in quartz sample tubes of 4 mm outer diameter (Wilmad-LabGlass, Vineland, NJ). EPR instrument settings were: center field, 3507.62 G; sweep width, 10.00 G; receiver gain, 5.02 × 104; modulation amplitude, 0.70 G; microwave frequency, 9.85 GHz; and nominal microwave power, 10.0 mW. To determine [Asc•−], 3-carboxy-PROXYL (3-CP; CAS No. 2154-68-9; Sigma-Aldrich, Saint Louis, MO) radical was used as a standard, taking into account saturation effects [29].
Ex vivo studies
Mice were treated with AscH− (4 g/kg) or NaCl (1.0 M) every day for 8 days. Blood was drawn 1 h after the last intraperitoneal dose. Thirty-day-old athymic nude mice were obtained from Harlan Sprague-Dawley (Indianapolis, IN). For the human ex vivo experiments, the blood samples were from the phase I trial approved by The University of Iowa Human Institutional Review Board and the Protocol Review and Monitoring Committee of the Holden Comprehensive Cancer Center at The University of Iowa Hospitals and Clinics on May 22, 2008 [10]. The trial was listed on www.clinicaltrials.gov under NCT01515046. Informed consent was documented by use of a written consent form approved by the Investigational Review Board and The University of Iowa.
In vivo studies
Thirty-day-old athymic nude mice were obtained from Harlan Sprague-Dawley (Indianapolis, IN). All of the nude mice protocols were reviewed and approved by the Animal Care and Use Committee of The University of Iowa. Each experimental group consisted of 12 to 16 mice. MIA PaCa-2 tumor cells (2 × 106) were delivered subcutaneously into the hind leg of nude mice. The tumors were allowed to grow until they reached between 3 mm and 4 mm in greatest dimension at 10 days at which time treatment was initiated. Mice were divided into four treatment groups and treated daily for 22 days. The groups included controls that received 1 M NaCl i.p.; ascorbate 4 g/kg i.p; MnT4MPyP 0.2 mg/kg i.p.; and ascorbate 4 g/kg + MnT4MPyP 0.2 mg/kg. Tumor size was measured every three to four days by means of a vernier caliper, and tumor volume was estimated according to the following formula: tumor volume = π/6 × L × W2, where L is the greatest dimension of the tumor, and W is the dimension of the tumor in the perpendicular direction. Animals were sacrificed by CO2 asphyxiation when the tumors reached 1000 mm3.
Statistical Analysis
Statistical significance was determined using an upper tailed, one-sided Student's t-test, with a value of p < 0.05 considered significant. Combination Index (CI) was calculated for AscH− and MnP to determine summation, antagonism, or synergy using the multiple drug interaction model [30]. Dose reduction index (DRI) values were also calculated as described [30, 31]. The two variables must be calculated from the same percent of cell killing [30, 31].
For the in vivo studies, the statistical analyses focused on the effects of different treatments on cancer progression. The primary outcome of interest was tumor growth over time. Tumor sizes (mm3) were measured throughout the experiments, resulting in repeated measurements across time for each mouse. A generalized estimating equations model was used to estimate and compare group-specific tumor growth curves. Pairwise comparisons were performed to identify specific group differences in the growth curves. All tests were two-sided and carried out at the 5% level of significance. Analyses were performed with SAS version 9.3 (SAS Institute Inc. Cary, NC).
RESULTS
Manganoporphyrins (MnPs) increase the generation of Asc•−, which is dependent on their reduction potential
Ascorbic acid exists predominantly (> 99.9 %) as the ascorbate monoanion (AscH−) at physiological pH (7.36–7.44). One-electron oxidation of AscH− forms the ascorbate radical (Asc•−). The ascorbate radical is a resonance stabilized tricarbonyl species that has a long half-life compared to most free radicals [32]. Typically, it is detectable by direct EPR; some Asc•− is always present in an aerated solution of AscH− [33]. Under our experimental conditions, ≈ 120 nM Asc•− was present in a 1.0 mM AscH− solution (Chelex-treated PBS, pH 7.4, 25 °C). The concentration of Asc•− increases in AscH− solutions in proportion to the oxidative flux in the system [12]. We hypothesized that MnPs would increase the steady-state level of Asc•− ([Asc•−]ss), consistent with an increased oxidative flux in the system. As seen in Figure 1, the reduction potential of an individual MnP relates to the rate of AscH− oxidation. MnT4MPyP and MnT2EPyP increase the [Asc•−]ss in solution (pH 7.5–7.6) in a dose-dependent manner. The reduction potential (E°′) of the Asc•−/AscH− couple is +282 mV [12] while the Mn3+ in MnT2EPyP, MnT4MPyP, and MnTBAP has reduction potentials of +228 mV [34], +60 mV [35], and −194 mV [36], respectively. The increase in the [Asc•−]ss upon introduction of MnT4MPyP and MnT2EPyP indicates that AscH− is able to effectively reduce both MnPs. Only a minimal increase in the [Asc•−]ss is observed upon introduction of MnTBAP, which is consistent with its much lower reduction potential. Thus, appropriate thermodynamics is an important consideration for redox active compounds that might accelerate AscH− oxidation.
Figure 1. Manganoporphyrins increase ascorbate radical indicating an increase in the rate of AscH− oxidation.
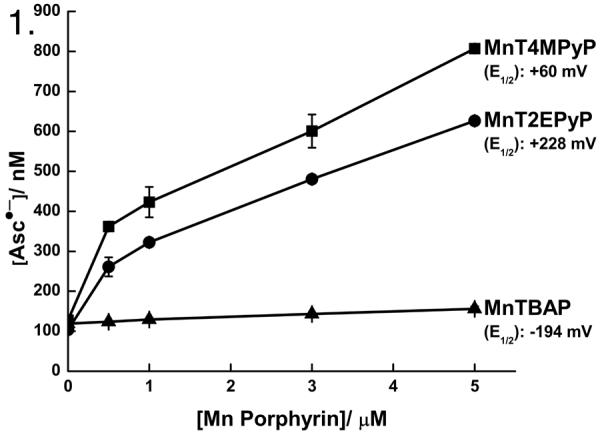
As seen by EPR spectroscopy, addition of MnPs to a solution of AscH− (1 mM) in PBS, increased [Asc•−] in a dose-dependent manner. This increase is dependent on the reduction potential of the individual MnPs. At lower concentrations of Asc•− the curves have a greater slope that decreases as [Asc•−] increases, which is consistent with an increased rate of AscH− oxidation and the second-order nature of the dismutation reaction of Asc•− [14]. n = 3.
Manganoporphyrins increase the oxygen consumption rate of ascorbate and generation of H2O2
To determine if the increase in [Asc•−]ss in the presence of MnPs would lead to an increase in the production of reactive oxygen species, we measured the rate of oxygen consumption and changes in accumulation of H2O2. A 1.0 mM solution of AscH− consumes oxygen at an initial rate of 2–5 nM s−1 in PBS. In the presence of MnT4MPyP (1 μM), this rate increases 10-fold (Figure 2A), while MnT2EPyP (1 μM) increases this rate 2-fold, Figure 2B. MnTBAP (1 μM) did not significantly alter the rate of oxygen consumption, Figure 2C.
Figure 2. Manganoporphyrins increase the rate of oxygen consumption, leading to generation of H2O2.
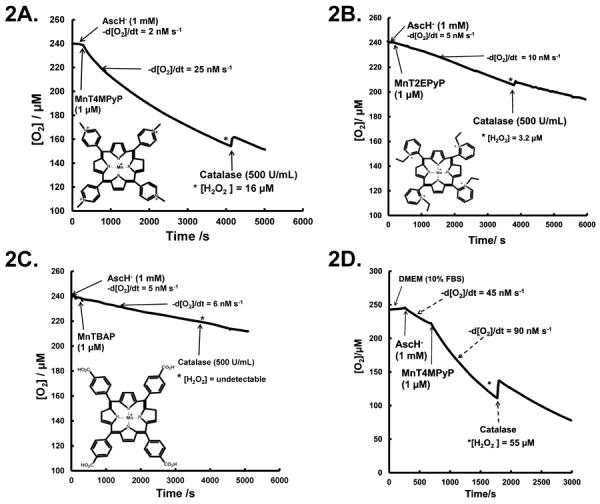
A. AscH− consumes oxygen at the rate of 2–5 nM s−1 in PBS. Addition of MnT4MPyP (1.0 μM) increases the rate of oxygen consumption to 22–25 nM s−1. Addition of catalase (500 U/mL) leads to a return of O2 with 16–20 μM H2O2 accumulating in solution. n = 3. Inset: MnT4MPyP molecular structure.
B. Addition of MnT2EPyP (1.0 μM) to AscH− (1 mM) solution increases the oxygen consumption rate from ≈ 5 nM s−1 to ≈ 10 nM s−1. Addition of catalase indicates 3–5 μM of H2O2 has accumulated in solution after 60 min. n = 3. Inset: MnT2EPyP molecular structure.
C. MnTBAP (1.0 μM) addition to AscH− (1.0 mM) solution does not alter the oxygen consumption rate. Addition of catalase does not return detectable amounts of oxygen demonstrating minimal H2O2 accumulation. n = 3. Inset: MnTBAP molecular structure.
D. In DMEM with 10 % FBS, oxygen consumption for AscH− (1 mM) is 20–45 nM s−1. Addition of MnT4MPyP increases oxygen consumption to 80–100 nM s−1. Addition of catalase 30 min after MnT4MPyP leads to a return of oxygen, indicating that 50–60 μM H2O2 has accumulated in solution. n = 3.
The oxidation of AscH− produces H2O2. If H2O2 accumulates, then addition of catalase will result in the return of oxygen. If all of the oxygen consumed accumulates as H2O2, then 50 % of the oxygen lost would be restored with the addition of catalase. Addition of catalase (500 U/mL) resulted in the return of 5–10 % of the oxygen consumed in the AscH− + MnT4MPyP or AscH− + MnT2EPyP solutions, Figure 2A, 2B. However, any return of oxygen in AscH−/MnTBAP solution was below the limit of detection by the Clark electrode, (< ≈ 2 μM). These results are consistent with our observations with [Asc•−]ss and consistent with the reduction potentials of the MnPs.
As a surrogate for extracellular fluid, we determined the rate of oxidation of AscH− in DMEM containing 10 % FBS. Because MnT4MPyP had the greatest effect on the rate of oxidation of AscH− in PBS, we examined MnT4MPyP as a catalyst in DMEM containing 10 % FBS. In this media, AscH− consumes oxygen at a rate of 12–50 nM s−1; this variability is typical, probably due to small changes in the content of catalytic metals [28]. This greater rate of oxygen consumption of AscH− in DMEM + FBS compared to PBS may be due to the presence of significant catalytic iron and other redox active metals in the DMEM and FBS; in addition, FBS contains heme proteins that can act as promiscuous peroxidases [37]. In a representative experiment with AscH− (1 mM) alone in DMEM with 10 % FBS, the oxygen consumption rate was 45 nM s−1; addition of MnT4MPyP increased this rate two-fold to 90 nM s−1, Figure 2D. After the addition of catalase (500 U/mL), about 20–25 % of the oxygen consumed was returned indicating that about 45 % of the oxygen consumed accumulated as H2O2. In these experiments, the accumulation of H2O2 was less than the loss of oxygen indicating that some of the H2O2 was reduced to H2O. Some MnPs have a catalase activity, which would also remove H2O2. However, the relatively long time frame of these experiments coupled with the clear accumulation of H2O2 indicates that the removal of H2O2 is a very slow process.
Manganoporphyrins increase ascorbate-induced cytotoxicity
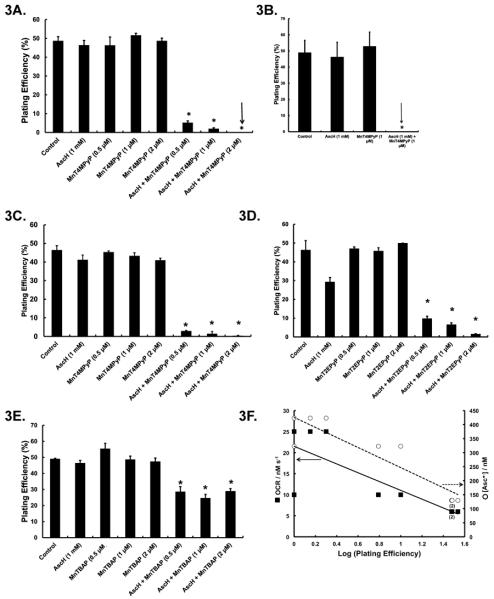
We hypothesized that the ability of MnPs to serve as catalysts for AscH− oxidation and associated generation of H2O2 would correlate with the ability of the MnPs to enhance AscH−-induced cytotoxicity in vitro. To determine this, we used concentrations (0.5–2 μM) of MnPs and AscH− (1 mM) that alone did not affect clonogenic survival in any of the human pancreatic cancer cell lines examined. MnT4MPyP was able to potentiate AscH− cytotoxicity most significantly as compared to the other two MnPs, Figure 3. The combination of AscH− (1 mM) with MnT4MPyP (0.5 μM) decreases plating efficiency of MIA PaCa-2 cells from 50 ± 2 % in controls to 5 ± 1 %, Figure 3A. As the dose of MnT4MPyP is increased in combination with AscH−, the plating efficiency decreased. When MIA PaCa-2 cells are exposed to 2 μM MnT4MPyP and 1 mM AscH−, no clones survive, Figure 3A. AsPC-1 cells were derived from ascites of a pancreatic cancer patient and are more resistant to gemcitabine [38] and AscH−-induced cytotoxicity compared to MIA PaCa-2 [6]. However, their susceptibility to AscH− and MnP co-treatment was similar to that of MIA PaCa-2 cells, Figure 3B. When co-treated with MnT4MPyP (1 μM) and AscH− (1 mM), no AsPC-1 clones survive. As seen in Figure 3C, combined treatment with MnT4MPyP and AscH− resulted in the same effect with Panc-1 cells.
Figure 3. Manganoporphyrins enhance AscH−-induced cytotoxicity in pancreatic cancer cell lines.
A. Treatment with AscH− or MnT4MPyP alone does not alter clonogenic survival of MIA PaCa-2 cells. However, the combination of AscH− (1 mM) and MnT4MPyP (0.5 μM) decreases plating efficiency to 5 ± 1 %. When MnT4MPyP (2 μM) was added to AscH−, no clones survived. n = 3. *p < 0.001 vs. controls.
B. MnT4MPyP (1 μM) or AscH− (1 mM) did not affect AsPC-1 clonogenic survival while the combination led to no surviving clones. n = 3. *p < 0.001 vs. control.
C. MnT4MPyP (0.5 – 2 μM) or AscH− (1 mM) did not affect Panc-1 clonogenic survival. However, addition of MnT4MPyP (0.5 μM) to AscH− (1 mM) decreased survival to 3 ± 0.2 %. Only 1 ± 0.1% clones survived exposure to 2 μM MnT4MPyP in the presence of AscH−. n = 3. *p < 0.001 vs. control.
D. MnT2EPyP (0.5, 1 or 2 μM) or AscH− (1 mM) does not alter MIA PaCa-2 survival. MnT2EPyP (0.5 μM) combined with AscH− (1 mM) decreases plating efficiency to 10 ± 0.5 %. When increased concentrations of MnT2EPyP are used, plating efficiency was reduced to ≤ 5 %. n = 3. *p < 0.001 vs. controls.
E. MnTBAP (0.5, 1 or 2 μM) does not alter MIA PaCa-2 plating efficiency. However, when MnTBAP was combined with AscH−, MIA PaCa-2 plating efficiency was decreased to 30 ± %. n = 3. *p < 0.001 vs. control.
F. The OCR of AscH− when combined with MnPs correlates with log (plating efficiency) in pancreatic cancer cell lines (solid line, R2 = 0.9, p < 0.05). In solution, [Asc•−] in the presence of MnPs (from Figure 1) strongly correlated with log (plating efficiency) in MIA PaCa-2, AsPC-1 and Panc-1 cells (hatched line, R2≈ 0.9, p < 0.05. The (2) below specific points on the graph indicate that two identical points at that value.
MnT2EPyP increases the [Asc•−] in solution but produces less H2O2 as compared to MnT4MPyP, Figure 2. The potentiating effect of MnT2EPyP on AscH− cytotoxicity in MIA PaCa-2 cells (Figure 3D) and in AsPC-1 (Supplemental Figure S1A) and Panc-1 cells (Supplemental Figure S1B), though significant, is not as marked as that of MnT4MPyP combined with AscH−. MnTBAP increases [Asc•−]ss in solution only modestly and has little effect on the OCR of AscH− in solution. It is, however, able to enhance AscH− cytotoxicity to a lesser extent (Figure 3E). This effect was also demonstrated in AsPC-1 (Supplemental Figure S1C) and Panc-1 cells (Supplemental Figure S1D). The decrease in plating efficiency of various pancreatic cancer lines by the addition of MnPs to AscH− correlates with the OCR (R2= 0.62, p = 0.01) (Figure 3F). Also, the plating efficiency correlates with the concentration of Asc•− present in solution after the addition of MnPs (R2= 0.82, p = 0.001) (Figure 3F). Together, these data demonstrate that the enhanced cytotoxicity of AscH− is a function of the MnP's reduction potential and their ability to enhance AscH− oxidation in solution.
The effects of MnP on AscH− cytotoxicity may be mediated by H2O2. The combination of AscH− (1 mM) and MnT4MPyP (1 μM) in DMEM + 10 % FBS, resulted in the accumulation of 55–60 μM H2O2 at 30 min. In addition, exposure of MIA PaCa-2 (Supplementary Figure 2A) and AsPC-1 cells (Supplementary Figure 2B) to a bolus dose of 60 μM H2O2 reduced their survival to levels similar to those seen with AscH− and MnT4MPyP co-treatment, suggesting indirectly that H2O2 is playing a significant role in the observed cytotoxicity.
Ascorbate and MnPs synergize to enhance cytotoxicity in vitro
To determine if MnPs were additive or synergistic to AscH−, the combination index (CI) and dose reduction index (DRI) for the three different cell lines were calculated [30, 31]. The CI allows for quantitative determination of drug interactions with CI < 1, = 1, and >1 indicating synergism, additive effects, or antagonism, respectively. The DRI was calculated as the molar ratio of AscH− alone vs. AscH− + MnT4MPyP required to produce the same level of cytotoxicity. For all combinations, the CI was < 0.1, indicating synergy (Supplemental information, Table 1). DRI indices for MnT4MPyP were between 4 × 105 and 8 × 106. Ascorbate DRI calculations demonstrated values in the 6–22 range (Supplemental information, Table 1) also suggesting synergy between AscH− and MnT4MPyP. The DRI values were less for AscH− as compared to MnT4MPyP in the same combination setting suggesting that AscH− is the main cytotoxic agent in this combination while MnT4MPyP is an adjuvant, acting mainly as a catalyst to enhance AscH−-induced cytotoxicity.
SOD activity does not alter AscH−-induced cytotoxicity
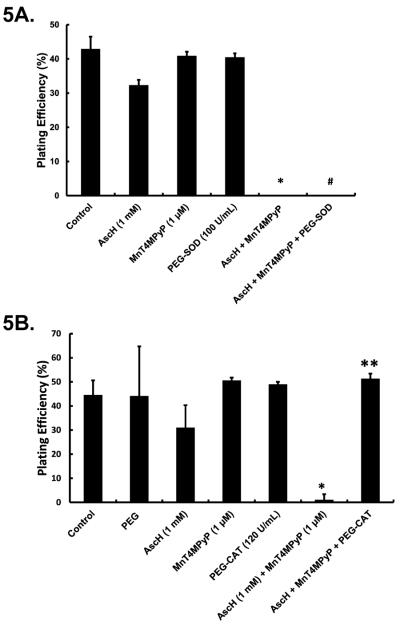
To determine if the SOD-mimicking functionality of the MnPs was responsible for the enhanced toxicity of AscH−, MIA PaCa-2 cells were exposed to polyethylene glycol-superoxide dismutase (PEG-SOD; 100 U/mL), PEG alone (100 U/mL), AscH− (1 mM), or the combination of PEG-SOD + AscH−. No changes in clonogenic survival were observed indicating that superoxide is not a major factor in ascorbate-induced cytotoxicity (Figure 4A). To further investigate the role of SOD activity, cells were transfected with the AdEcSOD to determine if overexpression of EcSOD would enhance AscH− toxicity. Western blots confirmed that EcSOD immunoreactive protein was absent in control and AdEmpty transfected cells, while abundant in the AdEcSOD transfected cells (Figure 4B). Transduction of cells with adenovirus vectors can decrease survival, as seen in Figure 4C. There were no differences in clonogenic survival in cells treated with the combination of AdEmpty and AscH−. Treatment of MIA PaCa-2 cells transfected with AdEcSOD with AscH− (1 mM) had little effect on clonogenic survival (Figure 4C). These data indicate that the SOD activity of MnPs is not responsible for the MnP-enhanced toxicity of AscH−.
Figure 4. SOD does not alter AscH−-induced cytotoxicity.
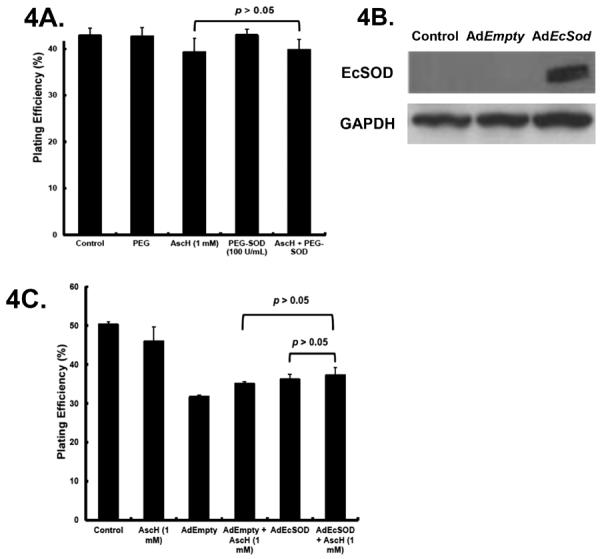
A. Cells were treated with AscH− (1 mM) and PEG-SOD (100 U/mL) alone or in combination for 60 min, washed and seeded for clonogenic survival. PEG-SOD did not alter clonogenic survival and combining PEG-SOD with AscH− did not alter clonogenic survival of MIA PaCa-2 cells. p > 0.05 comparing AscH− vs. AscH− + PEG-SOD.
B. MIA PaCa-2 cells transfected with AdEmpty or AdEcSOD were lysed and resolved on SDS-polyacrylamide gel and then blotted for the presence of EcSOD. GAPDH was used as a loading control. Lanes 1, 2 and 3 represent parental cells, cells infected with the AdEmpty vector and cells infected with the AdEcSOD vector, respectively.
C. MIA PaCa-2 cells were transfected with either AdEmpty or AdEcSOD at 100 MOI. There were minimal changes (p > 0.05) in plating efficiency in AdEmpty or AdEcSOD infected cells treated with AscH−.
Catalase reverses manganoporphyrin + ascorbate-induced cytotoxicity
AscH−-induced cytotoxicity may be mediated by H2O2 [6]. With the addition of a redox active metal like the Mn3+/Mn2+ couple of MnPs, the flux of O2•− may be altered, which could enhance toxicity in addition to the accumulation of H2O2. To probe for the free radical species involved in the MnP + AscH−-induced cytotoxicity, cells were treated with SOD or catalase. While AscH−, MnT4MPyP, PEG-SOD and PEG-catalase alone were not cytotoxic (Figure 5), few clones survived when cells were treated with both AscH− and MnT4MPyP (Figure 5). Addition of SOD did not reverse the AscH− + MnT4MPyP cytotoxicity (Figure 5A). However, PEG-catalase (120 U/mL) reversed the combined cytotoxicity of AscH− + MnT4MPyP (Figure 5B). These data suggest that while O2•− may not play a significant role, accumulation of H2O2 mediates the cytotoxicity of AscH− combined with the MnPs in our experimental setting.
Figure 5. H2O2 mediates MnP enhanced AscH−-induced cytotoxicity.
A. PEG-SOD (100 U/mL) did not reverse MnT4MPyP enhanced AscH−-induced cytotoxicity in MIA PaCa-2 cells, suggesting that superoxide is not directly responsible for the observed cytotoxicity. n =3. *p < 0.001 vs. controls. #p > 0.05 vs. AscH− + MnP.
B. Treatment with PEG-CAT (120 U/mL) reverses MnT4MPyP enhanced AscH−-induced cytotoxicity suggesting that H2O2 mediates the observed cytotoxicity. n = 3. *p < 0.001 vs. control. **p < 0.001 vs. AscH− + MnT4MPyP treated cells.
MnT4MPyP increases [Asc•−] ex vivo
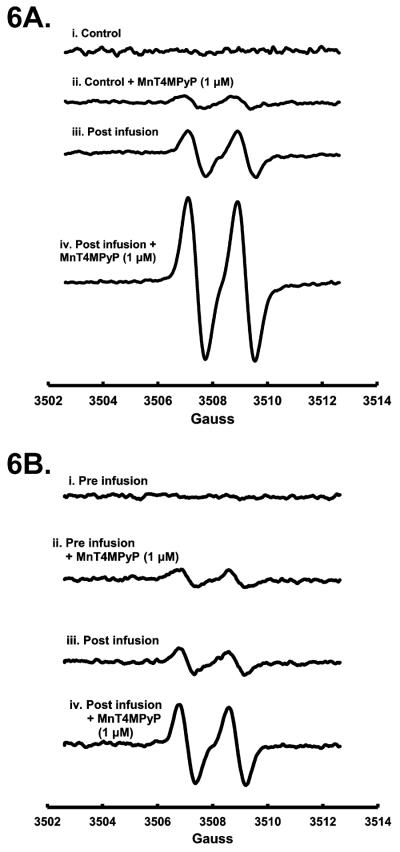
Infusions of pharmacological doses of AscH− (0.6–1.5 g/kg body weight) over an extended period of time result in peak plasma AscH− concentrations of 15–25 mM [8]. Achieving this plasma [AscH−] is central to cytotoxic AscH− therapy [4, 8, 10]. Oxidation of AscH− in extracellular fluid results in increased generation of H2O2 leading to cytotoxicity [3]. Enhanced oxidation of AscH− and increased steady-state levels of Asc•− are indicators of AscH− oxidation and resultant H2O2 production and cytotoxicity. MnT4MPyP increases [Asc•−] and the oxygen consumption rate of AscH− in solution, synergizes with AscH− in vitro, and increases AscH−-induced cytotoxicity in pancreatic cancer cell lines. To determine if MnT4MPyP increases the rate of AscH− oxidation ex vivo, whole blood [Asc•−] was measured by EPR from mice infused with pharmacological AscH−. In untreated mouse whole blood Asc•− is below detection limits (< 10 nM) (Figure 6A). Addition of MnT4MPyP (1.0 μM) increased Asc•− to 97 nM. Mice were infused with AscH− (4 g/kg) resulting in plasma levels of 29 mM AscH−; EPR detectable [Asc•−] increased to 350 nM in whole blood. However, addition of MnT4MPyP (1.0 μM) to this mouse whole blood ex vivo led to an increase in [Asc•−] to 1200 nM.
Figure 6. MnT4MPyP enhances ascorbate radical concentration in whole blood as seen by EPR spectroscopy.
A. In whole blood from mice, Asc•− is below the limit of detection. MnT4MPyP (1.0 μM) increased [Asc•−]ss to 97 nM. When mice were treated with AscH− (4 g/kg) resulting in a plasma level of [AscH−] of 29 mM, [Asc•−]ss was increased to 350 nM. With addition of MnT4MPyP (1.0 μM) to ascorbate treated mice, [Asc•−]ss increased more than 3-fold to 1200 nM. n = 3. (Hyperfine splitting of Asc•−, aH = 1.76 G.)
B. In whole blood from humans, Asc•− is also below the limit of detection. [Asc•−]ss increases to 82 nM upon addition of MnT4MPyP (1.0 μM) to human whole blood. After infusion of pharmacological AscH−(100 g), [AscH−] increased to 22 mM and [Asc•−]ss increased to 120 nM. Upon addition of MnT4MPyP (1.0 μM), [Asc•−]ss increased to 360 nM. n = 3.
We then obtained whole blood from human pancreatic cancer patients receiving pharmacological AscH− (50–125 g AscH− twice a week) as part of a phase I trial. Similar to trends seen in mice, Asc•− was not detected in whole blood drawn from patients prior to being infused with AscH− (Figure 6B). When MnT4MPyP (1.0 μM) was added to pre-infusion blood, EPR detectable Asc•− increased to 82 nM. Following intravenous infusion of pharmacological AscH− (100 g), EPR detectable Asc•− was increased to 120 nM. Addition of MnT4MPyP to post-infusion blood increased Asc•− in the whole blood to 360 nM. Thus, MnT4MPyP increased Asc•− in whole blood ex vivo from mice and from patients. This increased Asc•− in ex vivo whole blood indicates increased AscH− oxidation, similar to our in vitro observations; an increased AscH− oxidation will lead to an increased flux of H2O2.
MnT4MPyP enhances ascorbate-induced cytotoxicity in vivo
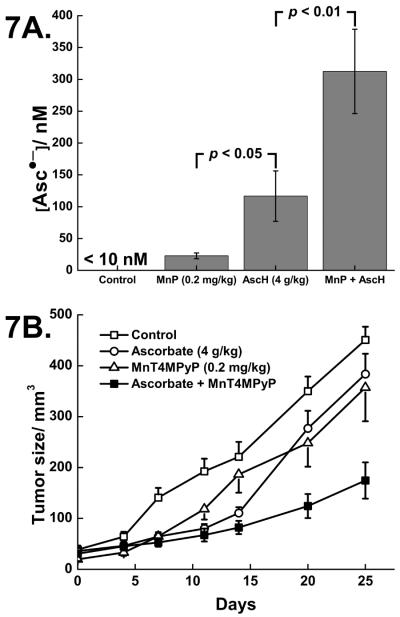
To determine if MnPs could enhance ascorbate-induced cytotoxicity in vivo, we treated mice with pre-established MIA PaCa-2 human pancreatic tumors. There were no differences in weight changes among the four treatment groups and none of the animals during the study had to be sacrificed for continued weight loss or cachexia. We also obtained whole blood from separate groups of mice treated with the same combinations. Asc•− was not detected in whole blood drawn from mice treated with saline (Figure 7A). When MnT4MPyP (0.2 mg/kg) was given i.p., EPR detectable Asc•− increased to 22 ± 5 nM. Following i.p. administration of AscH− (4 g/kg), EPR detectable Asc•− was increased to 120 ± 40 nM. In mice treated with both MnT4MPyP and AscH−, Asc•− increased to 310 ± 66 nM. Thus, MnT4MPyP enhanced AscH−-induced Asc•− in treated mice demonstrating increased AscH− oxidation.
Figure 7. MnT4MPyP enhances ascorbate-induced cytotoxicity and ascorbate radical concentration in vivo.
A. In whole blood from a separate group of mice, Asc•− is below the limit of detection. [Asc•]ss increases to 22 nM upon treatment with MnT4MPyP (0.2 mg/kg i.p.). After i.p. injection of pharmacological AscH− (4 g/kg), [AscH−] increased to 30 mM and [Asc•−]ss increased to 120 nM. Combining both treatments increased [Asc•−]ss to 310 nM. n = 3 for each determination. AscH− vs.MnT4MPyP, p < 0.05. AscH− vs. AscH− + MnT4MPyP, p < 0.01.
B. MnT4MPyP combined with pharmacological ascorbate decreased MIA PaCa-2 tumor growth in nude mice. The MnT4MPyP and ascorbate-treated animals had significantly slower tumor growth when compared to the control and ascorbate groups (*#p < 0.05, n = 12–16/group, means ± SEM). MIA PaCa-2 tumor cells (2 × 106) were delivered subcutaneously into the hind leg of nude mice. On day 25, there was nearly a 3-fold decrease in tumor growth in animals receiving the combination when compared to controls.
MnT4MPyP enhanced ascorbate-induced cytotoxicity as animals treated with the combination of ascorbate + MnT4MPyP had a slower rate of growth in tumors when compared to saline (controls) treatment (*p < 0.01) or ascorbate alone (#p < 0.05), Supplemental information, Table 2. The estimated tumor growth curve is displayed in Figure 7B. On day 25 of the study, the control group had a mean tumor volume of 491 mm3, while the ascorbate alone group had a mean tumor volume of 384 mm3. In mice treated with the combination of MnT4MPyP and ascorbate, mean tumor volume was 174 mm3.
DISCUSSION
Pharmacological AscH− induces tumor cell cytotoxicity in vitro and in vivo [3–6]; this toxicity is mediated by the generation of H2O2 [3, 4, 5, 6, 39]. With our goal to increase the flux of H2O2 from AscH− we hypothesized that MnPs combined with pharmacological ascorbate would increase the rate of oxidation of AscH−, leading to an increased flux of H2O2 enhancing cytotoxicity. MnPs are redox-active metal chelates that are being developed as SOD mimics [18]. The catalytic and thermodynamic properties of MnPs have been extensively investigated resulting in MnPs with a range of reduction potentials and have been used in both in vitro and in vivo experiments [18, 19, 34, 36]. Our study demonstrates that MnPs can increase [Asc•−] in solution, dependent on the [MnP] as well as the reduction potential of the individual MnP. The rate of oxygen consumption in a solution of AscH− is an indicator of its rate of oxidation. This oxidation leads to a flux of H2O2. MnPs increased the OCR of AscH− in solution and lead to the accumulation of H2O2. As observed with the increase in [Asc•−], the reduction potential of the MnP also correlated with the increase in the OCR as well as the production of H2O2.
Superoxide is an intermediate in the formation of H2O2 from AscH− [12]. However, we found that the ability to dismute O2•− has no effect on enhancement of AscH− cytotoxicity. Overexpression of SOD (either PEG-SOD or AdEcSOD) did not enhance AscH− cytotoxicity. Although O2•− is an intermediate in the generation of H2O2 from AscH−, it does not play a significant role in AscH−-induced cytotoxicity. Scavenging O2•− does not ameliorate MnP-enhanced AscH−- cytotoxicity. Accumulation of H2O2 does play a significant role in the cytotoxicity since scavenging H2O2 with catalase ameliorates the combined cytotoxicity of AscH− and MnPs. We also demonstrate a 300 % increase in the steady-state [Asc•−] with the addition of MnPs from mice infused with AscH− and in patients treated with pharmacologic AscH−.
Although our results are consistent with other studies demonstrating decreases in clonogenic survival with MnPs and AscH− [24, 25], our current study extends these previous observations by demonstrating the chemistry behind the observed biological effects. Most importantly, we demonstrate the effects of MnPs on AscH− oxidation in vitro, ex vivo, and in vivo. Studies previously carried out with MnPs have used AscH− as a reducing agent [25]. However, we use AscH− as the main cytotoxic agent and MnPs as catalysts to increase the flux of H2O2.
In summary, MnPs increased the rate of AscH− oxidation, the steady-state level of [Asc•−], and the rate of oxygen consumption, with MnT4MPyP having the greatest effect. MnPs synergistically enhanced AscH−-induced cytotoxicity in all pancreatic cancer cell lines studied. Catalase, but not SOD, reversed the cytotoxicity of the AscH− and MnPs combination, suggesting an H2O2-mediated mechanism. In addition, there was a marked increase in [Asc•−]ss in whole blood from mice upon the addition of MnPs and as well as in the blood from patients treated with pharmacologic AscH−. We conclude that MnPs can increase the rate of oxidation of AscH− leading to an increased flux of H2O2 resulting in increased AscH−-induced cytotoxicity. MnPs have the potential as adjuvants to pharmacological AscH− therapy.
Supplementary Material
Acknowledgments
Supported by NIH grants CA166800, GM073929, P42ES013661, the Medical Research Service, Department of Veterans Affairs and the Susan L. Bader Foundation of Hope.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A, Naishadham D. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78–88. doi: 10.3945/an.110.000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shacter E, Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci U S A. 2005;102(38):13604–9. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen P, Stone J, Sullivan G, Drisko JA, Chen Q. Anti-cancer effect of pharmacologic ascorbate and its interaction with supplementary parenteral glutathione in preclinical cancer models. Free Radic Biol Med. 2011;51(3):681–7. doi: 10.1016/j.freeradbiomed.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 5.Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR, Levine M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci U S A. 2007;104(21):8749–54. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du J, Martin SM, Levine M, Wagner BA, Buettner GR, Wang SH, Taghiyev AF, Du C, Knudson CM, Cullen JJ. Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin Cancer Res. 2010;16(2):509–20. doi: 10.1158/1078-0432.CCR-09-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olney KE, Du J, van 't Erve TJ, Witmer JR, Sibenaller ZA, Wagner BA, Buettner GR, Cullen JJ. Inhibitors of hydroperoxide metabolism enhance ascorbate-induced cytotoxicity. Free Radic Res. 2013;47(3):154–63. doi: 10.3109/10715762.2012.755263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffer LJ, Levine M, Assouline S, Melnychuk D, Padayatty SJ, Rosadiuk K, Rousseau C, Robitaille L, Miller WH., Jr. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol. 2008;19(11):1969–74. doi: 10.1093/annonc/mdn377. [DOI] [PubMed] [Google Scholar]
- 9.Monti DA, Mitchell E, Bazzan AJ, Littman S, Zabrecky G, Yeo CJ, et al. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS ONE. 2012;7:e29794. doi: 10.1371/journal.pone.0029794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welsh JL, Wagner BA, van't Erve TJ, Zehr PS, Berg DS, Halfdanarson TJ, Yee NS, Bodeker KL, Du J, Roberts LJ, Drisko J, Levine M, Buettner GR, Cullen JJ. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): Results from a phase I clinical trial. Cancer Chemotherapy and Pharmacology. 2013;71:765–75. doi: 10.1007/s00280-013-2070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300(2):535–43. doi: 10.1006/abbi.1993.1074. n. [DOI] [PubMed] [Google Scholar]
- 12.Williams NH, Yandell JK. Outer-sphere electron-transfer reactions of ascorbate anions. Aust J Chem. 1982;35:1133–1144. [Google Scholar]
- 13.Song Y, Buettner GR. Thermodynamic and kinetic considerations for the reaction of semiquinone radicals to form superoxide and hydrogen peroxide. Free Radic Biol Med. 2010;49(6):919–62. doi: 10.1016/j.freeradbiomed.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bielski BHJ, Comstock DA, Bowen RA. Ascorbic acid free radicals. I. Pulse radiolysis study of optical absorption and kinetic properties. J Am Chem Soc. 1971;93(22):5624–29. [Google Scholar]
- 15.Buettner GR, Jurkiewicz BA. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat Res. 1996;145(5):532–41. [PubMed] [Google Scholar]
- 16.Buettner GR, Jurkiewicz BA. Ascorbate free radical as a marker of oxidative stress: an EPR study. Free Radic Biol Med. 1993;14(1):49–55. doi: 10.1016/0891-5849(93)90508-r. [DOI] [PubMed] [Google Scholar]
- 17.Archibald FS, Fridovich I. The scavenging of superoxide radical by manganous complexes: in vitro. Arch Biochem Biophys. 1982;214(2):452–63. doi: 10.1016/0003-9861(82)90049-2. [DOI] [PubMed] [Google Scholar]
- 18.Batinic-Haberle I, Reboucas JS, Spasojevic I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal. 2010;13(6):877–918. doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batinic-Haberle I, Rajic Z, Benov L. A combination of two antioxidants (an SOD mimic and ascorbate) produces a pro-oxidative effect forcing Escherichia coli to adapt via induction of oxyR regulon. Anticancer Agents Med Chem. 2011;11(4):329–40. doi: 10.2174/187152011795677562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day BJ, Fridovich I, Crapo JD. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch Biochem Biophys. 1997;347(2):256–62. doi: 10.1006/abbi.1997.0341. [DOI] [PubMed] [Google Scholar]
- 21.Faulkner KM, Liochev SI, Fridovich I. Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J Biol Chem. 1994;269(38):23471–6. [PubMed] [Google Scholar]
- 22.Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86(16):6377–81. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorai T, Fishman AI, Ding C, Batinic-Haberle I, Goldfarb DS, Grasso M. Amelioration of renal ischemia-reperfusion injury with a novel protective cocktail. J Urol. 2011;186(6):2448–54. doi: 10.1016/j.juro.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Tian J, Peehl DM, Knox SJ. Metalloporphyrin synergizes with ascorbic acid to inhibit cancer cell growth through Fenton chemistry. Cancer Biother Radiopharm. 2010;25(4):439–48. doi: 10.1089/cbr.2009.0756. [DOI] [PubMed] [Google Scholar]
- 25.Ye X, Fels D, Tovmasyan A, Aird KM, Dedeugd C, Allensworth JL, et al. Cytotoxic effects of Mn(III) N-alkylpyridylporphyrins in the presence of cellular reductant, ascorbate. Free Radic Res. 2011;45(11–12):1289–306. doi: 10.3109/10715762.2011.616199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maliyackel AC, Otvos JW, Spreer LO, Calvin M. Photoinduced oxidation of a water-soluble manganese(III) porphyrin. Proc Natl Acad Sci U S A. 1986;83(11):3572–4. doi: 10.1073/pnas.83.11.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buettner GR, Need MJ. Hydrogen peroxide and hydroxyl free radical production by hematoporphyrin derivative, ascorbate and light. Cancer Lett. 1985;25(3):297–304. doi: 10.1016/s0304-3835(15)30009-4. [DOI] [PubMed] [Google Scholar]
- 28.Buettner GR. In the absence of catalytic metals ascorbate does not autoxidize at pH 7: ascorbate as a test for catalytic metals. J Biochem Biophys Methods. 1988;16(1):27–40. doi: 10.1016/0165-022x(88)90100-5. [DOI] [PubMed] [Google Scholar]
- 29.Buettner GR, Kiminyo KP. Optimal EPR detection of weak nitroxide spin adduct and ascorbyl free radical signals. J Biochem Biophys Methods. 1992;24(1–2):147–51. doi: 10.1016/0165-022x(92)90054-e. [DOI] [PubMed] [Google Scholar]
- 30.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 31.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–81. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 32.Buettner GR, Schafer FQ. Ascorbate (Vitamin C) as an antioxidant. In: May JM, Asard H, Smirnoff N, editors. Vitamin C:its Functions and Biochemistry in Animals and Plants. BIOS Scientific Publishers; 2004. pp. 173–188. [Google Scholar]
- 33.Buettner GR. Ascorbate autoxidation in the presence of iron and copper chelates. Free Radic Res Commun. 1986;1(6):349–53. doi: 10.3109/10715768609051638. [DOI] [PubMed] [Google Scholar]
- 34.Batinić-Haberle I, Spasojević I, Hambright P, Benov L, Crumbliss AL, Fridovich I. Relationship among redox potentials, proton dissociation constants of pyrrolic nitrogens, and in vivo and in vitro superoxide dismutating activities of manganese(III) and iron(III) water-soluble porphyrins. Inorganic Chemistry. 1999;38:4011–4022. [Google Scholar]
- 35.Batinic-Haberle I, Benov L, Spasojevic I, Fridovich I. The ortho effect makes manganese(III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin a powerful and potentially useful superoxide dismutase mimic. J Biol Chem. 1998;273(38):24521–8. doi: 10.1074/jbc.273.38.24521. [DOI] [PubMed] [Google Scholar]
- 36.Reboucas JS, Spasojevic I, Batinic-Haberle I. Pure manganese(III) 5,10,15,20-tetrakis(4-benzoic acid)porphyrin (MnTBAP) is not a superoxide dismutase mimic in aqueous systems: a case of structure-activity relationship as a watchdog mechanism in experimental therapeutics and biology. J Biol Inorg Chem. 2008;13(2):289–302. doi: 10.1007/s00775-007-0324-9. [DOI] [PubMed] [Google Scholar]
- 37.Wagner BA, Teesch LM, Buettner GR, Britigan BE, Burns CP, Reszka KJ. Inactivation of anthracyclines by serum heme proteins. Chem Res Toxicol. 2007;20(6):920–6. doi: 10.1021/tx700002f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bold RJ, Chandra J, McConkey DJ. Gemcitabine-induced programmed cell death (apoptosis) of human pancreatic carcinoma is determined by Bcl-2 content. Ann Surg Oncol. 1999;6(3):279–85. doi: 10.1007/s10434-999-0279-x. [DOI] [PubMed] [Google Scholar]
- 39.Espey MG, Chen P, Chalmers B, Drisko J, Sun AY, Levine M, et al. Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radic Biol Med. 2011;50(11):1610–9. doi: 10.1016/j.freeradbiomed.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.