Abstract
Purpose
To determine the effect of Alda-89 (an ALDH3 activitor) on (1) the function of irradiated (RT) submandibular gland (SMG) in mice, (2) its toxicity profile and (3) its effect on the growth of head and neck cancer (HNC) in vitro and in vivo.
Experimental Design
Adult mice were infused with Alda-89 or vehicle before, during and after RT. Saliva secretion was monitored weekly. Hematology, metabolic profile and post-mortem evaluation for toxicity were examined at the time of sacrifice. Alda-89 or vehicle was applied to HNC cell lines in vitro, and SCID mice transplanted with HNC in vivo with or without radiation; HNC growth was monitored. The ALDH3A1 and ALDH3A2 protein expression was evaluated in 89 HNC patients and correlated to freedom from relapse (FFR) and overall survival (OS).
Results
Alda-89 infusion significantly resulted in more whole saliva production and a higher percentage of preserved acini after RT compared to vehicle control. There was no difference in the complete blood count, metabolic profile, and major organ morphology between the Alda-89 and vehicle groups. Compared to vehicle control, Alda-89 treatment did not accelerate HNC cell proliferation in vitro, nor did it affect tumor growth in vivo with or without RT. Higher expression of ALDH3A1 or ALDH3A2 was not significantly associated with worse FFR or OS in either HPV-positive or HPV-negative group.
Conclusion
Alda-89 preserves salivary function after RT without affecting HNC growth or causing measurable toxicity in mice. It is a promising candidate to mitigate RT-related xerostomia.
Keywords: ALDH3A1, ALDH3A2, Alda-89, xerostomia, radiation, head and neck cancer
Introduction
Head and neck cancer (HNC) is the 4th most common cancer globally (1) and most of these patients require radiotherapy (RT) as part of their treatment (2). Despite the development of intensity-modulated radiotherapy (IMRT), which is often used to spare the parotid glands, submandibular glands (SMG) continue to be damaged from high dose RT due to their close proximity to the draining cervical lymph nodes. As a consequence, over 70% of HNC patients suffer from RT-related xerostomia or dry mouth, which severely impaired their quality of life (3).
Current treatments for RT-related xerostomia are mainly saliva substitutes that temporarily alleviate symptoms, but do not improve salivary function (4). Recently c-Kit+ salivary stem cells (SSC) have been identified from mature salivary glands in both mice and human and shown to improve saliva secretion when transplanted into recipient mice (5). This spurs an interest in the field to investigate the possibility of SSC therapy to restore salivary gland function to treat RT-induced xerostomia.
However, one major obstacle in developing SSC transplantation therapy is the rare number of SSC in in salivary glands. It has been shown that the overall SSC yield (defined as cells positive for SSC surface markers) by FACS analysis was 0.1–0.3% of total viable cells (5, 6). The rarity of these cells makes it very difficult to isolate enough viable cells for culture and for eventual transplanation therapy. Therefore, strategies that enhance SSC survival and enrichment in vivo during RT and allow them to proliferate and differentiate in the same gland after RT may be easier and more efficient to introduce into the clinic. This is especially important in patients who are about to start RT as part of their HNC treatment. We have previously reported that both adult human and murine SSCs express higher levels of ALDH3 isozymes compared to non-SSC (6). These findings are in accordance with other reports that ALDH is highly expressed in hematopoietic SCs (7–10), and is used as a stem cell marker in neuronal (11, 12), muscle (13), hepatic (14) and adipose tissue (15). We showed that in comparison to vehicle control, treatment of mice with Alda-89, a novel ALDH3 activator, resulted in a significant increase in the number of isolated c-Kit+/CD90+ SSC, more salispheres and larger salispheres with more proliferating cells, as verified by BrdU staining. We also verified the on-target effect of Alda-89 by confirming that it indeed enhanced ALDH3 activity in vivo. These findings indicate that activating ALDH3 with Alda-89 enhances SSC survival and proliferation in vivo (6).
Based on these data, we hypothesize that by preserving SSC survival during RT and enhancing their proliferation after RT; Alda-89 can mitigate salivary gland dysfunction from RT. Here, we show that Alda-89 infusion during RT minimizes RT damage to the SMG both functionally and morphologically compared to vehicle control. Alda-89 infusion does not result in any observable major organ toxicity in the treated animals. More importantly, Alda-89 treatment does not affect HNC cell line proliferation in vitro, nor does it affect tumor growth in vivo in SCID mice xenografts. In the small group of HNC patient evaluated, the expression of either ALDH3 isoforms does not significantly correlate with prognosis.
Materials and Methods
In vivo treatment with Alda-89 (ALDH3 activator)
All animal procedures were approved by the Stanford University Administrative Panel on Laboratory Animal Care (APLAC). The procedure is performed as previously described (6). Briefly, 4–5 weeks old female C57BL/6 mice or 4–6 weeks old SCID mice (Jackson Laboratory, Sacramento, CA) were used. Alda-89 (100 mg/kg/day, approximately 600 µM) or vehicle (PEG400/DMSO, 50:50) were delivered in a sustained fashion using an ALZET osmotic pump (DURECT Corporation, Cupertino, CA). The osmotic pumps contained 3.4 M of Alda-89 and delivered the drug at a rate of 0.15 µl/h continuously for up to 6 weeks. Pumps were implanted intra peritoneally into the mice under anesthesia.
Irradiation and saliva collection
One week after pump placement, the submandibular and upper neck region, containing the submandibular glands, were exposed to either a single dose of 15 Gy (one experiment) or 30 Gy in 5 consecutive daily fractions of 6 Gy (another experiment) with the rest of the body shielded. Saliva collection was performed at basal level (before pump placement), 1 week after pump placement, then 1, 2, 3, 4, 6 and 8 weeks after RT. 2 mg/kg pilocarpine injection (s.c.) was used to stimulate saliva production at each collection time point as previously described (6). Pumps were implanted intraperitoneally into the mice under anesthesia. The osmotic pumps contained 3.4 M of Alda-89 and delivered the drug at a rate of 0.15 µl/h continuously (calculated to deliver approximately 100mg/kg/day, which is equivalent to (0.1g/162)/(1kg/1kg/l) = 617 µM) for six weeks. The measured saliva secretion was normalized to the mouse body weight (at each measurement time point) and to the basal level.
Staining and quantification of acinar cells
At the time of sacrifice, SMGs were removed, fixed in formalin, and embedded in paraffin. Paraffin sections were deparaffined, rehydrated and stained with Hematoxylin and Eosin (HE) or Periodic acid Schiff (PAS, Sigma – Aldrich, INC, St. Louis, MO) following the manufacturer’s instructions. Ten randomly selected PAS stained images were acquired at 200x magnification using a Leica DM6000 B microscope (Leica Microsystems Inc., Bannockburn, IL). The percentage of intact acinar area to total measured area was quantified using Image J.
In vitro proliferation of head and neck cancer cell lines
HN5, Fadu, SQB20, Cal27, ICC8 and SCC1 were obtained from the American Type Culture Collection (ATCC). UM22B was obtainted from the Univeristy of Michigan (Courtesy Dr. Carey). SAS cells were purchased from Japanese Cell Culture Collection. SCC90 cell line was a gift from Dr. Robert Ferris (University of Pittsburgh, Pittsburgh, PA). Cell lines were maintained in DMEM supplemented with 10% fetal bovine serum. For the in vitro cell proliferation assays, cells were plated in equal cell numbers in the present of 60uM, 600uM Alda-89 or DMSO and counted during logarithmic growth phase with a hemacytometer.
Clonogenic survival assay
SAS cells were incubated with 200uM Alda-89 or DMSO 2 hours prior to RT (either single fraction of 8 Gy or a fractionated regimen of 3Gy/fraction/day for 4 consecutive days). Cells were then plated in triplicates at different densities ranging from 300 to 10,000 cells per dish in the presence of 200 µM Alda-89 or DMSO. Plating efficiency was determined by harvesting untreated cells. After 7–10 days, the cells were fixed and stained with a solution of 0.25% crystal violet in ethanol. Surviving fraction was determined by counting the number of colonies with >50 cells. Surviving fraction was normalized by the plating efficiency. The results represent the mean of triplicate with the error bars representing ±1 SD.
Xenograft study
Six-week old SCID mice were purchased from the Jackson Laboratory and five mice per group were used. SAS (2.5×106 cells/injection) were implanted into the flanks of each mouse. When the tumor volume reached approximately 100 mm3, Alzaret pumps containing either Alda-89 or DMSO were implanted intraperitoneally in the mice and allowed to release the drug up to 6 weeks at a rate of 0.15 µl/h continuously for a total dose of 100 mg/kg/day. Tumor size were measured every 1–2 days. Tumor volume was calculated by the formula (π × length × width × height)/6. In the xenograft irradiation study, when the tumor volume reached approximately 50mm3, Alzet pumps containing either Alda-89 or DMSO were implanted intraperitoneally in the mice. 1 week after the pump placement, when the tumor volume reached approximately 130mm3, 12 Gy was delivered to the tumor with the rest of the body shielded.
Blood test and pathology exam
Blood from Alda-89 and vehicle treated mice were obtained in heparinized syringes by cardiac puncture at terminal bleed. A complete blood count (CBC) and a comprehensive blood chemistry panel were analyzed by the Stanford Department of Comparative Medicine.
A complete necropsy was performed on representative mice by senior veteranerian, Dr. Richard Luong, from the Department of Comparative Medicine. All tissues were preserved and slides were prepared of the following organs: brain, liver, kidney, spleen, heart, adrenal gland, mesenteric lymph node, thyroid gland, thymus, trachea, lung, gastrointestinal tract (esophagus, stomach, duodenum, colon), pancreas, urinary bladder. All tissues were fit on 5 slides per organ set. The score for inflammation, necrosis and apoptosis according to a quantitative scale of 0 – 5 as outlined as follows: 0 = no significant lesion or within normal limits; +1 = minimal; +2 = mild; +3 = moderate; +4 = marked; +5 = severe.
Patients
Criteria for patient participation included (1) newly diagnosed head and neck squamous cell carcinoma (HNSCC), (2) available tissue block, and (3) willingness to sign an informed consent. All tumors were staged using the 2002 American Joint Committee on Cancer staging system (16).
Tissue microarray staining and scoring
The tissue microarray (TMA) was constructed from formalin-fixed paraffin-embedded samples of HNSCC as previously described (17). Immunoperoxidase stains for ALDH3A1 (citrate, 1:300; Abcam, Cambridge, MA) and ALDH3A2 (citrate, 1:200; Abcam, Cambridge, MA) were performed on 4 µm-thick sections of the TMA. The staining results were interpreted by a pathologist (CSK), who was blinded to the clinical data, and scored based on cytoplasmic staining as negative (blush or no tumor staining), weakly positive (< 70%) or strongly positive (> 70%). For the purpose of outcome analysis, the weakly and strongly positive groups were combined together into a single positive staining group.
Statistics
Data are expressed as mean ± standard errors (SEM). Statistical analysis of variance (ANOVA) and T-tests were use to compare the saliva secretion, acinar cell areas, clonogenic survival and tumor growth curve in vitro and in vivo. A p-value ≤ 0.05 is considered to be significant. Kaplan-Meier product-limit method was performed using the Statview (Analytical Software, Inc., Tallahassee, MI) statistical software as previously described (18). Log rank test was used to compare survival curves.
Results
Alda-89 protects SMG from radiation
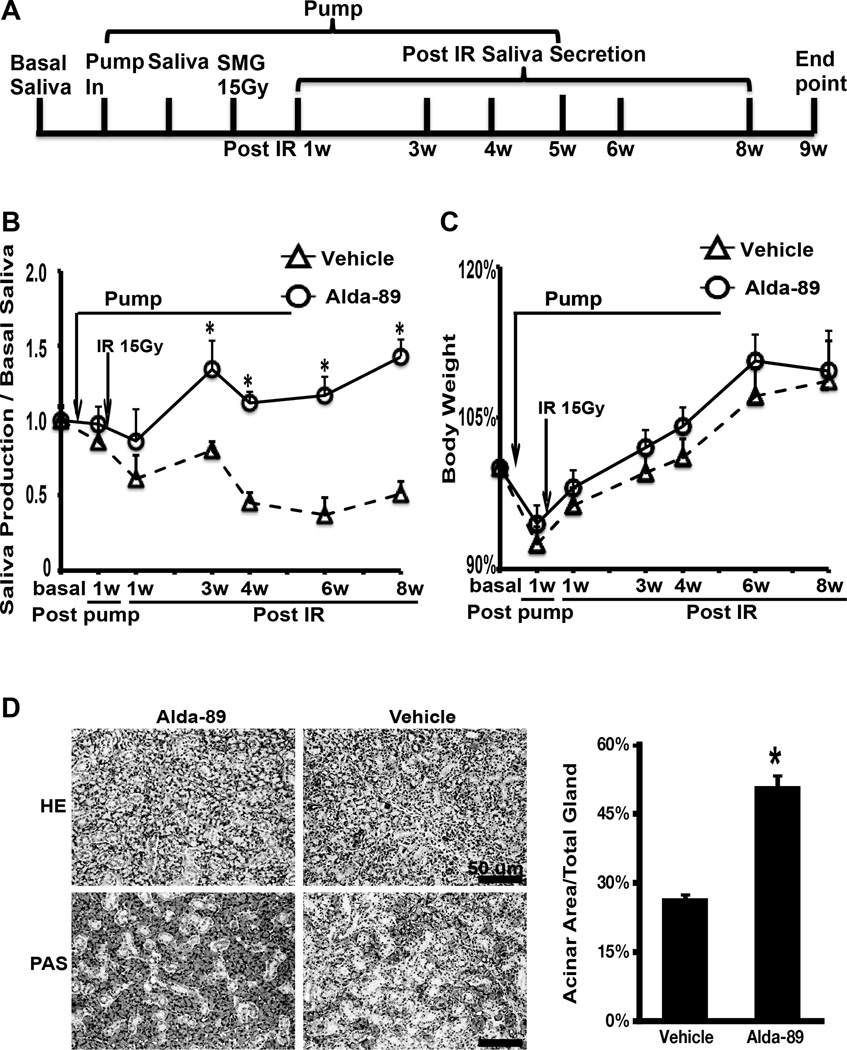
ALZET osmotic pumps were used to deliver Alda-89 at 34mg/kg/day (approximately 200 uM) or vehicle to C57BL/6 mice in a continuous manner one week before, during and one week after RT (2 weeks total). At this dose, there was a trend for a higher saliva production with Alda-89 treatment starting at week two (when drug delivery ended), but the difference was not statistically significant (Sup. Fig. 1). We hypothesize that both a higher dose and a longer treatment duration may be needed to observe a functional difference. Therefore, we proceeded to administer a higher dose of Alda-89 (100mg/kg/day, approximately 600 µM) one week before and five weeks after RT (Fig. 1A). At this dose, there was a significant improvement in saliva production in the Alda-89 treated mice (Fig. 1B). In fact, the Alda-89 treated group did not have any reduction in saliva production compared to baseline and this was maintained the same level until sacrificed after 8 weeks, while the vehicle control group continued to experience decline in saliva production for more than 50%, which stabilized after 6 weeks post RT. Importantly, Alda-89 treatment did not affect the general health of the mice as reflected in the body weight, which was similar between the two groups (Fig. 1C). A body weight reduction due to pump placement occurred in both groups, reflecting the stress of the procedure; however, all the mice regained their weight, which continued to climb during the observation period. H&E and PAS staining of the SMG tissues further confirmed that intact SMG acini structures were better preserved in the Alda-89-treated compared to vehicle-treated mice (Fig. 1D). The percentage of acinar area/ total area per high power field was 51.05% ± 2.64% in the Alda-89 group compared to 26.71% ± 1.24% in the vehicle group (Fig. 1E, p<0.05). The percentage of ainar area/total area per high power field in unirradiated SGMs typically ranged from 60% to 70%.
Figure 1. Alda-89 preserves submandibular gland function post radiation.
Panel A:Schematic representation of the experimental procedure. Saliva collection was performed at basal level (before pump placement), 1 week post pump placement, and 1, 3, 4, 6 and 8 weeks post-RT. Mice were euthanized at 9 weeks after RT. Panel B: Whole saliva measurement at different time points by treatment group normalized to the body weight and basal saliva level. Note that the curves diverge around week 3 after RT and the difference was statistitcally significant (* p < 0.05). Panel C: Mean body weight at different time points by treatment group. No significant difference was observed. Panel D: Representive HE staining (top panels, scale bar =100 um) and PAS staining (bottom panels, scale bar = 100um) of SMG tissue showing more intact acinars in the Alda-89 treated glands. Panel E: Quantification of the percent acinar area to total gland area in 10 randomly selected PAS stained images at 200x magnification. There was significantly more intact acini in the Alda-89 treated glands (*p<0.05).
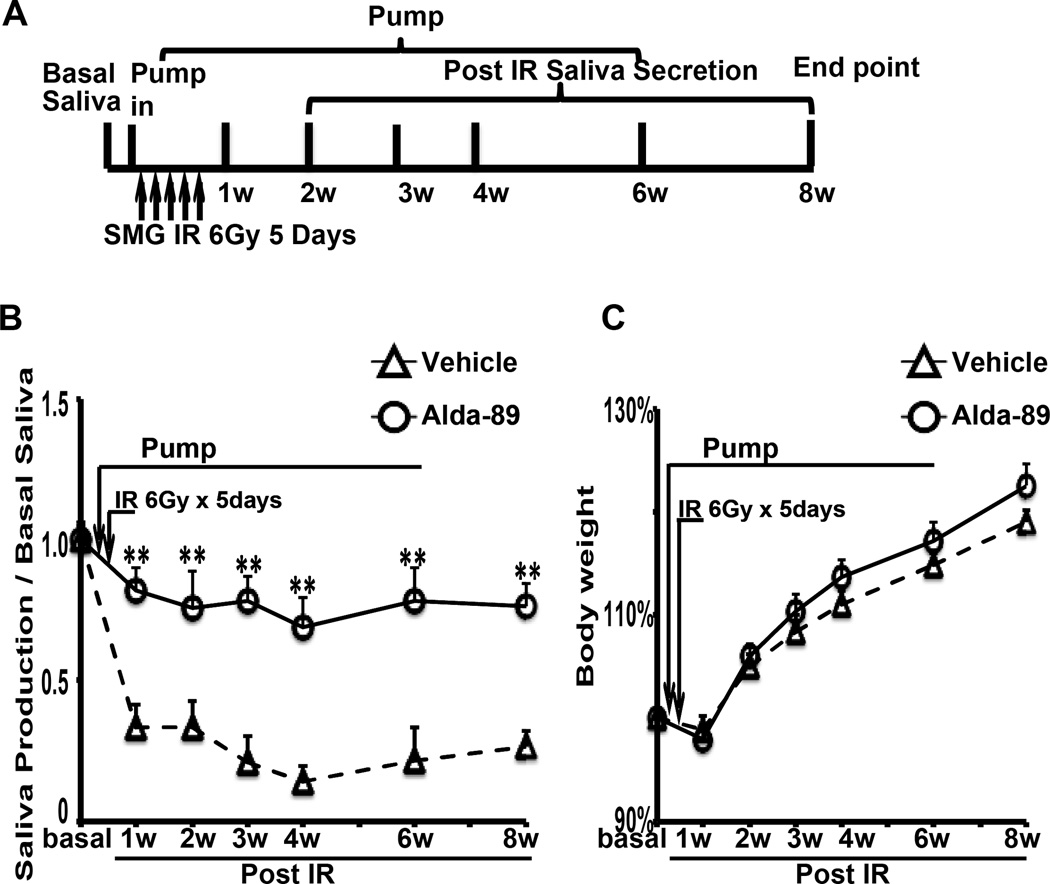
In addition to the above single-fraction experiment, we also performed fractionated radiation treatment (30 Gy total in 5 consecutive days with daily fraction of 6 Gy) on a different set mice receiving either Alda-89 or vehicle. The experimental schema is shown in Fig. 2A. Consistent with our finding for the single 15 Gy RT dose, Alda-89 significantly preserved the saliva flow in fractionally irradiated mice (Fig. 2B). All mice in both groups lost weight after irradiation but regained most of it over time with no significant difference between the two groups (Fig. 2C).
Figure 2. Alda-89 preserves submandibular gland function after fractionated radiation.
Panel A: Schematic representation of the experimental procedure. Saliva collection was performed at basal level (before pump placement), 1 week after the fist dose of RT, and 2, 3, 4, 6 and 8 weeks post-RT. Panel B: Whole saliva measurement at different time points by treatment group normalized to the body weight and basal saliva level. Note that the curves diverge around week 2 after RT and the difference was statistitcally significant (* p < 0.05). Panel C: Mean body weight at different time points by treatment group. No significant difference was observed.
Alda-89 does not accelerate HNC growth in vitro or in vivo
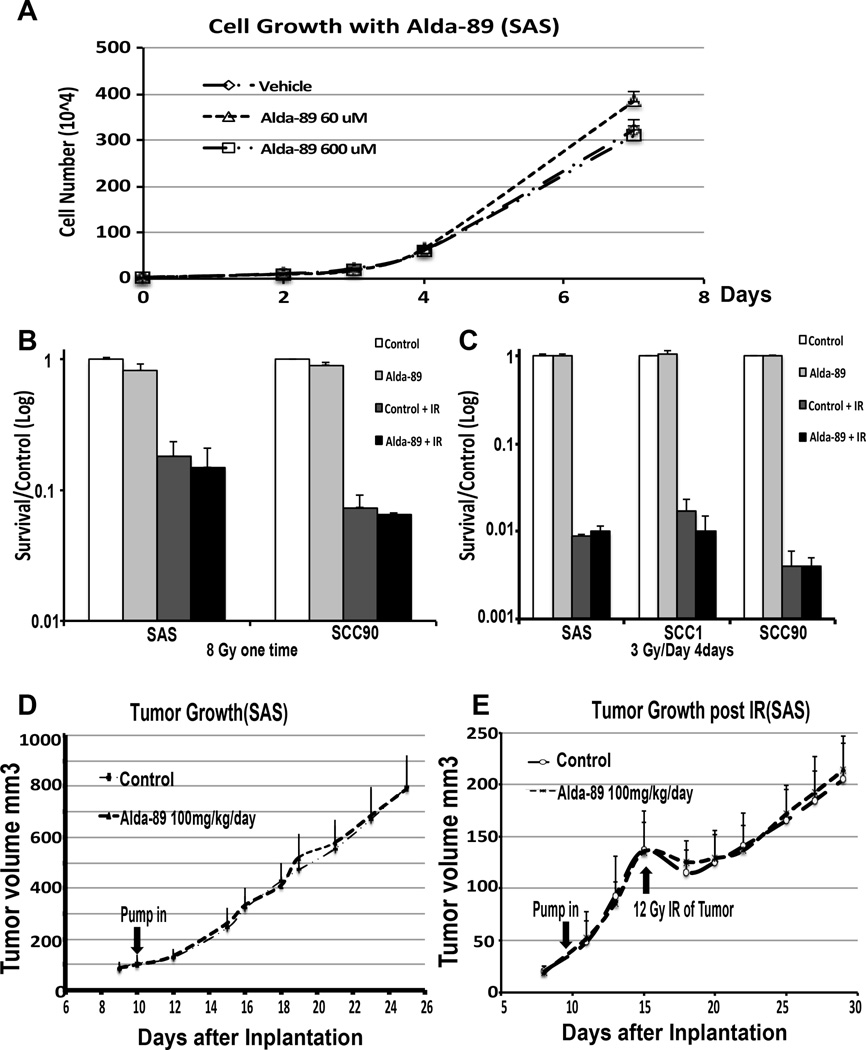
Aldh3Aa1 and Aldh3a2 proteins were expressed a different levels in the examined HNC cell lines, with high expression in the UM22B, Fadu, HN5, SCC1 and ICC8, medium in SAS and Cal27, and low in SCC90 and SQB20 (Sup Fig 2). To investigate the role of Alda-89 on HNC cell line proliferation in vitro, four head and neck squamous carcinoma cell lines with different ALDH3 expression level (3 HPV-negative lines: HN5, SCC1, and SAS and 1 HPV-positive line: SSC90) were plated in equal cell numbers in the presence of 60µM, 600µM Alda-89 or Vehicle. The growth rate was calculated by counting the cell numbers during logarithmic growth phase. In all cell lines tested, treatment with Alda-89 did not accelerate the growth rate as compared to vehicle control. There was also no difference in the growth rate between the high and low Alda-89 doses in these cell lines (Fig. 3A and Sup Fig. 3).
Figure 3. Alda-89 does not promote HNC growth in vitro or in vivo.
Panel A: Cell growth curves in culture for SAS cells treated with vehicle, 60 µM or 600 µM Alda-89. Panel B: Clonogenic survival assay showing that 8 Gy RT dramatically reduced the SAS and SCC90 cell survival normalized to the control group. Alda-89 treatment did not affect RT cell kill compared to vehicle control. Panel C: Clonogenic survival assay showing that fractionated RT of 3Gy/fraction/d for 4 consecutive days dramatically reduced the SAS, SCC90 and SCC1 cell survival normalized to the control group. Alda-89 treatment did not affect RT induced cell death compared to vehicle control. Panel D: SAS xenograft tumor growth curves over time. Treatment with Alda-89 at 100 uM/day did not accelerate the tumor growth in vivo compared to the vehicle. Panel E: SAS xenograft tumor growth curves over time after 12 Gy radiation. Treatment with Alda-89 at 100 mg/kg/day did not accelerate the tumor growth in vivo compared to the vehicle control.
To test the effect of Alda-89 on RT-induced cell kill, we performed clonogenic survival assay on HNC cell lines SAS and SCC90, which were pretreated with 200 µM Alda-89 or DMSO 2 hours before a single dose of 8 Gy). Cells were then plated in triplicates in the presence of 200 µM Alda-89 or DMSO. After 7–10 days, colonies were quantified to determine the surviving fraction. Alda-89 treatment did not protect cancer cells from RT; in fact, the Alda-89 treated cells had a non-significantly slightly lower surviving fraction than the vehicle treated cells either with or without RT, suggesting that Alda-89, at the concentration used, was potentially toxic to these cells (Fig. 3B).
We also performed clonogenic survival assay on SCC1, SCC90 and SAS, which were pretreated with 200 µM Alda-89 or DMSO 2 hours before fractionated RT of 3 Gy/fraction/d for 4 consecutive days. Cells were then plated in triplicate in the presence of 200 µM Alda-89 or DMSO. Similar to single dose of 8 Gy, Alda-89 treatment did not protect cancer cells from fractionated RT (Fig. 3C).
To evaluate the effect of Alda-89 on HNC growth in vivo, SAS tumor bearing SCID mice were exposed to either Alda-89 (100 mg/kg/day) or vehicle delivered via the Alzet osmotic pumps when the tumors reached approximately 100 mm3. Tumors size was measured every 1–2 day during Alda-89 infusion and then for another two weeks. The tumor growth curves of the vehicle and the Alda-89-treated mice practically overlapped, indicating that Alda-89 did not accelerate tumor growth in vivo (Fig. 3D).
To study the effect of tumor regrowth after RT, we irradiated the transplanted tumor xenograft with a single dose of 12 Gy with the rest of the body shielded. There was no difference in the tumor regrowth after RT between the two groups (Fig. 3E).
Alda-89 does not induce major organ toxicity in vivo
Since Alda-89 was delivered through an osmotic pump placed intraperitoneally, the entire mouse body was exposed to the drug. To assess for its potential toxicity, we obtained CBC and a comprehensive chemistry panel using blood from the terminal bleed. Although both groups showed hypochromic anemia and elevated absolute lymphocyte, monocyte and eosinophil counts, which were presumably related to the pump placement procedure, there was no significant difference in any measurement between the two groups (Table 1).
Table 1.
Lab Test for Representative mice Receiving Vehicle or Alda-89
| Reference range |
C1 | C2 | C3 | A1 | A2 | A3 | |
|---|---|---|---|---|---|---|---|
| CBC | |||||||
| WBC | 5.5–9.3 K/ul | 7.76 | 11.2 | 10.3 | 11.2 | 7.1 | 9.9 |
| HCT | 39.0–47.0 % | 36.5 | 41.1 | 42.3 | 42.1 | 36.2 | 35.5 |
| Platelet Estimate |
Adequate K/uL |
Adequate | Adequate | Adequate | Adequate | Adequate | Adequate |
| Chemistry Panel |
|||||||
| Glucose | 184–220 mg/dL |
160 | 198 | 208 | 224 | 173 | 176 |
| AST | 192–388 U/L |
51 | 102 | 50 | 67 | 62 | 38 |
| ALT | 76–160 U/L | 25 | 39 | 38 | 91 | 24 | 17 |
| Alkaline Phosphatase |
171–183 IU/L |
61 | 99 | 124 | 99 | 57 | 59 |
| GGT | N/A | 1 | 1 | 1 | 0 | 0 | 0 |
| Total Bilirubin |
N/A | 0 | 0 | 0 | 0 | 0 | 0 |
| BUN | 20.3–24.7 mg/dL |
14 | 27 | 29 | 21 | 19 | 22 |
| Creatinine | 0.1–1.1 mg/dL |
0 | 0.3 | 0.5 | 0.4 | 0.3 | 0.4 |
| Calcium | 8.9–9.7 mg/dL |
8.2 | 9.2 | 9.9 | 9.5 | 9 | 8.6 |
| Phosphorus | N/A | 5.1 | 6.6 | 6.9 | 7.7 | 7.2 | 6.6 |
| T.Protein | 5.0–6.2 g/dL |
3.9 | 5.5 | 5.4 | 5.1 | 4.6 | 4.5 |
| Albumin | 3.2–3.6 g/dL |
1.9 | 3.3 | 3.3 | 3.1 | 2.8 | 2.6 |
| Globulin | N/A | 2 | 2.2 | 2.1 | 2 | 1.8 | 1.9 |
| Electolyte Panel |
|||||||
| Sodium | 114–154 mmol/L |
157 | 156 | 155 | 155 | 157 | 152 |
| Potassium | 3.0–9,6 mmol/L |
4.7 | 5.6 | 5.1 | 5.8 | 4.9 | 4.8 |
| Chloride | N/A | 114 | 116 | 115 | 116 | 119 | 117 |
| Carbon Dioxide |
N/A | 18.4 | 20.8 | 22.2 | 24 | 23.7 | 21 |
C: Vehicle Control A: Alda-89 N/A not applicable
A complete necropsy was performed on representative mice, showing minimal tissue autolysis and normal microscopic appearance for all examined organs. All mice showed histologic evidence of chronic reactive peritonitis, which was consistent with intraperitoneal surgery and placement of the osmotic pumps.
ALDH3 protein expression did not correlate with prognosis in HNC patients
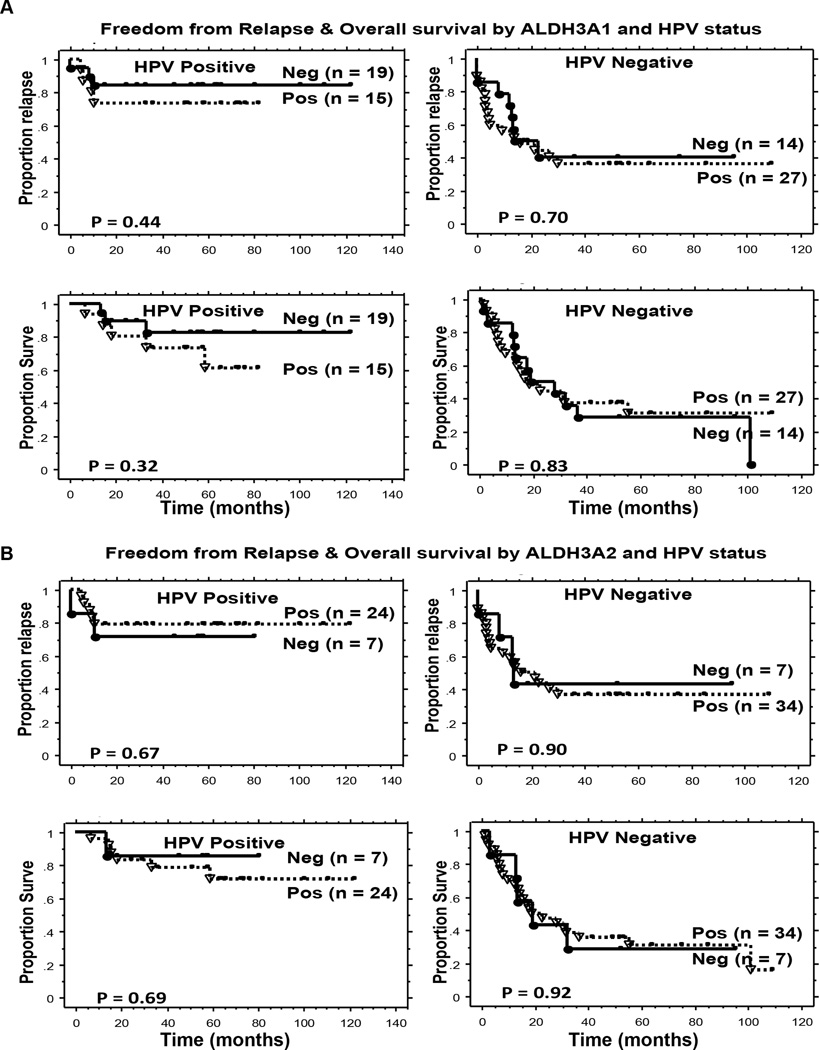
To further confirm that the expression of ALDH3 isozymes does not confer a worse outcome in HNC patients, we stained a tissue microarray containing 89 HNSCC with ALDH3A1 and ALDH3A2 specific antibodies. These patients with newly diagnosed HNSCC received their treatment at Stanford University with a median follow up of 64 months for living patients; Table 2 shows the patient, tumor and treatment characteristics. Since p16 and HPV status are an important independent prognostic factors in HNSCC, we also assessed the prognostic significance of ALDH3 isozymes by HPV status as previously determined by p16 immunohistochemistry and HPV pyrosequencing (17). 75 patients of the entire group had tumor assessable for both HPV status and ALDH3A1 expression: 34 patients with HPV-positive and 41 with HPV-negative tumors. As shown in Figure 4A, there was no statistically significant difference in FFR and OS between the ALDH3A1 positive and negative tumors for either HPV-positive or HPV-negative group.
Table 2.
Patient, Tumor, and Treatment Characteristics
| Parameter | ALDH3A1 | ALDH3A2 | |||||
|---|---|---|---|---|---|---|---|
| Negative (%) | Positive (%) | p-value | Negative (%) | Positive (%) | p-value | ||
| Age | < 60 ≥ 60 |
19 (21) 21 (24) |
27 (30) 22 (25) |
0.53 | 7 (8) 11 (13) |
35 (41) 32 (38) |
0.43 |
| Gender | Male Female |
38 (43) 2 (2) |
37 (42) 12 (13) |
0.02 | 16 (19) 2 (2) |
55 (65) 12 (14) |
0.72 |
| T-stage | 0–2 3–4 |
16 (18) 24 (27) |
24 (27) 25 (28) |
0.53 | 5 (6) 13 (15) |
32 (38) 35 (41) |
0.18 |
| N-stage | 0–1 2 3 |
5 (6) 27 (30) 8 (9) |
10 (11) 32 (36) 7 (8) |
0.53 | 2 (2) 11 (13) 5 (6) |
12 (14) 45 (53) 10 (12) |
0.41 |
| Stage | 2 3 4 |
0 (0) 4 (5) 36 (40) |
4 (5) 2 (2) 43 (48) |
0.11 | 0 (0) 1 (1) 17 (20) |
4 (5) 5 (6) 58 (68) |
0.54 |
| Site | OP Others |
30 (34) 10 (11) |
25 (28) 24 (27) |
0.03 | 14 (16) 4 (5) |
38 (45) 29 (34) |
0.17 |
| HPV* | Negative Positive Inevaluable |
14 (16) 19 (21) 7 (8) |
27 (30) 15 (17) 7 (8) |
0.07 | 7 (8) 7 (8) 4 (5) |
34 (40) 24 (28) 9 (11) |
0.57 |
|
Treat- ment |
CRT Surg+RT |
37 (42) 3 (3) |
33 (37) 16 (18) |
0.004 | 16 (19) 2 (2) |
51 (60) 16 (19) |
0.34 |
CRT: Chemoradiation, RT: Radiation
75 patients had tumor assessable for both HPV status (p16, HPV ISH and HPV pyrosequencing) and ALDH3A1 staining & 72 patients had tumor assessable for both HPV status (p16, HPV ISH and HPV pyrosequencing) and ALDH3A2 staining
Figure 4. Kaplan-Meier estimates of clinical outcomes by HPV and ALDH3 level.
Panel A: Freedom from relapse (Top) and overall survival (Bottom) in 75 HNC patients by ALDH3A1 expression and HPV status. Panel B: Freedom from relapse (Top) and overall survival (Bottom) of 72 HNC patients by ALDH3A2 expression and HPV status.
Similarly, 72 patients had tumor assessable for both HPV status and ALDH3A2 expression: 31 patients with HPV-positive and 41 patients with HPV-negative tumors. As shown in Figure 4B, there was no statistically significant difference in FFR and OS between the ALDH3A2 positive and negative tumors for either HPV-positive or HPV-negative group.
Discussion
There is an increasing interest in stem cell therapy to replenish salivary gland function after RT damage (5, 19, 20). One direction is to promote the survival and proliferation of the rare SSC population within the gland. Different growth factors or cytokines have been tested for this purpose, including the Keratinocyte Growth Factor (KGF) (21), also known as FGF7, which is a critical growth factor, supporting embryonic SMG epithelial bud proliferation and branching (22). However, since KGF is an epithelial growth factor, there is a theoretical concern that its administration before and during treatment can promote tumor growth or decrease the effectiveness of chemoradiation. Two randomized studies in HNC have shown that short-term administration of KGF did not compromise treatment cure rate; however, the duration and the dose of KGF used in these studies did not reduce radiation-related xerostomia (23).
Instead of focusing on growth factors, our group studied genes that are differentially up regulated in SSC compared to non-SSC counterparts and identified ALDH3A1 and ALDH3A2 as two genes that are differentially expressed at high levels in both adult human and murine SSCs (6). Aldehyde dehydrogenases are enzymes involved in oxidizing intracellular aldehydes. The family has 19 members in human with the most abundant and well-studied member being ALDH1 (24, 25), which has been found to be highly expressed in many adult tissue stem cells or progenitor cells, including hematopoietic, neuron, muscle, hepatic, adipose stem cells and progenitor cells (7–15). Cells with high ALDH activity as selected by the Aldefluor assay have been employed to treat ischemic limbs (26), myocardial infarction (22) and liver damage in animal models (14). Despite the fact that ALDH activity is considered a marker of stem cells, little is known about the exact role each ALDH isozyme plays in stem cells; this is partly because by modifying the aldehyde groups, ALDH family members play multiple functions in cells, including cholesterol and amino acid metabolism, alcohol and drug detoxification (27). In addition, ALDH enzymes are expressed in multiple cellular compartments, including the endoplasmic reticulum, mitochondria, cytosol and the nucleus, and there is compensation effect among the different family members, making it difficult to study the specific effect of a particular member (28).
As previously mentioned, we showed that Alda-89, a novel and specific ALDH3 activator, significantly increased the c-Kit+/CD90+ SSC number in vivo with associated increases in number and size of salispheres in culture (6). The mechanism by which activation of ALDH3 resulted in higher SCC numbers and salisphere formation has not yet been determined, but postulated to be due to better SSC survival and/or proliferation. This translated to improved salivary function and better preservation of acinar morphology, as shown in this study.
One draw back of using total stimulated saliva production to assess SGM function is the inability to distinguished contributions from SMG and other saliva glands such as parotid and minor salivary glands. However, currently we do not have a feasible way to directly quantify SMG contribution repeatedly. Previous studies have shown a direct relationship between the number of intact acini and saliva production after radiation (29). Therefore, we used this approach to indirectly assess SMG function. We found that Alda-89 treated mice had a significantly higher number of preserved acini than vehicle treated control animals.
In addition of being associated with normal tissue stem cells, elevated ALDH activity has been linked to different cancer stem cells, including head and neck squamous carinoma (30), lung (31), liver (32), pancreas (33), colon (25), breast (34), cervical (35) and prostate cancers (36). Specifically, these studies used the Aldefluor assay, which mainly evaluates the activity of ALDH1 (24). The relationship between ALDH3 expression or activity and cancer stem cell is less clear. ALDH3 subfamily includes ALDH3A1, which is mainly expressed in the cornea, lung, esophagus and stomach (37), and ALDH3A2, which is mainly found in the liver, and is expressed in many other tissues including kidney, intestine, stomach, skeletal muscles, skin, lung, pancreas, placenta, heart and brain (27). ALDH3A1 has been shown to be expressed at high level in breast cancer stem cells (38) and down-regulation of this enzyme resulted in reduced non-small cell lung cancer cell growth and motility (39). In contrast, neither ALDH3A2 expression nor activity has been linked to cancer development; mutations in this gene have been identified in humans and linked to a genetic condition known as the Sjogren-Larsson Syndrome (SLS), characterized by mental retardation, spasticity and itchthyosis (thick, scaly skin) (40). Function and expression of either enzyme has not been studied in head and neck squamous cell carcinoma. Ours is the first study to systematically evaluate the effect of activating ALDH3 in HNC cell lines and the results indicate that activation of ALDH3 with Alda-89 did not affect the pace of HNC growth in vitro or in vivo. In addition, it did not affect cell death by radiation or tumor regrowth after radiation in xenografts. Although 55% and 79% of the evaluated human HNSCC stained positive for ALDH3A1 and ALDH3A2, respectively, expression of these enzymes did not significantly correlate with either tumor relapse or overall survival. However, the number of patients in each subgroup is quite small and these results will need to be validated in a larger group of homogenously treated patients.
Conclusion
In summary, we have for the first time showed that administration of a specific ALDH3 activator, Alda-89, protected the SMG function from RT damage. The drug appears to be safe in treated mice when delivered for 6 weeks at the dose of 100mg/kg/day, and did not enhance tumor growth or protect tumor from RT. Future studies will focus on optimizing the drug delivery route, dose, duration of treatment and sequencing with radiotherapy. In addition, a larger study will be conducted to assess the relationship between treatment outcomes and the expression of these enzymes in human HNSCC.
Supplementary Material
Statement of Translational Relevance.
Xerostomia or dry mouth is the most common side effect in head and neck cancer (HNC) patients who receive radiation therapy. Due to the close proximity of the salivary glands to the cervical draining lymph nodes, they cannot be routinely spared from high dose radiation in many patients. Recently, adult stem cells have been identified in salivary glands and means that can protect these stem cells from radiation damage and allow them to subsequently regenerate have the promise of preserving or restoring salivary function. Previously our group has identified Alda-89 as a novel ALDH3 activator that could significantly enrich submandibular gland (SMG) stem cells in vivo. Here, we report that Alda-89 infusion significantly improves post-radiation SMG function in vivo without causing any measurable toxicity in treated animals. Most importantly, Alda-89 treatment does not result in accelerated growth of several HNC cell lines or tumor growth in a HNC xenograft model. Finally, neither ALDH3A1 nor ALDH3A2 protein expression in human HNC significantly correlated with prognosis. Altogether, these data indicate that short-term treatment with ALDH3 agonist can mitigate radiation-induced xerostomia without affecting tumor growth.
Acknowledgement
This work was supported by grant from the National Institute of Health (R21DE021167A1-01 [QTL, CSK, CHC, DMR, NX] and 5P01 CA067166-15 [QTL, AG, AK]. We thank Dr. Richard Luong (BVSc, DACVP) for mice necropsy evaluation.
Footnotes
Author Contributions:
Nan Xiao: Conception and design, aquisition of data, manuscript writing
Hongbin Cao: Aquisition of data, analysis and interpretation of data
Che-Hong Chen: Conception and design
Christina S. Kong: Aquisition of data, analysis and interpretation of data
Rehan Ali Aquisition of data, analysis and interpretation of data
Cato Chan: Administrative and material support
Davud Sirjani: Material support
Edward Graves: Conception and design
Albert Koong: Conception and design, manuscript writing
Amato Giaccia: Conception and design, manuscript writing
Daria Mochly-Rosen: Conception and design, manuscript writing
Quynh-Thu Le: Conception and design, manuscript writing, final approval of manuscript
Disclaimer: DMR and C-HC are co-founders of ALDEA Pharmaceuticals. However, none of the work described in this manuscript is supported by the company or done in collaboration with the company.
Reference
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 3.Lee NY, Le QT. New developments in radiation therapy for head and neck cancer: intensity-modulated radiation therapy and hypoxia targeting. Semin Oncol. 2008;35:236–250. doi: 10.1053/j.seminoncol.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sagowski C, Wenzel S, Metternich FU, Kehrl W. Studies on the radioprotective potency of amifostine on salivary glands of rats during fractionated irradiation: acute and late effects. Eur Arch Otorhinolaryngol. 2003;260:42–47. doi: 10.1007/s00405-002-0496-4. [DOI] [PubMed] [Google Scholar]
- 5.Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3:e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banh A, Xiao N, Cao H, Chen CH, Kuo P, Krakow T, et al. A novel aldehyde dehydrogenase-3 activator leads to adult salivary stem cell enrichment in vivo. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:7265–7272. doi: 10.1158/1078-0432.CCR-11-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storms RW, Trujillo AP, Springer JB, Shah L, Colvin OM, Ludeman SM, et al. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc Natl Acad Sci U S A. 1999;96:9118–9123. doi: 10.1073/pnas.96.16.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong L, Stojkovic M, Dimmick I, Ahmad S, Stojkovic P, Hole N, et al. Phenotypic characterization of murine primitive hematopoietic progenitor cells isolated on basis of aldehyde dehydrogenase activity. Stem Cells. 2004;22:1142–1151. doi: 10.1634/stemcells.2004-0170. [DOI] [PubMed] [Google Scholar]
- 9.Hess DA, Wirthlin L, Craft TP, Herrbrich PE, Hohm SA, Lahey R, et al. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107:2162–2169. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearce DJ, Taussig D, Simpson C, Allen K, Rohatiner AZ, Lister TA, et al. Characterization of cells with a high aldehyde dehydrogenase activity from cord blood and acute myeloid leukemia samples. Stem Cells. 2005;23:752–760. doi: 10.1634/stemcells.2004-0292. [DOI] [PubMed] [Google Scholar]
- 11.Corti S, Locatelli F, Papadimitriou D, Donadoni C, Salani S, Del Bo R, et al. Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells. 2006;24:975–985. doi: 10.1634/stemcells.2005-0217. [DOI] [PubMed] [Google Scholar]
- 12.Cai J, Cheng A, Luo Y, Lu C, Mattson MP, Rao MS, et al. Membrane properties of rat embryonic multipotent neural stem cells. Journal of neurochemistry. 2004;88:212–226. doi: 10.1046/j.1471-4159.2003.02184.x. [DOI] [PubMed] [Google Scholar]
- 13.Jean E, Laoudj-Chenivesse D, Notarnicola C, Rouger K, Serratrice N, Bonnieu A, et al. Aldehyde dehydrogenase activity promotes survival of human muscle precursor cells. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou P, Hohm S, Olusanya Y, Hess DA, Nolta J. Human progenitor cells with high aldehyde dehydrogenase activity efficiently engraft into damaged liver in a novel model. Hepatology. 2009;49:1992–2000. doi: 10.1002/hep.22862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estes BT, Wu AW, Storms RW, Guilak F. Extended passaging, but not aldehyde dehydrogenase activity, increases the chondrogenic potential of human adipose-derived adult stem cells. Journal of cellular physiology. 2006;209:987–995. doi: 10.1002/jcp.20808. [DOI] [PubMed] [Google Scholar]
- 16.Sobin LH, Hermanek P, Hutter RV. TNM classification of malignant tumors. A comparison between the new (1987) and the old editions. Cancer. 1988;61:2310–2314. doi: 10.1002/1097-0142(19880601)61:11<2310::aid-cncr2820611127>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 17.Kong CS, Narasimhan B, Cao H, Kwok S, Erickson JP, Koong A, et al. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. International journal of radiation oncology, biology, physics. 2009;74:553–561. doi: 10.1016/j.ijrobp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le QT, Sutphin PD, Raychaudhuri S, Yu SC, Terris DJ, Lin HS, et al. Identification of osteopontin as a prognostic plasma marker for head and neck squamous cell carcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:59–67. [PubMed] [Google Scholar]
- 19.Lombaert IM, Wierenga PK, Kok T, Kampinga HH, deHaan G, Coppes RP. Mobilization of bone marrow stem cells by granulocyte colony-stimulating factor ameliorates radiation-induced damage to salivary glands. Clin Cancer Res. 2006;12:1804–1812. doi: 10.1158/1078-0432.CCR-05-2381. [DOI] [PubMed] [Google Scholar]
- 20.Tatsuishi Y, Hirota M, Kishi T, Adachi M, Fukui T, Mitsudo K, et al. Human salivary gland stem/progenitor cells remain dormant even after irradiation. Int J Mol Med. 2009;24:361–366. doi: 10.3892/ijmm_00000240. [DOI] [PubMed] [Google Scholar]
- 21.Lombaert IM, Brunsting JF, Wierenga PK, Kampinga HH, de Haan G, Coppes RP. Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/progenitor pool. Stem Cells. 2008;26:2595–2601. doi: 10.1634/stemcells.2007-1034. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg Z, Myers C, Heim VM, Lathrop CA, Rebustini IT, Stewart JS, et al. FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development. 2005;132:1223–1234. doi: 10.1242/dev.01690. [DOI] [PubMed] [Google Scholar]
- 23.Brizel DM, Murphy BA, Rosenthal DI, Pandya KJ, Gluck S, Brizel HE, et al. Phase II study of palifermin and concurrent chemoradiation in head and neck squamous cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:2489–2496. doi: 10.1200/JCO.2007.13.7349. [DOI] [PubMed] [Google Scholar]
- 24.Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7:330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capoccia BJ, Robson DL, Levac KD, Maxwell DJ, Hohm SA, Neelamkavil MJ, et al. Revascularization of ischemic limbs after transplantation of human bone marrow cells with high aldehyde dehydrogenase activity. Blood. 2009;113:5340–5351. doi: 10.1182/blood-2008-04-154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasiliou V, Nebert DW. Analysis and update of the human aldehyde dehydrogenase (ALDH) gene family. Hum Genomics. 2005;2:138–143. doi: 10.1186/1479-7364-2-2-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson B, Brocker C, Thompson DC, Black W, Vasiliou K, Nebert DW, et al. Update on the aldehyde dehydrogenase gene (ALDH) superfamily. Hum Genomics. 2011;5:283–303. doi: 10.1186/1479-7364-5-4-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeilstra LJ, Vissink A, Konings AW, Coppes RP. Radiation induced cell loss in rat submandibular gland and its relation to gland function. International journal of radiation biology. 2000;76:419–429. doi: 10.1080/095530000138763. [DOI] [PubMed] [Google Scholar]
- 30.Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, et al. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 2010;32:1195–1201. doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ucar D, Cogle CR, Zucali JR, Ostmark B, Scott EW, Zori R, et al. Aldehyde dehydrogenase activity as a functional marker for lung cancer. Chem Biol Interact. 2009;178:48–55. doi: 10.1016/j.cbi.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lingala S, Cui YY, Chen X, Ruebner BH, Qian XF, Zern MA, et al. Immunohistochemical staining of cancer stem cell markers in hepatocellular carcinoma. Experimental and molecular pathology. 2010;89:27–35. doi: 10.1016/j.yexmp.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasheed ZA, Yang J, Wang Q, Kowalski J, Freed I, Murter C, et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102:340–351. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bortolomai I, Canevari S, Facetti I, De Cecco L, Castellano G, Zacchetti A, et al. Tumor initiating cells: development and critical characterization of a model derived from the A431 carcinoma cell line forming spheres in suspension. Cell Cycle. 2010;9:1194–1206. doi: 10.4161/cc.9.6.11108. [DOI] [PubMed] [Google Scholar]
- 36.Li T, Su Y, Mei Y, Leng Q, Leng B, Liu Z, et al. ALDH1A1 is a marker for malignant prostate stem cells and predictor of prostate cancer patients’ outcome. Laboratory investigation; a journal of technical methods and pathology. 2010;90:234–244. doi: 10.1038/labinvest.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lassen N, Bateman JB, Estey T, Kuszak JR, Nees DW, Piatigorsky J, et al. Multiple and additive functions of ALDH3A1 and ALDH1A1: cataract phenotype and ocular oxidative damage in Aldh3a1(−/−)/Aldh1a1(−/−) knock-out mice. The Journal of biological chemistry. 2007;282:25668–25676. doi: 10.1074/jbc.M702076200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang F, Song C, Ma Y, Tang L, Xu Y, Wang H. Effect of fibroblasts on breast cancer cell mammosphere formation and regulation of stem cell-related gene expression. International journal of molecular medicine. 2011;28:365–371. doi: 10.3892/ijmm.2011.700. [DOI] [PubMed] [Google Scholar]
- 39.Moreb JS, Baker HV, Chang LJ, Amaya M, Lopez MC, Ostmark B, et al. ALDH isozymes downregulation affects cell growth, cell motility and gene expression in lung cancer cells. Molecular cancer. 2008;7:87. doi: 10.1186/1476-4598-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Laurenzi V, Rogers GR, Hamrock DJ, Marekov LN, Steinert PM, Compton JG, et al. Sjogren-Larsson syndrome is caused by mutations in the fatty aldehyde dehydrogenase gene. Nature genetics. 1996;12:52–57. doi: 10.1038/ng0196-52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.