Abstract
Preparing to stop may ‘prime’ the neural mechanism for stopping and alter brain activity at the time of stopping. Much electroencephalography (EEG) research has studied the N2/P3 complex over fronto-central electrodes during outright stopping. Here we used differential reward of the stop and go processes in a stop signal task to study the sensitivity of these EEG components to preparation. We found that: 1) stopping was faster when it was rewarded, 2) the P3 amplitude was larger for successful vs. failed stopping and this difference was greater when stopping was rewarded over going, 3) the N2 component was only observed on failed stop trials, and 4) there was greater EEG coherence between fronto-central and occipito-parietal electrodes at 12 Hz during the initiation of a go response when stopping was rewarded over going. We propose that fronto-central cortical mechanisms active before and at the time of stopping are sensitive to preparation.
Introduction
Much research has addressed how initiated responses are stopped. Recent evidence from a handful of studies suggests that preparing to stop can influence the stopping process (Chikazoe et al., 2009; Greenhouse, Oldenkamp, & Aron, 2012; Jahfari, Stinear, Claffey, Verbruggen, & Aron, 2010; Leotti & Wager, 2010; Sinopoli, Schachar, & Dennis, 2011; Verbruggen & Logan, 2009a). Behaviorally, rewarding successful stopping induces response slowing in preparation for stopping and also speeds up the stopping process (Boehler, Hopf, Stoppel, & Krebs, 2012; Leotti & Wager, 2010; Sinopoli et al., 2011). This change in the speed of stopping could result from proactive adjustments in motor excitability by reducing the amount of inhibition required to override an initiated response without directly influencing the mechanism involved in stopping. In line with this hypothesis, the stopping process itself need not be affected by preparation. The difference in the speed of stopping instead could result from changes in the perceptual processing of the stop signal stimulus or reduced excitability associated with response execution processes. Alternatively, the stopping mechanism could be ‘primed’ to execute stopping more quickly, i.e. the changes in the speed of stopping could reflect changes within the stopping network. Indeed, several fMRI studies lend support to this idea and observed that preparing to stop activates the stopping network (Chikazoe et al., 2009; Jahfari et al., 2010; Swann et al., 2012; Vink et al., 2005; Vink, Ramsey, Raemaekers, & Kahn, 2006; Zandbelt & Vink, 2010; Zandbelt, Bloemendaal, Neggers, Kahn, & Vink, 2012). With fMRI it is difficult to make claims about whether these changes occur before the actual stop is implemented or at the actual time of stopping. No studies have utilized neuroimaging techniques with sufficient temporal resolution to investigated whether neural signatures at the time of stopping are influenced by preparation. Here, we used electroencephalography (EEG) and a modified stop signal task to test whether rewarding stopping over going vs. going over stopping changes the speed of stopping and the pattern of brain activity at the time of stopping.
The N2/P3 complex is a set of EEG event related potential (ERP) components that has, for over thirty years, been associated with reactive inhibitory control processes during the performance of the Go/NoGo and stop signal tasks (de Jong, Coles, Logan, & Gratton, 1990; Dimoska & Johnstone, 2008; Dimoska, Johnstone, & Barry, 2006; Huster et al., 2011; Huster, Westerhausen, Pantev, & Konrad, 2010; Kok, Ramautar, De Ruiter, Band, & Ridderinkhof, 2004; Liotti, Pliszka, Higgins, Perez, & Semrud-Clikeman, 2010; Ramautar, Kok, & Ridderinkhof, 2004; 2006; Schmajuk, Liotti, Busse, & Woldorff, 2006; van Boxtel, Molen, Jennings, & Brunia, 2001; van Gaal, Lamme, Fahrenfort, & Ridderinkhof, 2011). The established finding from these studies is that both the N2 and P3 components demonstrate fronto-central scalp topographies and are enhanced during motor inhibition.
The precise differential properties of the N2 and P3 components in motor inhibition are not clear. Recent evidence suggests that these components are signatures of separate processes associated with inhibitory control, with the former reflecting control over a response plan and the latter reflecting evaluation of motor response inhibition (Gajewski & Falkenstein, 2012; Huster, Enriquez-Geppert, Lavallee, Falkenstein, & Herrmann, 2012). One EEG study implemented a combined Go/NoGo and stop signal task and also incorporated different proportions of Go vs. reactive control trials (i.e. NoGo and stop trails) (Enriquez-Geppert, Konrad, Pantev, & Huster, 2010). This study found that the fronto-central N2 amplitude was larger for relatively infrequent events, regardless of whether the trial was a Go trial, a NoGo trial, or a Stop trial. In contrast, the P3 amplitude was the largest for successful stop trials, smaller for NoGo trials, and smallest for Go trials. This pattern was exaggerated when reactive control trials were less frequent. The implications of this functional dissociation between the N2 and P3 components are two-fold. First, these findings suggest that the N2 is a marker for relatively infrequent or unlikely events (Wessel, Danielmeier, Morton, & Ullsperger, 2012), possibly related to the conflict that arises from unexpectedly reconfiguring responses or an increased attentional demand required for such reconfiguration. Second, they suggest that the P3 component is selectively sensitive to reactive control processes. These results extended those of a previous study that also found increased P3 amplitude for infrequent relative to frequent stop trials (Ramautar et al., 2004), but see (Dimoska & Johnstone, 2008). However, all these previous studies only manipulated the likelihood of a stop signal, and therefore were unable to conclude whether differences in the P3 amplitude during successful stopping were due to the novelty of the stop signal or due to the influence of preparing to stop. This is an important distinction because in the former case, P3 amplitude differences could occur in the absence of any influence of preparation, while in the latter case differences in P3 amplitude could index preparedness to stop. Ruling out one of these possibilities would help to elucidate the functional significance of the P3 in stopping. A more precise characterization of the functional significance of the P3 would be useful in determining whether changes in stop signal task performance represent differences in novelty detection or processes associated with motor inhibition. Therefore, in addition to characterizing the influence of preparation on reactive stopping mechanisms, the current experiment has implications for determining the functional significance of the different N2 and P3 components.
Here, we set out to test whether preparing to stop influences brain mechanisms associated with the reactive stopping of initiated responses. We did this by measuring the N2/P3 complex that has previously been associated with reactive stopping. We developed a modified stop signal task that incorporated a points system. In one condition stopping was emphasized over going, and in the other condition going was emphasized over stopping. Importantly, the probability of a stop trial was the same in both conditions. We then compared the EEG signatures of interest between the two different conditions. Thus, it was possible to test if the N2/P3 complex that occurs during successful stopping relates to the speed of stopping, and hence demonstrates sensitivity to our reward manipulation. Such a finding would suggest that preparing to stop involves preparation of a brain mechanism involved in reactive stopping.
Method
Participants
Fifteen participants (9 female, 24.7 ± 8.5 years of age, 2 left-handed) were recruited from flyers posted on the University of California, San Diego campus. All participants were screened to rule out any neuropsychological or psychiatric disorders and were not taking any neuropsychiatric medication. One subject was excluded from the analysis due to a very high noise in their EEG data.
Task
We used a modified stop signal task with two conditions. In one condition more points were awarded for stopping than going and in the other condition the point contingencies were reversed (Figure 1). The task was administered in blocks of 12 trials. A 3 s fixation screen and then one of two possible instruction screens preceded each block. The instructions were either ‘going = 10 points / stopping = 1 point’ or ‘going = 1 point / stopping = 10 points’. These different point contingencies were selected to emphasize going over stopping (G > S) or stopping over going (S > G), respectively, and alternated from block to block. The starting instruction was counterbalanced across participants. Participants completed one block with each instruction as practice, and a total of 1200 trials in total during testing. This resulted in 50 blocks (600 trials) for each of the two points conditions during testing.
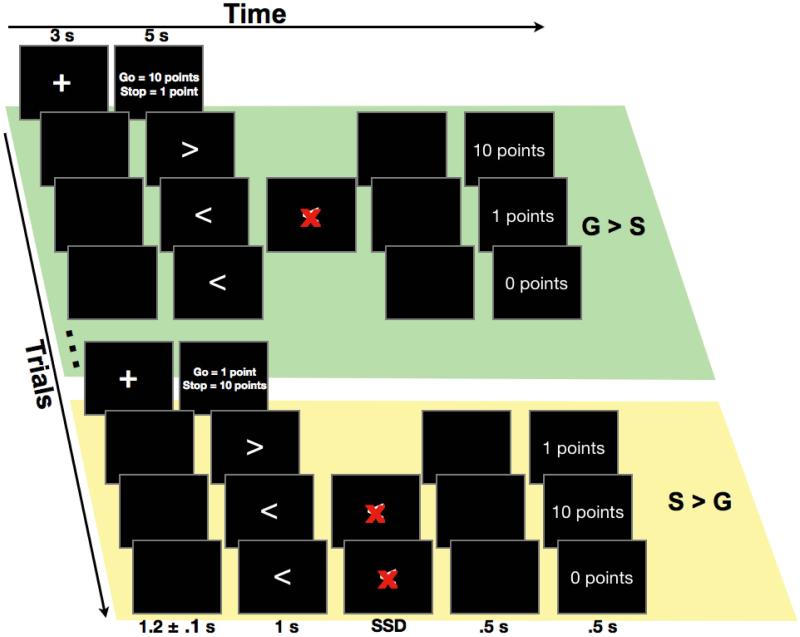
Figure 1.
The stop signal task was administered in blocks of twelve trials. An instruction screen indicated the number of points that could be earned for successful stopping and going quickly for the ensuing block (within the fastest 25% of the Go RT distribution). Either going quickly earned 10 points and successful stopping earned 1 point (G > S) or successful stopping earned 10 points and going quickly earned 1 point (S > G). The stop signal was a red ‘X’ that appeared over the Go target-arrow at a short and dynamically adjusted stop signal delay (SSD). At the end of each trial, the number of points earned was presented as feedback.
Each trial of the task consisted of the presentation of a left- or right-pointing white arrow stimulus presented in the center of a black computer screen for 1 s or until a response was made. On one third of trials, a red letter ‘X’ occluded the arrow at a brief delay. This ‘X’ served as a visual stop signal and remained on the screen until 1 s from target onset or until a response was made. The delay between the arrow and the stop signal (i.e. the stop signal delay, SSD) was dynamically adjusted in increments of 50 ms. Eight independent stop signal staircases were used. Two were mapped to the left arrow (starting at 150 and 200 ms) and two were mapped to the right arrow (starting at 150 and 200 ms) for each of the two instruction conditions. This resulted in 50 stop trials within each staircase, or 200 stop trials from each of the two different instruction conditions (400 stop trials in total).
All trials were followed by a 500 ms blank screen interval and then a 500 ms feedback screen that displayed the points earned on the immediately preceding trial (i.e. 0 points, 1 point, or 10 points). A jittered ITI was used (1.2 ± 0.1 s) to prevent anticipation of the target onset. Additionally, following every 6 blocks of the task, participants were presented with a feedback graph of their overall mean RT, averaged across the two different instruction conditions. This informed participants of their overall tendency to change the speed of responding across the testing session.
Participants were seated with their hands in their laps and their pinkies facing downwards. Button boxes were suspended beneath the edge of a desk on which the stimulus computer was positioned. This positioning necessitated an upward movement of the index fingers to make a response and permitted the recording of electromyography (EMG) from the first dorsal interosseous muscles of each hand. These EMG data will not be discussed here. Participants were instructed to respond quickly and accurately to the target arrows using the index fingers of their left and right hands and to try to stop to the stop signal. Participants were told that stopping would not be possible on approximately half of the stop trials due to the SSD adjustments, but that they should try to earn as many points as possible.
The point system was constructed to selectively manipulate the preparedness for stopping. For the G > S condition, 10 points were awarded on trials in which the correct Go RT fell within the fastest 25% of the cumulative Go RT distribution for that condition, and 1 point was awarded for successful stopping. For the S > G condition, 1 point was awarded on trials in which the correct Go RT fell within the fastest 25% of Go RTs for that condition, and 10 points were awarded for successful stopping. Otherwise, zero points were awarded for trials with slow Go RTs, choice errors, or failed stopping in both conditions. For the initial G > S and S > G blocks, the 25% RT cutoff was determined using the practice RT distributions.
This point system had two particularly important features. First, awarding points only for fast Go RTs and successful stopping encouraged participants to respond quickly and also to try to stop in both conditions. Second, the total number of points that could be earned was largely predetermined since points were awarded on only 25% of Go trials (i.e. the fastest 100 trials within each condition) and approximately 50% of stop trials (i.e. approximately 100 successful stop trials in each condition), based upon the dynamic staircase adjustments. Therefore, one condition was not expected to result in a greater number of points earned than the other.
Behavioral Analysis
Mean Go RT, total points earned, the mean SSD, the probability of successful inhibition (i.e. p(inhibit)), failed stop RT, and the stop signal reaction time (SSRT) were calculated separately for each of the two task conditions, i.e. S > G and G > S. SSRT is an estimate of the duration of the stopping process. Here, we used the integration method to calculate SSRT (Verbruggen & Logan, 2009b; Verbruggen, Chambers, & Logan, 2012). In brief, the integration SSRT is calculated by determining which RT values from the Go RT distribution fall at percentiles that correspond to the p(inhibit) for the three most common SSDs. Each SSD is subtracted from its corresponding Go RT value. The average of these differences is the integration SSRT.
EEG Acquisition
EEG data were sampled at 512 Hz, using a 32 + 8 channel Biosemi ActiveTwo system (Biosemi Instrumentation), with a CMS-DRL reference. Four extra electrodes were placed as follows: one on each mastoid (digitally linked, signal average used for subsequent re-referencing of the montage), one lateral to the left eye, and one below the left eye (as EOG’s to monitor eye movements.)
EEG Pre-processing and Analysis
The EEG data were preprocessed using a combination of EEGlab (Delorme and Makeig, 2004) (http://www.sccn.ucsd.edu/eeglab) and custom Matlab scripts in the following stages. First, the mean of each channel was removed. Second, the data were re-referenced to external electrodes placed on the left and right mastoids. Third, the data were filtered with a two-way least-squares FIR filter (low cutoff: 0.5 Hz, high cutoff: 30 Hz) using the EEGlab “eegfilt” function. Data were then binned into trial epochs, and trials with signal greater than 3 standard deviations from the mean probability distribution within each epoch were excluded from subsequent analysis (Delorme, Sejnowski, & Makeig, 2007). The remaining data was submitted to a temporal infomax independent component analysis (Bell & Sejnowski, 1995). Independent components that corresponded to stereotypical artifacts – eyeblinks, saccades, electrode artifacts – were identified using a published technique that compares favorably with other artifact rejection techniques (Jung et al., 2000). For each subject at least one component was found and removed which corresponded to eye movements/blinks. Visual inspection before and after rejection confirmed the effectiveness of this procedure. The channel-space EEG data was then reconstructed using only the non-artifact ICs using inverse matrix multiplication.
All the remaining stop trials were time-locked to the onset of the stop signal, and these epochs were then averaged within each of the two task conditions. Thus, it was possible to make direct comparisons between the two task conditions for the ERP of interest, i.e. the ERP to the stop signal.
In addition, as an exploratory analysis of the condition differences on Go trials, we assessed communication between frontal and posterior brain areas that exhibited ERP responses to the stop signal and were also sensitive to our points manipulation. To this end, we computed phase-locking value (PLV, (Lachaux, Rodriguez, Martinerie, & Varela, 1999) between fronto-central electrode Cz and occipital electrode Oz, separately for the G > S and S > G successful Go trials. These ROIs were selected based upon the scalp topographies of the ERP components on stop trials. To account for potential effects of volume conduction on the PLV, the continuous channel data was transformed to current-source density (CSD) using a MATLAB toolbox (Kayser & Tenke, 2006a; 2006b). These data were then transformed into frequency space using a Hilbert transform for each individual frequency (from 2 to 30 Hz). Subsequently, only the phase-angle information of the resulting transform was used to compute PLV separately for correct Go trials in each of the G > S and S > G conditions. To compute event-related PLV, the phase spectra were divided into 873 ms epochs starting from 500 ms before Go stimulus onset. This 500 ms pre-stimulus interval was used as a baseline and subtracted from the 373 ms post-stimulus interval. This post-stimulus interval was determined based upon the mean Go RT for the faster of the two task conditions (G > S). We restricted our analysis to this Go RT interval because we were interested in early fronto-occipital communication that preceded motor responses and was therefore less likely to be caused by the spread of cortical motor activity. Differences between the G > S and S > G conditions were tested with sample-wise t-tests (p < 0.05, two-sided, corrected for multiple comparisons in the time domain using the false discovery rate procedure, (Benjamini, Krieger, & Yekutieli, 2006).
Results
Behavior
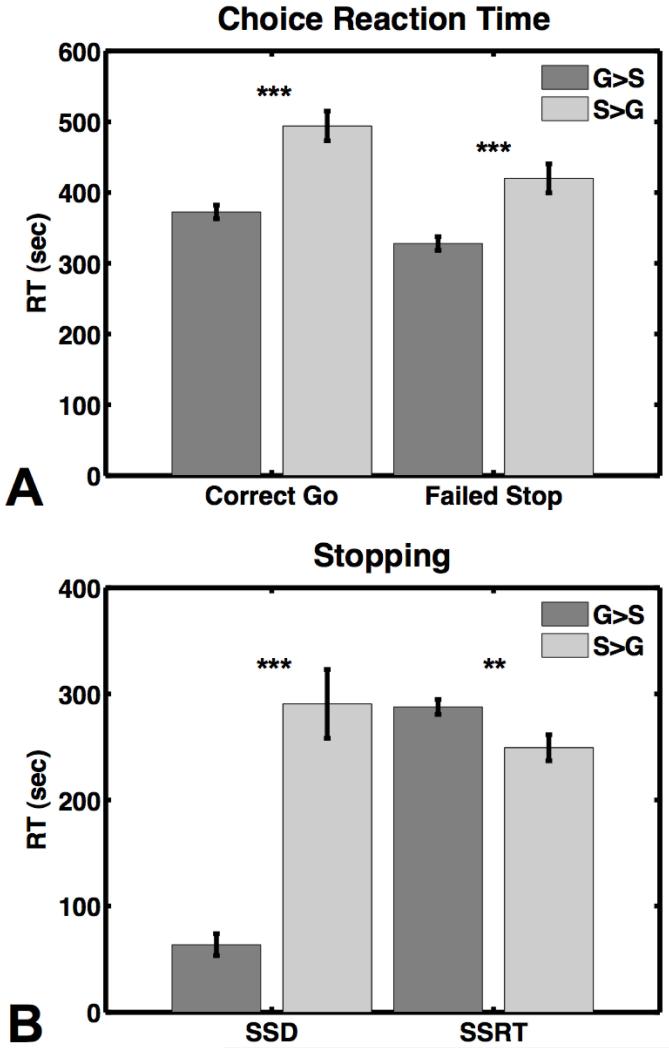
For stopping, the mean SSRT was significantly longer for the G > S condition (288 ± 26 ms) than the S > G condition (249 ± 46 ms), t(13) = 3.6, p < 0.005 (Figure 2a). This pattern of behavior confirmed that the reward manipulation was influencing subjects’ stopping behavior.
Figure 2.
A) Reaction time in milliseconds on Go trials and failed stop trials. B) The stop signal delay (SSD) and stop signal reaction time (SSRT). G > S = going quickly earned 10 points and successful stopping earned 1 point; S > G = successful stopping earned 10 points and going quickly earned 1 point. ** indicates significance at p < 0.01 and *** indicates significance at p < 0.001.
The mean SSD was significantly shorter for the G > S condition (63 ± 39 ms) than the S > G condition (291 ± 121 ms), t(13) = 7.6, p < 0.001 (Figure 2a). The mean p(inhibit) was also significantly smaller for the G > S condition (0.35 ± 0.14) than the p(inhibit) for the S > G condition (0.58 ± 0.05), t(13) = 5.7, p < 0.001, and the mean failed stop RT was significantly slower for the S > G condition (420 ± 20 ms) than the G > S condition (328 ± 9 ms), t(13) = 5.7, p < 0.0001 (Figure 2b). The pattern of p(inhibit) and failed stop RT also replicated the results of the previous study (Leotti & Wager, 2010). However, the p(inhibit) was relatively low in the G > S condition, and although every subject provided at least 44 successful stop trials, the resulting SSRT estimate may be unreliable (Verbruggen & Logan, 2009b).
The mean Go RT was significantly faster for the G > S condition (373 ± 36 ms) than the S > G condition (494 ± 78 ms), t(13) = 6.1, p < 0.001 (Figure 2b). The mean total points earned for the G > S condition was 1205 ± 249 and for the S > G condition was 1148 ± 66, and this was not a significant difference t(13) = 0.92, p = 0.4. Combined, this pattern of Go RT and points earned indicates that participants were modifying their proactive control strategies in accordance with the different point contingencies, i.e. they favored stopping over going in the stop-rewarded condition and vice versa.
EEG
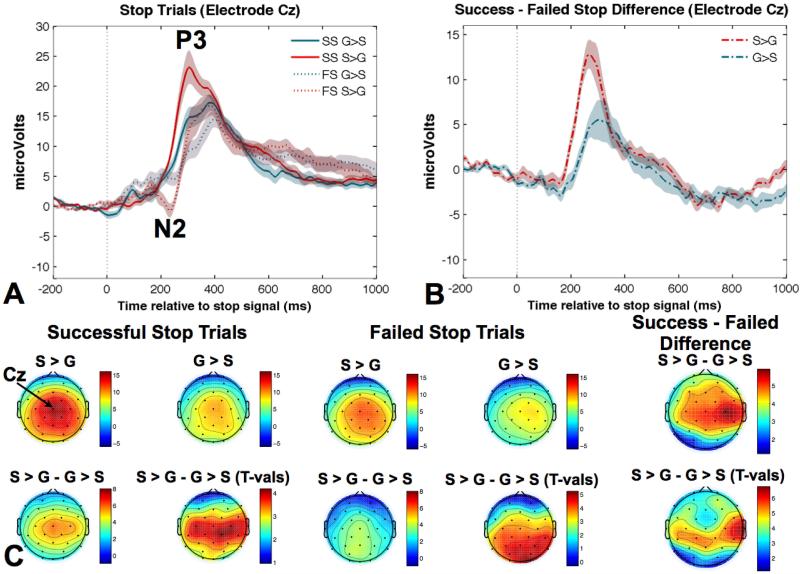
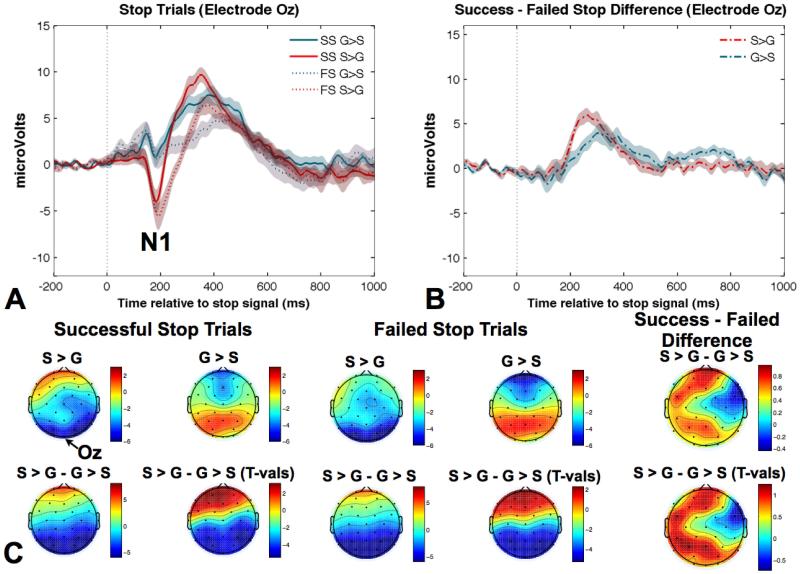
Three distinct ERP components were identified for successful and failed stop trials in both task conditions. These included the N2, the P3, and interestingly, the posterior visual N1. The fronto-central N2 peaked around 210 ms after the stop signal, centered over Cz (Figure 3). The P3 component peaked around 300 ms after the stop signal, with a fronto-central topography, centered over Cz (Figure 3). The posterior occipito-parietal N1 peaked first, approximately 190 ms after the onset of the stop signal, centered over electrode Oz (Figure 4).
Figure 3.
A) The average ERP measured at electrode Cz is presented for successful and failed stop trials for the two different task conditions. The N2 for failed stop trials and the P3 for all trial types are depicted. B) The ERP difference wave for successful - failed stopping is presented for the two different task conditions and shows a prominent difference between the two task conditions from ~200 to 300 ms after the stop signal. C) Scalp topographies for the peak amplitude of the fronto-central P3 are presented for successful and failed stop trials for each condition along with the difference and t-value maps. SS = successful stop; FS = failed stop; G > S = going quickly earned 10 points and successful stopping earned 1 point; S > G = successful stopping earned 10 points and going quickly earned 1 point.
Figure 4.
A) The average ERP measured at electrode Oz is presented for successful and failed stop trials for the two different task conditions. B) The ERP difference wave for successful – failed stopping is presented for the two different task conditions and indicates that there were no condition differences in the N2. C) Scalp topographies for the peak amplitude of the occipito-parietal N1 are presented for successful and failed stop trials for each condition along with the difference and t-value maps. The emergence of the fronto-central N2 is also visible for failed stopping. SS = successful stop; FS = failed stop; G > S = going quickly earned 10 points and successful stopping earned 1 point; S > G = successful stopping earned 10 points and going quickly earned 1 point.
For each subject, we derived the minimum (N1 and N2) and maximum (P3) peak amplitude of the ERPs at their corresponding electrode sites for successful and failed stop trials in each of the two task conditions. We ran separate ANOVA for each of the three peak ERP amplitudes with the factors condition (S > G vs. G > S) and type of stop trial (successful vs. failed).
Notably, we did not observe a fronto-central N2 component for successful stop trials in either the S > G or G > S condition, quantified at electrode Cz. However, we did observe an N2 at electrode Cz emerging around 200 ms for failed stop trials in both reward conditions, (Figure 3a). The N2 peak amplitude on failed stop trials was larger for the S > G than the G > S condition, t(13) = 3.6, p < 0.005.
As predicted, the P3 amplitude was larger for successful vs. failed stopping and was also larger for the S > G than G > S condition, F(1,13) = 117.3, p < 0.0001 and F(1,13) = 8.3, p < 0.05, respectively (Figure 3a). Moreover, there was a significant interaction, F(1,13) = 16.3, p < 0.01 (Figure 3c). Follow up t-tests indicated that the P3 amplitude difference between successful and failed stop trials was larger for the S > G condition than the G > S condition, t(13) = 4.0, p < 0.01, two-tailed.
The posterior N1 showed a much larger amplitude for the S > G condition for both successful and failed stop trials, F(1,13) = 25.7, p < 0.0001 (Figure 4a and b). Interestingly, in contrast to the chronologically later fronto-central P3, there was no main effect of successful vs. failed stopping and no interaction.
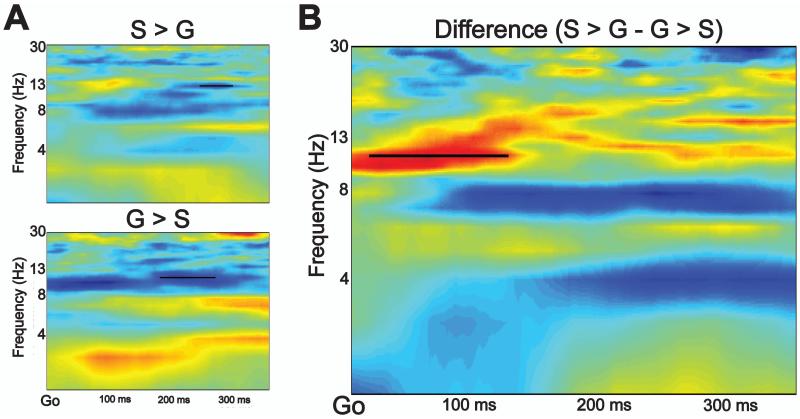
For our exploratory analysis of coherence between electrodes Cz and Oz on Go trials, PLV was significantly greater at 12 Hz for the S > G condition than the G > S condition (p < 0.05, FDR corrected) (Figure 5). This difference emerged shortly following Go stimulus onset and persisted for approximately 150 ms. Importantly, this is well before the mean Go RT for the G > S condition (373 ms), and the S > G condition (494 ms). There were no other significant differences between the two task conditions at any other time point or frequency.
Figure 5.
A) Phase coherence was calculated between electrodes Cz and Oz for the 373 ms following the target Go arrow for the S > G (top) and G > S (bottom) conditions. B) There was significantly greater phase coherence for the S > G than the G > S condition at 12 Hz, and this lasted for approximately 150 ms following Go target onset.
Discussion
Here, we manipulated the numbers of points that could be earned for stopping successfully vs. going quickly in a stop signal task while keeping the probability of a visual stop signal constant. We did this to determine whether neural activity at the time of stopping is influenced by preparation. To our knowledge, this is the only EEG study that has used a points system to manipulate the relative value of stopping vs. going to investigate signatures associated with successful stopping. Other EEG studies have only manipulated the likelihood of a stop signal and were therefore unable to disentangle the effects that result from the occurrence of an infrequent event (e.g. an oddball) from those that result from endogenous processes involved in preparing to stop (Dimoska & Johnstone, 2008; Enriquez-Geppert et al., 2010; Ramautar et al., 2004). Our experimental design ensured that the probability of a stop signal was identical between the two reward conditions. Therefore, any differences in the EEG signatures of interest between our task conditions cannot be attributed to differences in the probability of task stimuli. The effects we observed can only be attributed to the way in which subjects modified their behavior in accordance with the different task instructions.
We found that stopping was faster when it was rewarded over going and that the amplitude of a well-characterized ERP signature that emerges at the time of stopping, the fronto-central P3, was also sensitive to the reward manipulation. Specifically, the P3 amplitude was larger for successful than failed stopping and this difference was more pronounced when stopping was rewarded over going. Interestingly, we also observed that the occipito-parietal N1 component to the stop signal, commonly associated with visual attention (Luck, Woodman, & Vogel, 2000), showed increased amplitude when stopping was rewarded over going quickly. However, N1 amplitude did not differ between successful and failed stop trials. Thus, manipulating reward for stopping influenced brain activity over middle frontal cortex (indexed by the P3) that related to both stopping speed and success. This implies that preparing to stop facilitates stopping by influencing the dorsomedial frontal cortex and/or its connected regions.
Moreover, we observed greater coherence between fronto-central and occipito-parietal electrodes on Go trials during blocks in which stopping was valued over going. Such a signature may indicate communication between brain areas involved in the detection of a visual stop signal and brain areas involved in implementing outright stopping. Other studies have observed this type of prefrontal-occipital communication during adaptive behaviors, ostensibly to amplify early sensory information processing relevant to a motor task (Danielmeier, Eichele, Forstmann, Tittgemeyer, & Ullsperger, 2011; King, Korb, Cramon, & Ullsperger, 2010; Zanto, Rubens, Thangavel, & Gazzaley, 2011).
The P3
We found that the fronto-central P3 showed greater amplitude for successful vs. failed stop trials and that this difference was larger when successful stopping was rewarded over going. Notably, these changes in the P3 amplitude coincided with changes in SSRT. Therefore, the P3 may reflect a neural mechanism that is involved at the time of stopping and is also sensitive to preparation. Thus, preparing to stop could prime the dorsomedial frontal cortex for stopping.
Although the P3 peak occurred after the estimated completion of the stopping process for most subjects, the peak amplitude may result from earlier neural events. Importantly, differences in the P3 wave started to emerge around 200 ms after the stop signal, within the time frame of the stopping process. One previous study that also reported a P3 amplitude difference between successful and failed stopping noted that the difference reached significance approximately 20 ms before completion of the estimated SSRT for that study (Kok et al., 2004). Our results are in accordance with that previous finding.
It is commonly accepted that the P3 is comprised of two positive-going potentials that occur in close temporal proximity, an early P3a with a frontal distribution that peaks around 240 ms after a stimulus and a later P3b with a parietal distribution that peaks around 350 ms after a stimulus (N. K. Squires, Squires, & Hillyard, 1975). The P3 waves we observed here contain two peaks at the approximate latencies of the P3a and P3b. The P3a and P3b are believed to occur when working memory is updated with new information (Polich & Kok, 1995), and the P3a is hypothesized to reflect an attentional process that initiates the inhibition of ongoing activity (Polich, 2007). However, further evidence is required to substantiate this hypothesis. The larger difference between successful and failed stop trials that we observed for the S > G condition than the G > S condition may reflect a larger change in an underlying attention-driven process that may or may not itself be inhibitory. Such a putative mechanism could facilitate the initiation of the stop command and lead to faster stopping.
The N2
We observed a fronto-central N2 component time-locked to the stop signal for both task conditions at electrode Cz but only for failed stop trials (Figures 3a and 4c). This pattern replicates a previous study which observed changes in the fronto-central N2 for failed but not successful stopping (Kok et al., 2004) and extends the results of two previous studies that reported greater sensitivity to the stop signal for the P3 than the N2 component (Enriquez-Geppert et al., 2010; Ramautar et al., 2004). Thus, the fronto-central N2 may be sensitive to the reward manipulation implemented here, but only when there is a failure to stop. This leaves open the possibility that the N2 reflects error processing in the stop signal task, similar to the feedback-related negativity (Miltner, Braun, & Coles, 1997; Simons, 2010; Wessel et al., 2012). We note that the topography of the N2 we observed was not right lateralized as has been reported in some previous studies (e.g. Liotti, Pliszka, Higgins, Perez, & Semrud-Clikeman, 2010; Schmajuk, Liotti, Busse, & Woldorff, 2006). The fronto-central N2 component reported here may reflect a different mechanism than that which contributes to the right-frontal N2, although these signatures do sometimes co-occur (Enriquez-Geppert, Konrad, Pantev, & Huster, 2010).
The N1
We also detected a large difference between the two reward conditions in the posterior occipito-parietal N1 component, centered at electrode Oz. This component is believed to reflect visual attention processes (Luck et al., 2000), and in this case likely reflects differences in attention to the visual stop signal. Interestingly, we did not observe a difference between successful and failed stop trials in either reward condition. This pattern suggests that visual attention to the stop signal may not have related to stopping success, at least in this task. Moreover, it suggests that the differences in the P3 amplitude for successful vs. failed stopping are unlikely to have resulted from changes in visual attention.
Fronto-occipital Phase Coherence
In addition to the ERP measures of interest, we observed greater phase coherence between electrode Cz and Oz for the S > G condition than the G > S condition. This occurred within 150 ms of Go stimulus onset on successful Go trials and only at 12 Hz. Coherence in the alpha (8-12 Hz) frequency band between frontal and posterior electrodes has recently been identified as a candidate for top-down modulation of visual attention in working memory tasks (Bollinger, Rubens, Zanto, & Gazzaley, 2010; Capotosto, Babiloni, Romani, & Corbetta, 2009; Zanto et al., 2011; Zanto, Rubens, Bollinger, & Gazzaley, 2010). We observed EEG phase coherence between occipito-parietal brain areas, likely involved in the detection of the visual stop signal, and fronto-central areas implicated in outright stopping. Coherence of this kind is hypothesized to reflect neural communication (Fries, 2005). While we are unable to make claims about the direction of this communication, the difference between the S > G and G > S conditions suggests that it is sensitive to the points manipulation we used. Therefore, this early phase coherence (within 150 ms of the target stimulus) may reflect an anticipatory stopping-related strategy that is mediated by motivation.
Neural Mechanisms
Putative neural sources of the N2/P3 complex were recently identified in a study that utilized simultaneous EEG and functional MRI (Huster et al., 2011). This study reported that the N2/P3 complex was associated with increased BOLD signal within the pre-supplementary motor area (pre-SMA), striatum, anterior midcingulate, and anterior insula. These brain regions largely overlap with those believed to participate in a frontal-basal ganglia network for stopping outright (Aron et al., 2007). Considered together with our results, the P3 component may reflect activity changes at fronto-central cortical nodes within this network during stopping, e.g. the pre-SMA and its connected regions.
Conclusion
We implemented a modified stop signal task to investigate the effects of differentially rewarding either stopping or going on EEG signatures associated with response inhibition. We observed that rewarding stopping over going resulted in faster SSRT and a larger fronto-central P3. The P3 component exhibited increased amplitude for successful vs. failed stopping, and critically, this effect was greater when stopping was rewarded over going. This finding suggests that the P3 reflects a control process at the time of stopping that is dynamically adjusted with changing motivational states.
Acknowledgements
We would like to thank Adam R. Aron for discussions and feedback and the NIH (UCSD Institute for Neural Computation training grant) for funding.
References
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27(44):11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. doi:10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural computation. 1995;7(6):1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006 [Google Scholar]
- Boehler CN, Hopf J-M, Stoppel CM, Krebs RM. Motivating inhibition - reward prospect speeds up response cancellation. Cognition. 2012 doi: 10.1016/j.cognition.2012.07.018. doi:10.1016/j.cognition.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Bollinger J, Rubens MT, Zanto TP, Gazzaley A. Expectation-driven changes in cortical functional connectivity influence working memory and long-term memory performance. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30(43):14399–14410. doi: 10.1523/JNEUROSCI.1547-10.2010. doi:10.1523/JNEUROSCI.1547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capotosto P, Babiloni C, Romani GL, Corbetta M. Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29(18):5863–5872. doi: 10.1523/JNEUROSCI.0539-09.2009. doi:10.1523/JNEUROSCI.0539-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Hirose S, Yamashita K-I, Miyashita Y, Konishi S. Preparation to Inhibit a Response Complements Response Inhibition during Performance of a Stop-Signal Task. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29(50):15870–15877. doi: 10.1523/JNEUROSCI.3645-09.2009. doi:10.1523/JNEUROSCI.3645-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielmeier C, Eichele T, Forstmann BU, Tittgemeyer M, Ullsperger M. Posterior medial frontal cortex activity predicts post-error adaptations in task-related visual and motor areas. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(5):1780–1789. doi: 10.1523/JNEUROSCI.4299-10.2011. doi:10.1523/JNEUROSCI.4299-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong R, Coles MG, Logan GD, Gratton G. In search of the point of no return: the control of response processes. Journal of Experimental Psychology: Human Perception and Performance. 1990;16(1):164–182. doi: 10.1037/0096-1523.16.1.164. [DOI] [PubMed] [Google Scholar]
- Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. NeuroImage. 2007;34(4):1443–1449. doi: 10.1016/j.neuroimage.2006.11.004. doi:10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimoska A, Johnstone SJ. Effects of varying stop-signal probability on ERPs in the stop-signal task: do they reflect variations in inhibitory processing or simply novelty effects? Biological psychology. 2008;77(3):324–336. doi: 10.1016/j.biopsycho.2007.11.005. doi:10.1016/j.biopsycho.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Dimoska A, Johnstone SJ, Barry RJ. The auditory-evoked N2 and P3 components in the stop-signal task: indices of inhibition, response-conflict or error-detection? Brain and cognition. 2006;62(2):98–112. doi: 10.1016/j.bandc.2006.03.011. doi:10.1016/j.bandc.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Enriquez-Geppert S, Konrad C, Pantev C, Huster RJ. Conflict and inhibition differentially affect the N200/P300 complex in a combined go/nogo and stop-signal task. NeuroImage. 2010;51(2):877–887. doi: 10.1016/j.neuroimage.2010.02.043. doi:10.1016/j.neuroimage.2010.02.043. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends in Cognitive Sciences. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. doi:10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Gajewski PD, Falkenstein M. Effects of task complexity on ERP components in Go/Nogo tasks. International journal of psychophysiology: official journal of the International Organization of Psychophysiology. 2012 doi: 10.1016/j.ijpsycho.2012.08.007. doi:10.1016/j.ijpsycho.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Greenhouse I, Oldenkamp CL, Aron AR. Stopping a response has global or nonglobal effects on the motor system depending on preparation. Journal of Neurophysiology. 2012;107(1):384–392. doi: 10.1152/jn.00704.2011. doi:10.1152/jn.00704.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster RJ, Eichele T, Enriquez-Geppert S, Wollbrink A, Kugel H, Konrad C, Pantev C. Multimodal imaging of functional networks and event-related potentials in performance monitoring. NeuroImage. 2011;56(3):1588–1597. doi: 10.1016/j.neuroimage.2011.03.039. doi:10.1016/j.neuroimage.2011.03.039. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Enriquez-Geppert S, Lavallee CF, Falkenstein M, Herrmann CS. Electroencephalography of response inhibition tasks: Functional networks and cognitive contributions. International journal of psychophysiology: official journal of the International Organization of Psychophysiology. 2012 doi: 10.1016/j.ijpsycho.2012.08.001. doi:10.1016/j.ijpsycho.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Westerhausen R, Pantev C, Konrad C. The role of the cingulate cortex as neural generator of the N200 and P300 in a tactile response inhibition task. Human Brain Mapping. 2010;31(8):1260–1271. doi: 10.1002/hbm.20933. doi:10.1002/hbm.20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari S, Stinear CM, Claffey M, Verbruggen F, Aron AR. Responding with restraint: what are the neurocognitive mechanisms? Journal of cognitive neuroscience. 2010;22(7):1479–1492. doi: 10.1162/jocn.2009.21307. doi:10.1162/jocn.2009.21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2000;111(10):1745–1758. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2006a;117(2):348–368. doi: 10.1016/j.clinph.2005.08.034. doi:10.1016/j.clinph.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: II. Adequacy of low-density estimates. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2006b;117(2):369–380. doi: 10.1016/j.clinph.2005.08.033. doi:10.1016/j.clinph.2005.08.033. [DOI] [PubMed] [Google Scholar]
- King JA, Korb FM, Cramon, von DY, Ullsperger M. Post-error behavioral adjustments are facilitated by activation and suppression of task-relevant and task-irrelevant information processing. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30(38):12759–12769. doi: 10.1523/JNEUROSCI.3274-10.2010. doi:10.1523/JNEUROSCI.3274-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok A, Ramautar JR, De Ruiter MB, Band GPH, Ridderinkhof KR. ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology. 2004;41(1):9–20. doi: 10.1046/j.1469-8986.2003.00127.x. doi:10.1046/j.1469-8986.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Human Brain Mapping. 1999;8(4):194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leotti LA, Wager TD. Motivational influences on response inhibition measures. Journal of Experimental Psychology: Human Perception and Performance. 2010;36(2):430–447. doi: 10.1037/a0016802. doi:10.1037/a0016802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti M, Pliszka SR, Higgins K, Perez R, Semrud-Clikeman M. Evidence for specificity of ERP abnormalities during response inhibition in ADHD children: a comparison with reading disorder children without ADHD. Brain and cognition. 2010;72(2):228–237. doi: 10.1016/j.bandc.2009.09.007. doi:10.1016/j.bandc.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S, Woodman G, Vogel E. Event-related potential studies of attention. Trends in Cognitive Sciences. 2000;4(11):432–440. doi: 10.1016/s1364-6613(00)01545-x. [DOI] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, Coles MGH. Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a “generic” neural system for error detection. Journal of cognitive neuroscience. 1997;9(6):788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. doi:10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Kok A. Cognitive and biological determinants of P300: an integrative review. Biological psychology. 1995;41(2):103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Ramautar JR, Kok A, Ridderinkhof KR. Effects of stop-signal probability in the stop-signal paradigm: the N2/P3 complex further validated. Brain and cognition. 2004;56(2):234–252. doi: 10.1016/j.bandc.2004.07.002. doi:10.1016/j.bandc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Ramautar JR, Kok A, Ridderinkhof KR. Effects of stop-signal modality on the N2/P3 complex elicited in the stop-signal paradigm. Biological psychology. 2006;72(1):96–109. doi: 10.1016/j.biopsycho.2005.08.001. doi:10.1016/j.biopsycho.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Schmajuk M, Liotti M, Busse L, Woldorff MG. Electrophysiological activity underlying inhibitory control processes in normal adults. Neuropsychologia. 2006;44(3):384–395. doi: 10.1016/j.neuropsychologia.2005.06.005. doi:10.1016/j.neuropsychologia.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Simons RF. The way of our errors: theme and variations. Psychophysiology. 2010;47(1):1–14. doi: 10.1111/j.1469-8986.2009.00929.x. doi:10.1111/j.1469-8986.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- Sinopoli KJ, Schachar R, Dennis M. Reward improves cancellation and restraint inhibition across childhood and adolescence. Developmental psychology. 2011;47(5):1479–1489. doi: 10.1037/a0024440. doi:10.1037/a0024440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalography and clinical Neurophysiology. 1975;38(4):387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Swann NC, Cai W, Conner CR, Pieters TA, Claffey MP, George JS, Aron AR, et al. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: electrophysiological responses and functional and structural connectivity. NeuroImage. 2012;59(3):2860–2870. doi: 10.1016/j.neuroimage.2011.09.049. doi:10.1016/j.neuroimage.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel GJ, Molen MWVD, Jennings JR, Brunia CH. A psychophysiological analysis of inhibitory motor control in the stop-signal paradigm. Biological psychology. 2001;58(3):229–262. doi: 10.1016/s0301-0511(01)00117-x. [DOI] [PubMed] [Google Scholar]
- van Gaal S, Lamme VAF, Fahrenfort JJ, Ridderinkhof KR. Dissociable Brain Mechanisms Underlying the Conscious and Unconscious Control of Behavior. 2011 doi: 10.1162/jocn.2010.21431. http://dx.doi.org/10.1162/jocn.2010.21431. [DOI] [PubMed]
- Verbruggen F, Logan GD. Proactive adjustments of response strategies in the stop-signal paradigm. Journal of Experimental Psychology: Human Perception and Performance. 2009a;35(3):835–854. doi: 10.1037/a0012726. doi:10.1037/a0012726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Models of response inhibition in the stop-signal and stop-change paradigms. Neuroscience and biobehavioral reviews. 2009b;33(5):647–661. doi: 10.1016/j.neubiorev.2008.08.014. doi:10.1016/j.neubiorev.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Chambers CD, Logan GD. Fictitious inhibitory differences: How skewness and slowing distort the estimation of stopping latencies. Psychological Science. 2012 doi: 10.1177/0956797612457390. Retrieved June 11, 2012, from http://ccal-exeter.org/Resources/verbruggen_ssrt_psychscience.pdf. [DOI] [PMC free article] [PubMed]
- Vink M, Kahn RS, Raemaekers M, van den Heuvel M, Boersma M, Ramsey NF. Function of striatum beyond inhibition and execution of motor responses. Human Brain Mapping. 2005;25(3):336–344. doi: 10.1002/hbm.20111. doi:10.1002/hbm.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Ramsey NF, Raemaekers M, Kahn RS. Striatal dysfunction in schizophrenia and unaffected relatives. Biological Psychiatry. 2006;60(1):32–39. doi: 10.1016/j.biopsych.2005.11.026. doi:10.1016/j.biopsych.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Wessel JR, Danielmeier C, Morton JB, Ullsperger M. Surprise and error: common neuronal architecture for the processing of errors and novelty. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32(22):7528–7537. doi: 10.1523/JNEUROSCI.6352-11.2012. doi:10.1523/JNEUROSCI.6352-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandbelt BB, Vink M. On the role of the striatum in response inhibition. PloS one. 2010;5(11):e13848. doi: 10.1371/journal.pone.0013848. doi:10.1371/journal.pone.0013848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandbelt BB, Bloemendaal M, Neggers SFW, Kahn RS, Vink M. Expectations and violations: Delineating the neural network of proactive inhibitory control. Human Brain Mapping. 2012 doi: 10.1002/hbm.22047. doi:10.1002/hbm.22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Bollinger J, Gazzaley A. Top-down modulation of visual feature processing: the role of the inferior frontal junction. NeuroImage. 2010;53(2):736–745. doi: 10.1016/j.neuroimage.2010.06.012. doi:10.1016/j.neuroimage.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nature Neuroscience. 2011;14(5):656–661. doi: 10.1038/nn.2773. doi:10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]