Abstract
The moss, Physcomitrella patens is a non-seed land plant belonging to early diverging lineages of land plants following colonization of land in the Ordovician period in Earth’s history. Evidence suggests that mosses can be highly tolerant of abiotic stress. We showed previously that dehydration stress and abscisic acid treatments induced oscillations in steady-state levels of LEA (Late Embryogenesis Abundant) protein transcripts, and that removal of ABA resulted in rapid attenuation of oscillatory increases in transcript levels. Here, we show that other abiotic stresses like salt and osmotic stresses also induced oscillations in steady-state transcript levels and that the amplitudes of the oscillatory increases in steady-state transcript levels are reflective of the severity of the abiotic stress treatment. Together, our results suggest that oscillatory increases in transcript levels in response to abiotic stresses may be a general phenomenon in P. patens and that temporally dynamic increases in steady-state transcript levels may be important for adaptation to life in constantly fluctuating environmental conditions.
Keywords: abiotic stress, salt stress, osmotic stress, oscillations, gene expression
Introduction
Physcomitrella patens is a bryophyte belonging to early diverging lineages of land plants and one of the few extant primitive land plants with a publicly available genome sequence.1P. patens is a non-vascular plant, with a relatively simple morphology and single-cell layered anatomy, thereby requiring a constant co-equilibration of tissue water content with the environment.1-5 This may explain the restriction of mosses to refugial habitats.6 Such simple anatomical features necessitate the evolution of considerable intrinsic cellular and molecular mechanisms in response to abiotic stresses and there is evidence to suggest that P. patens is highly tolerant to abiotic stresses.5,7,8P. patens can survive moderate dehydration stress but it is not desiccation tolerant.8,9 However, pre-treatment of P. patens with abscisic acid (ABA) can confer desiccation tolerance.8-10 We showed previously that dehydration stress-induced oscillatory increases in the steady-state levels of two Group 3 LEA protein genes (Phypa_166566 and Phypa_211998), and that the amplitudes of the oscillatory increases are reflective of the severity of the dehydration stress.10 Additionally, we also showed that dehydration stress increased ABA levels in P. patens and that ABA can also induce dosage-dependent oscillatory increases in steady-state transcript levels of these Group 3 LEA protein genes. These data suggest, at least in the context of the moss, that dehydration-induced temporal dynamics in steady-state transcript levels that is responsive to the severity of the stress may contribute to survival under periodic fluctuations of water availability and confer evolutionary advantages during colonization of land by plants.10
In addition to its ability to survive moderate dehydration stress, P. patens has also been shown to be highly tolerant of salt and osmotic stress,7,11,12 as P. patens can survive exposure to 350 mM NaCl and 500 mM sorbitol.7 In this study, we aimed to address whether dosage-dependent oscillatory increases in steady-state transcript levels of stress-responsive genes are a more general phenomenon in moss, or whether it is restricted to dehydration stress. Our results showed that salt- and osmotic-stress also induced dosage-dependent oscillatory increases in steady-state transcript levels of Group 3 LEA protein genes, suggesting that temporal dynamics in steady-state transcript levels of abiotic stress-induced genes may be a general phenomenon in P. patens and that these oscillatory increases may contribute to abiotic stress tolerance.
Results and Discussion
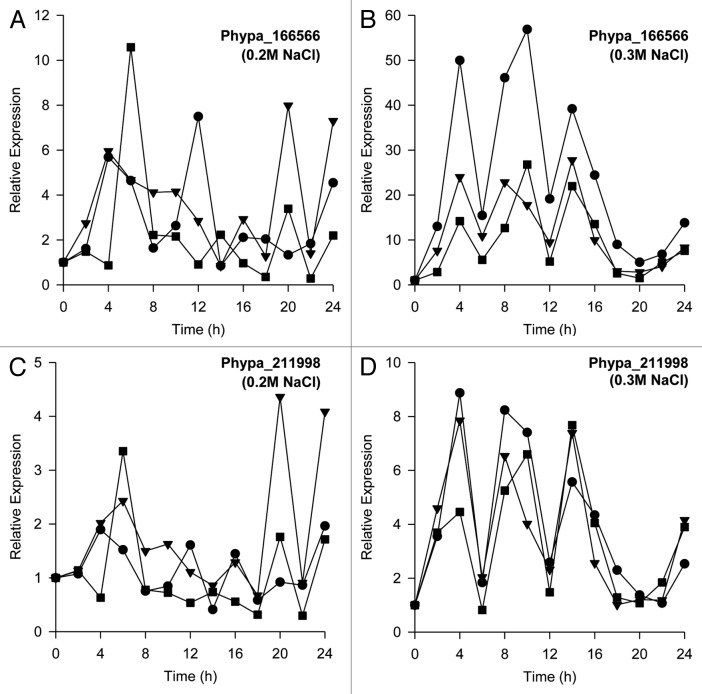
Physcomitrella patens has been shown to be highly tolerant of salt and osmotic stress,7,11,12 and they can survive exposure to 350 mM NaCl and 500 mM sorbitol.7 Microarray analysis has revealed substantial accumulation of two Group 3 LEA protein genes, Phypa_166566 and Phypa_211998, in response to various abiotic stress treatments.13 We previously showed using qPCR that dehydration stress-induced elevations in the steady-state transcript levels of Phypa_166566 and Phypa_211998 take the form of oscillations.10 Expression of these two Group 3 LEA protein genes was also upregulated by osmotic stress.14 However, Phypa_211998 was shown to be upregulated under osmotic stress and not salt stress.14 We were interested to determine whether salt- and osmotic-stress treatments can also induce temporally dynamic changes in the expression of these two Group 3 LEA protein genes. Figure 1 shows the effects of salt stress on steady-state transcript levels of Phypa_166566 (Fig. 1A and B) and Phypa_211998 (Fig. 1C and D). The results show that salt stress can induce oscillatory increases in the steady-state transcript levels of Phypa_166566 (Fig. 1A and B) and Phypa_211998 (Fig. 1C and D). Our data appears to be at odds with Cuming et al.14 who showed, using microarray analysis, that expression of Phypa_211998 is not upregulated by salt stress. However, it is important to note that the microarray data reflected gene expression at only one time interval (2 h) following salt-stress treatment.13 Additionally, we also observed dosage responsiveness of these two genes, as evident from the greater amplitudes of the temporally dynamic increases in transcript levels at higher salt concentration (0.3 M NaCl) (Fig. 1B and D) compared with a lower salt concentration (0.2 M NaCl) (Fig. 1A and C).
Figure 1. Salt stress-induced elevations in steady-state levels of the Group 3 LEA protein genes, Phypa_166566 and Phypa_211998 in Physcomitrella patens protonemata. Elevations in steady-state levels of Phypa_166566 induced by (A) 0.2 M NaCl and (B) 0.3 M NaCl (three independent biological replicates represented by closed symbols). Elevations in steady-state levels of Phypa_211998 induced by (C) 0.2 M NaCl and (D) 0.3 M NaCl (three independent biological replicates represented by closed symbols). Relative expression levels were determined by normalization of transcript levels of Phypa_166566 and Phypa_211998 with the geometric mean of stably expressed 18s rRNA, actin (Phypa_109052) and tubulin (Phypa_170860).
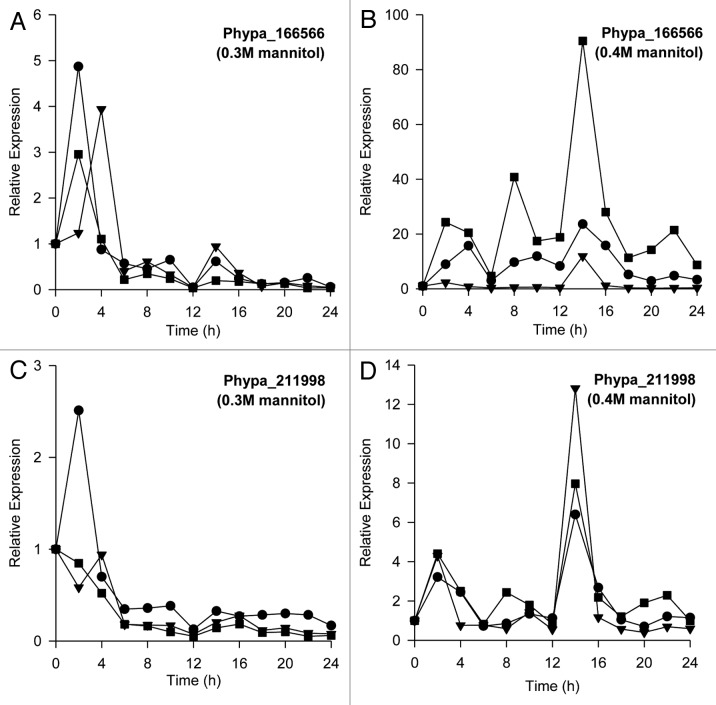
We also examined the effects of osmotic stress on changes in steady-state levels of these two Group 3 LEA protein genes (Fig. 2). We observed a transient elevation (within 2–4 h) in steady-state transcript levels of Phypa_166566 following exposure to 0.3 M mannitol (Fig. 2A). Greater upregulation of Phypa_166566 was observed when P. patens protonemata were treated with 0.4 M mannitol (Fig. 2B) and the elevations in steady-state levels of Phypa_166566 appear to be temporally dynamic (oscillatory) at 0.4 M mannitol (Fig. 2B). Similar variations in steady-state levels of Phypa_211998 transcripts were also observed when protonemata were subjected to osmotic stress treatment using 0.3 M (Fig. 2C) and 0.4 M mannitol (Fig. 2D).
Figure 2. Osmotic stress-induced elevations in steady-state levels of the Group 3 LEA protein genes, Phypa_166566 and Phypa_211998 in Physcomitrella patens protonemata. Elevations in steady-state levels of Phypa_166566 induced by (A) 0.3 M mannitol and (B) 0.4 M mannitol (three independent biological replicates represented by closed symbols). Elevations in steady-state levels of Phypa_211998 induced by (C) 0.3 M mannitol and (D) 0.4 M mannitol (three independent biological replicates represented by closed symbols). Relative expression levels were determined by normalization of transcript levels of Phypa_166566 and Phypa_211998 with the geometric mean of stably expressed 18s rRNA, actin (Phypa_109052) and tubulin (Phypa_170860).
We showed previously that dehydration stress and ABA can induce temporally dynamic changes in steady-state transcript levels of the Group 3 LEA protein genes, Phypa_166566 and Phypa_211998 that take the form of oscillations that is independent on circadian rhythms.10 Additionally, we also showed that the temporally dynamic nature of the steady-state transcript levels is responsive to the severity of dehydration stress or ABA dosage.10 Our current observations that other abiotic stresses like salt- and osmotic-stress can also induce oscillatory increases in steady-state levels of stress-induced transcripts suggest that non-circadian, temporally dynamic changes in stress responsive transcripts may be a general phenomenon in P. patens.
In mammals, non-circadian oscillations have been well-documented. For example, expression of the Hes1 gene, which encodes a basic helix-loop-helix (bHLH) transcriptional repressor, has been shown to oscillate, and the oscillatory expression is regulated by negative feedback and rapid degradation of the gene product.17 In neural progenitor cells, oscillations in Hes1 expression has been shown to contribute to the maintenance of the undifferentiated state,18 whereas oscillatory expression of Hes1 in mouse embryogenic cells contributes to heterogeneous responses,19 suggesting that oscillatory expression of Hes1 may have different functions in various cell types.
What might be the significance of such non-circadian oscillatory expression of genes in plants? Benfey and colleagues have demonstrated that non-circadian oscillations in gene expression may be important for developmental regulation of periodic root branching in Arabidopsis.15,16 Our observations of non-circadian oscillations in expression of two Group 3 LEA protein genes in response to dehydration stress10 and salt- and osmotic-stress (this study) that is reflective of the severity of the stress suggest that such a temporally dynamic system may confer responsiveness in speed and efficiency to abiotic stresses and may confer evolutionary advantages during colonization of land by plants. Future work will focus on understanding the underlying signaling mechanisms regulating this temporally dynamic abiotic stress-responsive gene expression system. The contribution of post-transcriptional stability of mRNA in the shaping of this temporally dynamic system should also be addressed. Additionally, it will be important to gain a global view of these oscillatory increases in transcriptional responses and to determine if levels of gene products also exhibit temporal dynamics.
Materials and Methods
Plants and growth conditions
Physcomitrella patens ecotype “Gransden 2004” was propagated on cellophane overlay plates containing BCDAT media (1 mM MgSO4, 1.84 mM KH2PO4 pH 6.5 adjusted with KOH), 10 mM KNO3, 45 µM FeSO4, 5 mM ammonium tartrate, 1 mM CaCl2 and supplemented with a trace element solution (Alternative TES- 10 µM H3BO3, 2 µM MnCl2, 0.22 µM CuSO4, 0.23 µM CoCl2, 0.19 µM ZnSO4, 0.1 µM Na2MoO4 and 0.17 µM KI) and 0.8% w/v agar as previously described (Nishiyama et al. 2000) under controlled conditions: light intensity (50 μmol s−1 m−2), 24 h light, 50% relative humidity (RH) and temperature (23°C) in a constant temperature growth room.
Salt- and osmotic-stress treatments
Ten to 12-d-old P. patens protonemata were used for salt- and osmotic-stress treatments over 24 h. Salt- and osmotic-stress treatments were performed by gently pipetting 1.5 ml of sterile liquid BCDAT medium containing 0.2 and 0.3 M NaCl (for salt stress) and 0.3 and 0.4 M mannitol (for osmotic stress) onto thin lawns of moss protonemata on cellophane overlays over BCDAT medium. Tissue samples were also harvested at regular 2 h time intervals (0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22 and 24 h) and snap-frozen using liquid N2 before RNA isolation.
RNA isolation, real-time, quantitative PCR (qPCR)
Total RNA was isolated, and qPCR was performed as previously described.10 qPCR reactions were run in triplicate for each experimental time interval using gene-specific primers (Table S1). Relative expression was determined by normalizing transcript levels using the geometric mean of three reference genes (18sRNA, actin: Phypa_109052, and tubulin: Phypa_170860).13 Three technical replicates were performed for each data point in the time series and the variations were < 3% for all genes tested.
Supplementary Material
Acknowledgments
This study was supported by a Science Foundation Ireland (SFI) Research Frontiers Programme Grant (08/SFI/EOB1087) to C.K.-Y.N.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22535
References
- 1.Rensing SA, Lang D, Zimmer AD, Terry A, Salamov A, Shapiro H, et al. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science. 2008;319:64–9. doi: 10.1126/science.1150646. [DOI] [PubMed] [Google Scholar]
- 2.Reski R. Molecular genetics of Physcomitrella. Planta. 1999;208:301–9. doi: 10.1007/s004250050563. [DOI] [Google Scholar]
- 3.Quatrano RS, McDaniel SF, Khandelwal A, Perroud PF, Cove DJ. Physcomitrella patens: mosses enter the genomic age. Curr Opin Plant Biol. 2007;10:182–9. doi: 10.1016/j.pbi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Charron AJ, Quatrano RS. Between a rock and a dry place: the water-stressed moss. Mol Plant. 2009;2:478–86. doi: 10.1093/mp/ssp018. [DOI] [PubMed] [Google Scholar]
- 5.Cho SH, von Schwartzenberg K, Quatrano R. The role of abscisic acid in stress tolerance. Ann Plant Rev. 2009;36:282–97. [Google Scholar]
- 6.Mishler BD, Oliver MJ. Putting Physcomitrella patens on the tree of life: the evolution and ecology of mosses. Ann Plant Rev. 2009;36:1–15. [Google Scholar]
- 7.Frank W, Ratnadewi D, Reski R. Physcomitrella patens is highly tolerant against drought, salt and osmotic stress. Planta. 2005;220:384–94. doi: 10.1007/s00425-004-1351-1. [DOI] [PubMed] [Google Scholar]
- 8.Koster KL, Balsamo RA, Espinoza C, Oliver MJ. Desiccation sensitivity and tolerance in the moss Physcomitrella patens: assessing limits and damage. Plant Growth Regul. 2010;62:293–302. doi: 10.1007/s10725-010-9490-9. [DOI] [Google Scholar]
- 9.Pressel S, Duckett JG. Cytological insights into the desiccation biology of a model system: moss protonemata. New Phytol. 2010;185:944–63. doi: 10.1111/j.1469-8137.2009.03148.x. [DOI] [PubMed] [Google Scholar]
- 10.Shinde S, Nurul Islam M, Ng CKY. Dehydration stress-induced oscillations in LEA protein transcripts involves abscisic acid in the moss, Physcomitrella patens. New Phytol. 2012;195:321–8. doi: 10.1111/j.1469-8137.2012.04193.x. [DOI] [PubMed] [Google Scholar]
- 11.Richardt S, Timmerhaus G, Lang D, Qudeimat E, Corrêa LGG, Reski R, et al. Microarray analysis of the moss Physcomitrella patens reveals evolutionarily conserved transcriptional regulation of salt stress and abscisic acid signalling. Plant Mol Biol. 2010;72:27–45. doi: 10.1007/s11103-009-9550-6. [DOI] [PubMed] [Google Scholar]
- 12.Ruibal C, Salamó IP, Carballo V, Castro A, Bentancor M, Borsani O, et al. Differential contribution of individual dehydrin genes from Physcomitrella patens to salt and osmotic stress tolerance. Plant Sci. 2012;190:89–102. doi: 10.1016/j.plantsci.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005;6:279–84. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- 14.Cuming AC, Cho SH, Kamisugi Y, Graham H, Quatrano RS. Microarray analysis of transcriptional responses to abscisic acid and osmotic, salt, and drought stress in the moss, Physcomitrella patens. New Phytol. 2007;176:275–87. doi: 10.1111/j.1469-8137.2007.02187.x. [DOI] [PubMed] [Google Scholar]
- 15.Moreno-Risueno MA, Benfey PN. Time-based patterning in development: The role of oscillating gene expression. Transcr. 2011;2:124–9. doi: 10.4161/trns.2.3.15637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreno-Risueno MA, Van Norman JM, Moreno A, Zhang J, Ahnert SE, Benfey PN. Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science. 2010;329:1306–11. doi: 10.1126/science.1191937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirata H, Yoshiura S, Ohtsuka T, Bessho Y, Harada T, Yoshikawa K, et al. Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science. 2002;298:840–3. doi: 10.1126/science.1074560. [DOI] [PubMed] [Google Scholar]
- 18.Shimojo H, Ohtsuka T, Kageyama R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron. 2008;58:52–64. doi: 10.1016/j.neuron.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi T, Mizuno H, Imayoshi I, Furusawa C, Shirahige K, Kageyama R. The cyclic gene Hes1 contributes to diverse differentiation responses of embryonic stem cells. Genes Dev. 2009;23:1870–5. doi: 10.1101/gad.1823109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.