Abstract
In a recent study, we demonstrated that although the auxin efflux carrier PIN-FORMED (PIN) proteins, such as PIN3 and PIN7, are required for the pulse-induced first positive phototropism in etiolated Arabidopsis hypocotyls, they are not necessary for the continuous-light-induced second positive phototropism when the seedlings are grown on the surface of agar medium, which causes the hypocotyls to separate from the agar surface. Previous reports have shown that hypocotyl phototropism is slightly impaired in pin3 single mutants when they are grown along the surface of agar medium, where the hypocotyls always contact the agar, producing some friction. To clarify the possible involvement of PIN3 and PIN7 in continuous-light-induced phototropism, we investigated hypocotyl phototropism in the pin3 pin7 double mutant grown along the surface of agar medium. Intriguingly, the phototropic curvature was slightly impaired in the double mutant when the phototropic stimulus was presented on the adaxial side of the hook, but was not impaired when the phototropic stimulus was presented on the abaxial side of the hook. These results indicate that PIN proteins are required for continuous-light-induced second positive phototropism, depending on the direction of the light stimulus, when the seedlings are in contact with agar medium.
Keywords: Arabidopsis, PIN, auxin, hypocotyl, phototropism
Text
Plants use several strategies to acclimate to the natural environment and phototropism is one of the most important mechanisms in their adaptation to the light environment. Molecular genetic studies have revealed several critical players that function during the early events of phototropism.1 The plant hormone auxin is one such key molecule, inducing curvature responses. According to the Cholodny-Went theory,2,3 auxin is asymmetrically distributed in response to unilateral blue-light irradiation. The auxin efflux carrier PIN-FORMED (PIN) proteins are thought to be involved in tropic responses. PIN3 plays a central role in regulating the lateral translocation of auxin because pin3 single mutants show defects in phototropism4 and PIN3 is asymmetrically localized in response to phototropic stimulation.5
We very recently reported that PIN1, PIN3 and PIN7 are necessary for pulse-induced hypocotyl phototropism, but not for continuous-light-induced phototropism in Arabidopsis.6 We used a highly sensitive method to induce hypocotyl phototropism, in which etiolated seedlings were grown on the surface of agar medium, which causes the hypocotyls to separate from the agar surface. Under these conditions, the shoots of the seedlings can move freely in any direction. Most researchers, including ourselves and other groups, have used Arabidopsis seedlings grown along the surface of agar medium,4,5,7 and it is possible that differences in experimental conditions have affected the results.
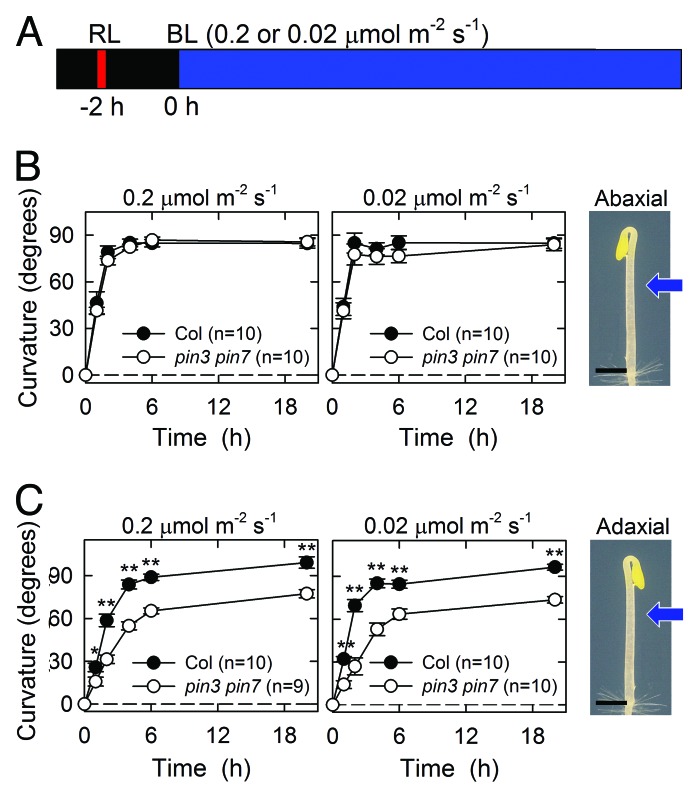
To extend our recent study and to evaluate further the possible involvement of PIN proteins in continuous-light-induced phototropism, we investigated hypocotyl phototropism in the pin3 pin7 double mutant, with a commonly used method in which the seedlings are grown along the surface of vertically oriented agar medium.8,9 Two-day-old etiolated seedlings were preirradiated with an overhead red light at 20 μmol m–2 s–1 for 2 min to enhance the phototropic response, and after 2 h, they were irradiated continuously with unilateral blue light (Fig. 1A). We used two fluence rates of blue light in this study: 0.2 μmol m–2 s–1 and 0.02 μmol m–2 s–1. The former is similar to the fluence rate used in our previous study.6 Because the relationship between the hook position and the direction of the phototropic stimulus affects the phototropic curvature of Arabidopsis hypocotyls,10 the phototropic stimulus was presented from the abaxial side (Fig. 1B) or the adaxial side (Fig. 1C) of the hook, and the hypocotyl curvature was determined at the indicated time points.
Figure 1. Hypocotyl phototropism induced by continuous blue-light irradiation. Two-day-old dark-grown seedlings were grown along the surface of vertically oriented agar medium. The Columbia (Col) strain and the pin3–5 pin7 (SALK_048791) double mutant were used in this study. (A) Experimental scheme of the investigation of hypocotyl phototropism. The seedlings were irradiated with overhead red light (RL) at 20 μmol m–2 s–1 for 2 min. Following incubation for 2 h, the seedlings were stimulated continuously with unilateral blue light (BL) at the indicated fluence rates. (B) Time courses of the hypocotyl phototropism induced by blue-light irradiation from the abaxial side of the hook. Hypocotyl curvature was determined at the indicated time points during the presentation of phototropic stimulation. The data shown are means ± SE. The image shown on the right illustrates the directional relationship between the hook and the phototropic stimulation. The blue arrow indicates the direction of the unilateral blue-light irradiation. Black bar, 1 mm. (C) Time courses of the hypocotyl phototropism induced with blue-light irradiation from the adaxial side of the hook. Asterisks indicate statistically significant differences between the Col strain and the pin3 pin7 double mutant (*p < 0.05, **p < 0.01). Other details are described above.
When unilateral blue-light stimulation was presented from the abaxial side of the hook, the wild-type hypocotyls showed phototropic curvature 1 h after the onset of stimulation, and reached the maximal level 2–4 h after the commencement of stimulation (Fig. 1B). Similar kinetics was observed in the pin3 pin7 double mutant, regardless of the light fluence rate, and there was no fundamental difference in the hypocotyl phototropism of the wild type and the mutant. When the phototropic stimulus was presented from the adaxial side of the hook, the wild-type hypocotyls bent to the horizontal level, regardless of the light fluence rate, although the maximum curvature was only induced after 4–6 h (Fig. 1C). Interestingly, the phototropic curvature was slightly impaired in the pin3 pin7 double mutant from the beginning of the phototropic response. These results indicate that PIN3 and PIN7 are not necessary for continuous-light-induced phototropism when the phototropic stimulus is presented from the abaxial side of the hook, but they are partly required for phototropism when the stimulus is presented from the adaxial side of the hook under the experimental conditions used here, in which the seedlings contact the surface of agar medium.
The impairment of the phototropic responses was observed in the pin3 pin7 double mutant under the experimental conditions described here, even when the continuous light stimulus was applied, whereas this phototropic defect was not observed in our previous study.6 Because the present investigation used seedlings grown along the surface of agar medium, it is possible that these growth conditions affect the phototropic responses. Therefore, PIN3- and PIN7-mediated auxin asymmetry might be required to overcome the friction that occurs when the hypocotyls are in contact with agar medium. Another possibility is that a directional relationship exists between the phototropic stimulus and the apical hook, which influences the phototropic responses, because the pin3 pin7 double mutant showed phototropic defects when the light stimulus was presented from the adaxial side but not when it was presented from the abaxial side (Fig. 1). Although we have not investigated the effects of directional differences in the light stimulus on the phototropism of the pin3 pin7 double mutant grown on the surface of agar medium used in our previous study,6 the present results indicate that the requirement for PIN proteins to generate auxin asymmetry depends on the direction of the phototropic stimulus during continuous-light-induced phototropism. In etiolated Arabidopsis seedlings, the distribution of auxin is asymmetrical in the hook region; auxin accumulates in the concave region of the apical hook.11 Therefore, it is apparently unnecessary to change the auxin asymmetry for the hypocotyl to bend toward the light source when the phototropic stimulus is presented from the abaxial side of the hook. The PIN3- and PIN7-mediated lateral transport of auxin may not be necessary under these conditions. In contrast, when the phototropic stimulus is presented from the adaxial side, the auxin asymmetry must be altered with a redistribution of the auxin. Under these conditions, PIN3- and PIN7-independent mechanisms may be insufficient to establish an auxin gradient, resulting in the phototropic impairment observed in the pin3 pin7 double mutant. It has been reported that PIN3 is localized preferentially to the convex region of the apical hook,12 which implies that PIN3 participates in the transport of auxin from the concave region to the convex region. Thus, the current study suggests that PIN3 and PIN7 are required for the regulation of the preferential auxin flow from the adaxial side to the abaxial side of the hook during the continuous-light-induced, second positive phototropism.
Our recent reports, including the present study, raise the question of how the auxin gradient is established during continuous-light-induced phototropism. It has not yet been clarified whether the asymmetrical distribution of auxin is involved in the PIN1-, PIN3- and PIN7-independent phototropism induced by continuous light irradiation. Although the asymmetry of auxin has not been explored in the pin1 pin3 pin7 triple mutant, an auxin gradient is at least established in the pin3 mutant, like that observed in the wild type during continuous-light-induced phototropism.6 Therefore, it is possible that PIN proteins other than PIN1, PIN3 and PIN7, or other types of auxin transporters such as the PIN-LIKES proteins13 and the ATP-BINDING CASSETTE subfamily B proteins14 are involved in the establishment of auxin asymmetry. Alternatively, auxin biosynthesis and/or metabolism might be regulated in response to continuous light stimulation to produce an auxin gradient. Further investigations are required to clarify how auxin gradients are established through PIN-dependent and PIN-independent mechanisms, and how these mechanisms are regulated by phototropic stimulation during continuous-light-induced phototropism.
Acknowledgments
We thank the Arabidopsis Biological Resource Center for providing the pin3–5 and pin7 (SALK_048791) mutants. This work was supported by the JST PRESTO program, the Japan Society for the Promotion of Science (JSPS) KAKENHI (no. 22570058); a Grant-in-Aid for Scientific Research on Innovative Areas “Plant Environmental Sensing,” (no. 23120510) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (to T.S.); and by KAKENHI (no. 24657027), a Grant-in-Aid for Challenging Exploratory Research (to K.H.).
Glossary
Abbreviations:
- PIN
PIN-FORMED
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22556
References
- 1.Sakai T, Haga K. Molecular genetic analysis of phototropism in Arabidopsis. Plant Cell Physiol. 2012;53:1517–34. doi: 10.1093/pcp/pcs111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Went FW, Thimann KV. Phytohormones 1937. New York: Macmillan. [Google Scholar]
- 3.Pedmale UV, Celaya RB, Liscum E. Phototropism: mechanism and outcomes. Arabidopsis Book. 2010;8:e0125. doi: 10.1199/tab.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–9. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- 5.Ding Z, Galván-Ampudia CS, Demarsy E, Łangowski L, Kleine-Vehn J, Fan Y, et al. Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat Cell Biol. 2011;13:447–52. doi: 10.1038/ncb2208. [DOI] [PubMed] [Google Scholar]
- 6.Haga K, Sakai T. PIN Auxin Efflux Carriers Are Necessary for Pulse-Induced But Not Continuous Light-Induced Phototropism in Arabidopsis. Plant Physiol. 2012;160:763–76. doi: 10.1104/pp.112.202432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagashima A, Uehara Y, Sakai T. The ABC subfamily B auxin transporter AtABCB19 is involved in the inhibitory effects of N-1-naphthyphthalamic acid on the phototropic and gravitropic responses of Arabidopsis hypocotyls. Plant Cell Physiol. 2008;49:1250–5. doi: 10.1093/pcp/pcn092. [DOI] [PubMed] [Google Scholar]
- 8.Sakai T, Wada T, Ishiguro S, Okada K. RPT2. A signal transducer of the phototropic response in Arabidopsis. Plant Cell. 2000;12:225–36. doi: 10.1105/tpc.12.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, et al. Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci U S A. 2001;98:6969–74. doi: 10.1073/pnas.101137598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khurana JP, Best TR, Poff KL. Influence of hook position on phototropic and gravitropic curvature by etiolated hypocotyls of Arabidopsis thaliana. Plant Physiol. 1989;90:376–9. doi: 10.1104/pp.90.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Johnson P, Stepanova A, Alonso JM, Ecker JR. Convergence of signaling pathways in the control of differential cell growth in Arabidopsis. Dev Cell. 2004;7:193–204. doi: 10.1016/j.devcel.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Zádníková P, Petrásek J, Marhavý P, Raz V, Vandenbussche F, Ding Z, et al. Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana. Development. 2010;137:607–17. doi: 10.1242/dev.041277. [DOI] [PubMed] [Google Scholar]
- 13.Barbez E, Kubeš M, Rolčík J, Béziat C, Pěnčík A, Wang B, et al. A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature. 2012;485:119–22. doi: 10.1038/nature11001. [DOI] [PubMed] [Google Scholar]
- 14.Titapiwatanakun B, Murphy AS. Post-transcriptional regulation of auxin transport proteins: cellular trafficking, protein phosphorylation, protein maturation, ubiquitination, and membrane composition. J Exp Bot. 2009;60:1093–107. doi: 10.1093/jxb/ern240. [DOI] [PubMed] [Google Scholar]