Abstract
Malformation is arguably the most crucial disease of mango (Mangifera indica L.) at present. It is receiving great attention not only because of its widespread and destructive nature but also because of its etiology and control is not absolutely understood. Recently, Fusarium mangiferae is found to be associated with mango malformation disease. There are indications that stress ethylene production could be involved in the disease. Here we have shown the first direct evidence of production of ethylene in pure culture of F. mangiferae obtained from mango. The study also revealed that all the isolates dissected from mango acquire morphological features of F. mangiferae showing most similarity to the features of species with accepted standard features. The isolates of F. mangiferae from mango were observed to produce ethylene in significant amounts, ranging from 9.28–13.66 n mol/g dry wt/day. The findings presented here suggest that F. mangiferae could contribute to the malformation of mango by producing ethylene and probably stimulating stress ethylene production in malformed tissue of mango. Ethylene might be produced through 2-oxoglutarate-dependent oxygenase-type ethylene-forming-enzyme (EFE) pathway in Fusarium sp, which needs to be investigated.
Keywords: ethylene, Fusarium mangiferae, gas liquid chromatography, mango malformation, mycelial mat and conidia
Introduction
Mango (Mangifera indica L.) is one of the most important commercial fruits grown in India and is cultivated in more than 100 countries spread over in five continents. The annual world production of mango is approximately 39,984,576 mt. India’s contribution to the world’s mango production is 17,650,000 mt from 2,502,000 ha and about 59,220.8 mt of mango is exported of approx. value of Rs. 16,292.1 lakhs during 2010–2011. In spite of the highest area (22,05,000 ha), the mango productivity is getting reduced from 1991–92 (8.1 mt/ha) to 2010–2011 (6.6 mt/ha).1 This is because mango production is badly hit by various diseases such as gummosis, internal necrosis, dieback, sooty-mold, black-tip, anthracnose, powdery mildew2 and, among those, malformation in mango is the most important and threatening disease of recent times causing considerable damage to the mango orchards worldwide.3,4 Mango malformation as recognized as early as 1891 by an expert mango grower from Darbhanga district in Bihar.5 Malformation is not only well-known in India but has also been confirmed in most mango-growing countries such as Pakistan, the Middlle East, Egypt, South Africa, Brazil, Sudan, Central America, Mexico, United States, Cuba, Malaysia, Australia, Israel, UAE and Bangladesh.6-10 There are several confusions in the literature to explain the nature of this malady because research efforts made up to now have not been able to determine its etiology. The complication of the disorder is ascribed by various factors like mites, fungal, viral and physiological factors.11 Present scenarios of mango malformation rely on the fact that Fusarium sp is having a wide acceptability in the scientific community as a causal agent of this disease. Recently, it has been reported that F. mangiferae is associated with mango malformation in Spain and Europe.3,12-14 However, physiological studies suggest that symptoms appear in malformed tissue resemble the symptoms caused by ethylene, which include leaf epinasty, abscission, formation of aerenchyma, suppression of apical dominance, hypertrophy of lenticels and increased gummosis and necrosis.2,15-20 Biotic stress (e.g., pathogen attack) elicits ethylene synthesis in plants.21 Ethylene production by Fusarium spp in vitro was measured under comparable conditions.22-25 Ethylene inducing protein (Nep1-like protein, NLP) that directly induces ethylene production in the attacked host. The NLPs and their homologs have been reported in many Fusarium sp.26 As shown in Figure 1, there are three routs of ethylene biosynthesis in plants, microorganism and fungi. Particularly, 2-oxoglutarate-dependent oxygenase-type EFE pathway occur entirely in fungi. Up to now, no evidence has been presented for the existence of 2-oxoglutarate- dependent oxygenase-type EFE pathway in plants.27 This study was designed to determine if F. mangiferae isolated from mango could produce ethylene in vitro, and thereby potentially contribute to malformation.
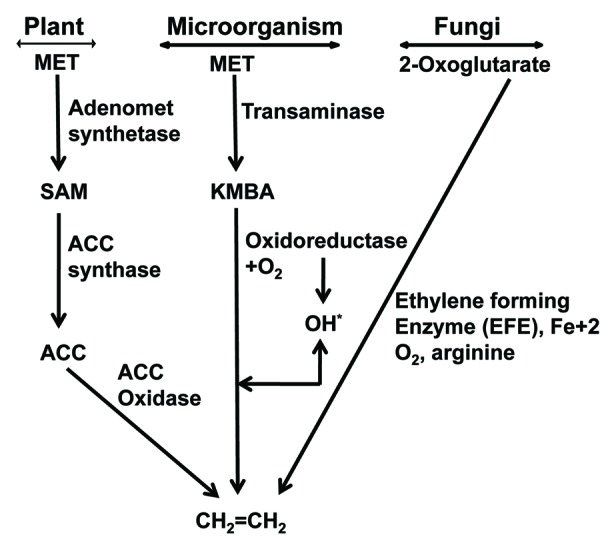
Figure 1. Ethylene biosynthesis pathways. First pathway, in higher plants, involves methionine (MET) as a precursor through the intermediates S-adenosyl methionine (SAM) and 1-aminocyclopropane-1-carboxylic acid (ACC). Second pathway described in bacteria where methionine is deaminated to produce α-keto γ-methylthiobutyric acid (KMBA), which is then oxidized to produce ethylene. Third one is found in range of fungi including Fusarium oxysporum in which 2-oxoglutarate is catalyzed by ethylene-forming enzyme (EFE). In addition to 2-oxoglutarate, the EFE of these organisms needs other amino acids such as arginine.
Results
The in vitro grown Fusarium culture obtained from malformed and healthy floral tissues of mango cultivars viz., Dashehri, Chausa, Amrapali, Mallika and Bombay Green grown in different districts, namely, Bareilly, Haldwani, Lucknow, Dehradun of India, exhibited most similarities to the accepted standard features of F. mangiferae (Table 1). The colonies developed from single-spore isolation methods revealed white plus light purple color on obverse side and purely light purple on reverse side of petri dishes after 14 d, which transformed to prominent purple color after 21 d. The sporulation started quickly after 3 d in mycelia mat of all isolates. Sclerotia- or chlamydospores-like fungal fragments were not observed in any isolate. The longitudinal section of hypha was septate. Conidia were slightly sickle shaped to straight. Macroconidia of all the isolates were four-celled (3-septate). Microconidia were in large number and were mostly fusiform, oval to elliptical in shape, partitioned with septa (0–1) and produced on polyphialides in false heads. Obovoid microconidia of F. mangiferae were also observed (Fig. 2). The size of micro- and macro-conidia conformed to the reported standards for the species ranging from 3.1–4 X 4.3–14.3 µM, 3.5–5 X 45–60 µM, respectively.
Table 1. Morphological and cultural characteristics of isolates of Fusarium mangiferae from Dashehri, Chausa, Amrapali, Mallika and Bombay Green cultivars of mango grown in Bareilly, Haldwani, Lucknow, Dehradun districts of India, recorded on CLA medium.
| Morphological characteristics |
Fusarium mangiferae |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolate-1 | Isolate-2 | Isolate-3 | Isolate-4 | Isolate-5 | Isolate-6 | Isolate-7 | Isolate-8 | Isolate-9 | Isolate-10 | |||||||
| Microconidia obovoid |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
||||||
| Microconidia oval to allantoid/or fusoid |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
||||||
| Sterile coiled hyphae |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
||||||
| Conidiophore originated erect |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
||||||
| Conidiogenous openings: ≤ 3 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
||||||
| Conidiogenous openings: ≥ 3 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
||||||
| Microconidia 0-septate |
+ |
- |
- |
- |
- |
- |
+ |
- |
+ |
- |
||||||
| Microconidia occasionally 1-septate |
- |
+ |
+ |
+ |
+ |
+ |
- |
+ |
- |
+ |
||||||
| Macroconidia 3-septate |
+ |
- |
- |
- |
- |
- |
+ |
- |
+ |
- |
||||||
| Macroconidia 3–5 septate |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
||||||
| Chlamydospore |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
||||||
| Polyphialides |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
||||||
| White + purple colony color in Petri dishes (obverse) |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
||||||
| Purple colony color in Petri dishes (reverse) |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
||||||
| Host specific | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ||||||
“+” indicates the presence of the characteristic; “-” indicates the absence of the characteristic and “?” Indicates that the characteristic has not been reported
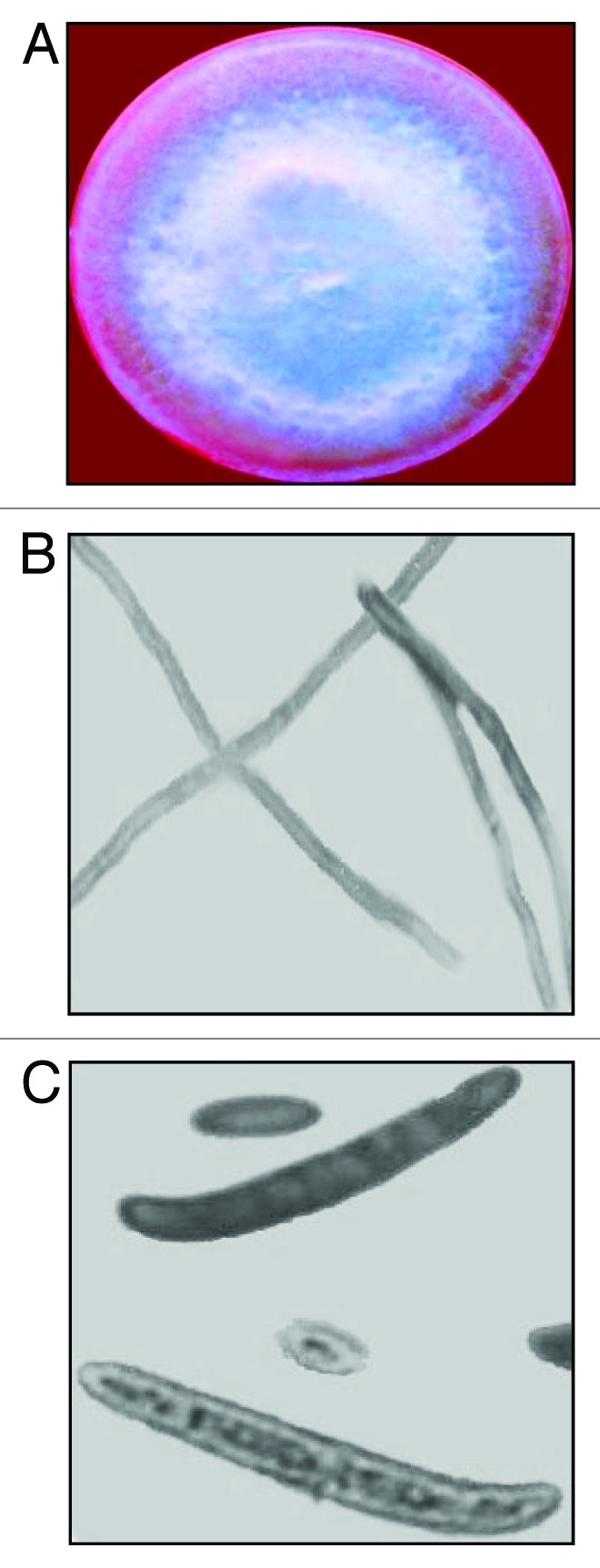
Figure 2. Morphological features of Fusarium mangiferae isolated from mango cultivars viz., Dashehri, Chausa, Amrapali, Mallika and Bombay Green grown in Bareilly, Haldwani, Lucknow, Dehradun districts of India, (A) White plus purple colony color in Petri dish (obverse). (B) Longitudinal septate hypha. (C) Three-septate (four-celled) typical macroconidium and obovoid microconidia.
All isolates of F. mangiferae tested produced ethylene (Table 2), ranging from 9.28–13.66 n mole/g dry wt/day. Ethylene was not recorded in the culture bottles containing uninoculated media (control) (Table 2).
Table 2. Production of Ethylene by isolates of Fusarium mangiferae from mango (Mangifera indica L.) cultivars viz., Dashehri, Chausa, Amrapali, Mallika and Bombay Green grown in Bareilly, Haldwani, Lucknow, Dehradun districts of India, growing on PDB. mango.
| Number of isolates | Concentration of ethylene (nano mole/g. dry wt./day) | Mean (nano mole/g dry wt/day) | ||
|---|---|---|---|---|
| |
R1 |
R2 |
R3 |
|
| Control |
00.00 |
00.00 |
00.00 |
00.00 |
| Isolate-1 |
12.40 |
14.48 |
14.10 |
13.66 |
| Isolate-2 |
11.01 |
08.30 |
09.82 |
09.71 |
| Isolate-3 |
09.02 |
10.00 |
11.25 |
10.09 |
| Isolate-4 |
09.93 |
11.12 |
10.05 |
10.37 |
| Isolate-5 |
08.55 |
10.67 |
09.42 |
09.55 |
| Isolate-6 |
12.70 |
11.25 |
11.53 |
11.83 |
| Isolate-7 |
10.32 |
09.50 |
13.14 |
10.99 |
| Isolate-8 |
09.57 |
09.27 |
11.17 |
10.00 |
| Isolate-9 |
13.26 |
12.48 |
12.57 |
12.77 |
| Isolate-10 |
09.02 |
08.97 |
09.84 |
09.28 |
| SEm ± Cd. at 5% CV |
0.5926474 1.738155 10.43215 |
|||
Discussion
Morphological examination of F. mangiferae isolated from malformed as well as healthy tissues of mango revealed that among the studied features, macro- and micro-conidia and conidiogenesis are the significant features used in the characterization of species. All the studied isolates were in conformity with the earlier used standard and displayed highly similar characteristics defined for F. mangiferae.30 The growth pattern, their colors, septate hypha, microconidia, macroconidia and transverse sections of hyphae were extremely specific to the described features of the species.
The production of ethylene by all 10 isolates F. mangiferae in this study is consistent with earlier work.25 Although endogenous ethylene levels in mango tissues were found to be higher in malformed tissues than the respective healthy tissues at different developmental stages of flowering in mang,.2,18,31,32 and Fusarium spp are capable of producing ethylene, the role of ethylene induction by F. mangiferae in causing mango malformation has not been successfully demonstrated. The present study provides further evidence that isolates of F. mangiferae produce ethylene, and the amounts produced can vary between isolates. These results suggest that F. mangiferae could contribute to the malformation of mango by producing ethylene and stimulating stress ethylene, production in malformed tissue of mango. The disease severity is reflected with the mean temperature preceding flowering. It is most severe where mean temperature remains between 10–15°C. It is mild where the corresponding temperature is 15–20°C, sporadic at 20–25°C and nil over 25°C. Influences of different temperatures have also been reported to affect growth and conidia germination of Fusaria. There was no growth of fungus at temperatures below 12°C and above 40°C, the temperatures of 25°C and 30°C were better suited for growth and sporulation of F. mangiferae. Therefore, we have an impression that endogenous ethylene content in mango tissues or ethylene produced by Fusaria itself may be affected with the trends of seasonal variation in temperature.32 However, the level of ethylene contributed by the F. mangiferae to stress ethylene pool: when invading the plant tissues needs to be investigated further. As shown in Figure 1, there are three routes of ethylene biosynthesis in plants, microorganism and fungi. Up to now, no evidence has been presented for the existence of 2-oxoglutarate-dependent oxygenase-type EFE in plants.27 This study provides the first direct evidence of ethylene production by F. mangiferae obtained from mango. Further, expression analysis of ethylene-forming-enzyme (EFE) will be taken into account for elucidating ethylene biosynthetic pathway in Fusarium sp (mangiferae) to narrate ethylene as a key player in malformation.
Materials and Methods
Morphological and cultural identification of F. mangiferae
Malformed and healthy samples of mango cultivars, namely, Dashehri, Chausa, Amrapali, Mallika and Bombay Green grown were collected from several locations of different districts, namely, Bareilly, Haldwani, Lucknow, Dehradun of India for isolation and identification of the fungi present. For isolation of the pathogen, small pieces of mango measuring about 5 mm long were cut using sterilized sharp scalpels. The pieces were then surface sterilized with 0.1% HgCl2 solution for 2 min, washed thrice with sterilized distilled water to remove the HgCl2, then placed on pre-sterilized blotting paper to remove excess moisture. Four to five surface-sterilized pieces were aseptically transferred with forceps onto Czepeck’s dox agar plates/slants.28 After the plates/slants were incubated at 27+1°C in BOD incubator for 3–4 d, the mycelial growth from the margin of the culture was aseptically removed for further purification on potato dextrose agar (PDA) plates/slants.29 A single-spore isolation technique was used for producing pure cultures of the Fusaria. Single spores were transferred from the axenic suspension culture to sterilized Petri plates/slants of PDA. From these parent cultures, mycelial growth from the culture margin was used for sub culturing as and when required. The parent cultures were maintained at 5 ± 1°C.13,28 The colonies of F. mangiferae were purified on carnation leaf agar (CLA) medium and the identification was done on the basis of typical macro and microconidia.29,30 Morphological and culture features such as colony color, shape and structure of mycelium, shape and structure of micro- and micro-conidia, number of septations micro- and micro-conidia, presence/absence of polyphialides and chlamydospores were recorded.
Quantification of ethylene
Five millimeter discs from the fungal mat of each isolate were cut from 10-d-old pure cultures of the F. mangiferae. Isolates obtained from mango (Mangifera indica L.) tissues and were inoculated aseptically into the sterilized culture bottles, each containing 100 ml of PDA. After inoculation, the bottles were closed with specially made screw caps fitted with serum stoppers. Two layers of parafilm were wrapped around the brim of the caps to make the bottles leak-proof. The culture bottles were then placed in a BOD incubator at 27 ± 1°C for 1 mo under constant shaking, at which time the ethylene content of the culture bottles was estimated by gas liquid chromatography (GLC). Four replicates were done for both control (uninoculated) as well as inoculated media for ethylene estimation.
Acknowledgments
RCP and MWA are thankful to U.P. Council of Agricultural Research, Lucknow, India for financial assistance in the form of a research project. Work on signal transduction and plant stress signaling in NT’s laboratory is partially supported by Department of Science and Technology (DST) and Department of Biotechnology (DBT), Government of India. M.W.A. is also thankful to Department of Science and Technology (DST) for funding under DST fast track scheme for young scientist.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22673
References
- 1.NBH Indian horticulture database. National Board of Horticulture, India. 2011. www.assamagribissiness.nic.in/database2011.pdf
- 2.Krishnan AG, Nailwal TK, Shukla A, Pant RC. Mango (Mangifera indica. L) malformation an unsolved mystery. Researcher. 2009;1:20–36. [Google Scholar]
- 3.Arif M, Pani DR, Zaidi NW, Singh US. PCR-based identification and characterization of Fusarium sp. associated with mango malformation. Biotechnol Res Int. 2011;2011:141649. doi: 10.4061/2011/141649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman Z, Freeman S, Biton I, Sa’ada D, Paz T, Maymon M, et al. Molecular diagnosis of mango malformation disease and phylogeny of Fusarium mangiferae. Phytoparasitica. 2012;40:287–97. doi: 10.1007/s12600-012-0224-6. [DOI] [Google Scholar]
- 5.Watt G. A Dictionary of Economic Products of India. Calcutta. Govt. Printing Press 1891; pp. 149. [Google Scholar]
- 6.Ploetz RC. Malformation: a unique and important disease of mango, Mangifera indica L. In: Summerell BA, Leslie SF, Backhouse D, Bryden WL, Bargus LW eds. Fusarium: Paul E Nelson Memorial Symposium. St Paul, MN: APS press 2001; 235-247. [Google Scholar]
- 7.Bains G, Pant RC. Mango malformation: Etiology and preventive measures. Physiol Mol Biol Plants. 2003;9:41–61. [Google Scholar]
- 8.Kvas M, Steenkamp ET, Al-Adawi AO, Deadman ML, Al-Jahwari AA, Marasas WFO, et al. Fusarium mangiferae associated with mango malformation in the Sultanate of Oman. Eur J Plant Pathol. 2008;121:195–9. doi: 10.1007/s10658-007-9231-8. [DOI] [Google Scholar]
- 9.Gamliel-Atinsky E, Sztejnberg A, Maymon M, Vintal H, Shtienberg D, Freeman S. Infection dynamics of Fusarium mangiferae, causal agent of mango malformation disease. Phytopathology. 2009;99:775–81. doi: 10.1094/PHYTO-99-6-0775. [DOI] [PubMed] [Google Scholar]
- 10.Haggag WM. Mango diseases in Egypt. Agric Biol J N Am. 2010;1:285–9. [Google Scholar]
- 11.Otero-Colina G, Rodríguez-Alvarado G, Fernández-Pavía S, Maymon M, Ploetz RC, Aoki T, et al. Identification and characterization of a novel etiological agent of mango malformation disease in Mexico, Fusarium mexicanum sp. nov. Phytopathology. 2010;100:1176–84. doi: 10.1094/PHYTO-01-10-0029. [DOI] [PubMed] [Google Scholar]
- 12.Ansari MW, Nailwal TK, Gomathi A, Singh AK, Bains G, Shukla A, et al. Singh, US, Pant RC. Mangiferin (1,3,6,7-tetrahydroxyxanthone-C2-β-D-glucoside), a phenolic metabolite of mango (Mangifera indica L.), affects germination of spore of Fusarium sp. J Plant Biol. 2005;32:155–9. [Google Scholar]
- 13.Ansari MW, Nainwal TK, Bains G, Shukla A, Singh US, Pant RC. Effect of ethrel on germination of spore of Fusarium sp isolated from Mangifera indica L. Pantnagar J of Res. 2008;6:275–8. [Google Scholar]
- 14.Lima CS, Pfenning LH, Costa SS, Abreu LM, Leslie JF. Fusarium tupiense sp. nov., a member of the Gibberella fujikuroi complex that causes mango malformation in Brazil. Mycologia 2012; PMID: 22675046; http://www.mycologia.org/content/early/2012/06 [DOI] [PubMed]
- 15.Abeles AL, Abeles FB. Biochemical pathology for stress-induced ethylene. Plant Physiol. 1973;50:496–8. doi: 10.1104/pp.50.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattoo AK, Suttle JC. The Plant Hormone Ethylene. CRC Press, London. Ist ed 1991; pp. 337. [Google Scholar]
- 17.Abeles FB, Morgan PW, Saltveit ME. Ethylene in Plant Biology. Academic Press. 2nd ed 1992; pp. 399. [Google Scholar]
- 18.Pant RC. Is ‘Stress ethylene’ the cause of mango (Mangifera indica L.) malformation? Physiol Mol Biol Plants. 2000;6:8–14. doi: 10.1007/s12298-014-0258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jennings JC, Patricia C, Bryan A, Anderson JD. Induction of ethylene biosynthesis and necrosis in weed leaves by a Fusarium oxysporum protein. Weed Sci. 2000;48:7–14. doi: 10.1614/0043-1745(2000)048[0007:IOEBAN]2.0.CO;2. [DOI] [Google Scholar]
- 20.Tao L, Dong HJ, Chen X, Chen SF, Wang TH. Expression of ethylene-forming enzyme (EFE) of Pseudomonas syringae pv. glycinea in Trichoderma viride. Appl Microbiol Biotechnol. 2008;80:573–8. doi: 10.1007/s00253-008-1562-7. [DOI] [PubMed] [Google Scholar]
- 21.Bleecker AB, Kende H. Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol. 2000;16:1–18. doi: 10.1146/annurev.cellbio.16.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Swart A, Kamerbeek GA. Different ethylene production in vitro by several species and formae speciales of Fusarium. Neth J Plant Pathol. 1976;82:81–4. doi: 10.1007/BF01976953. [DOI] [Google Scholar]
- 23.Swart A, Kamerbeek GA. Ethylene production and mycelium growth of the tulip strain of Fusarium oxysporum as influenced by shaking of and oxygen supply of the medium. Physiol Plant Pathol. 1977;39:38. [Google Scholar]
- 24.Graham JH, Linderman RG. Ethylene production by ectomycorrhizal fungi, Fusarium oxysporum f. sp. pini, and by aseptically synthesized ectomycorrhizae and Fusarium-infected Douglas-fir roots. Can J Microbiol. 1980;26:1340–7. doi: 10.1139/m80-222. [DOI] [PubMed] [Google Scholar]
- 25.Swart A, Kamerbeek GA. Ethylene Production and Mycelium Growth of the Tulip Strain of Fusarium oxysporum as Influenced by Shaking of and Oxygen Supply to the Culture Medium. Physiol Plant. 2010;39:1399–3054. [Google Scholar]
- 26.Bailey BA, Collins R, Anderson JD. Factors influencing the herbicidal activity of Nep1, a fungal protein that induces the hypersensitive response in Centaurea maculosa. Weed Sci. 2000;48:776–85. doi: 10.1614/0043-1745(2000)048[0776:FITHAO]2.0.CO;2. [DOI] [Google Scholar]
- 27.Araki S, Matsuoka M, Tanaka M, Ogawa T. Ethylene formation and phenotypic analysis of transgenic tobacco plants expressing a bacterial ethylene-forming enzyme. Plant Cell Physiol. 2000;41:327–34. doi: 10.1093/pcp/41.3.327. [DOI] [PubMed] [Google Scholar]
- 28.Keitt GW. Simple technique for isolating single spore strains of certain types of fungi. Phytopathology. 1951;5:266–9. [Google Scholar]
- 29.Nelson PE, Tousson TA, Marasas WFO. Fusarium species: An Illustrated Manual for Identification. University Park, The Penn. State, 1983. [Google Scholar]
- 30.Britz H, Steenkamp ET, Coutinho TA, Wingfield BD, Marasas WFO, Wingfield MJ. Two new species of Fusarium section Liseola associated with mango malformation. Mycologia. 2002;94:722–30. doi: 10.2307/3761722. [DOI] [PubMed] [Google Scholar]
- 31.Nailwal T, Gomathi A, Bains G, Shukla A, Pant RC. Mango (Mangifera indica L.) malformation: Role of stress ethylene and cyanide. Physiol Mol Biol Plants. 2006;12:163–5. doi: 10.1007/s12298-014-0258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rymbai H, Rajesh AM. Mango malformation: a review. Life Sci Leaf. 2011;22:1079–95. [Google Scholar]


