Abstract
Respiratory burst oxidase homologs (RBOHs) catalyze the reduction of oxygen to generate superoxide anion, a kind of reactive oxygen species (ROS). The ROS produced by RBOHs play essential roles in diverse processes, such as root hair development, stomata closure and signaling mechanisms in response to abiotic stimuli and during plant-pathogen interactions. Recently, we found that PvRbohB silencing in transgenic Phaseolus vulgaris roots had a negative impact on lateral root density. In this work, we show that the downregulation of PvRbohB affects both the growth and ROS levels in recently emerged lateral roots. In addition, we found that the PvRbohB promoter was activated during lateral root primordium initiation in the pericycle, and remained active throughout lateral root development. This study identifies RBOHs as potentially important players in lateral root development in P. vulgaris.
Keywords: RBOH, lateral roots, ROS, development, P. vulgaris
Plant NADPH oxidases (referred to as RBOHs) are one of the specific enzymes that produce reactive oxygen species (ROS) in plants. RBOHs have been localized to the plasma membrane of various plant cells, where they catalyze the conversion of diatomic oxygen to superoxide anion.1 These oxidases were initially thought to be associated with ROS production as part of defense mechanisms against pathogens and abiotic stimuli. However, loss-of-function of NADPH oxidase genes affects growth and development in diverse organisms, from fungi to plants and animals.2 For instance, depletion of RBOH in Solanum lycopersicum (tomato) plants reduced the chlorophyll content and apical dominance, and resulted in sterile and abnormal flowers.3 A reverse genetic approach in Nicotiana tabacum revealed that the polarized growth of pollen tubes requires NtRBOH activity.4 Furthermore, Foreman et al. (2003) demonstrated that the polarized growth of Arabidopsis thaliana root hairs depends on AtRBOHC-catalyzed ROS production in the apical region of root hairs.5
PvRbohB Silencing Affects Lateral Root Length
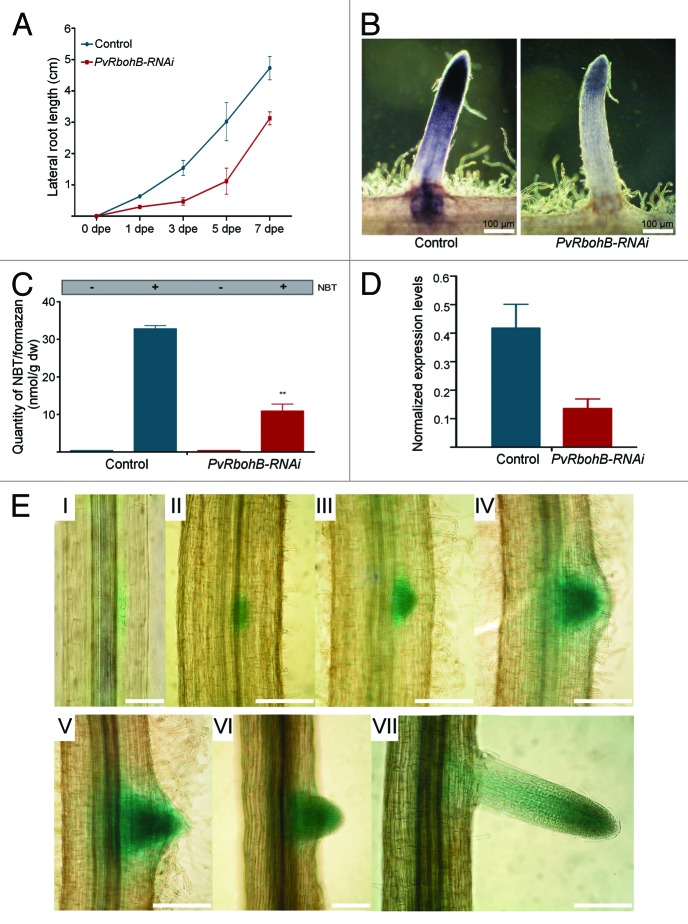
An increasing body of evidence indicates that ROS production by RBOHs is not only involved in the polarized growth of pollen tubes and root hairs, but also in the regulation of primary root growth. Tsukagoshi et al. (2010) demonstrated that altered ROS levels affect primary root growth.6 They found that the RBOH inhibitor dyphenilene iodonium (DPI) reduces the meristem cell number in the A. thaliana primary root.6 Accordingly, superoxide accumulation is mainly located in the meristematic region of A. thaliana plants and DPI treatment has a negative effect on ROS production and primary root growth.6,7 In addition, loss-of-function of AtRBOHC and AtRBOHF leads to a 20% and 35% reduction in primary root growth, respectively.5,8 We recently found that, in composite Phaseolus vulgaris plants, interference RNA (RNAi)-mediated silencing of PvRbohB reduced the growth of the main root (the hairy root equivalent of the primary root in wild-type plants) marginally, but not significantly. Nonetheless, a 15% reduction in lateral root (LR) density was detected in PvRbohB-RNAi roots.9 A more detailed inspection of this phenotype revealed that LR length was also decreased in PvRbohB-RNAi roots compared with control transgenic roots (Fig. 1A). In control transgenic roots harvested at 1 d post-emergence (dpe), LR length was 50% greater than in PvRbohB-RNAi roots. Similar differences (between 40–60%) in LR length remained between PvRbohB-RNAi and control transgenic roots collected at 1, 3, 5 and 7 dpe (Fig. 1A). Together, these observations imply that PvRbohB has a role in LR growth and development in P. vulgaris.
Figure 1. The involvement of PvRbohB in LR growth and development. (A) LR length in control (n = 13, blue line) and PvRbohB-RNAi (n = 15, red line) transgenic roots harvested at 1, 3, 5 and 7 dpe. (B) Detection of superoxide accumulation by NBT (0.1%) staining in control and PvRbohB-RNAi LRs. (C) Quantification of NBT/formazan precipitates per dry weight of LRs stained with NBT (as described by Ramel et al. 200915) as shown in (B). (D) PvRbohB transcript abundance in the apical region (~3 mm from the tip) of control and PvRbohB-RNAi LRs. Expression values were normalized to those of EF1α. Bars indicate means ± SEM from two independent biological replicates (n > 15 independent transgenic roots per biological replicate) with three technical repeats. (E) Promoter activity of PvRbohB, as assessed by bright field microscopy of transgenic roots expressing 1.8 kb of the PvRbohB promoter region fused to GUS and GFP (pPvRbohB:GUS-GFP). Promoter activity was detected by GUS staining during different stages of LR development in P. vulgaris roots (I–VII; n = 18). Scale bar, 100 µm.
Superoxide Accumulation Decreases in PvRbohB-Downregulated LRs
LRs are heterotrophic organs that form a plant root system. LR initiation takes place in pericycle cells adjacent to the xylem pole. In Arabidopsis, founder cells giving rise to lateral root primordia (LRP) become specified in the young differentiation zone.10 An analysis using epifluorescence microscopy revealed that hydrogen peroxide accumulates in LRP from A. thaliana plants.11 We found that superoxide, as assessed by nitroblue staining (NBT), accumulated in the apical region of emerging LRs of transgenic P. vulgaris plants (Fig. 1B); this distribution pattern is similar to that detected in the main root.9 Downregulation of PvRbohB transcript levels in the apical region (3 mm) of PvRbohB-RNAi lateral roots (Fig. 1D) resulted in lower superoxide levels in this region (Fig. 1B and C). This indicates that PvRBOHB makes an important contribution to the maintenance of superoxide levels in the apices of LRs, and this could be necessary for LR growth. Although ROS metabolism has not been deeply studied during LR initiation, a few reports suggest that ROS could perform crucial functions during LR development, as they do in the main root. It has recently been documented that LR formation in A. thaliana requires a capacity for redox regulation that is operated by NADPH-thioredoxin reductase C in the chloroplasts.12 Additionally, it has been proposed that the maintenance of cellular proliferation requires the accumulation of superoxide, whereas cellular differentiation depends on the accumulation of hydrogen peroxide.6
The PvRbohB Promoter is Active Throughout LR Development
LR organogenesis is an auxin-dependent process that requires the activity of several auxin transporters and cell cycle genes. The expression of some of these genes has been detected in the founder pericycle cells that will form LRs.13 Using P. vulgaris transgenic roots expressing the promoter sequence of PvRbohB in a transcriptional fusion to the molecular markers GUS and GFP (pPvRbohB:GUS-GFP), we found PvRbohB promoter activity during the initial mitotic divisions in the pericycle cells (Fig. 1E). Interestingly, histochemical GUS staining was also detected throughout LR development in pPvRbohB:GUS-GFP roots. Once LR emerged, GUS staining was restricted to the apical region (Fig. 1E), exhibiting a similar distribution pattern to that determined in the main root.9 The activity profile of the PvRbohB promoter provides crucial experimental evidence for the involvement of PvRbohB in LR development.
LR development is a complex process that requires cellular division within the founder pericycle cells.13 Consequently, cell wall dynamics must be modulated to allow cellular proliferation and eventually cell elongation. It is widely accepted that ROS can modulate cell wall extensibility. Hydrogen peroxide has been associated with lignification processes in an apoplastic peroxidase-dependent mechanism; furthermore, hydroxyl radicals are known to be involved in cell wall loosening.14 Our results demonstrate that PvRBOHB makes an important contribution to superoxide production during LR growth. PvRBOHB-mediated ROS production may participate in LR development by modulating cell wall dynamics. Additionally, specific levels of ROS may be necessary for maintaining the redox status during LR initiation. This study reveals a novel role for RBOHs in LR growth and development and prompts further investigation of the involvement of ROS in the LR development program.
Acknowledgments
This work was partially supported by Dirección General de Asuntos del Personal Académico–Universidad Nacional Autónoma de México (IN205609) and Consejo Nacional de Ciencia y Tecnología (153718 to C.Q.). Jesús Montiel was supported by a Consejo Nacional de Ciencia y Tecnología Ph.D. fellowship (199262).
Glossary
Abbreviations:
- dpe
days post-emergence
- DPI
diphenyleneiodonium
- GFP
green fluorescent protein
- GUS
beta glucuronidase
- LR
lateral root
- LRP
lateral root primordium
- NBT
nitroblue tetrazolium
- RBOHs
respiratory burst oxidase homologs
- ROS
reactive oxygen species
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22694
References
- 1.Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R. Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol. 2011;14:691–9. doi: 10.1016/j.pbi.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre J, Lambeth JD. Nox enzymes from fungus to fly to fish and what they tell us about Nox function in mammals. Free Radic Biol Med. 2010;49:1342–53. doi: 10.1016/j.freeradbiomed.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, et al. Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum Plant Cell 2004; 16:616-28.; PMID:14973161; 10.1105/tpc.019398. [DOI] [PMC free article] [PubMed]
- 4.Potocký M, Jones MA, Bezvoda R, Smirnoff N, Zárský V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol. 2007;174:742–51. doi: 10.1111/j.1469-8137.2007.02042.x. [DOI] [PubMed] [Google Scholar]
- 5.Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–6. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 6.Tsukagoshi H, Busch W, Benfey PN. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell. 2010;143:606–16. doi: 10.1016/j.cell.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Dunand C, Crèvecoeur M, Penel C. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol. 2007;174:332–41. doi: 10.1111/j.1469-8137.2007.01995.x. [DOI] [PubMed] [Google Scholar]
- 8.Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, et al. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003;22:2623–33. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montiel J, Nava N, Cárdenas L, Sánchez-López R, Arthikala MK, Santana O, et al. A Phaseolus vulgaris NADPH oxidase gene is required for root infection by rhizobia. Plant Cell Physiol. 2012;53:1751–67. doi: 10.1093/pcp/pcs120. [DOI] [PubMed] [Google Scholar]
- 10.Dubrovsky JG, Napsucialy-Mendivil S, Duclercq J, Cheng Y, Shishkova S, Ivanchenko MG, et al. Auxin minimum defines a developmental window for lateral root initiation. New Phytol. 2011;191:970–83. doi: 10.1111/j.1469-8137.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- 11.Tyburski J, Dunajska K, Tretyin A. Reactive oxygen species localization in Arabidopsis thaliana seedlings grown under phosphate deficiency. Plant Growth Regul. 2009;59:27–36. doi: 10.1007/s10725-009-9385-9. [DOI] [Google Scholar]
- 12.Ferrández J, González M, Cejudo F. Chloroplast redox homeostasis is essential for lateral root formation in Arabidopsis. Plant Signal Behav. 2012;7:1117–9. doi: 10.4161/psb.21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Smet I. Lateral root initiation: one step at a time. New Phytol. 2012;193:867–73. doi: 10.1111/j.1469-8137.2011.03996.x. [DOI] [PubMed] [Google Scholar]
- 14.Liszkay A, Kenk B, Schopfer P. Evidence for the involvement of cell wall peroxidase in the generation of hydroxyl radicals mediating extension growth. Planta. 2003;217:658–67. doi: 10.1007/s00425-003-1028-1. [DOI] [PubMed] [Google Scholar]
- 15.Ramel F, Sulmon C, Bogard M, Couée I, Gouesbet G. Differential patterns of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. BMC Plant Biol. 2009;9:28. doi: 10.1186/1471-2229-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]