Abstract
Natural selection is thought to have shaped the evolution of floral scent; however, unlike other floral characters, we have a rudimentary knowledge of how phenotypic selection acts on scent. We found that floral scent was under stronger selection than corolla traits such as flower size and flower color in weakly scented Penstemon digitalis. Our results suggest that to understand evolution in floral phenotypes, including scent in floral selection, studies are crucial. For P. digitalis, linalool was the direct target of selection in the scent bouquet. Therefore, we determined the enantiomeric configuration of linalool because interacting insects may perceive the enantiomers differentially. We found that P. digitalis produces only (S)-(+)-linalool and, more interestingly, it is also taken up into the nectar. Because the nectar is scented and flavored with (S)-(+)-linalool, it may be an important cue for pollinators visiting P. digitalis flowers.
Keywords: corolla color, floral scent, flower size, linalool, phenotypic selection, Penstemon digitalis, pollination
Phenotypic Selection on Floral Scent
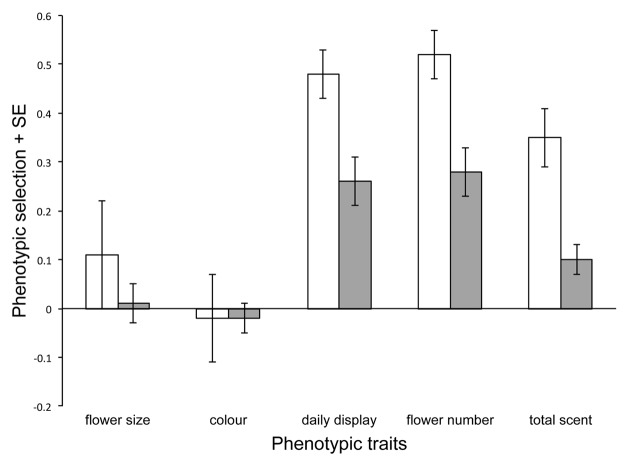
In our recent paper “Phenotypic selection to increase floral scent emission, but not flower size or color in bee-pollinated Penstemon digitalis” in New Phytologist,1 we found scent was under stronger selection than more commonly measured visual components of a floral display (Fig. 1). Scents are an important signal in plant-insect communication and therefore are expected to be under pollinator and antagonist-mediated selection.2,3 Animal-mediated selection on scent is predicted to be balancing due to conflicting selection pressures, if scents attract both mutualists and antagonists4 but directional if a scent simultaneously attracts pollinators and repels enemies.5 Of course, scent blends are generally composed of many volatiles and, thus, it is possible that different components of the blend will be under different forms of selection.6 However, due to the difficulty of conducting large-scale scent collections in the field, as well as the necessity of specialized equipment and expertise, our knowledge of the evolutionary ecology of scents is elementary. Therefore, incorporating floral chemistry into studies that include floral color, size and density of display should provide unbiased measurements of the relative importance of scent, particularly in systems in which it is not a conspicuous trait. Accordingly, we chose a weakly scented North American species of Penstemon (Plantaginaceae), a well-studied genus in pollination research, for which we had no strong expectations for scent to be a dominant floral character. We measured phenotypic selection on scents as well as other floral traits such as flower size, corolla color, display size and phenology in plants from three local populations of P. digitalis in New York. Although natural selection has been estimated for floral traits in many systems, the bulk of the research has been conducted on visual cues, mechanical fit traits and floral phenology.7 Thus, it is not only interesting and informative to measure selection on scents but it is also important to embed this knowledge within the framework of what we already know about floral evolutionary ecology.
Figure 1. Phenotypic selection on floral and inflorescence traits of Penstemon digitalis. White bars represent total selection (via direct selection and selection on correlated traits) while gray bars represent the targets of selection (direct selection) obtained from multivariate models that control for correlations among traits. Total and direct selection were significant for daily display size, total flower number and total scent. Flower size is the geometric mean of six floral measurements, floral color was estimated as the number × intensity of purple lines, daily display is the mean of display size, flower number is the total number of flowers produced and total scent is the sum of all relativized peak areas of 23 compounds emitted from the inflorescence. Traits not shown: first flower date, days flowering, plant height. Modified from table 3 (Parachnowitsch et al. 2012).1 [Author’s Note: Figure not cited in text]
For our study of P. digitalis, we took advantage of a common garden experiment for its relative ease of collecting floral scents from many individuals over a short time. We used the methods outlined by Lande and Arnold,8 which require phenotypic measurements of traits and a fitness estimate for each plant to determine which traits confer higher relative fitness. We measured eight traits [scent (n = 23 volatiles), flower size, corolla pigment, day of first flower, days flowering, daily display size, final height and total flower number] and estimated female fitness (seed set) to determine and compare phenotypic selection on scent, visual and phenological traits. Specifically, we used selection gradients obtained from multivariate regression analyses that control for correlations among traits to determine the direct targets of selection. That phenotypic selection on scent was stronger than for other corolla characters, such as floral color and size in P. digitalis, which was particularly interesting for two reasons. First, because pollinators frequently show consistent color preferences, floral color is generally hypothesized to be adaptive and a likely target of selection.9 Moreover, when corolla pigmentation varies, such as in P. digitalis [for example coefficients of variance (CV): CVpigment = 1.67, CVtotal scent = 0.69, CVlinalool = 0.75], this color variation may be maintained by conflicting or fluctuating selection, as suggested in other systems.10-12 Thus, although our work suggests that floral color variation in P. digitalis is selectively neutral,1,13,14 this was not the initial expectation. Second, our previous study found direct selection by pollinators on flower size in a nearby population,13 suggesting that it is an important trait for pollinator attraction in this system.
Of the entire blend of 23 scent compounds we found in P. digitalis, linalool was the target of selection.1 To determine which compounds were targets, we again used multivariate regression models but here we included individual compounds instead of total scent as a single-character state. Linalool is a common component of floral scents,15 suggesting that it plays important and diverse roles in floral communication.16 Our study demonstrates that this compound can also be under natural selection, and at least in P. digitalis, it is also the single target of selection from the scent bouquet. Our investigations suggested that linalool is a floral-specific volatile and therefore could be used as an odor cue by pollinators. However, linalool is a chiral compound with two enantiomeric forms that could influence insect behavior differentially and we had not determined which of the two enantiomers (or proportion of each) the flowers emitted. Therefore we returned in 2012 to assess the exact configuration of linalool emitted from P. digitalis flowers.
Linalool in Penstemon digitalis Flowers
Both insect mutualists and antagonists can potentially respond differently to the two optical isomers of linalool. Studies of the model insect, Manduca sexta, demonstrated two distinct olfactory processing channels for linalool, such that enantiomer-specific antennal receptor neurons project to different glomeruli in the antennal lobes of the moth’s brain.17 A differential perception by the insect potentially allows for alternate ecological functions of the enantiomers18 and, thus, differential natural selection on the two compounds. Therefore, to better understand how linalool might function in P. digitalis, we used a chiral column (Cyclosil-B, Agilent-J&W GC columns) to analyze its optical characteristics. We compared headspace from freshly cut P. digitalis flowers using SPME (solid-phase microextraction) with racemic linalool (Aldrich L260–2; 97% pure), (R)-(-)-linalool (Aldrich 62139, > 95% pure) and the odor of powdered coriander seed (Coriandrum sativum), which is dominated by (S)-(+)-linalool.19 Methods generally followed those we used previously (Note S1)1; collecting odors on 100 μm polydimethylsiloxane (PDMS) fibers from headspace chambers constructed using oven-baked glass scintillation vials with a gasket of nylon resin oven bag material (Reynolds, Inc.). For flower emissions, a 10 mL vial was packed with six cut flowers (from three populations we previously studied), to maximize the intensity of the linalool peak which can be weak for some plants.1 We used a 1.5 mL vial with approximately 0.5 g of coriander for comparison and collected scents from the two vials for 30 min. Using direct comparison of the GC-MS (gas chromatograph-mass spectrometer) output, we were able to determine that P. digitalis produces (S)-(+)-linalool only (Fig. 2). This likely has implications for plant-insect interactions in the system and most importantly will allow for manipulations of the compounds produced by P. digitalis in future experiments. For example, pollinators may use linalool as a cue after smelling it in nectar-rewarding flowers. Alternatively, linalool, like other monoterpenoids, can have repellent functions for antagonists in floral bouquets.20 Although we did not detect a relationship between linalool and predispersal seed predation in our common garden experiment,1 it is possible that the benefit of increased linalool is from its defensive rather than attractive function. Thus, further experimental tests with (S)-(+)-linalool in P. digitalis are necessary to address its functional role in the system.
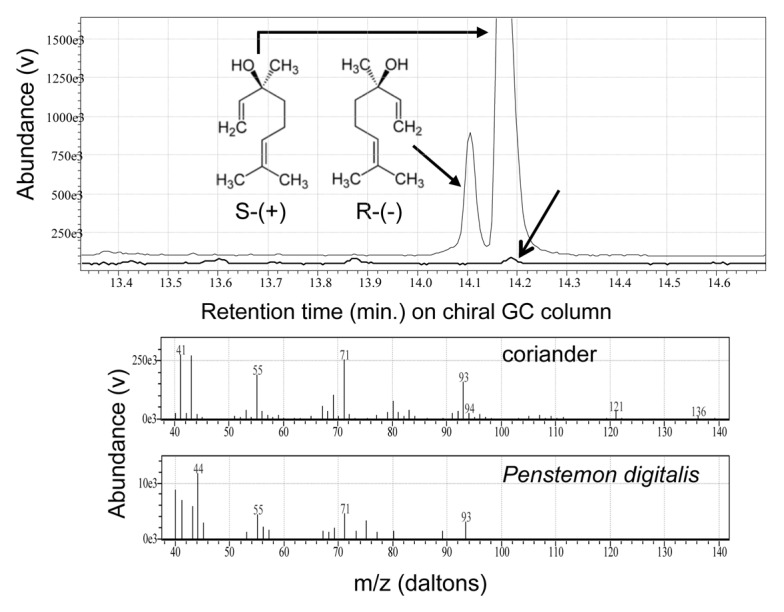
Figure 2. Enantiomeric configuration of linalool emitted by flowers of Penstemon digitalis. Upper panel: comparison of linalool emitted from coriander (upper GC trace) vs. cut P. digitalis flowers (lower trace) on a chiral GC column. Coriander shows a racemic mix dominated by (S)-(+)-linalool (right peak), whereas P. digitalis emits only that enantiomer (arrow). GC trace for (R)-(-)-linalool not shown (see text). Lower panel: EI-quadrupole mass spectra for (S)-(+)-linalool from coriander (above) and for linalool emitted by flowers of P. digitalis (below).
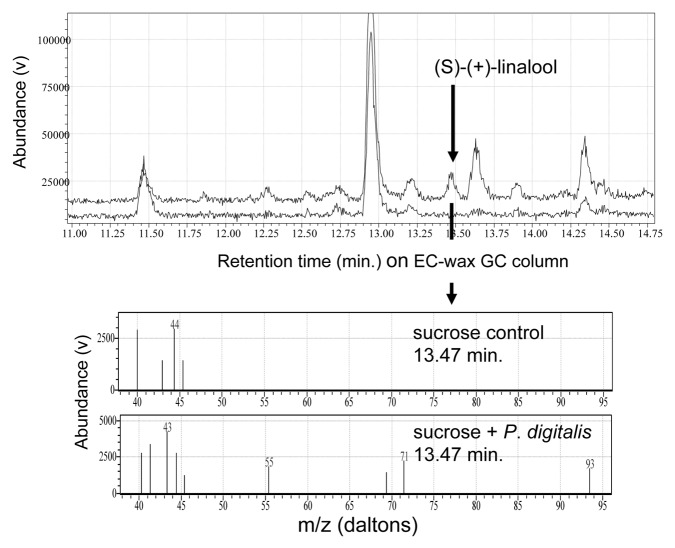
Because linalool is a polar compound, it could be absorbed into the nectar of P. digitalis if it is emitted from corolla tissue near the nectary.21 Thus, linalool not only would signal the presence of flowers, but also could indicate the nectar reward sought by pollinators. We tested this hypothesis by first trying to detect linalool in naturally occurring nectar samples. Unfortunately, P. digitalis does not produce large quantities of nectar and even with pooling from many individuals we were unable to detect scents from field-collected nectar samples. Thus, we used 3 µL of ~35% sucrose solution (approximately similar to the sanding crop of naturally produced nectar) inserted into > 40 flowers on eight fresh-cut stems from one population to demonstrate whether linalool would be passively taken up by nectar within the flower as in ref. 21. After 1 h, nectar was collected from the flowers using filter paper and scent was collected as previously but from 4 mL vials. We compared scent from this surrogate nectar with scent emitted from sucrose solution-soaked filter paper. Indeed, we found that the surrogate nectar was scented with linalool, suggesting that P. digitalis nectar is likely flavored as well as scented (Fig. 3). Thus, pollinators could learn to use linalool as an honest cue for the presence of reward in P. digitalis flowers.22
Figure 3. Evidence that Penstemon digitalis floral nectar is scented with (S)-(+)-linalool. Upper panel shows GC traces from SPME trapping of volatiles emitted by 35% sucrose solution on filter paper, either after 1 h incubation in the nectar tube of P. digitalis (upper trace) or from control filter papers (lower trace). The arrow indicates (S)-(+)-linalool, verified by MS and retention index on polar GC column using an authentic standard. Lower panel compares EI-quadrupole mass spectra from sucrose control (upper box) vs. flower-incubated sucrose (lower box), at the retention time (13.47 min) of (S)-(+)-linalool. The incubation of sucrose within the flower effectively reproduces the process of passive but selective absorption of polar volatiles by nectar in the corolla tubes of P. digitalis flowers.
The ecology and evolution of nectar in general and scented nectar in particular is poorly understood.21,23,24 Moreover, scented nectar may serve different functional roles in attraction for mutualists and repellence of antagonists.25,26 Therefore, as with linalool itself, direct tests of the function of linalool-scented nectar in P. digitalis are necessary to determine its role. However, the potential for linalool to act as an honest indicator of nectar-reward suggests that selection to increase linalool may be pollinator-mediated through their preference for higher rewarding plants in this system.
Conclusions
Our results suggest that by continuing to ignore floral scents, our overall perspective on natural selection on floral characters will remain skewed toward assuming the importance of visual characters. Moreover, as scent sampling methods, chemical analyses and data handling of the large data sets produced by measuring scent bouquets advance,27-29 large-scale field-based ecological experiments are becoming more attainable.6,30-32 Thus, future floral evolutionary research will likely increasingly incorporate scents to understand how integrated floral phenotypes evolve.
Acknowledgments
We thank all those involved with the original publication of the selection data, as well as Frantisek Baluska for the opportunity to expand our thoughts and results of Penstemon digitalis floral scent. GC-MS analyses of linalool were supported by NSF grant DEB-0746106 to R.A.R. and the 2012 field season was supported by Kungliga Vatenskapsakademien grant FOA11H-317 to A.L.P.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22704
References
- 1.Parachnowitsch AL, Raguso RA, Kessler A. Phenotypic selection to increase floral scent emission, but not flower size or colour in bee-pollinated Penstemon digitalis. New Phytol. 2012;195:667–75. doi: 10.1111/j.1469-8137.2012.04188.x. [DOI] [PubMed] [Google Scholar]
- 2.Dötterl S, Vereecken NJ. The chemical ecology and evolution of bee-flower interactions: a review and perspectives. Canadian Journal of Zoology-Revue Canadienne De Zoologie. 2010;88:668–97. doi: 10.1139/Z10-031. [DOI] [Google Scholar]
- 3.Schiestl FP. The evolution of floral scent and insect chemical communication. Ecol Lett. 2010;13:643–56. doi: 10.1111/j.1461-0248.2010.01451.x. [DOI] [PubMed] [Google Scholar]
- 4.Theis N, Lerdau M, Raguso RA. The challenge of attracting pollinators while evading floral herbivores: Patterns of fragrance emission in Cirsium arvense and Cirsium repandum (Asteraceae) Int J Plant Sci. 2007;168:587–601. doi: 10.1086/513481. [DOI] [Google Scholar]
- 5.Junker RR, Blüthgen N. Floral scents repel facultative flower visitors, but attract obligate ones. Ann Bot (Lond) 2010;105:777–82. doi: 10.1093/aob/mcq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiestl FP, Huber FK, Gómez JM. Phenotypic selection on floral scent: trade-off between attraction and deterrence? Evol Ecol. 2011;25:237–48. doi: 10.1007/s10682-010-9409-y. [DOI] [Google Scholar]
- 7.Harder LD, Johnson SD. Darwin’s beautiful contrivances: evolutionary and functional evidence for floral adaptation. New Phytol. 2009;183:530–45. doi: 10.1111/j.1469-8137.2009.02914.x. [DOI] [PubMed] [Google Scholar]
- 8.Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37:1210–26. doi: 10.2307/2408842. [DOI] [PubMed] [Google Scholar]
- 9.Frey FM. Opposing natural selection from herbivores and pathogens may maintain floral-color variation in Claytonia virginica (Portulacaceae) Evolution. 2004;58:2426–37. doi: 10.1111/j.0014-3820.2004.tb00872.x. [DOI] [PubMed] [Google Scholar]
- 10.Rausher MD. Evolutionary transitions in floral color. Int J Plant Sci. 2008;169:7–21. doi: 10.1086/523358. [DOI] [Google Scholar]
- 11.Irwin RE, Strauss SY. Flower color microevolution in wild radish: evolutionary response to pollinator-mediated selection. Am Nat. 2005;165:225–37. doi: 10.1086/426714. [DOI] [PubMed] [Google Scholar]
- 12.Caruso CM, Scott SL, Wray JC, Walsh CA. Pollinators, herbivores, and the maintenance of flower color variation: A case study with Lobelia siphilitica. Int J Plant Sci. 2010;171:1020–8. doi: 10.1086/656511. [DOI] [Google Scholar]
- 13.Parachnowitsch AL, Kessler A. Pollinators exert natural selection on flower size and floral display in Penstemon digitalis. New Phytol. 2010;188:393–402. doi: 10.1111/j.1469-8137.2010.03410.x. [DOI] [PubMed] [Google Scholar]
- 14.Parachnowitsch AL. Understanding natural selection on floral traits: Variation, agents and consequences. Dissertation. Ithaca, NY: Cornell, 2011. [Google Scholar]
- 15.Knudsen JT, Eriksson R, Gershenzon J, Stahl B. Diversity and distribution of floral scent. Bot Rev. 2006;72:1–120. doi: 10.1663/0006-8101(2006)72[1:DADOFS]2.0.CO;2. [DOI] [Google Scholar]
- 16.Raguso RA, Pichersky E. New perspectives in pollination biology: Floral fragrances. A day in the life of a linalool molecule: Chemical communication in a plant-pollinator system. Part 1: Linalool biosynthesis in flowering plants. Plant Species Biol. 1999;14:95–120. doi: 10.1046/j.1442-1984.1999.00014.x. [DOI] [Google Scholar]
- 17.Reisenman CE, Christensen TA, Francke W, Hildebrand JG. Enantioselectivity of projection neurons innervating identified olfactory glomeruli. J Neurosci. 2004;24:2602–11. doi: 10.1523/JNEUROSCI.5192-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reisenman CE, Riffell JA, Bernays EA, Hildebrand JG. Antagonistic effects of floral scent in an insect-plant interaction. Proc Biol Sci. 2010;277:2371–9. doi: 10.1098/rspb.2010.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casabianca H, Graff JB, Faugier V, Fleig F, Grenier C. Enantiomeric distribution studies of linalool and linalyl acetate. A powerful tool for authenticity control of essential oils. J High Resolut Chromatogr. 1998;21:107–12. doi: 10.1002/(SICI)1521-4168(19980201)21:2<107::AID-JHRC107>3.0.CO;2-A. [DOI] [Google Scholar]
- 20.Junker RR, Blüthgen N. Floral scents repel potentially nectar-thieving ants. Evol Ecol Res. 2008;10:295–308. [Google Scholar]
- 21.Raguso RA. Why are some floral nectars scented? Ecology. 2004;85:1486–94. doi: 10.1890/03-0410. [DOI] [Google Scholar]
- 22.Wright GA, Schiestl FP. The evolution of floral scent: the influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Funct Ecol. 2009;23:841–51. doi: 10.1111/j.1365-2435.2009.01627.x. [DOI] [Google Scholar]
- 23.Mitchell RJ. Heritability of nectar traits: Why do we know so little? Ecology. 2004;85:1527–33. doi: 10.1890/03-0388. [DOI] [Google Scholar]
- 24.Adler LS. The ecological significance of toxic nectar. Oikos. 2000;91:409–20. doi: 10.1034/j.1600-0706.2000.910301.x. [DOI] [Google Scholar]
- 25.Kessler D, Baldwin IT. Making sense of nectar scents: the effects of nectar secondary metabolites on floral visitors of Nicotiana attenuata. Plant J. 2007;49:840–54. doi: 10.1111/j.1365-313X.2006.02995.x. [DOI] [PubMed] [Google Scholar]
- 26.Adler LS, Irwin RE. Nectar alkaloids decrease pollination and female reproduction in a native plant. Oecologia. 2012;168:1033–41. doi: 10.1007/s00442-011-2153-3. [DOI] [PubMed] [Google Scholar]
- 27.Ranganathan Y, Borges RM. Reducing the babel in plant volatile communication: using the forest to see the trees. Plant Biol (Stuttg) 2010;12:735–42. doi: 10.1111/j.1438-8677.2009.00278.x. [DOI] [PubMed] [Google Scholar]
- 28.Tholl D, Boland W, Hansel A, Loreto F, Röse USR, Schnitzler J-P. Practical approaches to plant volatile analysis. Plant J. 2006;45:540–60. doi: 10.1111/j.1365-313X.2005.02612.x. [DOI] [PubMed] [Google Scholar]
- 29.van Dam NM, Poppy GM. Why plant volatile analysis needs bioinformatics--detecting signal from noise in increasingly complex profiles. Plant Biol (Stuttg) 2008;10:29–37. doi: 10.1055/s-2007-964961. [DOI] [PubMed] [Google Scholar]
- 30.Galen C, Kaczorowski R, Todd SL, Geib J, Raguso RA. Dosage-dependent impacts of a floral volatile compound on pollinators, larcenists, and the potential for floral evolution in the alpine skypilot Polemonium viscosum. Am Nat. 2011;177:258–72. doi: 10.1086/657993. [DOI] [PubMed] [Google Scholar]
- 31.Kessler A, Halitschke R, Poveda K. Herbivory-mediated pollinator limitation: negative impacts of induced volatiles on plant-pollinator interactions. Ecology. 2011;92:1769–80. doi: 10.1890/10-1945.1. [DOI] [PubMed] [Google Scholar]
- 32.Ibanez S, Dötterl S, Anstett MC, Baudino S, Caissard JC, Gallet C, et al. The role of volatile organic compounds, morphology and pigments of globeflowers in the attraction of their specific pollinating flies. New Phytol. 2010;188:451–63. doi: 10.1111/j.1469-8137.2010.03317.x. [DOI] [PubMed] [Google Scholar]