Abstract
Catharanthus roseus is an important source of pharmaceutically important Monoterpenoid Indole Alkaloids (MIAs). Accumulation of many of the MIAs is induced in response to abiotic stresses such as wound, ultra violet (UV) irradiations, etc. Recently, we have demonstrated a possible role of CrMPK3, a C. roseus mitogen-activated protein kinase in stress-induced accumulation of a few MIAs. Here, we extend our findings using Saccharomyces cerevisiae to investigate the role of CrMPK3 in giving tolerance to abiotic stresses. Yeast cells transformed with CrMPK3 was found to show enhanced tolerance to UV and heat stress. Comparison of CrMPK3 and SLT2, a MAPK from yeast shows high-sequence identity particularly at conserved domains. Additionally, heat stress is also shown to activate a 43 kDa MAP kinase, possibly CrMPK3 in C. roseus leaves. These findings indicate the role of CrMPK3 in stress-induced MIA accumulation as well as in stress tolerance.
Keywords: Catharanthus roseus, CrMPK3, abiotic stress, UV, heat, cold, Saccharomyces cerevisiae
Plants in natural conditions are exposed to different biotic and abiotic stress conditions. Plants respond to these stresses via several molecular and biochemical changes at the cellular level, including biosynthesis of a variety of secondary metabolites. Catharanthus roseus, a member of Apocynaceae family, produces a variety of secondary metabolites, especially monoterpenoid indole alkaloids (MIAs). Some of these MIAs are of high therapeutic values, such as the antihypertensive, ajmalicine, serpentine and anticancer vincristine, vinblastine.1 Many of the MIAs and transcripts of genes involved in MIAs biosynthesis show enhanced accumulation in response to stresses such as UV and plant stress hormone methyl jasmonate (MeJA).2-7
Mitogen-activated protein kinase (MAPK) signaling is an evolutionarily conserved mechanism in eukaryotes and involved in transducing extracellular signals to produce responses to the stress in question.8 In C. roseus involvement of protein phosphorylation as well as MAP kinase in stress-induced transcripts accumulation of key genes of MIA biosynthesis have been speculated.7,9 Recently, we have shown cloning and characterization of CrMPK3, a MAPK from C. roseus. CrMPK3 showed enhanced transcripts accumulation and MAPK activity in response to wounding, UV treatment and MeJA application in C. roseus. Further, transient overexpression of CrMPK3 showed higher transcripts level of key genes involved in MIA biosynthesis and higher accumulation of important MIAs (Fig. 1).10 Here, we extend functional characterization of CrMPK3 in S. cereviseae. Yeast has been used as a model system to substantiate the role of plant genes/protein in stress tolerance mechanisms since plants and yeast show significant similarity in signal transduction mechanism, particularly MAPK signaling mechanism.11-15
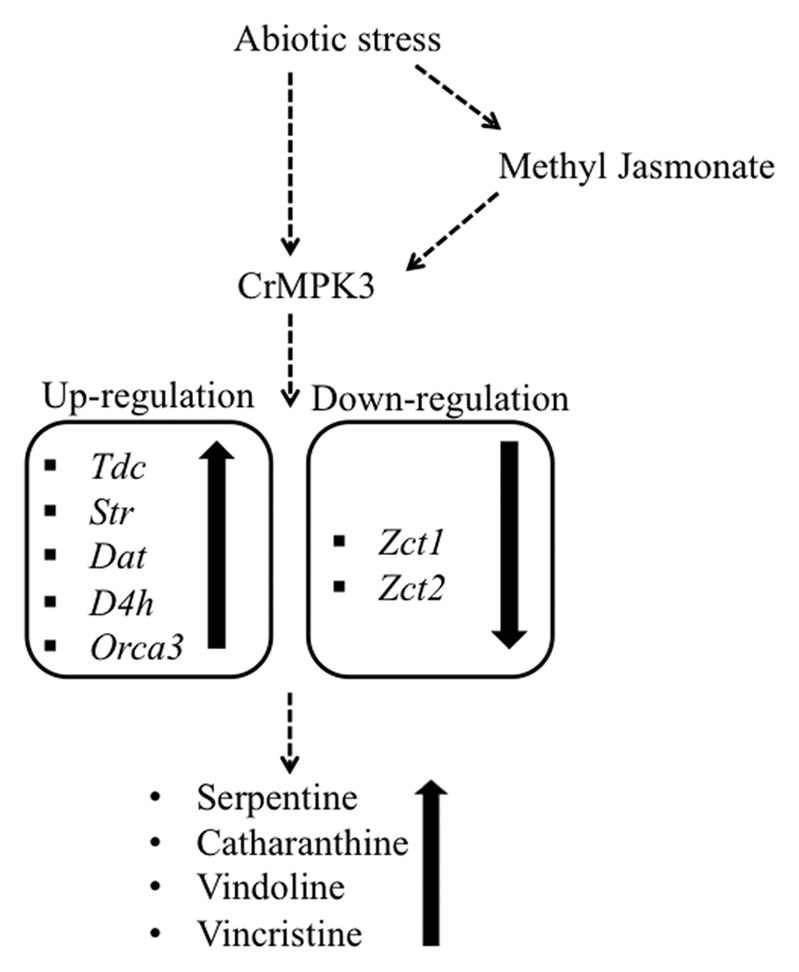
Figure 1. A simplified scheme depicting the role of CrMPK3 in stress-induced MIA accumulation in C. roseus. Dashed arrow indicates presence of either multiple steps or unknown components. Solid arrows indicate up- or downregulation.
Activation of CrMPK3 by wounding and UV exposure hinted at possible roles of CrMPK3 in abiotic stress tolerance. To support this hypothesis, we studied the effect of CrMPK3 overexpression and stress tolerance in budding yeast. CrMPK3 was cloned in pYES 2.1 under a galactose inducible promoter and resulting construct was transformed in a protease-deficient yeast strain BCY123. As a control, the same strain was transformed with vector alone. Yeast cells transformants with CrMPK3 and vector alone were subjected to UV, heat and cold stress treatment and then plated on nutrient media. As shown in figure (Fig. 2A and B), CrMPK3-transformed yeast exhibited better survival and growth than the vector-transformed controls in heat shock as well as UV stress. However, in case of cold stress, only a marginal difference in growth was observed (Fig. 2C). Since CrMPK3 protein activation was observed in response to UV exposure,10 better survivals of CrMPK3-transformed yeast during UV exposure substantiates the role of CrMPK3 in UV-mediated signaling. Further, as CrMPK3 rescued the yeast during heat shock, we studied MAPK activation in C. roseus leaves in response to heat shock. An in gel kinase assay showed a distinct ~43 kDa MAPK activity in response to heat shock in C. roseus leaves, which possibly indicates CrMPK3 activity in heat stress. The ability of yeast cells to resist heat shock and UV stress is related with cell wall integrity. In Saccharomyces cerevisiae, well-characterized SLT2, MAP kinase pathway is associated with cell wall integrity.16,17 When a sequence alignment of CrMPK3 and SLT2 was performed, a high domain identity was observed between CrMPK3 and SLT2 (Fig. 3). Owing to high homology and functional similarity, it could be inferred that overexpression of CrMPK3 might have been supplemental to the native SLT2 protein and, thus, enabling yeast to survive and grow better under stress conditions. Further, it indicates that CrMPK3 may have analogous function in planta to that of SLT2 in yeast, in addition to signaling for higher MIAs in response to stress.
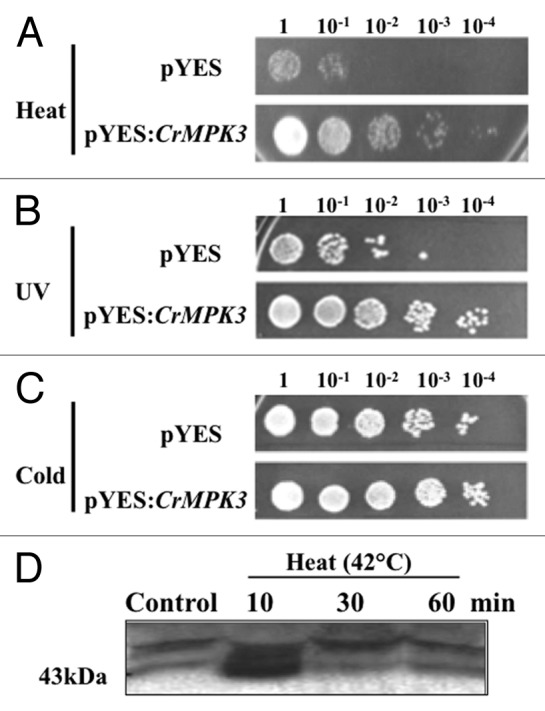
Figure 2. CrMPK3 provides tolerance to yeast against abiotic stresses. CrMPK3 was cloned in vector pYES2.1. BCY123 yeast cells were transformed either with pYES 2.1:CrMPK3 or vector alone (pYES2.1). The transformed overnight grown yeast cultures were diluted to OD600 = 0.5 in 2% Gal, 50 mM MES pH 5.5 and subjected to different stress treatments. 10 μl of original culture (OD600 = 0.5), along with different serial dilutions (10−1, 10−2, 10−3 and 10−4 cells), were spotted on dropout media lacking Uracil and containing 2% Galactose as inducer. (A) Heat shock was given by incubating yeast cultures in a water bath maintained at a temperature of 45°C for 30 min. (B) UV exposure by exposing the cultures to UV-C light for 45 min; (C) cold shock was given by incubating the cultures at 4°C for 1 h. The experiments were repeated three times with similar results. (D) Activation of MAP kinase by heat treatment in C. roseus leaves. C. roseus plants were subjected to heat stress and leaves were harvested at indicated time intervals post-treatment. Protein extract (20 μg) from these samples were tested for kinase in in-gel kinase assay using Mylein Basic Protein (MBP) as artificial substrate polymerized in SDS 10% (w/v) polyacrylamide gel. Autoradiogram represents in-gel phosphorylation of MBP.
Figure 3. Alignment of amino acid sequence of CrMPK3 (accession No: ABO84839.1) with SLT2 (accession No: CAA41954.1). Amino acids sequences of the conserved domains of MAPKs are marked with lines, roman numbers indicated conserved MAPK domains.
Acknowledgments
We thank Dr. Debasis Chattopadhyay, NIPGR, New Delhi for providing yeast strain BCY123 and pYES2.1 vector. S.K.R. and D.P.W. thank Council of Scientific and Industrial Research and University Grant Commission, India for research fellowships. This work is supported by core grant of National Institute of Plant Genome Research, New Delhi, India from Department of Biotechnology, India.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22716
References
- 1.van Der Heijden R, Jacobs DI, Snoeijer W, Hallard D, Verpoorte R. The Catharanthus alkaloids: pharmacognosy and biotechnology. Curr Med Chem. 2004;11:607–28. doi: 10.2174/0929867043455846. [DOI] [PubMed] [Google Scholar]
- 2.Vázquez-Flota FA, De Luca V. Jasmonate modulates development- and light-regulated alkaloid biosynthesis in Catharanthus roseus. Phytochemistry. 1998;49:395–402. doi: 10.1016/S0031-9422(98)00176-9. [DOI] [PubMed] [Google Scholar]
- 3.Ouwerkerk PBF, Memelink J. Elicitor-responsive promoter regions in the tryptophan decarboxylase gene from Catharanthus roseus. Plant Mol Biol. 1999;39:129–36. doi: 10.1023/A:1006138601744. [DOI] [PubMed] [Google Scholar]
- 4.Kumar S, Dutta A, Sinha AK, Sen J. Cloning, characterization and localization of a novel basic peroxidase gene from Catharanthus roseus. FEBS J. 2007;274:1290–303. doi: 10.1111/j.1742-4658.2007.05677.x. [DOI] [PubMed] [Google Scholar]
- 5.Jaggi M, Kumar S, Sinha AK. Overexpression of an apoplastic peroxidase gene CrPrx in transgenic hairy root lines of Catharanthus roseus. Appl Microbiol Biotechnol. 2011;90:1005–16. doi: 10.1007/s00253-011-3131-8. [DOI] [PubMed] [Google Scholar]
- 6.Memelink J, Verpoorte R, Kijne JW. ORCAnization of jasmonate-responsive gene expression in alkaloid metabolism. Trends Plant Sci. 2001;6:212–9. doi: 10.1016/S1360-1385(01)01924-0. [DOI] [PubMed] [Google Scholar]
- 7.Ramani S, Chelliah J. UV-B-induced signaling events leading to enhanced-production of catharanthine in Catharanthus roseus cell suspension cultures. BMC Plant Biol. 2007;7:61–77. doi: 10.1186/1471-2229-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinha AK, Jaggi M, Raghuram B, Tuteja N. Mitogen-activated protein kinase signaling in plants under abiotic stress. Plant Signal Behav. 2011;6:196–203. doi: 10.4161/psb.6.2.14701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menke FLH, Parchmann S, Mueller MJ, Kijne JW, Memelink J. Involvement of the octadecanoid pathway and protein phosphorylation in fungal elicitor-induced expression of terpenoid indole alkaloid biosynthetic genes in catharanthus roseus. Plant Physiol. 1999;119:1289–96. doi: 10.1104/pp.119.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raina SK, Wankhede DP, Jaggi M, Singh P, Jalmi SK, Raghuram B, et al. CrMPK3, a mitogen activated protein kinase from Catharanthus roseus and its possible role in stress induced biosynthesis of monoterpenoid indole alkaloids. BMC Plant Biol. 2012;12:134. doi: 10.1186/1471-2229-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain D, Roy N, Chattopadhyay D. CaZF, a plant transcription factor functions through and parallel to HOG and calcineurin pathways in Saccharomyces cerevisiae to provide osmotolerance. PLoS ONE. 2009;4:e5154. doi: 10.1371/journal.pone.0005154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Posas F, Chambers JR, Heyman JA, Hoeffler JP, de Nadal E, Ariño J. The transcriptional response of yeast to saline stress. J Biol Chem. 2000;275:17249–55. doi: 10.1074/jbc.M910016199. [DOI] [PubMed] [Google Scholar]
- 13.Pardo JM, Reddy MP, Yang S, Maggio A, Huh GH, Matsumoto T, et al. Stress signaling through Ca2+/calmodulin-dependent protein phosphatase calcineurin mediates salt adaptation in plants. Proc Natl Acad Sci USA. 1998;95:9681–6. doi: 10.1073/pnas.95.16.9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamel LP, Nicole MC, Sritubtim S, Morency MJ, Ellis M, Ehlting J, et al. Ancient signals: comparative genomics of plant MAPK and MAPKK gene families. Trends Plant Sci. 2006;11:192–8. doi: 10.1016/j.tplants.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez MC, Petersen M, Mundy J. Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol. 2010;61:621–49. doi: 10.1146/annurev-arplant-042809-112252. [DOI] [PubMed] [Google Scholar]
- 16.Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2005;69:262–91. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lesage G, Bussey H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2006;70:317–43. doi: 10.1128/MMBR.00038-05. [DOI] [PMC free article] [PubMed] [Google Scholar]



