Abstract
In a plant’s natural environment, the intensity of light can change rapidly due to sunflecks, cloudiness and intermittent shading. Fluctuations between high and low illumination phases expose the photosynthetic machinery to rapidly changing signals that can be overlapping or contradictory, and accordingly plants have developed astute acclimation strategies to maintain optimal photosynthetic performance in these conditions. Continuous exposure to high light induces an array of protective mechanisms at anatomical, chemical and molecular levels, but when high light phases are short, such as under fluctuating light conditions, the protective strategies that afford protection to constant high light are not employed by plants. One mechanism that is engaged under both constant and fluctuating high light is the photosynthetic control of the Cyt b6f complex, which prevents hyper-reduction of the electron transfer chain in order to protect PSI from photodamage. The PGR5 protein was recently shown to play an indispensable role in this protective mechanism. This review revisits the findings of earlier studies into photosynthetic control and places PGR5 within the broader context of photoprotection and light acclimation strategies.
Keywords: electron transfer, light acclimation, photosynthetic control, Photosystem I, PROTON GRADIENT REGULATION5, thylakoid membrane
Text
Experimental research into plant photosynthesis is largely performed on plant material that is grown in strictly controlled environments such as greenhouses and growth chambers. Cultivation of the prevailing plant model Arabidopsis thaliana (hereafter: Arabidopsis) in such artificial environments, which are generally characterized by unnaturally low light (~100–200 µmol photons m−2 s−1) delivered at constant intensity, is incongruent with its natural habitat, where it receives light intensities up to ten times higher, with frequent and unpredictable variations due to cloudiness, shading and sunflecks.1 The highly variable light environment requires an ability to maximize light-harvesting for photochemistry during the low light phases (clouds, shading from other vegetation), but a plant must also capitalise on sudden occurrences of high light, and must minimise photodamage of the photosynthetic machinery under all light intensities. Accordingly, abrupt and drastic light fluctuations necessitate multiple mechanisms facilitating required fine-tuning or major overhaul of photosynthesis, while at the same time buffering against the initiation of long-term light acclimation responses. Indeed, the light conditions occurring in nature challenge the capacity of a plant’s photosynthetic acclimation strategies, which are imperative for its growth and survival in natural conditions.
Our understanding of the structure2-5 and basic functions of many individual components of the photosynthetic machinery has developed to a level where we can now discern the specific molecular mechanisms and regulatory circuits necessary for successful growth of plants in fluctuating light. For a long time, the effects of rapid changes in light intensity were studied by shifting plants between different light qualities known to preferentially excite either photosystem II (PSII) or PSI, and then observing the subsequent impacts on photosynthetic parameters such as chloroplast ultrastructure, fluorescence parameters, expression of photosynthetic genes, protein composition and metabolic state.6-9 The use of these variations in light quality is relevant to some natural conditions, such as the early growth stages of dense plant populations where only low light intensities are available, and undoubtedly this approach has revealed some mechanistic details, for example the so-called “state transition” process. However, abrupt changes in the quantity of light rather than its quality are far more relevant to a plant’s natural environment, and this fact prompted us to examine the regulatory mechanisms that safeguard photosynthetic machinery function under continually changing light intensity. Our approach is to expose the plants to white light in repeating cycles composed of five minutes of low intensity (50 μmol photons m−2 s−1) followed by one minute of high (500 μmol photons m−2 s−1) intensity. Although the cloudiness, shading and sunflecks in nature are not so regular, we are confident that this system does mimic the natural light environments of Arabidopsis and other plants. Indeed, there is a distinct resemblance between the survival rates of Arabidopsis “light regulation mutants” grown in the fluctuating light in our growth chambers and those grown under natural conditions in Northern Sweden (see below).10
Our first experiments with plants grown under fluctuating light intensity11 have already revealed several acclimation mechanisms that have been largely neglected, or at least underestimated, in plants grown under constant light conditions. First, the stn7 mutant, which has no visible phenotype under constant light,11,12 showed a stunted growth phenotype compared with wild-type (WT) plants when grown under fluctuating light. The phenotype of the pgr5 mutant was even more severe, proving to be lethal under fluctuating light.11 Further investigations into the roles of these two regulatory proteins, STN7 and PGR5, under light fluctuations revealed that two distinct mechanisms are required for successful growth in these conditions; one for proper acclimation to the low illumination phases that primarily involves the STN7 kinase13 and another requiring the PGR5 protein as an indispensable regulatory component under the high light illumination phases (see below). The regulatory role of STN7 is beyond the scope of this article (but see7,11,13), but the role of the PGR5 protein in enabling the growth under fluctuating light via the maintenance photosynthetic control is discussed below in more detail.
Plants Possess Multiple Photoprotective Responses to Excess Light that are Ineffective in Protecting against Sudden High Light Peaks
When light intensity exceeds photosynthetic capacity for relatively long time periods, an array of photoprotective mechanisms is activated (reviewed in ref. 14). Photoreceptors, particularly phototropins, initiate chloroplast avoidance movements that relocate chloroplasts to the cell periphery where light absorption is much lower.15 Numerous morphological changes triggered by excess light include reduced leaf size and increases in leaf thickness,16 the number of stomata and the size of the palisade parenchyma cell layer.17 The amounts of cuticle waxes, trichomes and photoprotective pigments (antocyanins) in the epidermis are also increased, reducing the level of absorbed light by up to 40%. Additionally, thylakoid membranes in the chloroplasts of plants living under excess light are less appressed than those in the chloroplasts of shade plants.18
Several responses to high light also occur at the molecular level, such as the dissipation of excess light as heat via non-photochemical quenching (NPQ)14 to protect PSII and to dampen the increased rate of electron flow, and the acceleration of PSII complex turnover19 to maintain PSII functionality despite increased damage to the D1 protein.20,21 The activities of many auxiliary proteins are involved under high light in ensuring the multistep process of PSII turnover,22 including the SNT8 kinase that carries out phosphorylation of PSII core proteins.23 Nonetheless, high levels of light intensity still increase the rate of electron transfer that leads to the generation of reactive oxygen species (ROS) such as hydrogen peroxide, superoxide and singlet oxygen in the photosynthetic machinery24-26 that can cause oxidative damage to chloroplast proteins and lipids. To avoid ROS-mediated damage, plant cells employ a multi-layered network of enzymatic and non-enzymatic antioxidants to maintain ROS at basal levels,27 although the importance of antioxidants in counter-balancing ROS levels are perhaps more important as major hubs of redox signaling in plants cells.27 In particular, superoxide and hydrogen peroxide are important oxidative signals that regulate expression of photosynthetic genes.28,29 Some well-characterized examples ROS regulation include downregulated expression of the light-harvesting proteins30 and upregulation of ASCORBATE PEROXIDASE2 expression31,32 under high light. In addition to ROS, the redox states of the plastoquinone pool33 and stromal redox-active compounds34 have been reported regulate the expression of nuclear genes involved in photosynthesis. Phosphorylation of the major light-harvesting antenna (LHCII) proteins performed by SNT7 is also involved in translating changes in the redox states of the plastoquinone pool and the stroma into signals that regulate gene expression. These mechanisms for changing the expression of photosynthetic genes under high light aim to remodel the photosynthetic machinery to be more suited to the physiological demands of excess light energy. Indeed, in comparison to their low light-grown counterparts, plants grown in high light have lower levels of LHC proteins, whereas the amounts of Rubisco, Cytochrome (Cyt) b6f and PSII are increased.35,36
It is important to recognize that the above-mentioned photoprotective mechanisms, with the exception of NPQ, take a relatively long time to be activated and are therefore largely redundant if the duration of the high light peak is short, such as one minute in our fluctuating light set-up. We have found that the growth phenotype of wild type (WT) Arabidopsis under fluctuating light is almost identical to the phenotype of plants grown in moderate light, aside from slightly lower biomass in the former. Moreover, the visible characteristics typical to Arabidopsis grown under high light are not seen.13 Clearly the protection against the short phases of high light does not involve epidermal modifications such as increased waxes, pigments and trichomes. The applied high light peak is also too short to downregulate LHCII phosphorylation, as we have found that the level of LHCII phosphorylation is almost constant despite of the repeating high and low light phases.13 Under fluctuating light, WT plants have an increase in relative PSI content,10 but this is most likely an acclimation response to the low light that predominates in the fluctuating light system rather than to the fluctuations themselves, as this phenomenon is observed in plants grown under constant low light.37 It is clear, therefore, that photoprotection under sudden, short periods of high illumination intensity requires a specific mechanism different from that used to cope with constant high light, and this is plainly evident in the pgr5 mutant.10
Photosynthetic Control of Linear Electron Flow Provides Protection under Short-Term High Light Peaks
“Photosynthetic control” is, by definition, the regulation of the rate of photosynthetic electron transport, which is adjusted according to both the prevailing need for ATP and the capacity for ATP production (for review see ref. 38). Broadly speaking, photosynthetic control can be extended to include the control of nuclear gene expression in response to environmental cues, but according to the established concept it refers to the regulation of (linear) electron flow through acidification of the thylakoid lumen and the subsequent development of a transmembrane pH gradient. NPQ is a well-known example of photosynthetic control, as it decreases the rate of linear electron transfer by engaging the PsbS protein and the xanthophyll cycle, both of which are activated by lumen acidification, for the quenching of extra light energy as heat (qE). Another example of photosynthetic control has received far less attention, even though it was discovered as early as 1969 by Bernd Rumberg and Ulrich Siggel when they demonstrated that the mechanism that regulates light-induced lumen acidification and the decrease in linear electron transfer takes place between the two photosystems.39 In that pioneering study, as well as in a later report from Tikhonov et al. (1981), the reduction kinetics of P700 were used to monitor transmembrane ΔpH. Later on, however, the phenomenon of photosynthetic control was more specifically assigned to the Cyt b6f complex when Nishio and Whitmarsh (1993) showed that Cyt f reduction kinetics were also linked to lumenal pH. Despite these and many other studies, not to mention the fundamental importance of photosynthetic control for plant physiology, this concept has been consistently underrated, perhaps because the exact molecular mechanism that regulates electron flow via the Cyt b6f complex have not been well understood. In addition to Cyt b6f, the activity and mobility of plastocyanin are also suggested to be involved in regulating electron flow,40,41 therefore the reference to “photosynthetic control via the Cyt b6f complex” in this work encompasses the role of plastocyanin.
Strict control of linear electron flow in the thylakoid membrane is critical, above all, to protect PSI. Historically, PSI has been considered the more stable of the two photosystems because PSII is well known to be easily photodamaged, even under moderate light intensities.21 However, increasing evidence shows that PSI is in fact highly vulnerable and needs to be efficiently protected from a build-up of electrons in the ETC. The iron-sulfur clusters on the stromal side of the PSI complex are particularly susceptible to oxidative damage from an over-reduced electron transfer chain (ETC).42,43 Furthermore, unlike PSII that is maintained by a rapid and efficient repair cycle, photodamaged PSI is repaired extremely slowly, if indeed at all,42,44,45 such that a damaged PSI complex is practically lost, and synthesis of a replacement is a very slow process.46 Protecting PSI against over-reduction and photodamage is therefore a high priority of photosynthetic control. Johnson and Joliot (2011) recently showed that disruption of the transmembrane ΔpH in WT leaves by treatment with nigericin, followed by illumination of treated leaves under high light for 20 min, caused inactivation of around 70% of the PSI centers, while only 30% of the PSII complexes were damaged. This result shows that disruption of this mechanism poses a particular threat to PSI, and reiterates the importance of the pH gradient for photosynthetic control to protect PSI.
It has been suggested that cyclic electron transfer (CET) around PSI, which has a role in balancing the ATP/NADPH ratio, can operate as an electron safety valve to protect PSI from an over-reduced plastoquinone pool.47,48 This is, however, difficult to reconcile because CET would direct electrons back into an already overloaded ETC. The water-water cycle, in which stromal antioxidant enzymes scavenge ROS into water,25 has also been proposed to protect PSI from photodamage. While this should not be overlooked, we consider the main mechanism for PSI protection to be photosynthetic control of electron flow via the Cyt b6f complex. This control mechanism performed by Cyt b6f is evident in the redox state of PSI under high light illumination (see e.g., ref. 49), which is oxidised and not reduced, as one would expect if electrons flowed freely from to PSI from PSII. PSI is also oxidised during high light phases of a fluctuating light regime, as demonstrated by our measurements of the PSI redox state during repeating cycles of high light (one minute of 500 μmol photons m−2 s−1) and low light (5 min of 50 μmol photons m−2 s-1), i.e., under the conditions used in our fluctuating light chambers.10 The flow of electrons from PSII is therefore blocked by Cyt b6f before reaching PSI.
Our characterisations of the pgr5 mutant under fluctuating light have recently added another piece to puzzle. We found that the absence of PGR5 resulted in a loss of photosynthetic control, as shown from the highly reduced state of PSI that remained unchanged regardless of fluctuations in light intensity.10 This situation inhibited PSI function, and eventually decimated the entire PSI core. However, when the ETC was artificially blocked with DCMU, the high light-induced damage to PSI in the pgr5 mutant was largely avoided.10 These results provide evidence that the primary role of PGR5 lies in ensuring photosynthetic control by the means of regulating linear electron transfer.
The Photosynthetic Control and Beyond
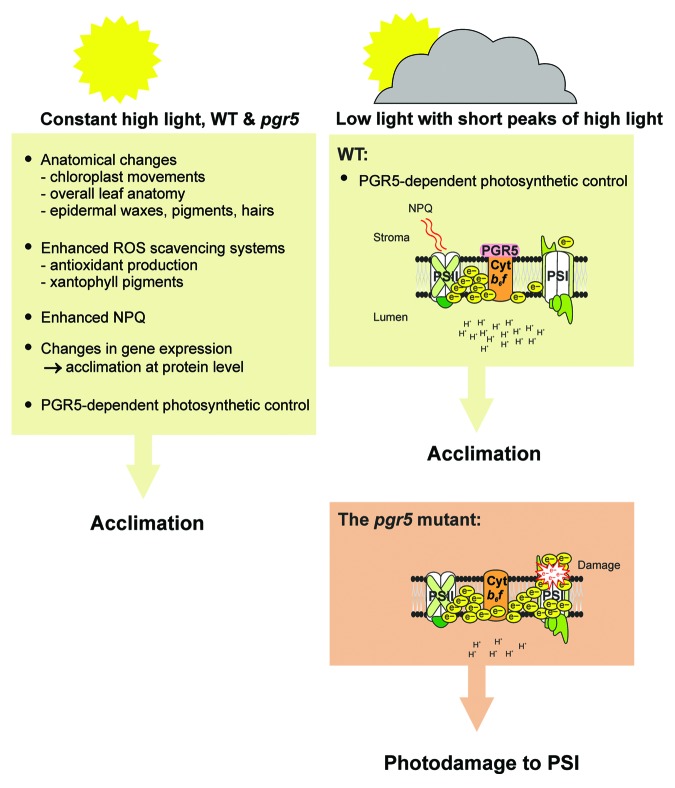
As discussed above, constant high light conditions induce a number of different photoprotective mechanisms at anatomical, chemical and molecular levels. By engaging these acclimation strategies, even the pgr5 mutant lacking photosynthetic control is fully capable of accumulating both photosystems and growing quite normally.10 In light of this, the fluctuating light regimes applied in our experiments have been designed to expose plants to high light phases for only short periods and low light for the remaining time, so that plants do not engage acclimation responses to high light. This system has enabled us to show that photosynthetic control by the Cyt b6f complex is driven by PGR5, and that this mechanism effectively protects PSI complexes from photodamage and subsequent degradation in the absence of other acclimation mechanisms (Fig. 1).
Figure 1. Mechanisms ensuring successful acclimation to either constant high light intensity or fluctuating light regime including short high light phases. Under constant high light the plant relies on a variety of protective mechanisms at the anatomical, chemical and molecular level. In contrast, these protective mechanisms are largely missing during short high light phases, and under these conditions PGR5-mediated photosynthetic control protects PSI from photodamage induced by a hyper-reduced electron transfer chain.
The primary and fundamental role of the PGR5-dependent photosynthetic control in relation to the other light acclimation mechanisms is unquestionable. We have tested the growth of a number of thylakoid acclimation mutants under our fluctuating light setup, and so far only the pgr5 mutant has exhibited a lethal phenotype under these conditions11 (and unpublished results from the Aro laboratory). The pgr5 mutant is also devoid of NPQ,10,50 which itself dependent on the development of transmembrane ΔpH. It is likely that the lack of NPQ partially reinforces the photodamage of PSI, as all the light energy entering the photosynthetic apparatus is induced to electron flow through the ETC. Nonetheless, the npq4 mutant does not show any distinct phenotype under fluctuating light,11 nor are its fluorescence parameters markedly disturbed.13 This indicates that loss of NPQ alone is not specifically destructive for photosynthetic electron transfer reactions under our fluctuating light setup. In an extension of the experiments with pgr5 under controlled light fluctuations, these mutants had even greater mortality rates under field conditions.10 This is significant, since previously only the stn7 and npq4 mutants have been shown to be defective (both showed lower fitness) under field conditions,1,51 however neither stn7 nor npq4 showed any visible phenotypical difference from WT plants under field conditions. This provides further support for the fact that NPQ and LHCII protein phosphorylation hold secondary importance for acclimation to fluctuating light compared with role of PGR5-dependent photosynthetic control.
Comparing the breadth of literature and research focusing on NPQ, CET and state transitions with that available on the photosynthetic control truly exposes the neglect of this fundamental regulatory mechanism. Photosynthetic control by the Cyt b6f complex prevents the over-reduction of the ETC, and thus protects PSI from photodamage, and this is imperative under natural conditions where light intensity increases abruptly, briefly and often. Although the indispensable role of the PGR5 protein in the maintenance of photosynthetic control has now been elucidated,10,52 further questions still remain to be answered: (1) What is the exact molecular mechanism of the PGR5 protein in regulating the photosynthetic control? For instance, is it integrated with the Q-cycle of the Cyt b6f complex? With which protein(s) of the Cyt b6f complex does PGR5 actually interact? (2) What contribution does ATP synthase make to photosynthetic control through its role in proton translocation? Interestingly, tobacco ATP synthase mutants recently showed enhanced capacity for photosynthetic control.53 (3) What is the significance of PSII photodamage in the scheme of photosynthetic control? PSII photoinhibition has been proposed as a mechanism to adjust the PSII activity level in order to meet the capacity of the ETC.40,54 This suggests that PSII photoinhibition and the PSII repair cycle are part of a deliberate regulation mechanism controlling the flux of electrons in ETC, rather than simply being a response to unwanted yet inevitable light-induced photodamage of PSII. To address these unknown factors, the wider scheme of the dynamics and regulation of the photosynthetic electron transfer chain must be reinvestigated in a modern context that takes into account all aspects of photosynthetic control as well as the lateral heterogeneity of the thylakoid membrane protein complexes.54-57 Moreover, the effects of environmental fluctuations on the distribution and interaction of the thylakoid protein complexes, and the subsequent impact on the functional dynamics of the electron transfer chain, remains to be elucidated.
Acknowledgments
Our research has been funded by the Academy of Finland [project numbers 138703 (M.S.), 260094 (M.T.), CoE projects 118637 (E.M.A.), 218157 (S.K.) and 130595 (S.K.)], as well as the European Union FP7 Marie Curie Initial Training Network (Project 215174) and the Finnish Doctoral Program in Plant Biology.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22741
References
- 1.Külheim C, Agren J, Jansson S. Rapid regulation of light harvesting and plant fitness in the field. Science. 2002;297:91–3. doi: 10.1126/science.1072359. [DOI] [PubMed] [Google Scholar]
- 2.Standfuss J, Terwisscha van Scheltinga AC, Lamborghini M, Kühlbrandt W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 A resolution. EMBO J. 2005;24:919–28. doi: 10.1038/sj.emboj.7600585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S. Architecture of the photosynthetic oxygen-evolving center. Science. 2004;303:1831–8. doi: 10.1126/science.1093087. [DOI] [PubMed] [Google Scholar]
- 4.Loll B, Kern J, Saenger W, Zouni A, Biesiadka J. Towards complete cofactor arrangement in the 3.0 A resolution structure of photosystem II. Nature. 2005;438:1040–4. doi: 10.1038/nature04224. [DOI] [PubMed] [Google Scholar]
- 5.Dekker JP, Boekema EJ. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim Biophys Acta. 2005;1706:12–39. doi: 10.1016/j.bbabio.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Wagner R, Dietzel L, Bräutigam K, Fischer W, Pfannschmidt T. The long-term response to fluctuating light quality is an important and distinct light acclimation mechanism that supports survival of Arabidopsis thaliana under low light conditions. Planta. 2008;228:573–87. doi: 10.1007/s00425-008-0760-y. [DOI] [PubMed] [Google Scholar]
- 7.Pesaresi P, Hertle A, Pribil M, Kleine T, Wagner R, Strissel H, et al. Arabidopsis STN7 kinase provides a link between short- and long-term photosynthetic acclimation. Plant Cell. 2009;21:2402–23. doi: 10.1105/tpc.108.064964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bräutigam K, Dietzel L, Kleine T, Ströher E, Wormuth D, Dietz KJ, et al. Dynamic plastid redox signals integrate gene expression and metabolism to induce distinct metabolic states in photosynthetic acclimation in Arabidopsis. Plant Cell. 2009;21:2715–32. doi: 10.1105/tpc.108.062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietzel L, Bräutigam K, Steiner S, Schüffler K, Lepetit B, Grimm B, et al. Photosystem II supercomplex remodeling serves as an entry mechanism for state transitions in Arabidopsis. Plant Cell. 2011;23:2964–77. doi: 10.1105/tpc.111.087049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suorsa M, Järvi S, Grieco M, Nurmi M, Pietrzykowska M, Rantala M, et al. PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell. 2012;24:2934–48. doi: 10.1105/tpc.112.097162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tikkanen M, Grieco M, Kangasjärvi S, Aro EM. Thylakoid protein phosphorylation in higher plant chloroplasts optimizes electron transfer under fluctuating light. Plant Physiol. 2010;152:723–35. doi: 10.1104/pp.109.150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellafiore S, Barneche F, Peltier G, Rochaix JD. State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature. 2005;433:892–5. doi: 10.1038/nature03286. [DOI] [PubMed] [Google Scholar]
- 13.Grieco M, Tikkanen M, Paakkarinen V, Kangasjärvi S, Aro EM. Steady-state phosphorylation of light-harvesting complex II proteins preserves Photosystem I under fluctuating white light. Plant Physiol. 2012 doi: 10.1104/pp.112.206466. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Wakao S, Fischer BB, Niyogi KK. Sensing and responding to excess light. Annu Rev Plant Biol. 2009;60:239–60. doi: 10.1146/annurev.arplant.58.032806.103844. [DOI] [PubMed] [Google Scholar]
- 15.Park YI, Chow WS, Anderson JM. Chloroplast movement in the shade plant tradescantia albiflora helps protect photosystem II against light stress. Plant Physiol. 1996;111:867–75. doi: 10.1104/pp.111.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra Y, Jänkänpää HJ, Kiss AZ, Funk C, Schröder WP, Jansson S. Arabidopsis plants grown in the field and climate chambers significantly differ in leaf morphology and photosystem components. BMC Plant Biol. 2012;12:6. doi: 10.1186/1471-2229-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim GT, Yano S, Kozuka T, Tsukaya H. Photomorphogenesis of leaves: shade-avoidance and differentiation of sun and shade leaves. Photochem Photobiol Sci. 2005;4:770–4. doi: 10.1039/b418440h. [DOI] [PubMed] [Google Scholar]
- 18.Boardman N. Comparative photosynthesis of sun and shade plants. Annu Rev Plant Physiol Plant Mol Biol. 1977;28:355–77. [Google Scholar]
- 19.Aro EM, Suorsa M, Rokka A, Allahverdiyeva Y, Paakkarinen V, Saleem A, et al. Dynamics of photosystem II: a proteomic approach to thylakoid protein complexes. J Exp Bot. 2005;56:347–56. doi: 10.1093/jxb/eri041. [DOI] [PubMed] [Google Scholar]
- 20.Aro EM, Virgin I, Andersson B. Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–34. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- 21.Tyystjärvi E, Aro EM. The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc Natl Acad Sci U S A. 1996;93:2213–8. doi: 10.1073/pnas.93.5.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulo P, Sirpiö S, Suorsa M, Aro EM. Auxiliary proteins involved in the assembly and sustenance of photosystem II. Photosynth Res. 2008;98:489–501. doi: 10.1007/s11120-008-9320-3. [DOI] [PubMed] [Google Scholar]
- 23.Rintamäki E, Salonen M, Suoranta UM, Carlberg I, Andersson B, Aro EM. Phosphorylation of light-harvesting complex II and photosystem II core proteins shows different irradiance-dependent regulation in vivo. Application of phosphothreonine antibodies to analysis of thylakoid phosphoproteins. J Biol Chem. 1997;272:30476–82. doi: 10.1074/jbc.272.48.30476. [DOI] [PubMed] [Google Scholar]
- 24.Hideg E, Kálai T, Hideg K, Vass I. Photoinhibition of photosynthesis in vivo results in singlet oxygen production detection via nitroxide-induced fluorescence quenching in broad bean leaves. Biochemistry. 1998;37:11405–11. doi: 10.1021/bi972890+. [DOI] [PubMed] [Google Scholar]
- 25.Asada K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–39. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- 26.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–8. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Foyer CH, Noctor G. Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal. 2009;11:861–905. doi: 10.1089/ars.2008.2177. [DOI] [PubMed] [Google Scholar]
- 28.Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, et al. Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol. 2006;141:436–45. doi: 10.1104/pp.106.078717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pogson BJ, Woo NS, Förster B, Small ID. Plastid signalling to the nucleus and beyond. Trends Plant Sci. 2008;13:602–9. doi: 10.1016/j.tplants.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Pursiheimo S, Mulo P, Rintamäki E, Aro EM. Coregulation of light-harvesting complex II phosphorylation and lhcb mRNA accumulation in winter rye. Plant J. 2001;26:317–27. doi: 10.1046/j.1365-313X.2001.01033.x. [DOI] [PubMed] [Google Scholar]
- 31.Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux PM. Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell. 1997;9:627–40. doi: 10.1105/tpc.9.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fryer MJ, Ball L, Oxborough K, Karpinski S, Mullineaux PM, Baker NR. Control of Ascorbate Peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J. 2003;33:691–705. doi: 10.1046/j.1365-313X.2003.01656.x. [DOI] [PubMed] [Google Scholar]
- 33.Allen J, Pfannschmidt T, Nilsson A. Photosynthetic control of chloroplast gene expression. Nature. 1999;397:625–8. doi: 10.1038/17624. [DOI] [Google Scholar]
- 34.Piippo M, Allahverdiyeva Y, Paakkarinen V, Suoranta UM, Battchikova N, Aro EM. Chloroplast-mediated regulation of nuclear genes in Arabidopsis thaliana in the absence of light stress. Physiol Genomics. 2006;25:142–52. doi: 10.1152/physiolgenomics.00256.2005. [DOI] [PubMed] [Google Scholar]
- 35.Bailey S, Walters RG, Jansson S, Horton P. Acclimation of Arabidopsis thaliana to the light environment: the existence of separate low light and high light responses. Planta. 2001;213:794–801. doi: 10.1007/s004250100556. [DOI] [PubMed] [Google Scholar]
- 36.Chow W, Anderson J. Photosynthetic responses of pisum-sativum to an increase in irradiance during growth. 2. thylakoid membrane-components. Aust J Plant Physiol. 1987;14:9–19. doi: 10.1071/PP9870009. [DOI] [Google Scholar]
- 37.Bailey S, Walters RG, Jansson S, Horton P. Acclimation of Arabidopsis thaliana to the light environment: the existence of separate low light and high light responses. Planta. 2001;213:794–801. doi: 10.1007/s004250100556. [DOI] [PubMed] [Google Scholar]
- 38.Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J. Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot. 2012;63:1637–61. doi: 10.1093/jxb/ers013. [DOI] [PubMed] [Google Scholar]
- 39.Rumberg B, Siggel U. pH changes in the inner phase of the thylakoids during photosynthesis. Naturwissenschaften. 1969;56:130–2. doi: 10.1007/BF00601025. [DOI] [PubMed] [Google Scholar]
- 40.Schöttler MA, Kirchhoff H, Weis E. The role of plastocyanin in the adjustment of the photosynthetic electron transport to the carbon metabolism in tobacco. Plant Physiol. 2004;136:4265–74. doi: 10.1104/pp.104.052324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirchhoff H, Schöttler MA, Maurer J, Weis E. Plastocyanin redox kinetics in spinach chloroplasts: evidence for disequilibrium in the high potential chain. Biochim Biophys Acta. 2004;1659:63–72. doi: 10.1016/j.bbabio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Sonoike K. Photoinhibition of photosystem I. Physiol Plant. 2011;142:56–64. doi: 10.1111/j.1399-3054.2010.01437.x. [DOI] [PubMed] [Google Scholar]
- 43.Sonoike K, Terashima I, Iwaki M, Itoh S. Destruction of photosystem I iron-sulfur centers in leaves of Cucumis sativus L. by weak illumination at chilling temperatures. FEBS Lett. 1995;362:235–8. doi: 10.1016/0014-5793(95)00254-7. [DOI] [PubMed] [Google Scholar]
- 44.Kudoh H, Sonoike K. Irreversible damage to photosystem I by chilling in the light: cause of the degradation of chlorophyll after returning to normal growth temperature. Planta. 2002;215:541–8. doi: 10.1007/s00425-002-0790-9. [DOI] [PubMed] [Google Scholar]
- 45.Scheller HV, Haldrup A. Photoinhibition of photosystem I. Planta. 2005;221:5–8. doi: 10.1007/s00425-005-1507-7. [DOI] [PubMed] [Google Scholar]
- 46.Schöttler MA, Albus CA, Bock R. Photosystem I: its biogenesis and function in higher plants. J Plant Physiol. 2011;168:1452–61. doi: 10.1016/j.jplph.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Rumeau D, Peltier G, Cournac L. Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant Cell Environ. 2007;30:1041–51. doi: 10.1111/j.1365-3040.2007.01675.x. [DOI] [PubMed] [Google Scholar]
- 48.Shikanai T. Cyclic electron transport around photosystem I: genetic approaches. Annu Rev Plant Biol. 2007;58:199–217. doi: 10.1146/annurev.arplant.58.091406.110525. [DOI] [PubMed] [Google Scholar]
- 49.Harbinson J, Woodward F. The use of light-induced absorbency changes at 820 nm to monitor the oxidation-state of P-700 in leaves. Plant Cell Environ. 1987;10:131–40. [Google Scholar]
- 50.Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell. 2002;110:361–71. doi: 10.1016/S0092-8674(02)00867-X. [DOI] [PubMed] [Google Scholar]
- 51.Frenkel M, Bellafiore S, Rochaix J, Jansson S. Hierarchy amongst photosynthetic acclimation responses for plant fitness. Physiol Plant. 2007;129:455–9. doi: 10.1111/j.1399-3054.2006.00831.x. [DOI] [Google Scholar]
- 52.Joliot P, Johnson GN. Regulation of cyclic and linear electron flow in higher plants. Proc Natl Acad Sci U S A. 2011;108:13317–22. doi: 10.1073/pnas.1110189108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rott M, Martins NF, Thiele W, Lein W, Bock R, Kramer DM, et al. ATP synthase repression in tobacco restricts photosynthetic electron transport, CO2 assimilation, and plant growth by overacidification of the thylakoid lumen. Plant Cell. 2011;23:304–21. doi: 10.1105/tpc.110.079111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tikkanen M, Grieco M, Nurmi M, Rantala M, Suorsa M, Aro EM. Regulation of the photosynthetic apparatus under fluctuating growth light. Philos Trans R Soc Lond B Biol Sci. 2012;367:3486–3493. doi: 10.1098/rstb.2012.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andersson B, Anderson JM. Lateral heterogeneity in the distribution of chlorophyll-protein complexes of the thylakoid membranes of spinach chloroplasts. Biochim Biophys Acta. 1980;593:427–40. doi: 10.1016/0005-2728(80)90078-X. [DOI] [PubMed] [Google Scholar]
- 56.Anderson JM, Aro EM. Grana stacking and protection of photosystem-ii in thylakoid membranes of higher-plant leaves under sustained high irradiance - an hypothesis. Photosynth Res. 1994;41:315–26. doi: 10.1007/BF00019409. [DOI] [PubMed] [Google Scholar]
- 57.Trissl HW, Wilhelm C. Why do thylakoid membranes from higher plants form grana stacks? Trends Biochem Sci. 1993;18:415–9. doi: 10.1016/0968-0004(93)90136-B. [DOI] [PubMed] [Google Scholar]