Abstract
Calcium-dependent protein kinases (CDPKs) modulate plant development and growth and are important regulators of biotic and abiotic stress responses. Recently it was found that simultaneously silencing Nicotiana attenuata NaCDPK4 and NaCDPK5 (IRcdpk4/5 plants) results in accumulation of exceptionally high JA levels after wounding or simulated herbivory treatments, which in turn induced high levels of defense metabolites that slowed the growth of Manduca sexta, a specialist insect herbivore. To investigate the mechanism by which NaCDPK4 and NaCDPK5 regulate JA accumulation, we analyzed the transcript levels of all important enzymes involved in JA biosynthesis, but these genes showed no differences between wild-type and IRcdpk4/5 plants. Moreover, the dynamics of JA were similar between these plants, excluding the possibility of decreased degradation rates in IRcdpk4/5 plants. To gain insight into the mechanism by which NaCDPK4 and NaCDPK5 regulate JA biosynthesis, free fatty acids, including C18:3, and (9S,13S)-12-oxo-phytodienoic acid (OPDA), two important precursors of JA were quantified at different times before and after wounding and simulated herbivore feeding treatments. We show that after these treatments, IRcdpk4/5 plants have decreased levels of C18:3, but have enhanced OPDA and JA levels, suggesting that NaCDPK4 and NaCDPK5 have a role in the early steps of JA biosynthesis. The possible role of NaCDPK4 and NaCDPK5 regulating AOS and AOC enzymatic activity is discussed.
Keywords: CDPK, jasmonic acid, defense, herbivore, insect, wounding
Jasmonic acid (JA) has been intensively studied for its important role in regulating plant responses to wounding and herbivore feeding.1,2 In unelicited mature leaves, JA is maintained at very low levels, however, upon wounding or herbivore feeding its biosynthesis is induced within a few minutes.2-4 The first step of JA synthesis is the cleavage of α-linolenic acid (18:3Δ9,12,15, 18:3) from chloroplast membranes, and this reaction is catalyzed by a glycerolipase class A enzyme (GLA1).5-9 A 13-lipoxygenase (13-LOX) functions in the next step of JA synthesis forming 13(S)-hydroperoxy-octadecatrienoic acid [13S-(OOH)-18:3], and thereafter it is further converted to (9S,13S)-12-oxo-phytodienoic acid (OPDA) by allene oxide synthase (AOS) and allene oxide cyclase (AOC). OPDA is transported from the plastid into the peroxisome where after three cycles of β-oxidation JA is formed.10
Although the JA biosynthesis pathway has been intensively studied, how the activity of JA biosynthetic enzymes is regulated remains largely unknown. After wounding or herbivore feeding, elevation of JA is usually seen within a few minutes.11 The rapid increase of JA levels is most likely resulted from activation of constitutively expressed enzymes by certain posttranslational modifications. Two MAPK, salicylic acid-induced protein kinase (SIPK) and wound-induced protein kinase (WIPK) are rapidly activated by wounding or Manduca sexta feeding and these MAPKs are necessary for the induction of JA in response to herbivory.12,13 A recent study on wild tobacco, Nicotiana attenuata, suggested that substrate supply for JA-production is controlled by SIPK, most likely by changes in NaGLA1 activity.8 In contrast to SIPK, the same study revealed that WIPK is critically important for the conversion of 13S-(OOH)-18:3 into OPDA. In addition to MAPKs, CDPKs have been also implicated in JA regulation. CDPKs were found to have elevated transcript levels after wounding in maize (Zea mays) and tobacco, and after herbivore attack in wild tobacco N. attenuata.13-15 Simultaneously silencing NaCDPK4 and NaCDPK5 in N. attenuata resulted in high JA levels in stems of unelicited plants (Heinrich, Hettenhausen, Lange, Wünsche, Fang, Baldwin, and Wu, in press), and leads to over-accumulation of JA after wounding (W+W) or simulated herbivory (W+OS) treatment.16 The high accumulations of JA not only confer increased resistance to attack from M. sexta larvae but also induce enhanced SIPK and WIPK activity. However, how NaCDPK4 and NaCDPK5 function as strong suppressors of stress-induced JA accumulation remains unclear. The transcript levels of JA biosynthesis enzymes were not influenced by silencing NaCDPK4 and NaCDPK5 and also the dynamics of synthesis and degradation of JA appeared similar among these plants: both wild-type (WT) and IRcdpk4/5 plants reached their highest levels of JA 0.5 h after the treatment and declined to almost basal levels after 3 h.16 This implies that over-accumulation of JA in IRcdpk4/5 did not result from impaired JA metabolism but from the enhanced JA biosynthesis activity.
The release of trienoic fatty acids from cell membranes is considered one of the earliest steps in JA biosynthesis. To further understand how NaCDPK4 and NaCDPK5 control JA biosynthesis, we measured levels of free fatty acids in unelicited leaves and following W+OS treatment in WT and IRcdpk4/5 leaves. A high percentage of the detected saturated fatty acids, which were likely extracted from the cuticular waxes8 and do not contribute to JA production, were unchanged after silencing NaCDPK4 and NaCDPK5. Importantly, the unsaturated fatty acids, 16:3 and 18:3 had significantly lower levels in non-elicited IRcdpk4/5 compared with those in WT plants and remained unchanged even after the treatment (Fig. 1). Plants silenced in the expression of SIPK had reduced GLA1 activity and consequently lower levels of free 18:3 and JA. In contrast, IRcdpk4/5 was shown to have higher SIPK activity and increased JA levels after W + W and W + OS treatment.16 The lack of increase in free fatty acids following elicitation suggests that, after they are released from the chloroplast membrane, free 18:3 is very rapidly converted to 13S-(OOH)-18:3 by13-LOX. The reduced levels of free 16:3 and 18:3 that were detected in IRcdpk4/5 plants most likely resulted from increased speed of turnover of the released free fatty acids into the JA pathway in IRcdpk4/5 plants.
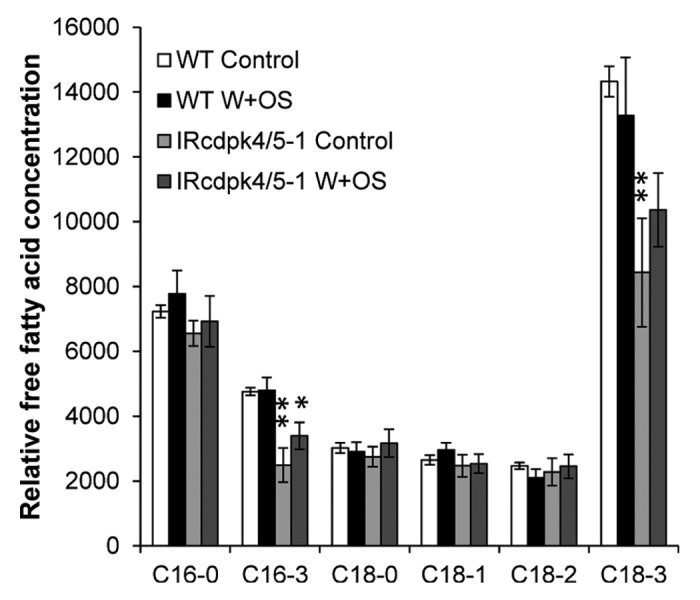
Figure 1. Free fatty acid levels in wild-type (WT) and IRcdpk4/5 plants. Wild-type (WT) and IRcdpk4/5 (line IRcdpk4/5–1) plants were wounded with a pattern wheel, and 20 µL of M. sexta OS (W+OS) were immediately applied to wounds. Thirty minutes after the treatment leaves were harvested and free fatty acids were isolated and quantified (mean ± SE); non-treated plants served as controls. Asterisks indicate significant differences between WT and IRcdpk4/5 plants (n = 5; Student’s t-test; **, p < 0.01.
Next, we monitored changes of JA and its precursors 13S-(OOH)-18:3 and OPDA within a short time interval after W+OS elicitation (0–30 min; Figure 2). As early as 5 min after W+OS treatment, we detected higher JA levels in IRcdpk4/5 compared with those in WT plants (Fig. 2A). We did not observe large differences in the amount of 13S-(OOH)-18:3, and 13S-(OOH)-18:3 contents tended to be even slightly lower in IRcdpk4/5 than in WT plants (Fig. 2B); in contrast, the levels of OPDA were significantly greater (about 1 fold) in IRcdpk4/5 (Fig. 2C). Therefore, it is probable that the enzymes converting 13S-(OOH)-18:3 to OPDA, namely, AOS (allene oxide synthase) and AOC (allene oxide cyclase), have elevated activity in IRcdpk4/5, although they do not show different transcript levels between WT and IRcdpk4/5 plants.16
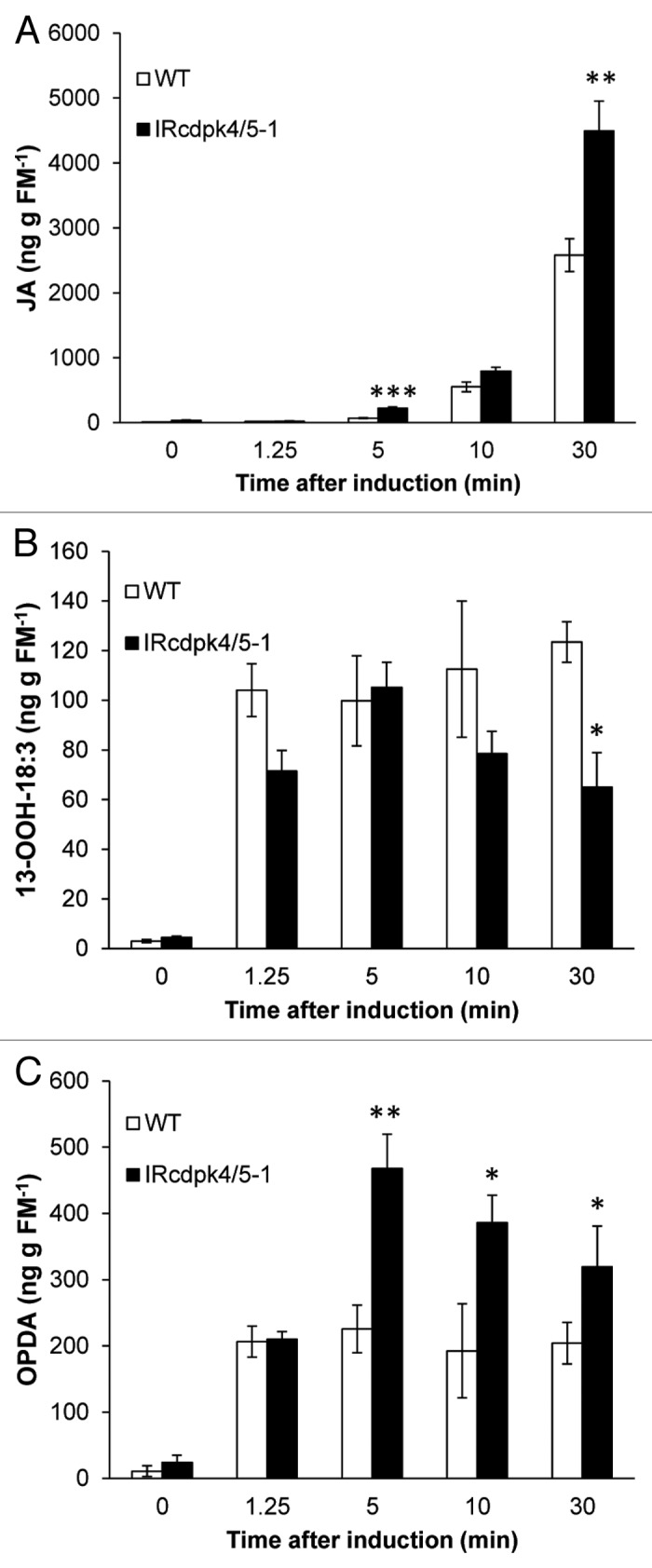
Figure 2. IRcdpk4/5 plants have elevated levels of OPDA after W+OS elicitation. Wild-type (WT) and IRcdpk4/5 (line IRcdpk4/5–1) plants were wounded with a pattern wheel, and 20 µL of M. sexta OS (W+OS) were immediately applied to wounds. Contents (mean ± SE) of (A) JA, (B) 13-(OOH)-18:3, and (C) OPDA were determined in leaves sampled after indicated times. Asterisks indicate significant differences between WT and IRcdpk4/5 plants (n = 5; Student’s t-test; *, p < 0.05; **, p < 0.01).
In summary, the exact mechanism by which NaCDPK4 and NaCDPK5 affect JA biosynthesis remains unclear. Despite having higher SIPK activity which was previously shown to control GLA1 activity, IRcdpk4/5 plants accumulated less free 18:3 fatty acid. Moreover, these plants had higher levels of the JA intermediate OPDA, but accumulated about 75% more JA 30 min after W+OS treatment. These data suggest that NaCDPK4 and NaCDPK5 probably control at least the activity of AOS and AOC. Further studies are required to determine how NaCDPK4 and NaCDPK5 influence the activity of JA biosynthetic enzymes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the Max Planck Society for funding and Dr. Mario Kallenbach for technical assistance.
Glossary
Abbreviations:
- CDPK
calcium-dependent protein kinase
- MAPK
mitogen-activated protein kinase
- SIPK
salicylic acid-induced protein kinase
- WIPK
wound-induced protein kinase
- OS
oral secretions
- JA
jasmonic acid
- OPDA
(9S,13S)-12-oxo-phytodienoic acid
- GLA1
glycerolipase A1
- LOX
lipoxygenase
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22784
References
- 1.Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot (Lond) 2007;100:681–97. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu JQ, Baldwin IT. New insights into plant responses to the attack from insect herbivores. Annu Rev Genet. 2010;44:1–24. doi: 10.1146/annurev-genet-102209-163500. [DOI] [PubMed] [Google Scholar]
- 3.Howe GA, Jander G. Plant immunity to insect herbivores. Annu Rev Plant Biol. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- 4.Wu JQ, Baldwin IT. Herbivory-induced signalling in plants: perception and action. Plant Cell Environ. 2009;32:1161–74. doi: 10.1111/j.1365-3040.2009.01943.x. [DOI] [PubMed] [Google Scholar]
- 5.Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–81. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- 6.Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K. The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell. 2001;13:2191–209. doi: 10.1105/tpc.010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellinger D, Stingl N, Kubigsteltig II, Bals T, Juenger M, Pollmann S, et al. DONGLE and DEFECTIVE IN ANTHER DEHISCENCE1 lipases are not essential for wound- and pathogen-induced jasmonate biosynthesis: redundant lipases contribute to jasmonate formation. Plant Physiol. 2010;153:114–27. doi: 10.1104/pp.110.155093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kallenbach M, Alagna F, Baldwin IT, Bonaventure G. Nicotiana attenuata SIPK, WIPK, NPR1, and Fatty Acid-Amino Acid Conjugates Participate in the Induction of Jasmonic Acid Biosynthesis by Affecting Early Enzymatic Steps in the Pathway. (vol 152, pg 96, 2010) Plant Physiol. 2010;152:1760. doi: 10.1104/pp.109.149013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonaventure G, Schuck S, Baldwin IT. Revealing complexity and specificity in the activation of lipase-mediated oxylipin biosynthesis: a specific role of the Nicotiana attenuata GLA1 lipase in the activation of jasmonic acid biosynthesis in leaves and roots. Plant Cell Environ. 2011;34:1507–20. doi: 10.1111/j.1365-3040.2011.02348.x. [DOI] [PubMed] [Google Scholar]
- 10.Delker C, Stenzel I, Hause B, Miersch O, Feussner I, Wasternack C. Jasmonate biosynthesis in Arabidopsis thaliana--enzymes, products, regulation. Plant Biol (Stuttg) 2006;8:297–306. doi: 10.1055/s-2006-923935. [DOI] [PubMed] [Google Scholar]
- 11.Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 2001;125:711–7. doi: 10.1104/pp.125.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kandoth PK, Ranf S, Pancholi SS, Jayanty S, Walla MD, Miller W, et al. Tomato MAPKs LeMPK1, LeMPK2, and LeMPK3 function in the systemin-mediated defense response against herbivorous insects. Proc Natl Acad Sci USA. 2007;104:12205–10. doi: 10.1073/pnas.0700344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Hettenhausen C, Meldau S, Baldwin IT. Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell. 2007;19:1096–122. doi: 10.1105/tpc.106.049353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon GM, Cho HS, Ha HJ, Liu JR, Lee HSP. Characterization of NtCDPK1, a calcium-dependent protein kinase gene in Nicotiana tabacum, and the activity of its encoded protein. Plant Mol Biol. 1999;39:991–1001. doi: 10.1023/A:1006170512542. [DOI] [PubMed] [Google Scholar]
- 15.Szczegielniak J, Klimecka M, Liwosz A, Ciesielski A, Kaczanowski S, Dobrowolska G, et al. A wound-responsive and phospholipid-regulated maize calcium-dependent protein kinase. Plant Physiol. 2005;139:1970–83. doi: 10.1104/pp.105.066472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang DH, Hettenhausen C, Baldwin IT, Wu J. Silencing Nicotiana attenuata calcium-dependent protein kinases, CDPK4 and CDPK5, strongly up-regulates wound- and herbivory-induced jasmonic acid accumulations. Plant Physiol. 2012;159:1591–607. doi: 10.1104/pp.112.199018. [DOI] [PMC free article] [PubMed] [Google Scholar]


