Abstract
Several microbial molecules are released to the extracellular space in vesicle-like structures. In pathogenic fungi, these molecules include pigments, polysaccharides, lipids, and proteins, which traverse the cell wall in vesicles that accumulate in the extracellular space. The diverse composition of fungal extracellular vesicles (EV) is indicative of multiple mechanisms of cellular biogenesis, a hypothesis that was supported by EV proteomic studies in a set of Saccharomyces cerevisiae strains with defects in both conventional and unconventional secretory pathways. In the human pathogens Cryptococcus neoformans, Histoplasma capsulatum, and Paracoccidioides brasiliensis, extracellular vesicle proteomics revealed the presence of proteins with both immunological and pathogenic activities. In fact, fungal EV have been demonstrated to interfere with the activity of immune effector cells and to increase fungal pathogenesis. In this review, we discuss the impact of proteomics on the understanding of functions and biogenesis of fungal EV, as well as the potential role of these structures in fungal pathogenesis.
Keywords: Extracellular vesicles, pathogenic fungi, proteomics
1. Introduction
In most eukaryotic cells, secretion is a sophisticated biological process that functions to traffic proteins to the plasma membrane and/or to the extracellular space [1]. Eukaryotic secretion occurs through mechanisms that are finely regulated at different cellular levels [1, 2]. The literature about secretory mechanisms used by eukaryotic cells is abundant and several comprehensive reviews in this field are available [1-5].
During secretion, the plasma membrane is, in general, the final barrier to be traversed by eukaryotic molecules destined to the extracellular space. Fungal and plant cells, however, differ from most eukaryotes, since they are surrounded by a complex and very dynamic cell wall [6, 7]. Extracellular molecules in plants and fungi have been first described more than fifty years ago [8] and many of them were characterized in detail during the last decades (reviewed in [9]). The observation of extracellular molecules in fungal and plant cells implies that trans-cell wall secretion is mandatory in these organisms. Indeed, the fact that the final step of secretion in fungi and plants is the traversal of the cell wall brings additional complexity to the secretory mechanisms used by these cells. A similar rational is valid for cell wall-containing prokaryotes. Mechanisms of trans-cell wall secretion in bacteria, however, have been extensively discussed in recent years and will not be the topic of this review [10-12]. Trans-cell wall secretion in plants is also the focus of some recent, excellent reviews [7, 13, 14]. In this article, we will focus on vesicular mechanisms of secretion in fungi, highlighting the contribution of proteomics to the understanding of how fungal molecules reach the extracellular space.
2. Export of macromolecules in fungi requires EV
The mechanisms by which molecules produced by fungal cells are transported across the cell wall remained unknown until very recently. The cell wall is mainly composed of polysaccharides and proteins [6], but early reports suggested that lipids could be transitory components of this cellular compartment [15, 16]. Moreover, studies combining electron microscopy and the freeze-etching technique suggested the association of membrane vesicles with the cell wall of fungi four decades ago [17, 18]. Approximately thirty years later, a cell-wall lipid was fully characterized in a fungal species for the first time, and its association with cell-wall vesicles was again proposed [19]. Together, these reports suggested that cell-wall lipids could be components of carrier vesicles involved in the transport of macromolecules to the extracellular space. This hypothesis, raised in many of these early reports, was experimentally confirmed only about five years ago in the human pathogen Cryptococcus neoformans [20]. In this organism, polysaccharide-containing vesicular structures were isolated from culture supernatants, supporting the hypothesis that vesicular cell-wall transport could represent a fungal solution for the delivery of extracellular components, as illustrated in Figure 1. After the initial description and further confirmation of the existence extracellular vesicles (EV) in C. neoformans [20-31], analogous compartments were found in Paracoccidioides brasiliensis [32-35], Histoplasma capsulatum [21-23], Sporothrix schenckii [23], Candida albicans [23], C. parapsilosis [23], Saccharomyces cerevisiae [23, 36, 37], and Malassezia sympodialis [38]. Similar findings were observed for plant cells [39].
Figure 1.
The complexity of the fungal cell wall illustrated in the C. neoformans model. Molecules destined to the extracellular space are expected to traverse this dense and complex network that includes proteins, polysaccharides, pigments, and lipids. Fungal vesicles, here denominated ‘virulence bags’, are bilayered vesicle membranes that have been proposed as molecular carriers traversing the cell wall. These carriers vary in dimension and biochemical properties. GXM, glucuronoxylomannan; GalXM, galactoxylomannan. Reproduced with permission from Cryptococcus: From Human Pathogen to Model Yeast Editors: Joseph Heitman, Thomas R. Kozel, Kyung J. Kwon-Chung, John R Perfect, and Arturo Casadevall. ASM, 2011. The Cell Wall of Cryptococcus, Gilbert NM, Lodge JK, and Specht CA.
In eukaryotes, protein secretion or exocytosis follows a conventional endoplasmic reticulum-trans-Golgi network-plasma membrane route, where a finally coordinated network of vesicle transport promotes vesicular fusion with the plasma membrane and release of cargo to the extracellular space [1]. The characterization of fungal EV implied the existence of alternative mechanisms of secretion by which vesicles would be transported through the plasma membrane. EV can have multiple mechanisms of biogenesis [4], which usually results in compositional divergence. In this regard, recent proteomic studies suggest that fungal EV have complex and still obscure mechanisms of biogenesis that may share similarities with those described in mammalian cells [23, 24, 35, 37]. The principal features of EV produced by fungi will be discussed in the next sections.
3. Characterization of fungal EV in different species: a great molecular diversity
In C. neoformans, EV were first identified with classic cell biology tools. By transmission electron microscopy, vesicles that were isolated from culture supernatants were demonstrated to contain bilayered membranes. They manifested various sizes and morphologies, including electron-dense and electron-lucid vesicles, vesicular structures with membrane-associated electron-dense regions, and vesicles containing hyper-dense structures resembling a dark pigment [20, 24]. These vesicles were found to be associated with inner and external layers of the cell wall [20], suggesting an involvement in trans-cell wall traffic. Serological approaches revealed the presence of polysaccharides and proteins that were recognized by sera from human individuals infected with C. neoformans [20, 24]. Mass spectrometry (MS) analysis of lipids revealed the presence of sterols and glucosylceramide [20], a glycolipid component of the fungal cell wall [19].
A more comprehensive characterization of C. neoformans EV was only achieved when vesicle samples were analyzed by proteomic-based approaches [24]. More than seventy proteins were identified in the C. neoformans EV. Surprisingly, most of these proteins lacked the characteristic signal peptide required for conventional secretion [24]. Protein classification revealed very diverse cellular processes; most of them were not related to conventional secretory mechanisms. This observation, which had precedents in mammalian systems [40, 41], suggested that fungal EV also derived from unconventional and/or still unknown pathways of secretion.
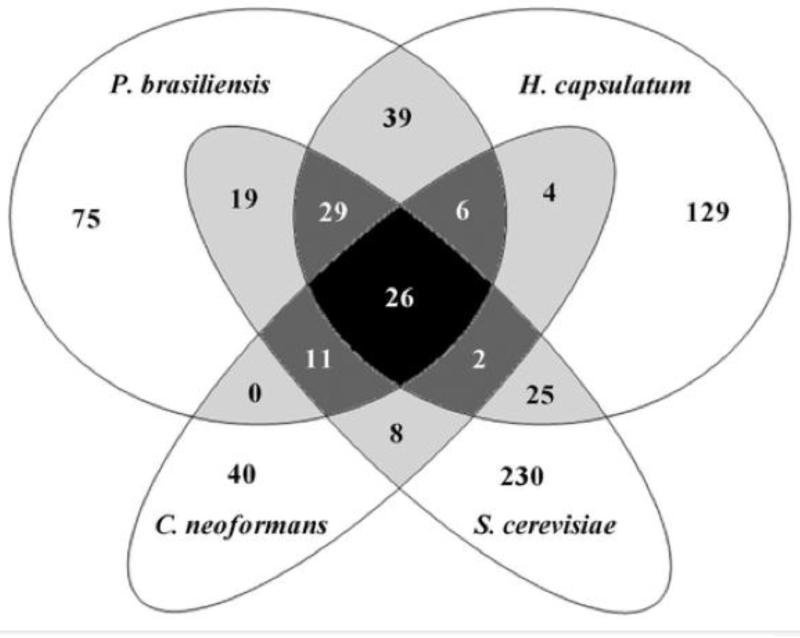
The combined use of serology, biochemistry, and MS led to the identification of polysaccharides, phospholipids, neutral lipids, and seventy-six proteins as EV components secreted by C. neoformans [20-22, 24-26, 42]. Under conditions of higher sensitivity, however, similar samples from other species were analyzed and the results revealed an interesting complexity in the composition of fungal EV (Table 1). In the human dimorphic pathogen H. capsulatum, the number of proteins validated was much higher (n = 283) than that found for C. neoformans [23]. Protein categorization, however, revealed many similarities in vesicle samples from both species. Similarly, the S. cerevisiae EV proteome using samples produced by eight different strains had many similarities to the protein categorization observed for C. neoformans and H. capsulatum [37]. Finally, proteomic analysis of vesicles released to the extracellular space by the human pathogen P. brasiliensis allowed characterization of 205 proteins [35]. In that study, the EV proteomes of P. brasiliensis, C. neoformans, H. capsulatum, and S. cerevisiae were finely compared, revealing an effective overlapping in protein composition (Figure 2). Indeed, twenty-six proteins were identified in all four species analyzed, while seventy-two proteins were common to P. brasiliensis and at least two other species [35]. Proteomic analysis of EV produced by C. albicans revealed that a similar protein classification is also observed for this fungal pathogen (G. Vargas and L. Nimrichter, unpublished observations). On the other hand, EV proteomic analyses have demonstrated interesting species-specific particularities. For instance, the cell division proteins septins were described in P. brasiliensis EVs, but not in similar preparations from other species [35]. In S. cerevisiae, five GPI-anchored proteins (aspartyl protease, endoglucanase and three GPI-anchored proteins of unknown functions) that are absent in EVs from H. capsulatum, P. brasiliensis and C. neoformans were characterized [37], suggesting the existence of species-specific mechanisms of export of this class of glycoproteins. UDP-glucuronic acid decarboxylase and UDP-glucose dehydrogenase, two enzymes that are essential for the metabolism of glucuronic acid, were only identified in C. neoformans EVs [24], a finding that is probably related with the abundance of this carbohydrate residue in the cryptococcal capsule. These particularities may suggest species-specific, EV-related physiological events. However, as well pointed-out by Vallejo and colleagues [35], the comparative analysis between EVs produced by different species has to be extremely careful, considering that the variations in experimental conditions and amount of sample used in each study could affect the identification of different fungal orthologues.
Table 1.
General properties of fungal EV and experimental conditions used for proteomic characterization
| Fungal species | General characteristics of EVs |
Number of strains used in proteomic studies |
Purification method |
Experimental conditions for proteomic analysis1 |
Proteins identified (n) |
Reference |
|---|---|---|---|---|---|---|
| C. neoformans | Bilayered, spherical vesicles; 20 to 400 nm; variable electron density |
2 | Ultracentrifugation of cell-free culture supernatants (100,000 g, 1h) |
Shotgun proteomics by LC- MS/MS using an electrospray ionization-linear ion trap-mass spectrometer |
76 | [20, 24, 26] |
| H. capsulatum | Bilayered, spherical vesicles; 10 to 350 nm; variable electron density |
1 | Ultracentrifugation of cell-free culture supernatants (100,000 g, 1h) |
Shotgun proteomics by LC- MS/MS using an electrospray ionization-linear ion trap-mass spectrometer |
283 | [23] |
| S. cerevisiae | Bilayered, spherical vesicles; 50 to 250 nm; variable electron density |
8 | Ultracentrifugation of cell-free culture supernatants (100,000 g, 1h) |
Shotgun proteomics by LC- MS/MS using an electrospray- linear ion trap mass spectrometer |
400 | [37] |
| P. brasiliensis | Bilayered, spherical vesicles; 20 to 200 nm; variable electron density |
1 | Ultracentrifugation of cell-free culture supernatants (100,000 g, 1h) |
Shotgun proteomics by LC- MS/MS using an electrospray- linear ion trap- mass spectrometer |
205 | [32, 35] |
LC-MS/MS, liquid chromatography-tandem mass spectrometry
Figure 2.
Comparative analysis of proteins identified in EV obtained from different fungal species. Overlapping groups are shown in gray/black. Reproduced with permission from [35].
EV produced by fungi mostly contain proteins related to diverse metabolic processes. Other classes found in vesicle samples mainly included cell organization and biogenesis, molecular transporters, carbohydrate metabolism, stress response, protein biosynthesis and degradation, among others [23, 24, 35, 37]. It is worth mentioning that proteins required for cell-wall modeling, including different glucanases and glucanosyl transferases, were consistently identified in fungal EV (n = 24 in S. cerevisiae, for instance) [37]. This observation may be related to regular processes of cell wall synthesis, but also to still unknown mechanisms of vesicle passage through this cellular barrier. Stress-related proteins, as well as peptidases, vacuolar and secretory proteins have been repeatedly identified by proteomic approaches [23, 24, 35, 37]. Noteworthy, many of the proteins found in fungal EV play moonlight functions. Enolase, for instance, has been found in EV from all the species examined so far [23, 24, 35, 37]. This enzyme, which plays a key and well-known role in glycolysis, interacts with plasminogen and promotes adhesion to host cells in the P. brasiliensis model [43]. In C. albicans, enolase is an effective immunogen with promising perspectives of application in both prophylactic and diagnostic protocols [44-46]. Similarly, other glycolytic enzymes with moonlight functions were described in fungal vesicles [17, 23, 24, 35, 37].
Although the protein composition of fungal EV is multifaceted in many aspects, most of the proteins identified in these preparations are putatively functionally connected with one or more molecules within the vesicular proteome [37], as illustrated for S. cerevisiae vesicles (Figure 3). Of note, proteins targeted to the endoplasmic reticulum and the secretory pathway represent the minority of the molecules carried by fungal EV [23, 24, 35, 37]. This information and the fact that molecules that do not contain any molecular tag directing them to conventional processes of secretion (e.g. polysaccharides and pigments) are components of fungal EV [20, 24, 26, 33] strongly suggests, once more, the participation of unconventional and maybe still unknown mechanisms of secretion and vesicle biogenesis. Therefore, one can conclude that the still embryonic proteomic and biochemical studies on EV produced by fungi revealed the existence of membrane-containing compartments at least composed of lipids, polysaccharides, and proteins that, in general, did not engage the conventional pathway of secretion.
Figure 3.
Illustration of the functional interrelationship in the collection of vesicular proteins obtained from S. cerevisiae culture supernatants. For protein identification according with each individual code, see [37]. Credits to Oliveira and colleagues, PLoS ONE 5(6): e11113. doi:10.1371/journal.pone.0011113.
4. Biogenesis of fungal EV: the S. cerevisiae model
The discovery of EV produced by fungi was based on studies with the C. neoformans model [20], as mentioned before. This fungus, however, is well known to produce massive amounts of extracellular polysaccharides, which hinder proteomic protocols. S. cerevisiae, on the other hand, is an efficient producer of EV [23, 37] that has been serving as a model for studies on secretion mechanisms in eukaryotes for the last thirty years. In this regard, the pioneering work by Schekman and colleagues with S. cerevisiae unraveled the role of the SEC genes and secretory vesicles in conventional, post-Golgi secretion [47]. S. cerevisiae had also been fundamental for studies on the role of the endosomal sorting complex required for transport (ESCRT) pathway, which is a key mediator of multivesicular bodies (MVB) biogenesis [48]. Upon fusion of MVB with the plasma membrane, EV defined as exosomes are released by eukaryotic cells [49], which consists of a key pathway of unconventional secretion [4]. Therefore, defects in MVB formation are expected to influence exosome biogenesis and unconventional mechanisms of secretion. Another distinctive attribute of the S. cerevisiae system is the availability of mutants with defects in classic and/or unconventional pathways of secretion [50].
Mutant strains with defective MVB formation [48] were natural candidates to evaluate whether exosomal formation was related to EV in yeast cells; sec mutants [51] were selected to evaluate whether events of the post-Golgi conventional secretion were required for the formation of fungal EV. Semi-quantitative proteomic analysis of vesicular protein abundance strongly suggested that compartments related to the MVB-derived pathway of exosomal formation were required for formation of EV in S. cerevisieae [36, 37]. For instance, mutants with defects in MVB formation produced vesicles in which the abundance of more than 30% of the protein set was modified relative to similar samples produced by wild type cells [37]. Interestingly, vesicles produced by one of the MVB mutants (snf7Δ) had significantly decreased levels of proteins that were not related to processes associated to secretion or MVB function, including cyclophilin [37]. This protein supposedly interacts with thirty-four other molecules in S. cerevisiae cells, including components of the secretory machinery and cell wall assembly [52, 53]. This observation efficiently illustrates the hypothesis that vesicular proteins with no apparent connections with secretory mechanisms may be required for vesicle formation, which adds considerable complexity to the understanding of how fungal EV are generated.
Vesicle release was not turned off in any of the S. cerevisiae MVB mutants [37]. This observation probably reflects the fact that, in eukaryotes, multiple cellular pathways are required for formation of EV, including different mechanisms of unconventional secretion [2-4]. For instance, in Dictyostelium discoideum, Pichia pastoris, S. cerevisiae and C. neoformans, it has been suggested that Golgi reassembly and stacking protein (GRASP), a regulator of unconventional protein secretion, is required for secretory mechanisms that may involve EV, autophagy genes, early endosomal compartments, and MVBs [4, 54-57]. In fact, a S. cerevisiae mutant lacking GRASP expression showed a decreased content of EV, in comparison with wild type cells [37]. In different eukaryotes, GRASP is primarily Golgi-associated, although other cellular sites have been suggested [58, 59]. Therefore, the decreased efficacy of the S. cerevisiae GRASP mutant to produce EV may suggest an importance of Golgi components in extracellular vesicle formation. Accordingly, conventional, post-Golgi secretory pathways are apparently also involved with the biogenesis of fungal EV. In S. cerevisiae, mutation of one of the SEC genes (SEC4) was associated with a delayed release of vesicles to the extracellular space [37]. This finding is in agreement with the view that fungal EV may not correspond uniquely to conventional exosomes, as recently proposed [60]. Nonetheless, it is noteworthy to mention that post-Golgi vesicles are targeted to and fuse with the plasma membrane [1, 51]. Therefore, it remains elusive how defects in Sec proteins would affect vesicle transport across the cell wall. The observation that not all secretory vesicles fuse with the plasma membrane (reviewed in [61]) may support findings with the S. cerevisiae sec4Δ mutant [37] and with a C. neoformans sec6Δ mutant that also showed altered EV release [60]. Altogether, these findings are in agreement with the view that extracellular release of vesicles in fungi is a multifactorial cellular mechanism of high complexity [5, 21, 22, 36, 42], which may involve significant redundancy. However, the possibility that the mutations tested until know are affecting different types of vesicles cannot be ruled out, since the methods currently used for EV purification do not discriminate between vesicles of different origins.
The studies discussed above make clear the complexity associated with the biogenesis of fungal EV. A similarly obscure perspective is associated with the mechanisms by which fungal vesicles traverse the cell wall, which are virtually unknown. In this context, the need for new experimental tools seems crucial. In this sense, Huang and colleagues [28] recently generated a C. neoformans strain bearing a fluorescent vesicle marker. Marker selection was based on previous proteomic studies that identified the C. neoformans 14-3-3 protein as one of the most abundant proteins in EV [24]. For direct visualization of vesicles, the green fluorescence protein (GFP) was fused to the C-terminus of 14-3-3. The expression of the 14-3-3-GFP fusion gene was driven by the actin-1 promoter, and the construct containing the fusion gene was successfully introduced into C. neoformans. The generation of a fluorescent EV marker has already allowed functional studies of pathogenesis [28]. Nevertheless, this experimental tool seems to be extremely useful in studies for investigating traversal of the cell wall by fungal vesicles, as well as in experiments focused on the cellular biogenesis of vesicles that are further targeted to the extracellular milieu.
5. Biological functions of fungal EV: the ‘virulence bags’
The combined use of serology, biochemistry, proteomics, and lipidomics led to the identification of polysaccharides, phospholipids, neutral lipids, and proteins as extracellular vesicle components in fungi [20, 24-26, 33-35, 37, 38]. Therefore, it is now well accepted that fungal EV are carriers of distinct molecules to the extracellular space. In fungal pathogens, this array of molecules includes a wide range of virulence determinants [20, 23, 24, 33-35]. The vesicular transport in fungi may even include the release of nucleic acids to the extracellular space [31], as described for mammalian exosomes [62]. Therefore, the concentration of such a complex array of molecules in vesicular compartments could influence the interaction of fungal pathogens with host cells, as illustrated in a number of studies. For instance, the principal virulence factor of C. neoformans is exported through vesicular traffic [20]. A similar glycan is exported to the extracellular space in secretory vesicles produced by P. brasiliensis [33]. In C. neoformans and H. capsulatum, vesicular components reacted with immune serum from patients with cryptococcosis and histoplasmosis [23, 24], respectively, revealing an association of the vesicular products with serological markers produced during disease. Finally, in the Paracoccidioides model, anti-α-Gal antibodies isolated from paracoccidioidomycosis patients efficiently recognized vesicle components containing α-Gal epitopes, which are highly immunogenic to humans [32, 63].
In addition to inducing humoral responses, EV isolated from different fungal pathogens were recently reported to interfere with the physiology of host cells [27] and with the course of animal infection [28]. It has been suggested that C. neoformans produce EV during tissue infection [20]. In addition, in vitro studies demonstrated that mammalian macrophages can incorporate cryptococcal EV, resulting in increased levels of tumor necrosis factor alpha (TNF-α), interleukin-10 (IL-10), transforming growth factor β (TGF-β), and the antimicrobial compound nitric oxide [27]. This response apparently required receptor-mediated internalization of the EV, as inferred from the kinetics of vesicle distribution after exposure to the phagocytes. The modulation of macrophage physiology by C. neoformans vesicles resulted in more effective killing of the fungus [27], but the effects of cryptococcal vesicles on host cells are apparently not restricted to macrophages. In the presence of purified EV, C. neoformans showed enhanced adherence to human brain microvascular endothelial cells (HBMECs) and increased efficacy in traversing the blood-brain barrier [28]. However, in HBMECs the cryptococcal vesicles appeared to fuse with cell membrane inducing the redistribution of lipid raft components. The C. neoformans vesicles also caused global effects during infection. Vesicle administration to mice before infection with C. neoformans enhanced brain colonization [28]. In M. sympodialis, the vesicular delivery of allergens was associated in increased levels of IL-4 and TNF-α in vitro and in vivo [38]. Therefore, it is now clear that fungal EV are efficient modulators of cellular responses (Figure 4), a property that is likely related to the mechanisms by which vesicular components are made accessible to host cells. In this sense, it has been recently demonstrated that serum albumin mediates destabilization of fungal EV [30], which is likely to occur in vivo.
Figure 4.
A model of interaction of fungal EV with host cells, using C. neoformans as a prototype. Vesicles delivered by fungal cells reach the extracellular environment and interact with host cells. Membrane fusion was observed in HBMECs [28] and receptor-mediated internalization by macrophages has been suggested [27]. The consequences of the exposure of host cells to fungal EV are listed on the right. Cap, capsule; CW, cell wall; Cyt, cytoplasm. In HBMECs, fungal vesicles appear in green (GFP); the plasma membrane was stained in red with a rhodamine-conjugated antibody to the surface marker CD44, and the nucleus was stained in blue with DAPI. For details, see [28]. In macrophages, fungal EV were stained in red with the DiI dye; the plasma membrane was stained in green with FITC-labeled cholera toxin subunit B, and the nucleus was stained in blue with DAPI. For details, see [27]. Credits of the BMEC panel to Huang and colleagues, PLoS ONE 7(11): e48570. doi:10.1371/journal.pone.0048570.
In other models, exosomal preparations from dendritic cells are being investigated as potential anticancer vaccines [64]. In fungi, experimental evidence suggests that EV can induce antimicrobial activity in macrophages [27], but also increase fungal pathogenesis in vivo [28]. Fungal cells indeed export several molecules with conflicting pathogenic functions. For instance, GXM, the major C. neoformans virulence factor and also a vesicular component, protects the fungus against phagocytosis and inhibits leukocyte migration (reviewed in [65]). However, this polysaccharide, in its capsular form, is also an efficient inducer of the activation of the alternative pathway of the complement system, which results in the deposition of opsonins in the capsule and increased phagocytosis [65]. Such example reveals the enormous complexity in predicting whether fungal EV would contribute to enhancement of pathogenesis or induce protective immune responses. Therefore, the combination of apparently contradictory results [27, 28] and a highly complex molecular composition [17, 20, 23-26, 31-35, 37] turns the response of host cells to EV preparations virtually unpredictable. In this context, new models to investigate the functions of fungal EV during infection are crucial.
6. Conclusion and future thoughts
The advances in the understanding of the biochemical and functional properties of fungal EV in the last five years are incontestable. Several questions, however, remain unanswered, and some of them are fundamental for the comprehension of how fungal EV are formed and how they impact fungal pathogenesis. Proteomic tools have been extremely useful for the determination of the composition of fungal EV [23, 24, 35, 37], but results obtained so far have not discriminated between vesicle populations of different cellular origins. Therefore, a first challenge in the field is the development of methods allowing separation of vesicle populations with different biochemical and physical chemical properties for new proteomic determinations and further comparison with existing data. Centrifugation gradients have been tested, but the yields were unsatisfactory and the efficacy of separation was questionable [20, 25]. As an alternative approach quantitative proteomic analysis of vesicles derived from several strains defective in different components of conventional and unconventional secretory pathways could expand the catalog of proteins secreted by each route. These proteins could be further used as molecular markers to differentiate distinct vesicle populations. A second challenge is the establishment of protocols to evaluate whether fungal EV are produced in vivo. Until now, attempts to isolate fungal vesicles from host tissues and fluids have been unsuccessful, which may have a connection with the proposition that fungal EV are short-lived in vivo [30]. Nevertheless, the development of fungal strains producing fluorescent vesicles [28] will likely bring considerable progress to the field. A third challenge is to unravel how fungal vesicles traverse the cell wall. So far, this is probably one of the most puzzling topics within the field of fungal EV. However, the advance of new microscopy tools such as electron tomography suggests that it is now possible to examine the architecture of the fungal cell in a deeper level of detail, which will probably improve the comprehension of how fungal vesicles are released extracellularly. Another challenge is to understand the cellular signals that trigger vesicle secretion and markers that sort proteins into the vesicles. The recent advances in the analysis of protein post-translational modifications, such as phosphorylation, acetylation, and ubiquitination, open a great opportunity to study these events. For instance, exosomes from a B cell-like cell line was shown to have ubiquitinated proteins [66]; however, whether ubiquitination is required to sort proteins into exosomes and its mechanism is still a question to be answered. Finally, it remains unknown whether fungal EV can act as vehicles of intercellular communication, as described for mammalian EV [67]. Assays aiming at determining whether fungal vesicles can be transferred from one cell to another promoting regulation of signaling pathways and modification of gene expression will probably be useful to address this question. In addition, the demonstration that fungal EV can modify the status of activation of immune cells [27, 38] strongly suggests that these molecular cargo carriers can interfere with different pathways regulating the physiology of mammalian cells.
Highlights.
-
►
The discovery of fungal extracellular vesicles as trans-cell wall carriers of proteins, lipids, and polysaccharides.
-
►
The use of proteomics for compositional and functional characterization of fungal extracellular vesicles.
-
►
The need for new tools for understanding vesicle biogenesis and functions in vivo.
Acknowledgements
We thank Rosana Puccia (UNIFESP, Brazil) and Jennifer Lodge (Washington University School of Medicine) for sharing figures that were originally published by their groups. We are also thankful to Arturo Casadevall and Joshua Nosanchuk (Albert Einstein School of Medicine -Yeshiva University) for collaboration in many projects focused on fungal EV and current and past members of the Rodrigues and Nimrichter laboratories for their efforts on vesicles studies. MLR and LN are supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP, Brazil), and Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ, Brazil). ICA was supported by grants 1R01AI070655-04, 3R01AI070655-04S1, 5S06GM08012-37, 8G12MD007592, 2G12RR008124-16A1, and 2G12RR008124-16A1S1 from the National Institutes of Health. We are grateful to the Biomolecule Analysis Core Facility at the Border Biomedical Research Center/UTEP (NIH grants 8G12MD007592, 2G12RR008124-16A1, and 2G12RR008124-16A1S1) for the access to LC-MS instruments used in many of the proteomic analyses described in this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Stephens C. Protein secretion: getting folded proteins across membranes. Current biology: CB. 1998;8:R578–81. doi: 10.1016/s0960-9822(07)00366-1. [DOI] [PubMed] [Google Scholar]
- [2].Nickel W. Pathways of unconventional protein secretion. Current opinion in biotechnology. 2010;21:621, 6. doi: 10.1016/j.copbio.2010.06.004. [DOI] [PubMed] [Google Scholar]
- [3].Nickel W. The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. European journal of biochemistry/FEBS. 2003;270:2109–19. doi: 10.1046/j.1432-1033.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- [4].Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nature reviews Molecular cell biology. 2009;10:148–55. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- [5].Rodrigues ML, Nosanchuk JD, Schrank A, Vainstein MH, Casadevall A, Nimrichter L. Vesicular transport systems in fungi. Future microbiology. 2011;6:1371, 81. doi: 10.2217/fmb.11.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nimrichter L, Rodrigues ML, Rodrigues EG, Travassos LR. The multitude of targets for the immune system and drug therapy in the fungal cell wall. Microbes and infection/Institut Pasteur. 2005;7:789–98. doi: 10.1016/j.micinf.2005.03.002. [DOI] [PubMed] [Google Scholar]
- [7].Samuels L, McFarlane HE. Plant cell wall secretion and lipid traffic at membrane contact sites of the cell cortex. Protoplasma. 2012;249(Suppl 1):S19–23. doi: 10.1007/s00709-011-0345-7. [DOI] [PubMed] [Google Scholar]
- [8].Wickerham LJ. Evidence of the production of extracellular invertase by certain strains of yeasts. Archives of biochemistry and biophysics. 1958;76:439–48. doi: 10.1016/0003-9861(58)90169-3. [DOI] [PubMed] [Google Scholar]
- [9].Finkelman MA. Yeast strain development for extracellular enzyme production. Bioprocess technology. 1990;8:185–223. [PubMed] [Google Scholar]
- [10].Kerr JR. Gram-negative bacterial protein secretion. The Journal of infection. 2000;40:121–6. doi: 10.1016/s0163-4453(00)80002-x. [DOI] [PubMed] [Google Scholar]
- [11].Thanassi DG, Hultgren SJ. Multiple pathways allow protein secretion across the bacterial outer membrane. Current opinion in cell biology. 2000;12:420–30. doi: 10.1016/s0955-0674(00)00111-3. [DOI] [PubMed] [Google Scholar]
- [12].Lee VT, Schneewind O. Protein secretion and the pathogenesis of bacterial infections. Genes & development. 2001;15:1725–52. doi: 10.1101/gad.896801. [DOI] [PubMed] [Google Scholar]
- [13].Jurgens G, Geldner N. Protein secretion in plants: from the trans-Golgi network to the outer space. Traffic. 2002;3:605–13. doi: 10.1034/j.1600-0854.2002.30902.x. [DOI] [PubMed] [Google Scholar]
- [14].Ding Y, Wang J, Wang J, Stierhof YD, Robinson DG, Jiang L. Unconventional protein secretion. Trends in plant science. 2012;17:606–15. doi: 10.1016/j.tplants.2012.06.004. [DOI] [PubMed] [Google Scholar]
- [15].Kanetsuna F, Carbonell LM, Moreno RE, Rodriguez J. Cell wall composition of the yeast and mycelial forms of Paracoccidioides brasiliensis. Journal of bacteriology. 1969;97:1036–41. doi: 10.1128/jb.97.3.1036-1041.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cox RA, Best GK. Cell wall composition of two strains of Blastomyces dermatitidis exhibiting differences in virulence for mice. Infection and immunity. 1972;5:449–53. doi: 10.1128/iai.5.4.449-453.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Takeo K, Uesaka I, Uehira K, Nishiura M. Fine structure of Cryptococcus neoformans grown in vivo as observed by freeze-etching. Journal of bacteriology. 1973;113:1449–54. doi: 10.1128/jb.113.3.1449-1454.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Takeo K, Uesaka I, Uehira K, Nishiura M. Fine structure of Cryptococcus neoformans grown in vitro as observed by freeze-etching. Journal of bacteriology. 1973;113:1442–8. doi: 10.1128/jb.113.3.1442-1448.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rodrigues ML, Travassos LR, Miranda KR, Franzen AJ, Rozental S, de Souza W, et al. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infection and immunity. 2000;68:7049–60. doi: 10.1128/iai.68.12.7049-7060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rodrigues ML, Nimrichter L, Oliveira DL, Frases S, Miranda K, Zaragoza O, et al. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryotic cell. 2007;6:48–59. doi: 10.1128/EC.00318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nosanchuk JD, Nimrichter L, Casadevall A, Rodrigues ML. A role for vesicular transport of macromolecules across cell walls in fungal pathogenesis. Communicative & integrative biology. 2008:1, 37–9. doi: 10.4161/cib.1.1.6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rodrigues ML, Nimrichter L, Oliveira DL, Nosanchuk JD, Casadevall A. Vesicular Trans-Cell Wall Transport in Fungi: A Mechanism for the Delivery of Virulence-Associated Macromolecules? Lipid insights. 2008;2:27–40. doi: 10.4137/lpi.s1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Albuquerque PC, Nakayasu ES, Rodrigues ML, Frases S, Casadevall A, Zancope-Oliveira RM, et al. Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cellular microbiology. 2008;10:1695–710. doi: 10.1111/j.1462-5822.2008.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, et al. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryotic cell. 2008;7:58–67. doi: 10.1128/EC.00370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Oliveira DL, Nimrichter L, Miranda K, Frases S, Faull KF, Casadevall A, et al. Cryptococcus neoformans cryoultramicrotomy and vesicle fractionation reveals an intimate association between membrane lipids and glucuronoxylomannan. Fungal genetics and biology: FG & B. 2009;46:956–63. doi: 10.1016/j.fgb.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Eisenman HC, Frases S, Nicola AM, Rodrigues ML, Casadevall A. Vesicle-associated melanization in Cryptococcus neoformans. Microbiology. 2009;155:3860–7. doi: 10.1099/mic.0.032854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Oliveira DL, Freire-de-Lima CG, Nosanchuk JD, Casadevall A, Rodrigues ML, Nimrichter L. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infection and immunity. 2010;78:1601–9. doi: 10.1128/IAI.01171-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Huang SH, Wu CH, Chang YC, Kwon-Chung KJ, Brown RJ, Jong A. Cryptococcus neoformans-derived microvesicles enhance the pathogenesis of fungal brain infection. PloS one. 2012;7:e48570. doi: 10.1371/journal.pone.0048570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Robertson EJ, Wolf JM, Casadevall A. EDTA inhibits biofilm formation, extracellular vesicular secretion, and shedding of the capsular polysaccharide glucuronoxylomannan by Cryptococcus neoformans. Applied and environmental microbiology. 2012;78:7977–84. doi: 10.1128/AEM.01953-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wolf JM, Rivera J, Casadevall A. Serum albumin disrupts Cryptococcus neoformans and Bacillus anthracis extracellular vesicles. Cellular microbiology. 2012;14:762–73. doi: 10.1111/j.1462-5822.2012.01757.x. [DOI] [PubMed] [Google Scholar]
- [31].Nicola AM, Frases S, Casadevall A. Lipophilic dye staining of Cryptococcus neoformans extracellular vesicles and capsule. Eukaryotic cell. 2009;8:1373–80. doi: 10.1128/EC.00044-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vallejo MC, Matsuo AL, Ganiko L, Medeiros LC, Miranda K, Silva LS, et al. The pathogenic fungus Paracoccidioides brasiliensis exports extracellular vesicles containing highly immunogenic alpha-Galactosyl epitopes. Eukaryotic cell. 2011;10:343–51. doi: 10.1128/EC.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Albuquerque PC, Cordero RJ, Fonseca FL, Peres da Silva R, Ramos CL, Miranda KR, et al. A Paracoccidioides brasiliensis glycan shares serologic and functional properties with cryptococcal glucuronoxylomannan. Fungal genetics and biology: FG & B. 2012;49:943–54. doi: 10.1016/j.fgb.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vallejo MC, Nakayasu ES, Longo LV, Ganiko L, Lopes FG, Matsuo AL, et al. Lipidomic analysis of extracellular vesicles from the pathogenic phase of Paracoccidioides brasiliensis. PloS one. 2012;7:e39463. doi: 10.1371/journal.pone.0039463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vallejo MC, Nakayasu ES, Matsuo AL, Sobreira TJ, Longo LV, Ganiko L, et al. Vesicle and vesicle-free extracellular proteome of Paracoccidioides brasiliensis: comparative analysis with other pathogenic fungi. Journal of proteome research. 2012;11:1676–85. doi: 10.1021/pr200872s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Oliveira DL, Nakayasu ES, Joffe LS, Guimaraes AJ, Sobreira TJ, Nosanchuk JD, et al. Biogenesis of extracellular vesicles in yeast: Many questions with few answers. Communicative & integrative biology. 2010;3:533–5. doi: 10.4161/cib.3.6.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Oliveira DL, Nakayasu ES, Joffe LS, Guimaraes AJ, Sobreira TJ, Nosanchuk JD, et al. Characterization of yeast extracellular vesicles: evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PloS one. 2010;5:e11113. doi: 10.1371/journal.pone.0011113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gehrmann U, Qazi KR, Johansson C, Hultenby K, Karlsson M, Lundeberg L, et al. Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses--novel mechanisms for host-microbe interactions in atopic eczema. PloS one. 2011;6:e21480. doi: 10.1371/journal.pone.0021480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Regente M, Corti-Monzon G, Maldonado AM, Pinedo M, Jorrin J, de la Canal L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS letters. 2009;583:3363–6. doi: 10.1016/j.febslet.2009.09.041. [DOI] [PubMed] [Google Scholar]
- [40].Looze C, Yui D, Leung L, Ingham M, Kaler M, Yao X, et al. Proteomic profiling of human plasma exosomes identifies PPARgamma as an exosome-associated protein. Biochemical and biophysical research communications. 2009;378:433–8. doi: 10.1016/j.bbrc.2008.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Palazzolo G, Albanese NN, G DIC, Gygax D, Vittorelli ML, Pucci-Minafra I. Proteomic analysis of exosome-like vesicles derived from breast cancer cells. Anticancer research. 2012;32:847–60. [PubMed] [Google Scholar]
- [42].Casadevall A, Nosanchuk JD, Williamson P, Rodrigues ML. Vesicular transport across the fungal cell wall. Trends in microbiology. 2009;17:158–62. doi: 10.1016/j.tim.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nogueira SV, Fonseca FL, Rodrigues ML, Mundodi V, Abi-Chacra EA, Winters MS, et al. Paracoccidioides brasiliensis enolase is a surface protein that binds plasminogen and mediates interaction of yeast forms with host cells. Infection and immunity. 2010;78:4040–50. doi: 10.1128/IAI.00221-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pitarch A, Diez-Orejas R, Molero G, Pardo M, Sanchez M, Gil C, et al. Analysis of the serologic response to systemic Candida albicans infection in a murine model. Proteomics. 2001;1:550–9. doi: 10.1002/1615-9861(200104)1:4<550::AID-PROT550>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- [45].Montagnoli C, Sandini S, Bacci A, Romani L, La Valle R. Immunogenicity and protective effect of recombinant enolase of Candida albicans in a murine model of systemic candidiasis. Medical mycology: official publication of the International Society for Human and Animal Mycology. 2004;42:319–24. doi: 10.1080/13693780310001644653. [DOI] [PubMed] [Google Scholar]
- [46].Li W, Hu X, Zhang X, Ge Y, Zhao S, Hu Y, et al. Immunisation with the glycolytic enzyme enolase confers effective protection against Candida albicans infection in mice. Vaccine. 2011;29:5526–33. doi: 10.1016/j.vaccine.2011.05.030. [DOI] [PubMed] [Google Scholar]
- [47].Schekman R. Charting the secretory pathway in a simple eukaryote. Molecular biology of the cell. 2010;21:3781–4. doi: 10.1091/mbc.E10-05-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Developmental cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- [49].van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. Journal of biochemistry. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- [50].Goffeau A. Four years of post-genomic life with 6,000 yeast genes. FEBS letters. 2000;480:37–41. doi: 10.1016/s0014-5793(00)01775-0. [DOI] [PubMed] [Google Scholar]
- [51].Lyman SK, Schekman R. Polypeptide translocation machinery of the yeast endoplasmic reticulum. Experientia. 1996;52:1042–9. doi: 10.1007/BF01952100. [DOI] [PubMed] [Google Scholar]
- [52].Costanzo M, Baryshnikova A, Bellay J, Kim Y, Spear ED, Sevier CS, et al. The genetic landscape of a cell. Science. 2010;327:425–31. doi: 10.1126/science.1180823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schuldiner M, Collins SR, Thompson NJ, Denic V, Bhamidipati A, Punna T, et al. Exploration of the function and organization of the yeast early secretory pathway through an epistatic miniarray profile. Cell. 2005;123:507–19. doi: 10.1016/j.cell.2005.08.031. [DOI] [PubMed] [Google Scholar]
- [54].Kinseth MA, Anjard C, Fuller D, Guizzunti G, Loomis WF, Malhotra V. The Golgi-associated protein GRASP is required for unconventional protein secretion during development. Cell. 2007;130:524–34. doi: 10.1016/j.cell.2007.06.029. [DOI] [PubMed] [Google Scholar]
- [55].Manjithaya R, Anjard C, Loomis WF, Subramani S. Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. The Journal of cell biology. 2010;188:537–46. doi: 10.1083/jcb.200911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Duran JM, Anjard C, Stefan C, Loomis WF, Malhotra V. Unconventional secretion of Acb1 is mediated by autophagosomes. The Journal of cell biology. 2010;188:527–36. doi: 10.1083/jcb.200911154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kmetzsch L, Joffe LS, Staats CC, de Oliveira DL, Fonseca FL, Cordero RJ, et al. Role for Golgi reassembly and stacking protein (GRASP) in polysaccharide secretion and fungal virulence. Molecular microbiology. 2011;81:206–18. doi: 10.1111/j.1365-2958.2011.07686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Vinke FP, Grieve AG, Rabouille C. The multiple facets of the Golgi reassembly stacking proteins. The Biochemical journal. 2011;433:423–33. doi: 10.1042/BJ20101540. [DOI] [PubMed] [Google Scholar]
- [59].Abrahamsen H, Stenmark H. Protein secretion: unconventional exit by exophagy. Current biology: CB. 2010;20:R415–8. doi: 10.1016/j.cub.2010.03.011. [DOI] [PubMed] [Google Scholar]
- [60].Panepinto J, Komperda K, Frases S, Park YD, Djordjevic JT, Casadevall A, et al. Sec6-dependent sorting of fungal extracellular exosomes and laccase of Cryptococcus neoformans. Molecular microbiology. 2009;71:1165–76. doi: 10.1111/j.1365-2958.2008.06588.x. [DOI] [PubMed] [Google Scholar]
- [61].Stephens DJ, Pepperkok R. Illuminating the secretory pathway: when do we need vesicles? Journal of cell science. 2001;114:1053–9. doi: 10.1242/jcs.114.6.1053. [DOI] [PubMed] [Google Scholar]
- [62].Lasser C. Exosomal RNA as biomarkers and the therapeutic potential of exosome vectors. Expert opinion on biological therapy. 2012;12(Suppl 1):S189–97. doi: 10.1517/14712598.2012.680018. [DOI] [PubMed] [Google Scholar]
- [63].Macher BA, Galili U. The Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochimica et biophysica acta. 2008;1780:75–88. doi: 10.1016/j.bbagen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Mignot G, Roux S, Thery C, Segura E, Zitvogel L. Prospects for exosomes in immunotherapy of cancer. Journal of cellular and molecular medicine. 2006;10:376–88. doi: 10.1111/j.1582-4934.2006.tb00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zaragoza O, Rodrigues ML, De Jesus M, Frases S, Dadachova E, Casadevall A. The capsule of the fungal pathogen Cryptococcus neoformans. Advances in applied microbiology. 2009;68:133–216. doi: 10.1016/S0065-2164(09)01204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Buschow SI, Liefhebber JM, Wubbolts R, Stoorvogel W. Exosomes contain ubiquitinated proteins. Blood cells, molecules & diseases. 2005;35:398–403. doi: 10.1016/j.bcmd.2005.08.005. [DOI] [PubMed] [Google Scholar]
- [67].Schneider A, Simons M. Exosomes: vesicular carriers for intercellular communication in neurodegenerative disorders. Cell and tissue research. 2012 doi: 10.1007/s00441-012-1428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]