Abstract
TGF-β has limited effects on ovarian cancer cells but its contributions to ovarian tumor growth might be mediated through elements of the tumor microenvironment. In the present study, we tested the hypothesis that TGF- modulates ovarian cancer progression by modulating the contribution of cancer-associated fibroblasts (CAFs) that are present in the microenvironment. Transcriptome profiling of microdissected stromal and epithelial components of high-grade serous ovarian tumors and TGF-β-treated normal ovarian fibroblasts identified versican (VCAN) as a key upregulated target gene in CAFs. Functional evaluations in co-culture experiments demonstrated that TGF-β enhanced the aggressiveness of ovarian cancer cells by upregulating VCAN in CAFs. VCAN expression was regulated in CAFs through TGF-β receptor type II and SMAD signaling. Upregulated VCAN promoted the motility and invasion of ovarian cancer cells by activating the NF-κB signaling pathway and by upregulating expression of CD44, MMP9, and the hyaluronan-mediated motility receptor (HMMR). Our work identified a TGF-β-inducible gene signature specific to CAFs in advanced high-grade serous ovarian tumors, and showed how TGF-β stimulates ovarian cancer cell motility and invasion by upregulating the CAF-specific gene VCAN. These findings suggest insights to develop or refine strategies for TGF-β-targeted therapy of ovarian cancer.
Keywords: TGF-β, versican, ovarian cancer, cancer-associated fibroblast, tumor microenvironment
Introduction
Advanced ovarian cancer is the fifth most common form of cancer in women in the United States (1). Researchers estimated the diagnosis of 22,280 new cases of and 15,500 deaths owing to ovarian cancer in 2012 (2), making it the most lethal gynecologic malignancy. In particular, ovarian cancer is noted for its initial chemotherapy sensitivity. However, more than 75% of ovarian cancers recur within 12–24 months (3).
Ovarian cancer cells are known to lose responsiveness to inhibitory growth signals exerted by transforming growth factor β (TGF-β) (4, 5). Authors have reported on mechanisms of lost TGF-β responsiveness, including downregulation of the TGF-β receptors and alterations in the expression of genes involved in the TGF-β signaling pathways in ovarian cancer cells (6). Whereas previous studies focused on evaluating the effect of TGF-β on ovarian cancer growth, recent studies indicated that TGF-β is at least partly responsible for activation of fibroblasts in cases of a number of different cancer types (7–9). Primarily composed of fibroblasts and extracellular matrix (ECM) proteins as well as endothelial cells and lymphocytic infiltrates, the tu mor microenvironment directly affects cell growth, migration, and differentiation via the action of secreted proteins, cell-cell interactions, and matrix remodeling (10, 11). TGF-β may indirectly affect ovarian tumor growth by modulating the secretion of stroma-specific mediators in the tumor microenvironment.
The purpose of this study was to use a whole-genome oligonucleotide array platform to perform transcriptome profiling of the fibroblastic stromal and epithelial components microdissected from a series of advanced-stage, high-grade serous ovarian adenocarcinomas and of TGF-β–treated ovarian fibroblasts in vitro to identify a TGF-β-regulated gene signature in cancer-associated fibroblasts (CAFs). We further investigated the molecular mechanism by which one of the TGF-β-upregulated genes, VCAN, which codes for the ECM proteoglycan versican, promotes ovarian cancer progression and delineated the functional role of VCAN in modulation of ovarian cancer cell motility and invasion potential.
Materials and Methods
Cell lines and culture conditions
The human ovarian adenocarcinoma cell lines ALST, HeyA8, OVCA3, OVCA420, OVCA429, OVCA433, and SKOV3ipluc, which cultured in RPMI 1640 medium supplemented with 10% FBS, were used in this study. All of the cell lines were tested for mycoplasma contamination and authenticated using microsatellites panel. The telomerase-immortalized human normal ovarian fibroblast (NOF) line NOF151-hTERT and primary ovarian CAF lines were cultured in MCDB 105/199 medium (1:1) supplemented with 10% FBS, and 1 ng/mL epidermal growth factor.
Microdissection and microarray analysis of tissue samples
RNA was extracted from microdissected frozen ovarian tissue samples, which included tumor epithelial components (n = 35), normal ovarian surface epithelial (OSE) cells (n = 6), stromal CAFs (n = 28), and normal stromal fibroblasts (n = 8). Microdissection was performed by fixing tissue sections in 70% ethanol and then staining them with 1% methyl green to visualize the histologic features. During dissection, areas of interest in the sections were carefully outlined. Areas with immune cell and blood vessel infiltration were excluded to minimize contamination (Supplementary Figs. S1A and S1B). Patient samples were collected from the Ovarian Cancer Repository under protocols approved by The University of Texas MD Anderson Cancer Center IRB. RNA samples were amplified, labeled, and hybridized onto GeneChip Human Genome U133 Plus 2.0 microarrays (Affymetrix) according to the manufacturer’s protocol. After hybridization, arrays were washed and stained using a GeneChip Fluidics Station 450 and then scanned using a GeneChip Scanner 3000 7G (Affymetrix).
Statistical analysis
SPSS (version 17; IBM Corporation) and Prism (version 5.0; GraphPad Software) software programs were used to perform the statistical tests. All in vitro experiments were repeated independently in triplicate. Two-tailed Student t-test was used to test differences in sample means for data with normally distributed means. The Mann-Whitney U test was used for analysis of nonparametric data. t-test and Benjamini-Hochberg FDR multiple testing corrections were used for microarray data analysis. P-values less than 0.05 were considered statistically significant.
Results
TGF-β-modulated ovarian cancer cell motility in the presence of ovarian fibroblasts
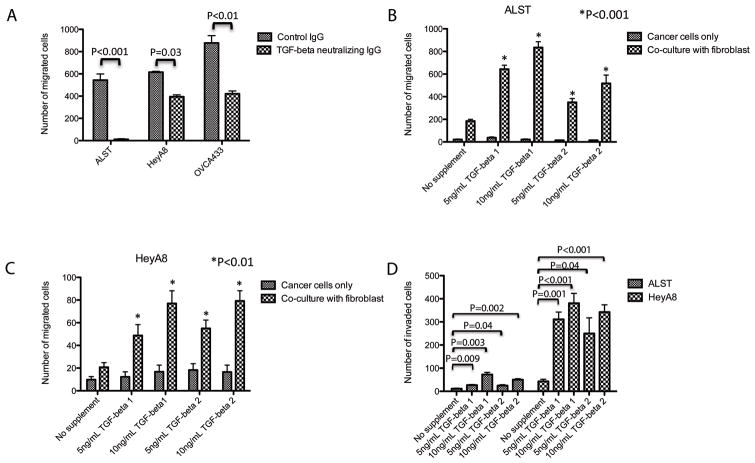
To evaluate the effect of TGF-β on ovarian cancer cell motility and invasion potential in the presence of ovarian stromal fibroblasts, high-grade serous ovarian cancer cell lines ALST, HeyA8, and OVCA433 cells were co-cultured with a telomerase immortalized human ovarian fibroblast cell line, NOF151-hTERT, in the presence of an anti-TGF-β antibody. The results of migration assays demonstrated that inclusion of the TGF-β-neutralizing antibody abrogated the stimulating effect of fibroblasts on ovarian cancer cell motility (Fig. 1A), suggesting that in the tumor microenvironment, TGF-β was able to modulate the behavior of ovarian cancer cells via the fibroblasts.
Figure 1.
TGF-β modulates ovarian cancer cell motility in the presence of fibroblasts. (A) In a co-culture system of normal fibroblasts and ovarian cancer cells, TGF-neutralizing antibody inhibited cancer cell motility, suggesting that the motility is TGF-β-dependent. Co-culture of fibroblasts with (B) ALST and (C) HeyA8 cells demonstrated that the presence of ovarian fibroblasts is required for TGF-β1- and 2-modulated cancer cell motility. TGF-β had no effect on cancer cell motility in the absence of fibroblasts. (D) ALST and HeyA8 cells were co-cultured with fibroblasts. In the presence of TGF-β and ovarian fibroblasts, cancer cells demonstrated a significant increase in invasion potential.
To better understand the role of TGF-β, we measured the concentrations of TGF-β 1 and 2 in the ALST, NOF151-hTERT co-culture system. We harvested conditioned culture medium from the co-culture system at 24 and 48 hours and measured the concentrations of TGF-β using ELISA. We detected higher concentrations of both TGF-β 1 and 2 in the co-culture system when compared to the culture with either cell types alone (Supplementary Tables S1A and S1B). However, the extent of the increases in TGF-β concentration led us to believe that they resulted mainly from increased cell numbers rather than induction of TGF-β expression by co-culture. We validated this result by co-culturing ovarian cancer cells and fibroblasts and then performing TGF-β expression analysis. We did not detect a significant increase in TGF-β mRNA expression in either cell type (data not shown). Also, ELISA results demonstrated that the presence of the TGF-β-neutralizing antibody significantly decreased the detectable amount of TGF-β in the system.
To further determine whether fibroblasts mediated the effect of TGF-β on ovarian cancer cell behavior, we performed a co-culture study using NOF151-hTERT fibroblasts and ALST and HeyA8 cells in the presence of exogenous TGF-β 1 and TGF-β 2. While TFG-β had no effect on the motility of either cancer cell line in the absence of fibroblasts (Figs. 1B and 1C), but the motility of both cell lines increased significantly (P < 0.01) when they were co-cultured with the fibroblasts. These data suggest that ovarian fibroblasts mediate the indirect stimulating effect of TGF-β on ovarian cancer cell motility.
We also assayed the invasion potential of ALST and HeyA8 cells co-cultured with ovarian fibroblasts. In the presence of TGF-β, the cancer cells in the co-culture system demonstrated increased invasion potential (Fig. 1D).
Differential expression of TGF-β and its receptors in epithelial and stromal cells in normal and malignant ovarian tissue samples
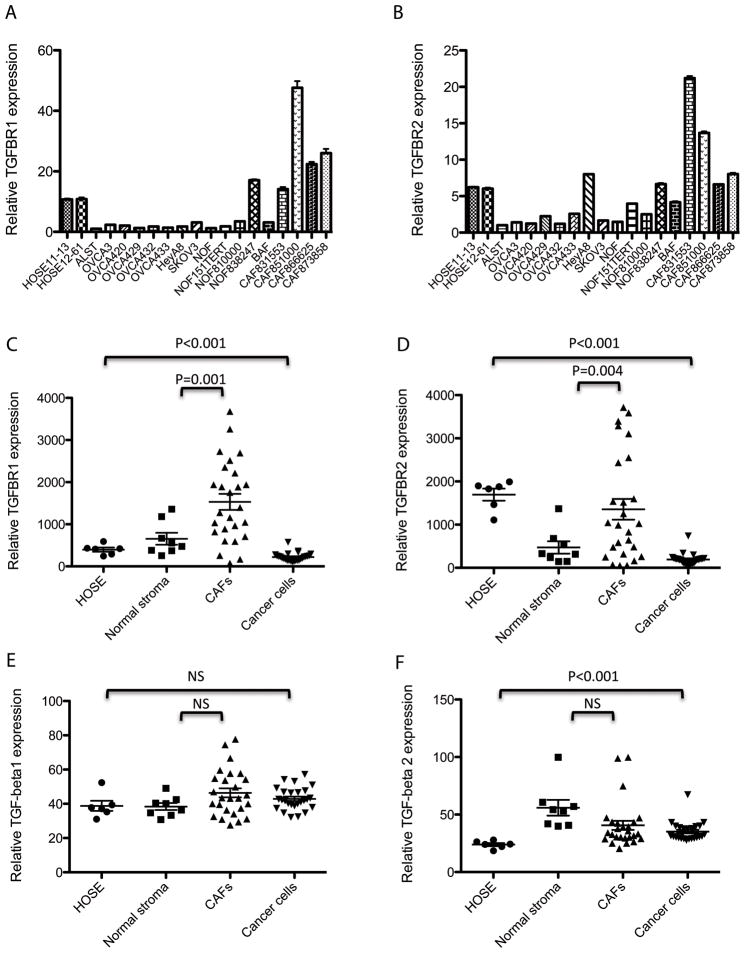
To identify the molecular mechanism that conferred TGF-β non-responsiveness in ovarian cancer cells, we first performed qRT-PCR analysis of TGF-β receptor type 1 and 2 expression in a panel of high-grade serous ovarian cancer cell lines, OSE cells, NOFs, and ovarian CAFs. We observed significantly lower expression of the TGF-β receptors in the cancer cells than in the OSE cells (Figs. 2A and 2B), suggesting that the non-responsiveness to TGF-β resulted in part from the downregulation of the receptors. To further validate downregulation of receptors expression in ovarian cancer cells in vivo, we examined the receptor expression in transcriptome profiles generated from laser-microdissected epithelial and stromal fibroblastic components of both normal and malignant ovarian tissue samples (Figs. 2C and 2D). We found that epithelial tumor cells had significantly lower levels of TGF-β receptor type 1 and 2 expression than did normal OSE cells (P < 0.001). Also, to evaluate the protein expression and localization of the TGF-β receptors, we performed immunolocalization of TGF-β receptors type 1 and 2 in ovarian tumor and normal ovarian tissue sections. Specifically, we stained sections of high-grade serous ovarian tumors (n = 15) and normal ovarian tissue (n = 8) for both receptors and quantified the staining intensity (Supplementary Figs. S2A–S2D). The results demonstrated lower TGF-β receptor type 1 and 2 protein expression in ovarian cancer cells than in normal ovarian epithelial cells (P < 0.001) (Supplementary Figs. S2E and S2F). Unlike in normal surface epithelium, ovarian cancer cells had no distinct cell surface staining for the receptors. The expression of the two receptors in ovarian cancer stroma by CAFs was visualized under high magnification (Supplementary Figs. S2G and S2H).
Figure 2.
Expression levels of TGF-β and its receptors in various tissue types. Expression levels of (A) TGF-β receptor type 1 and (B) type 2 in OSE cells, ovarian cancer cells, NOFs and CAFs were examined. A significantly lower Expression of the TGF-β receptors in the cancer cells than that in the OSE cells was observed. Plots showing the expression levels of (C) TGF-β receptor type 1 and (D) type 2 in transcriptome profiles generated from laser-microdissected epithelial and stromal fibroblastic components of both normal and malignant ovarian tissue samples. Plots showing expression levels of (E) TGF-β1 and (F) TGF-β2 in transcriptome profiles generated from laser-microdissected ovarian tissue samples. The data suggest that both tumor cells and CAFs are sources of TGF-β1 and 2 in the ovarian tumor microenvironment.
Our transcriptome profiling data on microdissected CAFs and NOFs tissue samples demonstrated that CAFs expressed significantly higher levels of both type of receptors than did NOFs (P = 0.001 and P = 0.004, respectively) (Figs. 2C and 2D). We observed the same result in cultured primary CAFs (Figs. 2A and 2B). These data suggested that CAFs in the ovarian tumor microenvironment may be more responsive to TGF-β than are NOFs in normal ovaries.
Next, we evaluated the TGF-β expression in different ovarian tissue components using transcriptome profiles from laser-microdissected ovarian tissue samples. The results demonstrated that all of the cell types had comparable levels of TGF-β1 expression (Fig. 2E). Also, tumor and stromal cells (NOFs and CAFs) had comparable levels of TGF-β2 expression, whereas normal OSE cells expressed slightly lower levels of TGF-β2 than did the other cell types (Fig. 2F). These data suggested that both ovarian cancer cells and CAFs are sources of TGF-β in the ovarian tumor microenvironment.
Identification of a TGF-β-responsive gene signature in cancer associated fibroblasts
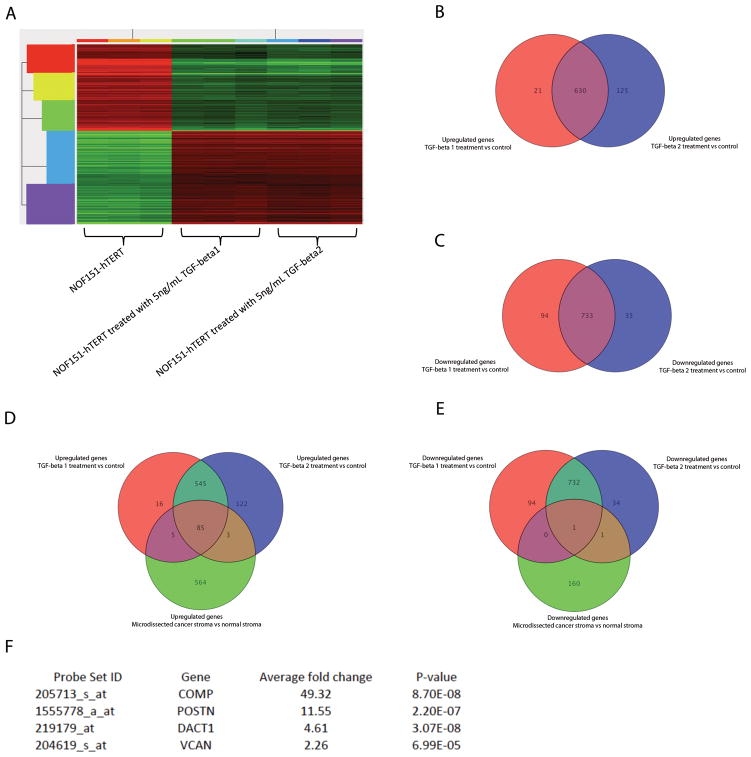
To identify genes whose expression is regulated by TGF-β in ovarian fibroblasts, we generated transcriptome profiles from TGF-β1- and TGF-β2-treated NOF151-hTERT fibroblasts (Gene Expression Omnibus GES40266). We identified 651 upregulated and 827 downregulated genes in TGF-β1-treated NOF151-hTERT cells, and 755 upregulated and 768 downregulated genes in TGF-β2-treated cells, showing more than twofold differences when compared to untreated cells (P < 0.05) (Fig. 3A). We also demonstrated that a majority of the differentially expressed genes were common to the two treatment profiles (Figs. 3B and 3C). These results suggested that TGF-β1 and 2 may have very similar effects on NOF151-hTERT fibroblasts.
Figure 3.
Identification of a TGF-β-responsive gene signature in ovarian fibroblasts. (A) Heat map showing a TGF-β-induced gene expression signature in NOF151-hTERT fibroblasts treated with TGF-β. Venn diagrams showing a high degree of overlap of TGF-β1- and 2-induced genes. (B) More than 83% of upregulated genes and (C) more than 88% of downregulated genes were common to both TGF-β1 and 2 treatments. Venn diagram showing that (D) 85 upregulated and (E) 1 downregulated probe set were common to the gene signatures for CAF by comparing transcriptome profiles generated from microdissected ovarian NOFs and CAFs and the gene signature induced by TGF-β, representing genes that are CAF-specific and regulated by TGF-β (See also Supplementary Table S1). (F) Four TGF-β-induced, CAF-specific genes were chosen for further validation.
Next, we obtained a gene signature for CAFs by comparing transcriptome profiles generated from microdissected ovarian NOFs and CAFs (Gene Expression Omnibus GES40595). We identified 657 upregulated and 162 downregulated genes that showed significant differences between the two types of fibroblasts. We then compared the gene signature with that generated from TGF-β-treated NOF151-hTERT fibroblasts. We identified 85 upregulated probe sets and 1 downregulated probe set that were common to the two signatures (Figs. 3D and 3E), suggesting that these sets represent CAF-specific genes whose expression is regulated in the TGF-β-rich ovarian tumor microenvironment. In the GeneChip Human Genome U133 Plus 2.0 microarray, a gene is represented by multiple probe sets on the chip, and 11 probe pairs (perfect match and mismatch) are included within each probe set to interrogate a given sequence. Using the probe set IDs, a list of 71 upregulated genes was generated for further analysis (Supplementary Table S3).
Validation of the patterns of expression of TGF-β-responsive genes in NOFs
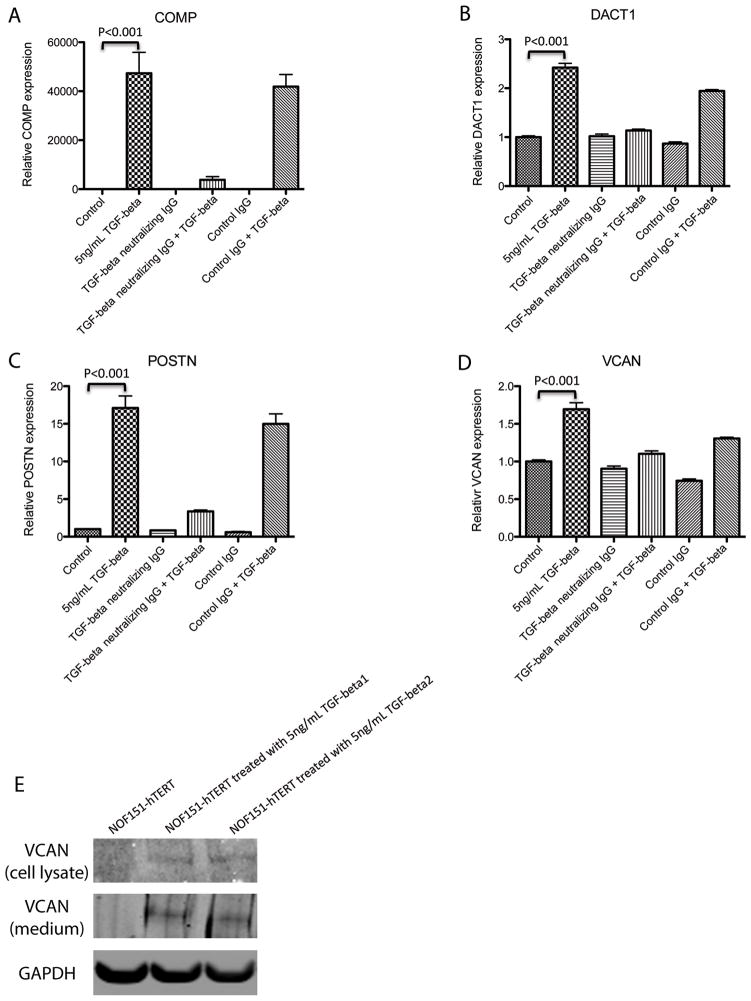
In this study, we focused on how TGF-β modulates the motility and invasion potential of ovarian cancer cells via fibroblasts. Among the 71 upregulated genes, 4 non-collagen and ECM-related genes (Fig. 3F) were chosen for validation because of their potential ability to modify the ECM or behavior of surrounding cancer cells, which may subsequently affect the motility and invasion potential of cancer cells. Specifically, we selected VCAN, periostin (POSTN), and cartilage oligomeric matrix protein (COMP), which code for secretory proteins produced by fibroblasts; and dapper, antagonist of β-catenin, homolog 1 (DACT1), which is implicated to be involved in the development of fibrosis by interacting with Wnt signaling, for validation in TGF-β-treated NOFs using qRT-PCR (Figs. 4A–4D). Treatment with TGF-β1 significantly increased the expression of all four genes (P < 0.001). The TGF-β-induced increase was attenuated in the presence of a TGF-β-neutralizing antibody, however. These results confirmed that upregulation of the four genes in ovarian fibroblasts was mediated by TGF-β. VCAN, a poor prognostic marker for ovarian cancer (12), was selected for further studies. We confirmed upregulation of VCAN protein expression by Western blot analysis. Protein from NOF151-hTERT cell lysate and concentrated conditioned medium was used for analysis. We detected significant increases in the amounts of intracellular and secreted VCAN protein in TGF-β-treated NOF151-hTERT fibroblasts (Fig. 4E).
Figure 4.
TGF-β mediates ovarian cancer cell behavior by inducing stromal factors. TGF-β induction of (A) COMP, (B) DACT1, (C) POSTN, and (D) VCAN in ovarian fibroblasts was validated using qRT-PCR analysis. Treatment with TGF-β1 significantly increased the expression levels of them in NOF151-hTERT cells (P < 0.001). In the presence of TGF-β-neutralizing antibody, upregulation of the four genes was inhibited, suggesting that the induction was TGF-β-specific. (E) Western blot analysis validating the induction of VCAN protein expression in NOF151-hTERT fibroblasts by TGF-β. Increased VCAN protein expression was detected in both lysates of and conditioned medium from TGF-β-treated fibroblasts.
Induction of SMAD signaling pathway-mediated VCAN expression by TGF-β
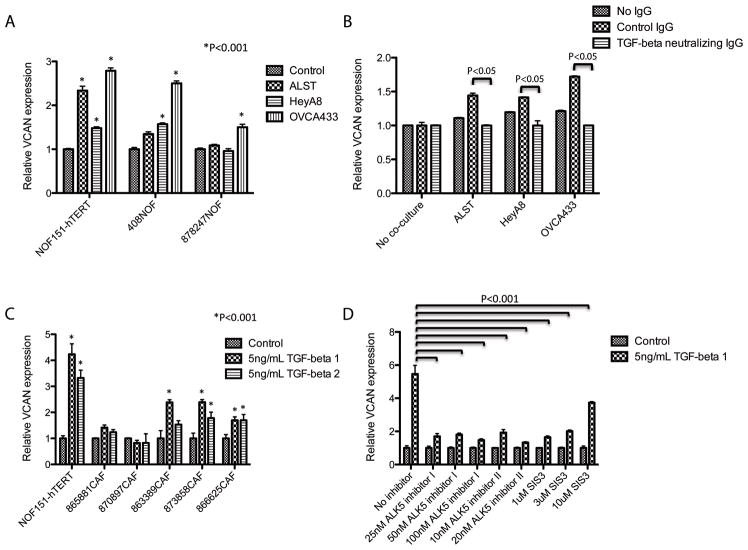
In a co-culture of NOF151-hTERT fibroblasts with ovarian cancer cell lines (A2780, ALST, and OVCA433), we observed significantly higher levels of VCAN mRNA expression in the fibroblasts (P < 0.001), suggesting that mediators secreted by ovarian cancer cells transcriptionally upregulate VCAN expression in fibroblasts (Fig. 5A). To determine whether induction of VCAN expression was mediated by TGF-β produced by the ovarian cancer cells, we introduced TGF-β-neutralizing antibody into the co-culture system. Experimental results demonstrated that NOF151-hTERT fibroblasts had significantly lower expression of VCAN than did the control (P < 0.05) (Fig. 5B). To further evaluate the effect of TGF-β on VCAN expression, qRT-PCR analysis was performed on TGF-β-treated NOF151-hTERT fibroblasts and CAF lines. TGF-β significantly increased VCAN expression in the NOF151-hTERT cells (P < 0.001) (Fig. 5C). Furthermore, TGF-β1 induced significant increases in VCAN expression in three of the CAF lines (P < 0.001).
Figure 5.
Ovarian cancer cell-derived TGF-β induces VCAN expression in fibroblasts. (A) In a co-culture experiment, VCAN expression in fibroblasts was induced by the presence of cancer cells. (B) In the co-culture, the effect of cancer cells on upregulation of VCAN in the fibroblasts was abrogated in the presence of TGF-β-neutralizing antibody, suggesting that TGF-β secreted by cancer cells is required for VCAN induction. (C) A graph showing that exogenous TGF-β was able to induce VCAN expression in normal fibroblasts and some CAF lines. (D) A graph showing the effect of SMAD pathway inhibitors on TGF-β-mediated VCAN expression in NOF151-hTERT fibroblasts. The presence of SMAD inhibitors abrogated the effect of TGF-β.
To determine whether induction of VCAN expression in ovarian fibroblasts by TGF-β is mediated by the TGF-β receptors and SMAD signaling, we examined this induction in the presence of inhibitors. The results demonstrated that the TGF-β receptor inhibitors ALK5 inhibitor I and II and SMAD3-specific inhibitor SIS3 significantly abrogated induction of VCAN expression in NOF151-hTERT fibroblasts (Fig. 5D). We validated these results in an additional primary CAF line (Supplementary Figure S3). These data confirmed that the TGF-β receptors and SMAD signaling regulated VCAN expression in the ovarian fibroblasts.
Mediation of TGF-β enhancement of ovarian cancer motility by upregulation of VCAN expression in neighboring stromal fibroblasts
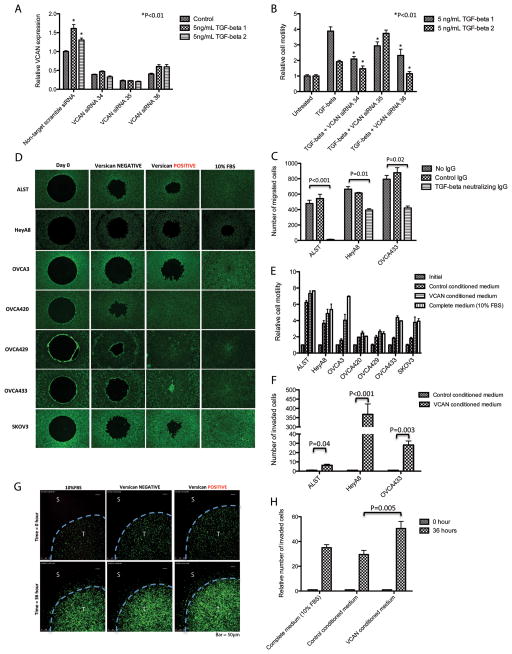
We observed that ALST cell motility was significantly increased when co-cultured with TGF-β-treated ovarian fibroblasts. To confirm that the increase in motility was VCAN-dependent, we repeated the co-culture experiment using NOF151-hTERT fibroblasts transfected with VCAN-specific siRNA. TGF-β treatment did not upregulate VCAN mRNA in siRNA-transfected cells (Fig. 6A). Subsequently, using a Transwell co-culture migration assay, we demonstrated that TGF-β-induced motility of ovarian cancer cells was reduced when co-cultured with VCAN-silenced NOF151-hTERT fibroblasts. TGF-β1 and 2-induced ALST cell motility was significantly abrogated in co-culture with VCAN siRNA-transfected fibroblasts (P < 0.01) (Fig. 6B).
Figure 6.
TGF-β-induced upregulation of VCAN in ovarian stromal fibroblasts enhances ovarian cancer cell motility. (A) VCAN-specific siRNA was transfected into NOF151-hTERT to inhibit the effect of TGF-β on VCAN upregulation. (B) While TGF-β enhanced cancer cell motility in the presence of fibroblasts, the enhancement was inhibited when VCAN-targeting siRNA-transfected fibroblasts were used, suggesting that VCAN is an essential stromal factor responsible for enhanced cancer cell motility. (C) TGF-β-modulated cancer cell motility was validated by inclusion of a TGF-β-neutralizing antibody in the co-culture system. (D) Wound healing assay was performed and (E) cell motility was quantified. Enhanced cell motility was observed in all VCAN-treated cancer lines. (F) Matrigel invasion assay was performed, a significant increase in invasion potential was observed in VCAN-treated cancer cells. (G) Images from live cell imaging of HeyA8 ovarian cancer cells in 0.2% type I collagen matrix. Increased cell invasion was observed in the 3D culture system with VCAN (T: tumor; S: stroma). (H) The relative numbers of invasive cells in the different setups were determined by measuring the fluorescence emitted by GFP-labeled HeyA8 cells. Cells invaded and moved further away from their original locations in the 3D matrix in the presence of conditioned medium with VCAN.
Polyclonal anti-VCAN antibody was also used to block the effect of TGF-β-induced VCAN on ALST, HeyA8, and OVCA433 cell motility. In the presence of anti-VCAN antibody, TGF-β-induced cell motility was attenuated (Fig. 6C). These data suggested that TGF-β-induced VCAN expression in ovarian fibroblasts is essential for enhancement of ovarian cancer cell motility.
To further confirm the direct effect of VCAN on ovarian cancer cell motility, wound healing assays were performed with ovarian cancer cell lines ALST, HeyA8, OVCA3, OVCA420, OVCA429, OVCA433, and SKOV3 treated with VCAN-positive or -negative conditioned medium (Figs. 6D and 6E). The results demonstrated that treatment of the ovarian cells with VCAN significantly increased cell motility. These results supported our hypothesis that TGF-β-induced ovarian cancer cell motility is mediated by fibroblast-derived VCAN.
In addition, we evaluated the effects of VCAN on ALST, HeyA8, and OVCA433 cells’ invasion potential using a Matrigel invasion assay. The results demonstrated that in the presence of exogenous VCAN, the invasion potential increased significantly (Fig. 6F). The effect of VCAN on ovarian cancer cell invasion was also confirmed using a three-dimensional (3D) culture model and confocal microscopy (Figs. 6G and 6H).
Signaling events contributing to the effects of VCAN on ovarian cancer cells
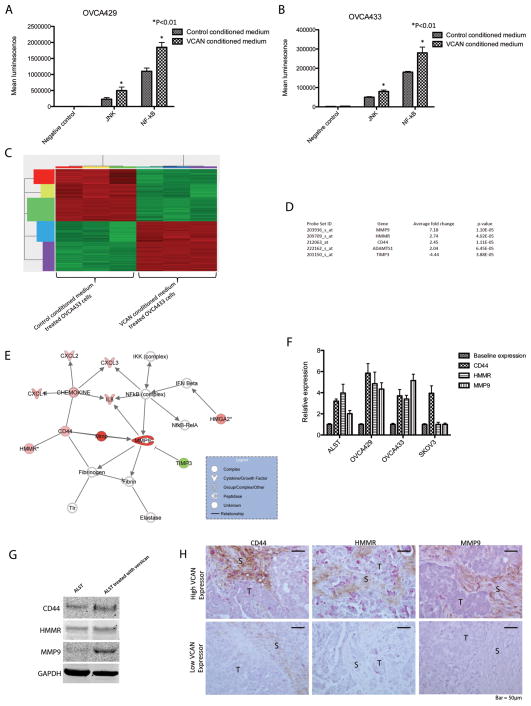
To identify signaling molecules and events involved in mediation of VCAN’s effect on ovarian cancer cell motility and invasion potential, we conducted luciferase reporter assays for seven the major signaling pathways, ERK, Wnt, Notch, JNK, PKC, NF-κB and PI3K, which are frequently involved in the progression of human cancers, in the VCAN-conditioned medium-treated ovarian cancer cell lines OVCA429 and OVCA433. We observed that the JNK and NF-κB pathways were significantly activated (P < 0.01) (Figs. 7A and 7B).
Figure 7.
Signaling events contributing to the effects of VCAN on ovarian cancer cells. Luciferase reporter assay was performed using (A) OVCA429 and (B) OVCA433 cell lines. Results for both cell lines suggested that the JNK and NF-κB signaling pathways were activated upon treatment with VCAN. (C) Transcriptome profiling of VCAN-treated OVCA433 cells revealed 288 differentially expressed genes (See also Supplementary Table S3). (D) Five genes identified in the microarray experiment. These genes have been shown to related to cell motility and ECM degradation. (E) Strong association among of CD44, HMMR, and MMP9 with the NF-κB signaling pathway was identified, suggesting that these VCAN-activated genes may be tightly regulated with each other. (F) The effect of exogenous VCAN on CD44, HMMR, and MMP9 expression in ovarian cancer cell lines was validated using qRT-PCR analysis. (G) Increased CD44, HMMR, and MMP9 protein expression in ALST ovarian cancer cells by VCAN treatment was validated via Western blot analysis. (H) Immunolocalization was performed to demonstrate the association between stromal VCAN expression and HMMR, CD44, and MMP9 expression in ovarian cancer cells in vivo. The results showed that in tissue sections obtained from the same patient, regions with intense stromal VCAN staining (brown) frequently had high levels of HMMR, CD44, and MMP9 expression (pink). (T: tumor; S: stroma).
To identify downstream target genes for VCAN signaling, we transcriptome profiled VCAN-treated OVCA433 cells. We identified 288 genes that were differentially expressed in VCAN-treated OVCA433 cells and control cells (Gene Expression Omnibus GES40241) (Fig. 7C; Supplementary Table S2). Five of these genes, which are involved in either cell motility or ECM degradation were chosen for validation. Expression of four of the genes—matrix metallopeptidase 9 (MMP9), hyaluronan (HA)-mediated motility receptor (HMMR), a disintegrin and metalloproteinase with thrombospondin motifs 1 (ADAMTS1), and CD44—was upregulated, whereas that of the fifth gene—tissue inhibitor of metalloproteinase 3 (TIMP3)—was downregulated (Fig. 7D). Ingenuity Pathway Analysis (Ingenuity Systems) showed a strong association among these genes and the VCAN-activated signaling pathways that we identified (Fig. 7E).
To further validate the effect of exogenous VCAN on MMP9, HMMR, CD44, ADAMTS1, and TIMP3 expression in ovarian cancer cells, qRT-PCR analysis were performed on ovarian cancer cell lines ALST, OVCA429, OVCA433, and SKOV3 treated with VCAN. We confirmed upregulation of CD44 expression in all four cell lines and VCAN-induced upregulation of MMP9 and HMMR expression in all cell lines but SKOV3 (Fig. 7F). Furthermore, we validated the protein expression of CD44, HMMR, and MMP9, whose expression are regulated by VCAN, in ALST (Fig. 7G) and OVCA433 (data not shown) cells. Increased expression levels of CD44, HMMR, and MMP9 were observed in VCAN-treated ovarian cancer cells.
To demonstrate the association between stromal VCAN expression and HMMR, CD44, and MMP9 expression in ovarian cancer cells in vivo, immunolocalization of these proteins was performed on tissue sections obtained from high-grade serous ovarian cancer patients. The results showed that regions with intense stromal VCAN staining frequently corresponded to regions with high levels of HMMR, CD44, and MMP9 protein expression, particularly at the stroma-cancer interface (Fig. 7H). This suggests that CAF-derived VCAN modulates ovarian cancer cell motility and invasion potential via activation of HMMR, CD44, and MMP9.
Discussion
Ovarian cancer can be classified into type I and type II tumors based on their pathways of tumorigenesis (13). While type I tumors tend to be low-grade neoplasms, type II tumors are high-grade neoplasms. High-grade serous ovarian carcinomas are type II surface epithelial tumors which show frequent p53 mutation and in the clinical standpoint, they are aggressive tumors which progress rapidly. Owning to the fact that high-grade serous carcinoma is the most common type of ovarian cancer, our study focused on this specific type of cancer.
In this study, we identified a TGF-β-regulated gene signature in CAFs and investigated the molecular mechanism by which TGF-β upregulated VCAN. TGF-β regulates many cellular processes, including cell growth, differentiation, adhesion, migration, and apoptosis (14). Through TGF-β receptors and SMAD signaling, TGF-β activates or represses specific target genes (15, 16). The mechanisms involved in the resistance of ovarian cancer cells to the antiproliferative effects of TGF-β remain unclear and controversial (6). However, our findings reported therein demonstrated that ovarian cancer cells express significantly lower levels of both TGF-β receptors than do normal OSE cells, suggesting that downregulation of the receptors may play a key role in conferring resistance to TGF-β in ovarian cancer cells. We also found that ovarian CAFs have significantly higher levels of both receptors than do NOFs and ovarian cancer cells, suggesting that CAFs in the ovarian tumor microenvironment are more responsive to TGF-β ligands than are other cell types.
Multiple in vivo studies have shown that the TGF-β ligand-enriched pro-inflammatory tumor microenvironment particularly created by the recruited macrophages plays a key role in tumor initiation and progression (16–19). TGF-β activates stromal cells, including immune cells, and fibroblasts, which subsequently modulate neighboring epithelial cell growth and oncogenic potential. This supports our finding that TGF-β enhanced ovarian tumor cell motility and invasion potential in the stromal-epithelial cell co-culture system. Because CAFs express high levels of both TGF-β receptor types 1 and 2, enhanced TGF-β signaling in these cells may modulate the motility and invasion potential of the adjacent ovarian cancer cells.
Recent studies demonstrated that TGF-β is in part responsible for activating fibroblasts in a number of cancer types (7–9). A majority of the TGF-β-responsive genes identified in our study represent ECM proteins and have been implicated to support cancer stem cell growth or bone and cartilage morphogenesis. For example, POSTN is required for breast cancer stem cell maintenance and facilitation of breast cancer cell metastasis (20, 21). Also, COMP interacts with other ECM proteins to enhance cartilage organization and assembly (22) or protect HeLa cells against apoptosis (23). Furthermore, DACT1 is implicated to be involved in development of dermal fibrosis (24). These data suggest that these CAF-derived ECM proteins may develop a niche in the TGF-β-rich ovarian tumor microenvironment, which supports ovarian stem cell growth. In addition, the niche may provide a rigid microenvironment, which has been demonstrated to facilitate tumor invasion and metastasis (25, 26).
VCAN is another ECM-related gene implicated to play a crucial role in ovarian tumor progression. VCAN has been shown to bind HA, which subsequently activates CD44-mediated downstream signaling (27). Previous studies showed that VCAN-treated cancer cells incorporated VCAN and HA into a pericellular matrix that promoted their motility and that the effect correlated with the cancer cells’ ability to express the HA receptor, CD44 (28, 29). Despite these findings, however, the functional role of stromal VCAN in ovarian cancer progression has yet to be thoroughly evaluated.
Using a co-culture model, we demonstrated in the present study that TGF-β-induced ovarian cancer cell motility was abrogated by silencing of VCAN in NOFs using siRNAs and a VCAN-blocking antibody and that TGF-β-induced VCAN expression was attenuated by the addition of a TGF-β-neutralizing antibody or in the presence of a TGF-β receptor and SMAD3-specific inhibitors. These data confirm that TGF-β modulates the invasion potential of ovarian cancer cells via upregulation of CAF-derived VCAN, which most likely results from transcriptional activation of the VCAN promoter through the potential Sp1 and AP1 binding sites (Supplementary Fig. S4A).
Our data suggest that induction of increased ovarian cancer cell migration or invasion potential by stromal VCAN may be mediated through binding of VCAN to CD44, which subsequently activates the NF-κB and JNK signaling pathways in ovarian cancer cells and increases CD44, HMMR, and MMP9 production. Upregulation of CD44 may activate the autocrine loop for VCAN and HA mediated signaling in ovarian cancer cells. Also, upregulation of HMMR may activate HA mediated signaling pathways, which have been shown to enhance tumor cells’ focal adhesion, migration, and cell cycle progression (30). In the present study, CD44 was identified in the microarray study by a probe set that detects CD44 isoforms 1–7. Although not examined in this study, the eight CD44 isoforms are known to be differentially expressed in different tissue types, an interesting aspect for inclusion in future studies. Upregulation and activation of MMP9, which has been shown to be activated by the NF-κB pathway and downregulation of TIMP3, respectively, may facilitate the breakdown and remodeling of the ECM and the invasion potential of tumor cells, which may further contribute to tumor progression and subsequently affect patient survival rates.
Because epithelial-to-mesenchymal transition (EMT) is a key phenotypic change that contributes to cell motility, we sought to determine whether EMT was induced in the proposed model, First, we co-cultured ovarian cancer cell lines with fibroblasts in the absence or presence of a TGF-β-neutralizing antibody. We then extracted RNA from ovarian cancer cells and examined the expression levels of an epithelial marker (E-cadherin) and mesenchymal markers (N-cadherin and vimentin). If EMT is induced in cancer cells by co-culture with fibroblasts in the presence of TGF-β, a decreased level of E-cadherin and increased levels of N-cadherin and vimentin should be observed in the control group when compared to the TGF-β-neutralizing antibody group. However, our experimental results did not support this (Supplementary Figs. S5A–S5C).
We also studied the protein expression levels of EMT markers in ovarian cancer cell lines using Western blot analysis. Cells were treated with exogenous VCAN and expression levels of E-cadherin, N-cadherin, and vimentin were examined. We observed no significant changes in EMT marker expression levels between VCAN treated and untreated cancer cells.
Kang et al. (31) found that cross-talk between TGF-β and EGF receptor (EGFR) signaling induced EMT in liver cancer cells. In that study, expression of transmembrane 4 L6 family member 5 (TM4SF5), an EMT inducer, was upregulated in liver cancer cells by exogenous TGF-beta 1 and EGFR signaling pathways were involved. However, in our ovarian cancer model, we observed VCAN-induced EGFR expression similar to that described by Sheng et al. (32) but did not observe significant EMT under the experimental conditions. We speculated that the main difference between the two models was their responses to TGF-β. Specifically, whereas liver cancer cells respond directly to exogenous TGF-β and have TM4SF5 expression induced, ovarian cancer cells have minimal response to exogenous TGF-β. This also explained the negative EMT phenotypes of ovarian cancer cells when co-cultured with fibroblasts. Though ovarian cancer cells were provided with a TGF-β enriched environment in the co-culture system, our study demonstrated that EMT was not induced and that exogenous TGF-β had little effect on ovarian cancer cell motility. In our proposed model, whereas EGFR activation by VCAN may increase cancer cell motility, EMT may not be directly involved.
In conclusion, we describe herein a model of TGF-β-modulated molecular cross-talk between ovarian cancer cells and CAFs in the ovarian tumor microenvironment (Supplementary Fig. S4B). Upregulation of expression of TGF-β-responsive genes such as VCAN in CAFs, together with the loss of the inhibitory effect of TGF-β in ovarian cancer cells, may result in tumor progression. Understanding the role of TGF-β as a mediator in epithelial-mesenchymal interaction in ovarian cancer may aid the development of new treatment strategies for ovarian cancer and lengthen survival durations in patients.
Supplementary Material
Acknowledgments
Grant Support: This study was supported in part by grants R01CA133057 and RC4CA156551 and MD Anderson Ovarian Cancer SPORE grant P50CA083639 from the National Institutes of Health and by the MD Anderson Cancer Center Support Grant CA016672.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.Wu X, Groves FD, McLaughlin CC, Jemal A, Martin J, Chen VW. Cancer incidence patterns among adolescents and young adults in the United States. Cancer Causes Control. 2005;16:309–20. doi: 10.1007/s10552-004-4026-0. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. The New England journal of medicine. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 4.Yamada SD, Baldwin RL, Karlan BY. Ovarian carcinoma cell cultures are resistant to TGF-beta1-mediated growth inhibition despite expression of functional receptors. Gynecol Oncol. 1999;75:72–7. doi: 10.1006/gyno.1999.5535. [DOI] [PubMed] [Google Scholar]
- 5.Baldwin RL, Tran H, Karlan BY. Loss of c-myc repression coincides with ovarian cancer resistance to transforming growth factor beta growth arrest independent of transforming growth factor beta/Smad signaling. Cancer Res. 2003;63:1413–9. [PubMed] [Google Scholar]
- 6.Sunde JS, Donninger H, Wu K, Johnson ME, Pestell RG, Rose GS, et al. Expression profiling identifies altered expression of genes that contribute to the inhibition of transforming growth factor-beta signaling in ovarian cancer. Cancer Res. 2006;66:8404–12. doi: 10.1158/0008-5472.CAN-06-0683. [DOI] [PubMed] [Google Scholar]
- 7.Guido C, Whitaker-Menezes D, Capparelli C, Balliet R, Lin Z, Pestell RG, et al. Metabolic reprogramming of cancer-associated fibroblasts by TGF-beta drives tumor growth: Connecting TGF-beta signaling with “Warburg-like” cancer metabolism and L-lactate production. Cell Cycle. 2012:11. doi: 10.4161/cc.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco OE, Jiang M, Strand DW, Peacock J, Fernandez S, Jackson RS, 2nd, et al. Altered TGF-beta signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 2011;71:1272–81. doi: 10.1158/0008-5472.CAN-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ao M, Franco OE, Park D, Raman D, Williams K, Hayward SW. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67:4244–53. doi: 10.1158/0008-5472.CAN-06-3946. [DOI] [PubMed] [Google Scholar]
- 10.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annual review of pathology. 2006;1:119–50. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 11.Tlsty TD, Hein PW. Know thy neighbor: stromal cells can contribute oncogenic signals. Curr Opin Genet Dev. 2001;11:54–9. doi: 10.1016/s0959-437x(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh S, Albitar L, LeBaron R, Welch WR, Samimi G, Birrer MJ, et al. Up-regulation of stromal versican expression in advanced stage serous ovarian cancer. Gynecologic oncology. 2010;119:114–20. doi: 10.1016/j.ygyno.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shih Ie M, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–8. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 16.Drabsch Y, Ten Dijke P. TGF-beta signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev. 2012 doi: 10.1007/s10555-012-9375-7. [DOI] [PubMed] [Google Scholar]
- 17.Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A. 2004;101:4966–71. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mak P, Leav I, Pursell B, Bae D, Yang X, Taglienti CA, et al. ERbeta impedes prostate cancer EMT by destabilizing HIF-1alpha and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell. 2010;17:319–32. doi: 10.1016/j.ccr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 20.Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–9. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Ouyang G. Periostin: a bridge between cancer stem cells and their metastatic niche. Cell Stem Cell. 2012;10:111–2. doi: 10.1016/j.stem.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Haleem-Smith H, Calderon R, Song Y, Tuan RS, Chen FH. Cartilage oligomeric matrix protein enhances matrix assembly during chondrogenesis of human mesenchymal stem cells. J Cell Biochem. 2012;113:1245–52. doi: 10.1002/jcb.23455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gagarina V, Carlberg AL, Pereira-Mouries L, Hall DJ. Cartilage oligomeric matrix protein protects cells against death by elevating members of the IAP family of survival proteins. J Biol Chem. 2008;283:648–59. doi: 10.1074/jbc.M704035200. [DOI] [PubMed] [Google Scholar]
- 24.Bayle J, Fitch J, Jacobsen K, Kumar R, Lafyatis R, Lemaire R. Increased expression of Wnt2 and SFRP4 in Tsk mouse skin: role of Wnt signaling in altered dermal fibrillin deposition and systemic sclerosis. J Invest Dermatol. 2008;128:871–81. doi: 10.1038/sj.jid.5701101. [DOI] [PubMed] [Google Scholar]
- 25.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–22. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–94. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- 28.Ricciardelli C, Russell DL, Ween MP, Mayne K, Suwiwat S, Byers S, et al. Formation of hyaluronan- and versican-rich pericellular matrix by prostate cancer cells promotes cell motility. J Biol Chem. 2007;282:10814–25. doi: 10.1074/jbc.M606991200. [DOI] [PubMed] [Google Scholar]
- 29.Gardner MJ, Catterall JB, Jones LM, Turner GA. Human ovarian tumour cells can bind hyaluronic acid via membrane CD44: a possible step in peritoneal metastasis. Clin Exp Metastasis. 1996;14:325–34. doi: 10.1007/BF00123391. [DOI] [PubMed] [Google Scholar]
- 30.Hall CL, Lange LA, Prober DA, Zhang S, Turley EA. pp60(c-src) is required for cell locomotion regulated by the hyaluronan receptor RHAMM. Oncogene. 1996;13:2213–24. [PubMed] [Google Scholar]
- 31.Kang M, Choi S, Jeong SJ, Lee SA, Kwak TK, Kim H, et al. Cross-talk between TGFbeta1 and EGFR signalling pathways induces TM4SF5 expression and epithelial-mesenchymal transition. The Biochemical journal. 2012;443:691–700. doi: 10.1042/BJ20111584. [DOI] [PubMed] [Google Scholar]
- 32.Sheng W, Wang G, Wang Y, Liang J, Wen J, Zheng PS, et al. The roles of versican V1 and V2 isoforms in cell proliferation and apoptosis. Mol Biol Cell. 2005;16:1330–40. doi: 10.1091/mbc.E04-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellenrieder V. TGFβ regulated gene expression by Smads and Sp1/KLF-like transcription factors in cancer. Anticancer Res. 2008;28:1531–9. [PubMed] [Google Scholar]
- 34.Jungert K, Buck A, Buchholz M, Wagner M, Adler G, Gress TM, et al. Smad-Sp1 complexes mediate TGFβ-induced early transcription of oncogenic Smad7 in pancreatic cancer cells. Carcinogenesis. 2006;27:2392–401. doi: 10.1093/carcin/bgl078. [DOI] [PubMed] [Google Scholar]
- 35.Li JM, Datto MB, Shen X, Hu PP, Yu Y, Wang XF. Sp1, but not Sp3, functions to mediate promoter activation by TGF-β through canonical Sp1 binding sites. Nucleic acids research. 1998;26:2449–56. doi: 10.1093/nar/26.10.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.