Abstract
Although females suffer twice as much as males from stress-related disorders, sex-specific participating and pathogenic cellular stress mechanisms remain uncharacterized. Using corticotropin-releasing factor receptor 2–deficient (Crhr2−/− ) and wild-type (WT) mice, we show that CRF receptor type 2 (CRF2) and its high-affinity ligand, urocortin 1 (Ucn1), are key mediators of the endoplasmic reticulum (ER) stress response in a murine model of acute pancreatic inflammation. Ucn1 was expressed de novo in acinar cells of male, but not female WT mice during acute inflammation. Upon insult, acinar Ucn1 induction was markedly attenuated in male but not female Crhr2−/− mice. Crhr2−/− mice of both sexes show exacerbated acinar cell inflammation and necrosis. Electron microscopy showed mild ER damage in WT male mice and markedly distorted ER structure in Crhr2−/− male mice during pancreatitis. WT and Crhr2−/− female mice showed similarly distorted ER ultrastructure that was less severe than distortion seen in Crhr2−/− male mice. Damage in ER structure was accompanied by increased ubiquitination, peIF2, and mistargeted localization of vimentin in WT mice that was further exacerbated in Crhr2−/− mice of both sexes during pancreatitis. Exogenous Ucn1 rescued many aspects of histological damage and cellular stress response, including restoration of ER structure in male WT and Crhr2−/−mice, but not in females. Instead, females often showed increased damage. Thus, specific cellular pathways involved in coping and resolution seem to be distinct to each sex. Our results demonstrate the importance of identifying sex-specific pathogenic mechanisms and their value in designing effective therapeutics.
INTRODUCTION
Classically, the stress response involves central neurohormonal activation of the corticotropin-releasing-factor (CRF) system via the hypothalamic–pituitary– adrenal (HPA) axis. The effectors and responders consist of the neuropeptides CRF, urocortins 1–3 (Ucn1–3), and two G protein–coupled receptors, CRF type 1 (CRF1) and CRF type 2 (CRF2). The ligand CRF is primarily responsible for regulating and/or initiating stress responses via activation of the HPA axis (1), whereas the urocortins play a vital role in the peripheral stress response. Even though they are ubiquitously present throughout mammalian tissues, CRF, Ucn1–3 and their receptors are variably expressed in the skin (2), skeletal muscle (3), immune system (4), lung (5), heart (6), genitourinary system (7) and gastrointestinal (GI) system (8).
Accumulating evidence demonstrates localized, cellular expression of Ucn1 in response to GI inflammation (9–14). CRF and the urocortin ligands bind CRF1 and CRF2 with varying affinities. Ucn1 binds with equal affinities to both CRF1 and CRF2, but binds with a ten-fold higher affinity to CRF1 than to CRF (15). Others and we have shown that Ucn1 and CRF2 are required for normal resolution of GI inflammatory diseases like colitis (9,13,14,16,17). The lack of CRF2 in the GI tract leads to increased inflammation and delayed healing (9,13,18), perpetuating the insult. Patients with inflammatory bowel diseases (IBD) have higher incidences of acute and chronic pancreatitis (19), but the precise role of the CRF system in the inflammatory process or cellular signaling is unclear.
Recently, sex differences in Ucn1 signaling have been described in the midbrain and may result in different HPA axis–associated CRF responses to emotional stress between the sexes (20,21). The CRF system has been shown to be vitally important to embryo implantation (22,23) and maternal–fetal stress patterns, further suggesting sex-defined responses. Moreover, females are more prone to stress-related disorders, which include inflammatory and functional gastrointestinal (GI) diseases (20,24,25).
Acute pancreatitis, a common inflammatory-mediated disease of the GI tract, confers significant morbidity and mortality in up to 30% of patients whose disease progresses to systemic, multiorgan dysfunction (26–28). Although the progression of the disease is unpredictable, the pathogenic mechanisms of pancreatitis have largely been identified in animal models. The disease is a complex process regulated by intracellular and extracellular stress cascades. Intracellular acinar pathways resulting in premature activation of degradative enzymes, disrupted inflammatory signaling nuclear factor-κB (NF-κB), calcium-signaling defects, endoplasmic reticulum (ER) stress defects and dysfunctional secretory pathways have all been identified mechanisms that contribute to the pathogenesis of pancreatitis (26,29–33). Although the CRF system has been linked to protein secretion by acinar and β cells in the regulation of glycemia (34–36), during inflammation, where Ucn1 and CRF2 play important roles, the role of this system on acinar cell secretion has not been studied. Furthermore, the cellular mechanism(s) that lead to disrupted acinar cell function or resultant cellular stress-coping mechanisms during an inflammatory insult are unclear.
We sought to understand the role of acinar Ucn1 and CRF2 in the pathogenesis of pancreatic inflammation. Specifically, we wished to delineate the molecular mechanisms and cellular pathways by which CRF2 and Ucn1 mediate the actions of an inflammatory insult in a sex-specific manner. To do this, we used a caerulein-induced model of acute pancreatitis in mice. We also examined the differences between sexes in response to the stress of inflammation.
MATERIALS AND METHODS
Animal Studies and Pancreatitis Model
Crhr2−/− (C57/bl6 background) mice were a generous gift from Mary Stenzel-Poore, Oregon Health Sciences University, Portland, OR, USA (37). Crhr2+/− × Crhr2+/− mice were bred to obtain Crhr2+/+ (wild-type [WT]), Crhr2+/− and Crhr2−/− male and female mice. Littermates 8–10 wks of age weighing ~20–25g were used for all experiments described. The mice were housed in a temperature- and light-controlled room (22°C; 12-h light/12-h dark cycle) and were bred at a University of California, San Francisco (UCSF) housing facility. They had ad libitum access to food and water. Six, once hourly, subcutaneous injections of caerulein, a cholecystokinin analogue (Sigma-Aldrich, St. Louis, MO, USA; C-9026, 50 μg/kg), were used to induce pancreatitis according to a previously defined experimental pancreatitis model (30). Saline was injected as a vehicle control. Astressin 2B (A2B), a CRF2-specific antagonist (Sigma-Aldrich; A5227, 30 μg/kg), or Ucn1 (Sigma-Aldrich; U6631, 30 μg/kg) was injected intraperitoneally 30 min before the first caerulein injection. Subsequently, Ucn1 and A2B were injected hourly together with caerulein. Mice were euthanized 1 h after the last injection. All in vivo experiments were repeated at three independent times by using littermates from all three genotypes, for a total of 6–12 animals/group/genotype. The Institutional Animal Care and Use Committee at UCSF approved all procedures.
Immunohistochemistry and Histological Evaluation
Mice were transcardially perfused with 4% paraformaldehyde (PFA); pancreas were removed, postfixed in 4% PFA, washed with PBS, followed by ethanol dehydration and paraffin embedding. Pancreatic tissue was sectioned at 5 μm, deparaffinized in xylene and rehydrated in ethanol series. The following primary antibodies and dilutions were used: anti-Ucn1 (Sigma-Aldrich; U4757, rabbit, 1:300), anti-amylase (sheep, 1:1,000), anti-ubiquitin (Cell Signaling, Davers, MA, USA; 3933, rabbit, 1:1,000), anti-peIF2α (Cell Signaling, 119A11, rabbit, 1:100), and anti-Vimentin (DakoCytomation, Carpinteria, CA, USA; M0725, mouse, 1:100). Secondary antibodies used were species-specific FITC or Rhodamine Red™-X (Jackson ImmunoResearch, West Grove, PA, USA; 115-297-003, goat, 1:200) fluorescent antibodies, and sections were viewed by confocal microscopy. Serial sections were stained with hematoxylin and eosin to determine histopathological changes and analyzed using ImageJ64 software (NIH, Bethesda, MD, USA). A histologist scored coded tissue sections for necrosis, vacuolization, polymorphonuclear (PMN) cell infiltration and zymogen degranulation (0–5 scale) as described previously (30).
Western Blot Analysis
Tissue samples were homogenized in lysis buffer and processed for Western blotting as described (38). Total protein (40 μg) transferred to PVDF membranes (Immobilon-FL, Millipore, Billerica, MA, USA) was incubated with anti-pERK1/2 (Santa Cruz Biotechnology Inc., Dallas, TX, USA; E-4, mouse, 1:1,000) and anti-ERK2 (Santa Cruz Biotechnology Inc.; C-14, rabbit, 1:5,000) and visualized with Alexa Fluor® 680 (Li-COR Biosciences, Lincoln, NE, USA; 1:20,000) and goat IRDye™800 (Li-COR Biosciences). Blots were analyzed with the Odyssey Infrared Imaging System (Li-COR Biosciences), and densitometry was reported as previously described (38).
Enzyme-Linked Immunsorbent Assay (ELISA) for Detection of Serum Urocortin 1
Ucn1 concentration (pg/mL) in murine serum and pancreatic tissue lysate was quantified by using the Urocortin Fluorescent EIA Kit (Phoenix Pharmaceuticals, Burlingame, CA, USA; FEK-019-15) according to the manufacturer’s protocol. Resultant relative fluorescent units were measured at 450 nm.
Electron Microscopy
Control and experimental male and female mice were deeply anesthetized by using Nembutal and transcardially perfused with phosphate-buffered saline (PBS), followed by 2% glutaraldehyde/2% formaldehyde in 0.1 mol/L phosphate-buffered solution (39). Pancreatic tissue was cut into small blocks and fixed overnight. Postfixation was done with 1% OsO4, en bloc staining with 2% uranyl acetate, followed by ethanol dehydration and embedding in epoxy resin. Semithin and ultrathin sections were cut and stained with toluidine blue and uranyl acetate for examination under the light microscope and electron microscope, respectively. Electron micrographs were taken at 120 kV with a JEOL 1400/charge-coupled device (CCD) digital camera.
Cell Culture and Amylase Secretion Assay
Pancreatic acinar cells (AR42J) were cultured in F-12K nutrient mixture, Kaighn’s modification, + 2 mmol/L l-glutamine, + 10% fetal bovine serum (FBS). Cells were seeded onto poly-d-lysine coated plates at a density of 2 × 105 cells/well. Cells were preincubated for 30 min at 37°C in the presence or absence of 10−7 mol/L Ucn1 (Sigma-Aldrich; U6631) or 10−6 mol/L CRF2 receptor antagonist followed by treatment for 15 min with vehicle or 10−7 mol/L caerulein (Sigma-Aldrich; C-9026). Amylase content in the medium and cell lysate was measured by using Infinity amylase reagent (Thermo Fisher Scientific Inc., Waltham, MA, USA) as described (40). The secreted amylase component was expressed as a percentage of the total amylase content (medium + cell lysate) and normalized to total protein concentration determined by using a bicinchoninic acid (BCA) colorimetric assay. See Supplementary Methods for method for measuring intracellular calcium.
Semiquantitative RT-PCR
RNA was isolated from rat pancreatic tissue or AR42J acinar cells treated with either vehicle or caerulein by using TRIzol (Invitrogen/Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol. RT-PCR was performed by using gene-specific primers for rat Ucn1 (forward: 5′-CTCCT GGTAGCGTTGCTGC-3′; reverse: 5′-GCCCACCGAATCGAATATGATGC-3′) for 30 cycles with the following cycling parameter: 95°C for 30 s, 66°C for 30 s, 72°C for 30 s by using conditions described elsewhere (14). Cyclophilin (forward: 5′-TGCAGACGCCGCTGTCTC-3′; reverse: 5′-TGCTCTCCTGAGCTACAG-3′; annealing temperature 60°C) was selected as an unrelated housekeeping gene for normalization; and band intensities were quantified by using ImageJ64 software (NIH). Primer sets for CRF, Ucn2 and CRF receptors are provided in Supplementary Table S1.
Statistical Analysis
Data are shown as mean ± standard error of mean (SEM). Group differences were analyzed by using analysis of variance (ANOVA) followed by Bonferroni posttests (Prism; GraphPad Software Inc.). Student t test was used to compare two groups when appropriate. Two-sided p values ≤0.05 were considered statistically significant.
All supplementary materials are available online at www.molmed.org.
RESULTS
Ucn1 Is Induced De Novo during Acute Pancreatitis
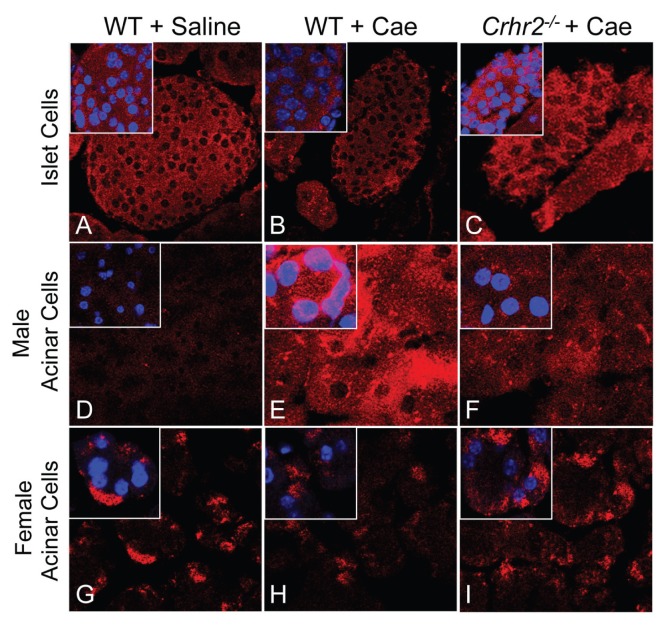
Although β-cell glucose regulation has been linked to the CRF system and Ucn1 expression has been reported in islet cells during periods of stress (34–36), the role of the CRF system in exocrine acinar cells, which show context-dependent protein secretion, has not been established. To examine the role of Ucn1 and CRF2 in acinar cells during pancreatic stress and inflammation, we used caerulein to induce pancreatitis in mice. Our results showed that under normal physiologic conditions (baseline), Ucn1 was localized in islet cells, but not in acinar cells (Figures 1A, D, Supplementary Figure S1A). After acute pancreatitis was induced, male WT and Crhr2+/− mice displayed de novo, context-dependent induction of Ucn1 in acinar cells, a new and novel finding (Figure 1E, Supplementary Figures S1A–C), but baseline islet cell localization of Ucn1 did not change (Figures 1B, C). In contrast, male Crhr2−/− mice showed attenuated de novo Ucn1 induction in acinar cells (Figure 1F). However, male Crhr2+/− or WT mice treated with a CRF2-specific antagonist, A2B, retained their ability to induce acinar Ucn1 expression (Supplementary Figures S1A, C). Surprisingly, and unlike their male littermates, female mice showed no significant change in acinar Ucn1 expression after inflammation was induced (Figures 1G–I). Baseline Ucn1 was evident in control female WT mice, and pancreatitis did not change its expression in either WT or Crhr2−/− mice (Figures 1G–I).
Figure 1.
De novo induction of Ucn1 in exocrine acinar cells. Pancreatic sections were stained with anti-Ucn1 antibody and visualized under a confocal microscope. Representative sections are shown from saline-treated WT (A, D, G), caerulein-treated (+ Cae) WT (B, E, H), and caerulein-treated Crhr2−/− (C, F, I) pancreatic sections (60×; inset magnification: 173×) from male and female mice (n = 6/group).
Because Ucn1 can have endocrine, paracrine or autocrine effects, we determined if circulating levels of Ucn1 also changed during acute pancreatitis. Unlike local levels of Ucn1 in the pancreatic acinar cells, serum concentration of Ucn1 did not vary significantly among control, WT, and Crhr2−/− male mice (Supplementary Figure S1D), or between Crhr2+/−mice and WT mice pretreated with astressin 2B (WT+A2B) (Supplementary Figure S1D). Since Ucn1 binds both CRF receptors with equal affinity and can exert its effect via activation of either receptor, we next ascertained if these two receptors were present in the pancreas. At baseline, low levels of CRF2 were present and its messenger RNA (mRNA) levels increased during pancreatitis in WT male and female mice (Supplementary Figure S1E). As expected, CRF2 mRNA was not detected in pancreas of Crhr2−/− male and female mice (Supplementary Figure S1E). Interestingly, mRNA for CRF1 receptor was not detected in pancreas of either WT or Crhr2−/− male and female mice, whereas CRF1 was present in brain mRNA, which served as a positive control (Supplementary Figure S1E).
CRF2 Deficiency Renders Males and Females Susceptible to Acute Pancreatitis
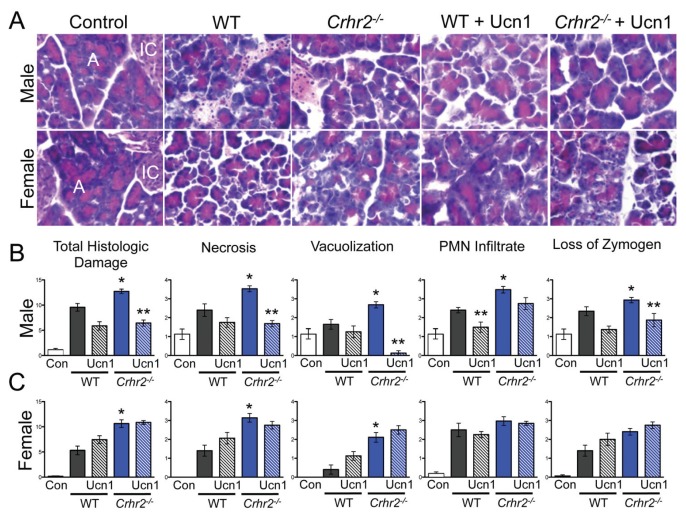
Because CRF2 actions are antiinflammatory (9,13), we predicted that CRF2 deficiency would exacerbate inflammation. To test this prediction, histopathology scores were obtained from control and pancreatitis tissues. Pancreatitis resulted in significant pancreatic histologic damage in WT mice of both sexes (Figures 2A–C). As predicted, histological damage was further increased in Crhr2−/− mice of both sexes (Figures 2A–C, blue bars). Histologic damage was similarly exacerbated in male Crhr2+/− and WT+A2B-treated mice (Supplementary Figures S2A, B). Necrosis, vacuolization, polymorphonuclear (PMN) cell infiltration and zymogen degranulation were significantly increased in male Crhr2−/− mice, whereas only necrosis and vacuolization were significantly increased in females (Figure 2C). Pancreatic PMN infiltration, quantified by MPO activity, increased to similar levels in male and female WT and male Crhr2+/− mice after caerulein treatment (Supplementary Figures S3A, B), but in WT+A2B mice, pancreatic MPO activity increased by two-fold over that in WT mice (Supplementary Figure S3B). In female Crhr2−/− mice, pancreatic MPO activity remained similar to that of female WT mice, correlating with the degree of PMN infiltration. These findings suggest that CRF2 is protective during acute inflammation, but indicate that males and females respond differently to stressful conditions when CRF2 and/or Ucn signaling is disrupted.
Figure 2.
CRF2 actions are key determinants of an inflammatory insult. Representative light micrographs (40×; A, acinar cells, IC, islet cells) of H&E stained pancreas sections from male and female saline-treated WT, caerulein-treated WT, and caerulein-treated Crhr2−/−mice (A). Graphical representation of histologic damage scores graded by a pathologist is shown. Duplicate sections were scored for necrosis, vacuolization, polymorphonuclear cell infiltration and zymogen granule formation in vehicle-treated WT (Con, white bars), caerulein-treated WT (grey bars) and caerulein-treated Crhr2−/− (blue bars) mice. Severity scores increased in all histopathologic categories for male Crhr2−/− mice (*p < 0.05 versus WT) (B). Total score, necrosis and vacuolization increased in female Crhr2−/− mice (*p < 0.05) (C). Ucn1 treatment (hashed grey and blue bars) significantly decreased all scores for male Crhr2−/− except PMN infiltrate, which was decreased in male WT mice (**p < 0.05). Ucn1 treatment either worsened histologic damage in female WT and Crhr2−/− mice or had no effect on variables examined (C). Statistical analyses were performed between same-sex groups by using ANOVA, followed by Bonferroni posttests. Data represent score ± SEM of 12 mice/group.
Ucn1 Is Induced De Novo in Cultured Acinar (AR42J) Cells
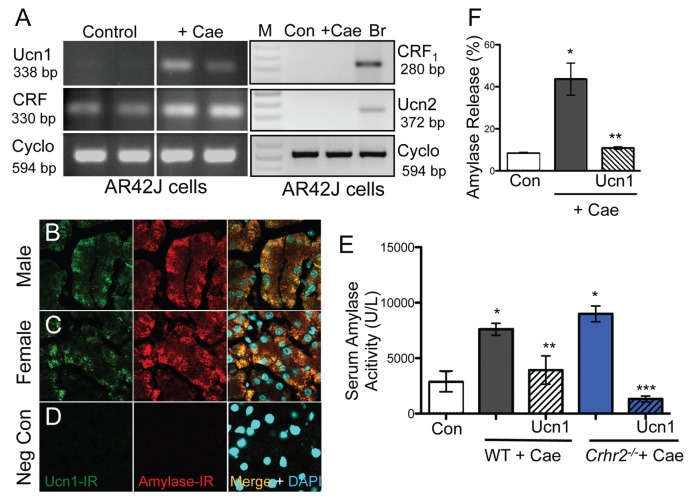
To confirm our in vivo finding that Ucn1 is induced de novo in acinar cells and is not being released from elsewhere via innervation found in the acinar cells, we determined the presence of Ucn1 mRNA by RT-PCR in cultured pancreatic acinar cells (AR42J), with or without caerulein treatment. As in vivo, Ucn1 mRNA was undetectable at baseline (control) in AR42J cells, but was highly induced upon caerulein treatment (Figure 3A, Supplementary Figure S4A), confirming that Ucn1 is expressed locally in the acinar cells. Importantly, CRF was expressed under basal conditions in AR42J cells (Figure 3A) and showed a two-fold increase in mRNA (Supplementary Figure S4A). Furthermore, neither CRF1 nor Ucn2 were expressed under basal conditions or after caerulein treatment in acinar cells (Figure 3A). We predicted that this de novo increase in Ucn1 during inflammation would alter function. To test this, we measured changes in intracellular calcium (Ca2+) responses in AR42J cells at baseline and after pretreatment with caerulein. As expected, treatment of AR42J cells with 100 nmol/L Ucn1or CRF evoked a robust Ca2+ response (Supplementary Figures S4B, C). Caerulein pretreatment abolished Ucn1-evoked Ca2+ responses (Supplementary Figure S4B, blue line), whereas CRF-evoked Ca2+ responses remained largely unchanged (Supplementary Figure S4C, red line). These results suggest that de novo induction of Ucn1 is of functional significance during stressful conditions and that small increases in CRF mRNA levels do not result in measurable functional changes. Furthermore, Ucn1-evoked changes in Ca2+ responses were attenuated after pretreatment of cells with A2B, a CRF2-specific antagonist, but remained at par with control cells after pretreatment with CP154526, a CRF1-specific antagonist (Supplementary Figure S4D). Thus, this further confirms that Ucn1 actions in acinar cells are mediated via CRF2, but not CRF1 (Supplementary Figure S4D).
Figure 3.
Ucn1 colocalizes with amylase in acinar cells. Ucn1 mRNA was induced de novo in acinar AR42J cells treated with 10−7 mol/L caerulein for 1 h. CRF mRNA expression was present under basal conditions and was not induced de novo. Ucn2 and CRF1 mRNA were not detected at baseline (Con) or after caerulein treatment in acinar cells, but were present in brain (Br) mRNA. Cyclophilin was used as a normalization control (A). Caerulein treatment increased amylase-IR in acinar cells in WT mice and colocalized with Ucn1-IR in male and female mice (B, C), respectively (63×); sections stained without any primary antibody served as negative controls (D). Serum amylase activity increased over basal (control) levels (*p < 0.05) in WT (grey bars) and Crhr2−/− (blue bars) mice (E), and Ucn1 treatment (hashed grey and blue bars) significantly decreased amylase release (**p < 0.05 versus WT; ***p < 0.05 versus Crhr2−/−). Amylase release from AR42J cells (F). Caerulein treatment increased amylase release (*p < 0.05) and 10−7 mol/L Ucn1 treatment decreased release (**p < 0.05). Statistical analyses were by ANOVA. Data represent mean ± SEM of three replicates per group.
Ucn1 Treatment Reduces Hyperamylasemia and Amylase Release in Acinar Cells
Increased amylase release from acinar cells reflects acinar cell damage and is an early pathologic feature in animal and human models of pancreatitis (26). We confirmed that after caerulein treatment, amylase immunoreactivity (amylase-IR) was increased in the acinar cells and colocalized with Ucn1-IR in both male and female mice (Figures 3B, C), but not in negative controls (Figure 3D). Pancreatitis resulted in hyperamylasemia in WT and Crhr2−/−mice (Figure 3E). Because Ucn1 was induced de novo in the exocrine acinar cells of WT, but not Crhr2−/− mice, we reasoned that Ucn1 might rescue and stabilize some, if not all, aspects of acinar cell damage. As predicted, exogenous Ucn1 decreased serum amylase levels in WT and Crhr2−/−mice (Figure 3E, hatched bars). This finding is further supported by our in vivo result that acute caerulein treatment of AR42J cells increased amylase release into the medium and Ucn1 treatment of AR42J cells significantly decreased caerulein-induced amylase release (Figure 3F). These findings suggest that an acinar cell–specific, de novo induction of Ucn1, is vital to cellular coping responses during inflammation.
Ucn1 Treatment Reduces Pancreatic Inflammation Only in Male Mice
Because Ucn1 has known protective effects during inflammation in many peripheral organ systems (6,9,17,41), we wondered if this was true in the pancreatic tissue as well. The ability of Ucn1 to reduce acinar amylase release prompted us to investigate if exogenous Ucn1 would rescue Crhr2−/− mice from the sequelae of pancreatitis. As predicted, Ucn1 administered concurrently with caerulein decreased the overall severity of histologic damage in male Crhr2−/−mice (Figure 2B); that is, progression to necrosis, vacuolization and zymogen de-granulation all improved. In male WT mice, Ucn1 decreased the number of PMN cell infiltrate, but otherwise had little effect on inflammation severity (Figure 2B). In sharp contrast to Ucn1’s effect in male Crhr2−/− mice, Ucn1 showed no protective effect in female Crhr2−/− mice (Figure 2C), suggesting that Ucn1 confers an antiinflammatory or protective effect in male mice only.
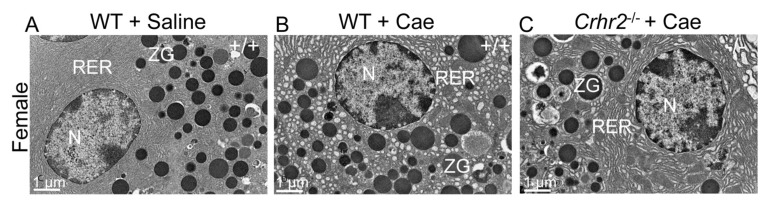
CRF2 Deficiency Results in Changes to the Ultrastructure of the Endoplasmic Reticulum
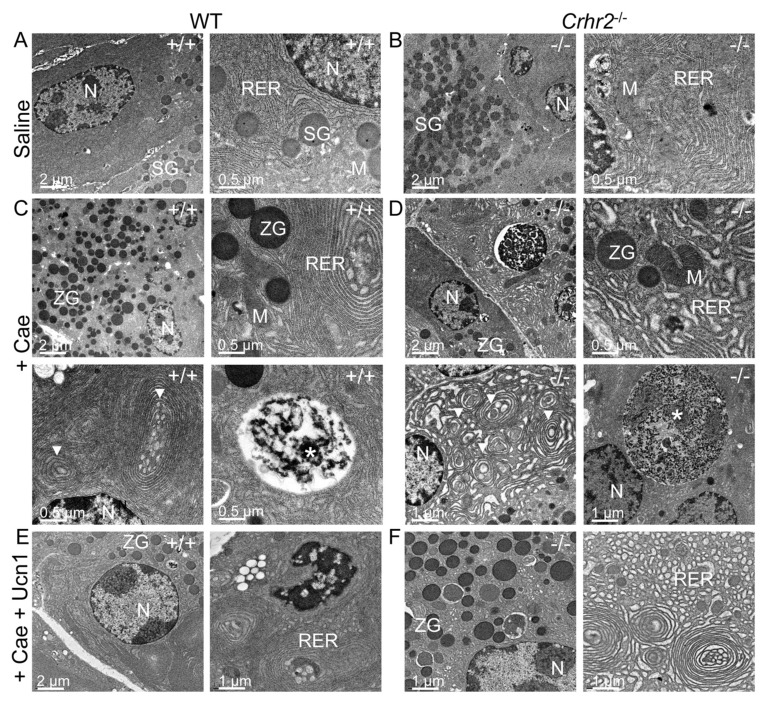
Dysfunctional pancreatic exocrine secretion during pancreatitis manifests in pathologic zymogen formation and the release of activated peptidases (42). The increased vacuolization and loss of zymogen granules we observed in Crhr2−/− mice suggested that CRF2 was involved in maintaining cellular organelle integrity and affected cellular protein machinery. Electron microscopy showed no discernable differences in acinar cell ultrastructure between male and female WT mice (Figures 4A, 5A), or Crhr2−/−mice, at baseline (Figure 4B). Pancreatitis increased peripheral nuclear chromatin condensation and mitochondrial size similarly in male WT and Crhr2−/− mice (Figure 4C, D). In male WT mice, there was minor disruption of the rough ER and a few autophagic compartments were discernable (Figure 4C), but the rough ER of male Crhr2−/− mice was strikingly distorted, with numerous whorls and intraluminal inclusion bodies (Figure 4C versus 4D). The male Crhr2−/−mice also had many large cytoplasmic autophagic compartments that contained aggregates of ribosomes and rough ER membrane components (Figure 4D), suggesting increased ER stress. Pancreatitis resulted in distorted rough ER ultrastructure in the male Crhr2+/− mice, and to a lesser degree in WT+A2B mice (Supplementary Figure S4). In contrast to the male mice, female WT and Crhr2−/− mice displayed similarly distorted ER structure during pancreatitis (Figure 5B, C). However, female WT mice displayed worse ER damage than male WT mice, whereas female Crhr2−/− mice displayed markedly less damaged ER than their male Crhr2−/− mice (Figures 5B and C versus 4B and C). Thus, the baseline response to the stress of inflammation appears to be more tempered in females and more dramatic and fast in males.
Figure 4.
CRF2 is central to the maintenance of organelle integrity and the endoplasmic reticulum stress responses. Representative electron microscopy (EM) shows normal acinar cell ultrastructure in saline-treated WT (A) or saline-treated Crhr2−/− (B) mice (n = 4/group) with normal appearing acinar cell ultrastructure. During pancreatitis, WT male mice show a marked increase in zymogen granule (ZG) formation, mild expansion of rough ER and intraluminal material (C), consistent with ER stress. Infrequent, small autophagic bodies (*) containing densely staining material were found. During pancreatitis, Crhr2−/− mice show markedly dilated rough ER (D); ER whorls and increased intraluminal inclusion bodies were visible, all of which were increased relative to what was seen in WT pancreatitis. Frequent, multiple large autophagic bodies (white *) that contained numerous ribosomes and membrane components were seen. (M, mitochondria; N, nucleus; RER, rough endoplasmic reticulum; SG, secretory granules; ZG, zymogen granule). Exogenous Ucn1 treatment during pancreatitis markedly reduces ER ultrastructure damage and number of autophagic bodies in both WT and Crhr2−/− male mice (E, F).
Figure 5.
Lack of Crhr2−/− in female mice results in a milder ER phenotype. WT female controls show normal organelle ultrastructure (A) similar to their male littermates. When pancreatitis is induced, females, unlike their male counterparts, show milder ER ultrastructure damage in both WT (B) and Crhr2−/− (C) genotypes. (N: nucleus, RER: rough endoplasmic reticulum, ZG: zymogen granule).
Ucn1 and CRF2 Alter the ER Stress Pathway in Males Alone
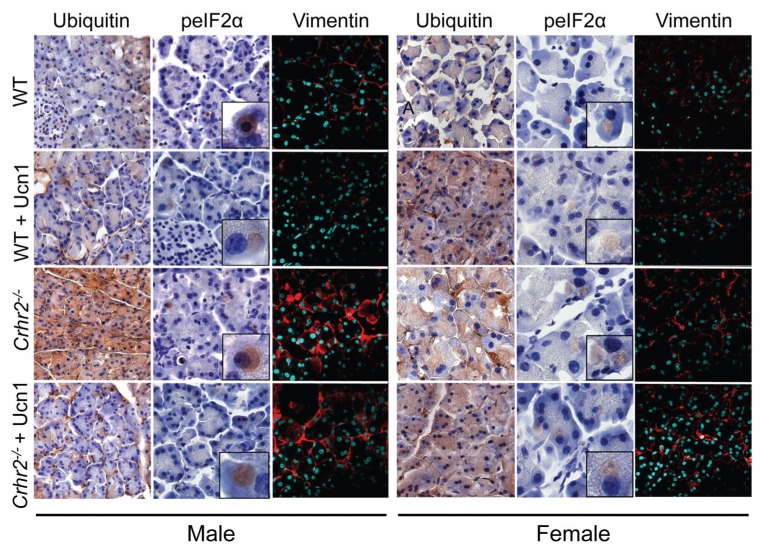
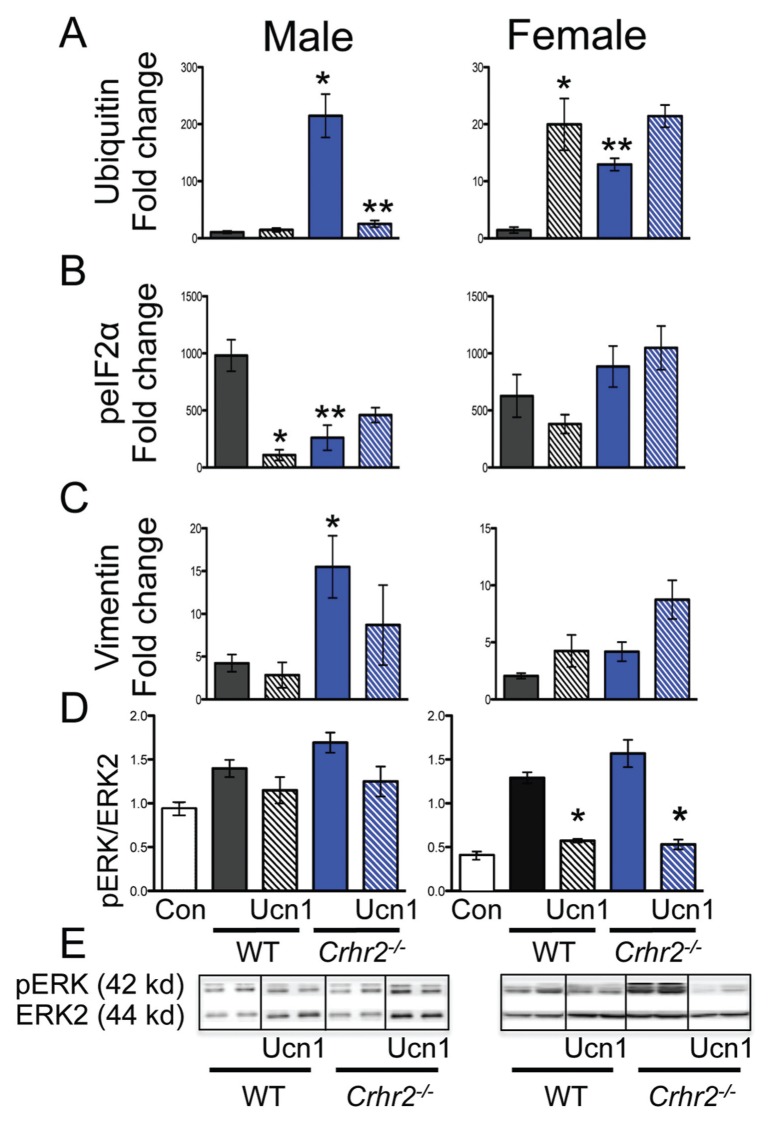
The ER stress response is activated early in the development of acute pancreatitis (31). This response may be characterized in several steps that include activation of the unfolded protein response (UPR) to inhibit protein translation. The UPR aids in directing misfolded proteins toward ubiquitin-dependent degradation (43). We therefore examined whether ER distortion in Crhr2−/− mice contributes to the UPR and ubiquitinates proteins for degradation. We found that caerulein treatment increased accumulation of ubiquitinated proteins by 10-fold in male WT mice, and by more than 220-fold in Crhr2−/− mice (Figures 6, 7A). In female Crhr2−/− mice, the accumulation of ubiquitinated proteins increased by only 10-fold over that in female WT mice (Figures 6, 7A). As predicted, exogenous Ucn1 markedly decreased ubiquitination in male Crhr2−/− mice, but did not affect ubiquitination in female WT or Crhr2−/−mice (Figures 6, 7A).
Figure 6.
CRF2 disrupts the ER stress response. Pancreatic sections immunostained for ER stress pathway proteins. Representative light micrographs (60×; inset: 300×; A, acinar cells, IC, islet cells) immunostained for ubiquitin and peIF2α in WT and Crhr2−/− male and female control mice or mice treated with caerulein alone or caerulein + Ucn1 are shown. Mistargeting of vimentin is evident in Crhr2−/− mice of both sexes (magnification: 63×; nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI); n = 6/group).
Figure 7.
ER stress response is restored after Ucn1 treatment. Graphical representation of fold-change in staining intensity for ubiquitin (A), peIF2α (B), and vimentin (C) over saline-treated controls is shown (*p < 0.05, WT versus Crhr2−/−). Simultaneous Ucn1 treatment decreased ubiquitin levels in male Crhr2−/− mice (** p < 0.05) and peIF2α levels in male WT mice (*p < 0.05) (A, B). Western blot analysis of pancreatic lysates was performed (D, E), and representative Western blot from two mice per group is shown (E). Quantitation of pERK1/2 normalized to total ERK showed significant increases in pERK1/2 in male and female WT and Crhr2−/− mice (D, *p < 0.05). Ucn1 treatment did not affect pERK levels in male WT or Crhr2−/− mice, but significantly (**p < 0.05) reduced pERK levels in female WT and Crhr2−/− mice. Statistical analyses were performed between same-sex groups by using ANOVA. Data represent mean ± SEM of 12 mice per group.
To reduce demand on the protein-folding machinery, cells increase phosphorylation of eIF2α (peIF2α), resulting in reduced protein translation (44). We postulated that uncontrolled protein ubiquitination in Crhr2−/− mice might be due to the inability to increase peIF2α. During pancreatitis, expression of peIF2α was localized in distinct compartments of acinar cells in WT and Crhr2−/− mice (Figures 6, 7B), but induction of peIF2α was markedly compromised in Crhr2−/− mice (Figures 6, 7B). As expected, exogenous Ucn1 decreased pancreatitis-induced peIF2α in male WT mice, suggesting decreased UPR. Ucn1 did not significantly alter peIF2α levels in male Crhr2−/− mice (Figures 6, 7B). We next examined if restoration of ubiquitination and peIF2α signaling in male mice was accompanied by improved ER ultrastructure after Ucn1 treatment. Indeed, in male WT and Crhr2−/− mice, Ucn1 treatment improved ER ultrastructure defects (Figures 4E, F); notably, the distortion seen in Crhr2−/−mice improved dramatically.
Conversely, in female WT and Crhr2−/−mice, peIF2α levels did not vary significantly (Figures 6, 7B). Female WT and Crhr2−/− mice had increases in pancreatitis-induced peIF2α, and remarkably, Ucn1 treatment had little effect on peIF2α levels (Figures 6, 7B). The results of these experiments suggested two things: first, that CRF2 deficiency resulted in cellular organelle architectural defects that Ucn1 may be able to rescue in males; and second, that CRF2 regulates stress responses not only at the organ level, functioning as a neuromodulator, but also at the organelle level, functioning to maintain organelle integrity. The difference between males and females is surprising, but suggests that females may have more redundant ER stress pathways to maintain homeostasis.
Ucn1 Treatment Restores Vimentin Mistargeting in Male, but Not Female Crhr2−/− Mice
Cellular microenvironments and appropriate subcellular localization are important in fine-tuning cellular responses (45) and provide resistance against stressors. Vimentin, a highly conserved and widely expressed intermediate filamentous protein, is involved in facilitating cytoskeletal cross-talk and cellular targeting of proteins (45). We identified vimentin as a CRF2-interacting protein by coimmunoprecipitation and mass spectrometry (S Mahajan and A Bhargava, unpublished results). Because vimentin is often found associated with either the nucleus or the ER and is integral to cell viability, we ascertained if lack of CRF2 altered pancreatic vimentin expression or its localization. We found that at baseline, vimentin was expressed perinuclearly in male and female WT mice (Figure 6). During pancreatitis, vimentin translocated to the plasma membrane in male WT mice and its expression increased 5-fold (Figures 6, 7C). In male Crhr2−/− mice, vimentin expression increased 15-fold, and its expression was mistargeted to the intracellular matrix. Ucn1 treatment during pancreatitis decreased total vimentin expression and decreased its intracellular expression in male WT, and to a lesser degree in male Crhr2−/− mice (Figures 6, 7C).
During pancreatitis, in female WT and Crhr2−/− mice, vimentin expression increased modestly and discrete plasma membrane localization was not visible (Figures 6, 7C). Unlike in males, Ucn1 treatment did not restore the perinuclear localization of vimentin, or decrease its expression in females. These findings, together with the ultrastructure, ubiquitination, and peIF2α results, show that dysfunctional CRF2/Ucn1 circuit dramatically affects ER stress responses in male, but not in female, mice.
Ucn1 Modulates Extracellular Signal-Regulated Kinase (ERK) Signaling Responses in Female Mice Only
Downstream modulators of ER stress include the mitogen-activated protein kinase pathway (MAPK/ERK) (46). Others and we have shown that ERK signaling modulates the inflammatory response during colitis (9,41), and that colon-specific knockdown of CRF2 by RNA interference markedly attenuates phosphorylation of ERK (pERK) (9). To determine whether CRF2 deficiency compromised ERK signaling in either sex during pancreatitis, we quantified increases in pERK levels in pancreatic lysates by Western blot analysis. We found that during pancreatitis, pERK levels tended to increase modestly in male WT and Crhr2−/− mice over baseline, and were not affected by Ucn1 treatment (Figures 7D, E). In female WT and Crhr2−/− mice, pERK levels increased significantly over baseline, but unlike in males, Ucn1 significantly decreased pERK levels (Figures 7D, E). Although ERK phosphorylation is associated with early pancreatic damage, the pathway is dynamic and may be temporally activated and deactivated (47,48). It may be that Ucn1 and/or CRF2 recruit distinct MAPK signaling pathways in males and females.
DISCUSSION
As our understanding of GI inflammatory diseases improves, we find that neurohormonal stress responders, in particular the CRF system, play an important role. Others and we have shown that Ucn1 and CRF2 are required for resolution of GI inflammatory diseases like gastritis and colitis (9,13,14,16,17,31,49). Loss of CRF2 leads to increased inflammation, delayed healing, and exacerbates the inflammatory insult (9,18,43). Using acute pancreatitis as another model of inflammation, we found that CRF2 deficiency renders both males and females more susceptible to inflammation.
As the pathobiology of acute pancreatitis becomes better understood, the precise nature of inflammation that leads to acinar cell dysfunction has yet to be established. Our confocal microscopy and RT-PCR results show that Ucn1, but not its closely related neuropeptide hormones, CRF and Ucn2, is induced de novo in the exocrine pancreatic acinar cells when acute pancreatitis is induced, whereas the source of Ucn1 in the islet cells remains to be established. Furthermore, while CRF2 mRNA levels are increased during pancreatitis in WT mice, CRF1 mRNA is undetectable during pancreatitis, suggesting that Ucn1 does not mediate its effect via CRF1 in this model of pancreatitis. CRF1 and CRF2 expression on a subset of β cells has been previously reported (35,50). In agreement with our findings, the same study did not find CRF1 expression in acinar cells, but reported presence of CRF1 and CRF2 in β cells and MIN6 cells (50). Most components of histologic damage were increased in male Crhr2−/− mice relative to their WT littermates, whereas only necrosis and vacuolization were increased in female Crhr2−/− mice relative to their WT littermates. Interestingly, Ucn1 reduced the extent of histologic damage in male Crhr2−/− mice more effectively than in male WT mice, whereas Ucn1 treatment had no effect in either female WT or Crhr2−/− mice. Our finding that loss of CRF2 has a particularly profound effect on PMN cell infiltration suggests that CRF2 is crucial to recruitment of immune cells in both males and females and shows a particularly novel function for CRF2 in regulating immune cell function. Others have proposed that the CRF receptors act in a regulatory loop that aids in local containment of inflammation (41). Acutely, this autoregulatory loop might aid in maintaining homeostasis at the local level.
Autophagy of the exocrine pancreas plays an important role in the progression of pancreatitis, but cellular and molecular defects that lead to autophagy are unclear. An early pathologic feature of pancreatitis is increased amylase release from acinar cells (26). In our experiments, caerulein-induced pancreatitis resulted in hyperamylasemia in male and female WT and Crhr2−/− mice. Furthermore, Ucn1 treatment decreased amylase release in vivo and in vitro, suggesting that disruption of Ucn1-CRF2 signaling contributes to pathologic secretory mechanisms during acute pancreatitis.
Recent evidence suggests that acinar cell damage is caused by at least two mutually exclusive events—the first is independent of trypsinogen activation, and the second is immune cell dependent (27). In males, Ucn1 had more profound effects in rescuing inflammation in Crhr2−/− mice than in WT mice, suggesting that Ucn1 can still act via a mutated CRF2 receptor. Possibly, these Crhr2−/− mice that lack transmembrane domains 3 and 4 (37) may express this deficient receptor at different levels in females than in males, accounting for a differential response to Ucn1 in males. The lack of CRF1 suggests that Ucn1 actions during pancreatitis are not mediated by compensatory changes in CRF1. Furthermore, increase in Ucn1, and not CRF, in acinar cells is of functional consequence as after caerulein treatment, Ucn1 was unable to evoke a Ca2+ response, whereas addition of CRF continued to evoke a robust Ca2+ response. We have previously shown how these two ligands can mediate differential trafficking and signaling responses via the same receptor in a context-dependent manner (38). But the fact that the WT females did not respond to exogenous Ucn1 treatment, unlike their WT male counterparts, would suggest that another, yet identified receptor might mediate some of the effects of Ucn1 in the periphery. Alternatively, the CRF2 receptor can interact with distinct ancillary proteins in a sex-specific manner during stress of inflammation. Our findings show that only a subset of variables contributing to pancreatic damage are CRF2 dependent and that they vary between the sexes, whereas other variables are distinct sex-dependent responses, as summarized in Supplementary Figure S6.
The pancreatic acinar cell is rich in ER, with the richness reflecting its role in digestive enzyme secretion and also making it an attractive model to study ER and organelle ultrastructure in response to inflammatory stress. ER stress and vacuolization contribute to the increased autophagy seen during pancreatitis (51). Here, we show for the first time that disruption in the signaling of Ucn1 and its high-affinity receptor, CRF2, results in gross morphological distortion of the acinar ER ultrastructure, which is accompanied by sex-specific changes in the ER stress signaling components. Previously, ER stress has been studied in the context of alcohol consumption (52); and the results showed that alcohol consumption leads to an increased UPR response, accompanied by disorganized and disrupted ER (53). Because alcohol can have pleiotropic effects on cell and organelle function, disrupted ER in that scenario is not surprising. We did not expect to find gross rough ER distortion or dilation in Crhr2−/− and Crhr2+/− mice, or after pharmacological inhibition (A2B treatment). Our ultrastructure findings clearly show that CRF2 dysfunction results in cellular organelle defects. CRF2 is well established as a central regulator of the stress response, functioning as a neuromodulator (54), but our ER ultrastructure data show a significant role of CRF2 at the organelle level, where the appropriate function of Ucn/CRF2 signaling might be to protect organelle integrity. Alcohol ingestion is a main contributor to pancreatitis in males (52), yet not everyone who consumes alcohol develops pancreatitis. In those who do, the phenotypic effects are variable. Alcohol is a known modulator of the CRF system (54,55) and disrupts the ER stress response (52).
The signaling events that accompany ER stress are not well characterized. Our study shows that ER disruption in male Crhr2−/− mice is accompanied by significant ubiquitination and that Ucn1 significantly reduces the ubiquitinated protein levels in male mice, but not females. Moreover, we think that uncontrolled protein ubiquitination in male Crhr2−/− mice might be due to the inability to regulate phosphorylation status of eIF2α. Not only may protein synthesis and secretion be affected, but protein mistargeting also appears to occur in Crhr2−/− mice; all of these findings are consistent with rough ER disruption. We found that perinuclear staining of the filamentous protein vimentin was restored in male mice after Ucn1 treatment, whereas in females, discrete perinuclear expression of vimentin was lacking to start with, and pancreatitis only modestly increased its expression. Furthermore, in Crhr2−/− mice that still express a mutated version of CRF2 receptor, other proteins required for normal signaling can be sequestered or squelched. This observation supports our findings that interaction between CRF2 and vimentin might be critical for a microdomain locale of the CRF2 and that vimentin might be critical for microdomain locale of the CRF2 complex and downstream activation of signaling pathways. Our MAPK/ERK signaling results indicate that phosphorylation of ERK1/2 occurs in females alone, reinforcing the notion that cellular stress coping and inflammatory resolution pathways are distinct in males and females. These results also suggest that to combat inflammation, Ucn1 uses MAPK/ERK-dependent pathways in females. Thus, ER architectural distortion after an inflammatory insult in Crhr2−/− mice and Crhr2+/−mice, and after pharmacological inhibition of CRF2 in WT mice, confirmed that basic intracellular stress responses of non-immune cells are different between the sexes. This difference is linked to the local presence or absence of CRF2 and Ucn1.
The role of the sex hormones testosterone and estrogen in pancreatic inflammation is unequivocal. Estrogen can have protective effects on cultured acinar cells and works to decrease acinar cell apoptosis in a dose-dependent manner (56). Estrogen replacement therapy worsens pancreatitis outcomes in females with another comorbidity, such as hyperlipidemia (57,58). Low levels of testosterone, on the other hand, result in worsened survival outcomes in male patients with pancreatic adenomas (59). Here we show that females are resistant to protective effects of exogenous Ucn1 administration, suggesting that estrogen or testosterone are not the primary contributors to inflammation, and do not significantly interfere with CRF2/Ucn1 signaling. Taken together, our findings suggest that Ucn1 administration could rescue key deleterious aspects of pancreatitis in males.
Twice as many women as men suffer from stress-related disorders, including anxiety, depression and gastrointestinal diseases (20,24,25). The classic “flight or fight” stress response involves neurohormonal activation of the CRF system via the HPA axis. Perturbations in the CRF system have been implicated in preeclampsia, a life-threatening maternal–fetal disease in which delivery of a preterm infant is often the only cure for significant maternal–fetal stress (22,23). Despite the known preponderance of stress-related diseases in females, the use of female animal subjects is perpetually lacking and the resultant sex-specific molecular pathogenesis in disease responses remains vastly understudied. Furthermore, as the dynamic relationship between stress and inflammation has become evident, CRF receptor antagonists and related molecular targets have been intensely studied and used as promising therapeutic targets for these pathophysiologic mechanisms. Most CRF-based therapeutics, though promising in male animal subjects, fail in clinical trials.
CONCLUSION
Our findings demonstrate a distinct role for CRF2 and Ucn1 signaling in mediating the stress-related exacerbation of pancreatic inflammation, and show that the cellular mechanisms by which the CRF system propagates its actions are sex-specific. We provide evidence that suggests the reason for CRF-based clinical treatment failures may not lie in the therapeutics themselves, but in the sex-specific response to the therapeutics. Finally, in the greater evolutionary scheme, our observations are an example of how males and females differently maintain homeostasis and temper stress responses to inflammation.
Supplemental Data
ACKNOWLEDGMENTS
This work was supported through grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01-DK080787 and R01-DK080787-02S1 (A Bhargava). E Kubat was a recipient of a T-32 NIH-NIDDK training grant DK07573-22/23. We thank Victoria Lyo for her expertise with the caerulein model and Pallavi Mhaske for technical help. We thank David Scheel in the German lab, UCSF for excellent technical help with paraffin sections. We thank Pamela Derish in the Department of Surgery at UCSF for critical reading of the manuscript. We thank Mary Stenzel-Poore at Oregon Health Sciences University for the generous gift of the WT and Crhr2−/− mice breeding pairs.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Muglia L, Jacobson L, Dikkes P, Majzoub JA. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995;373:427–32. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
- 2.Slominski A, et al. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15:1678–93. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- 3.Chen A, Blount A, Vaughan J, Brar B, Vale W. Urocortin II gene is highly expressed in mouse skin and skeletal muscle tissues: localization, basal expression in corticotropin-releasing factor receptor (CRFR) 1- and CRFR2-null mice, and regulation by glucocorticoids. Endocrinology. 2004;145:2445–57. doi: 10.1210/en.2003-1570. [DOI] [PubMed] [Google Scholar]
- 4.Baigent SM. Peripheral corticotropin-releasing hormone and urocortin in the control of the immune response. Peptides. 2001;22:809–20. doi: 10.1016/s0196-9781(01)00395-3. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Xu Y, Zhou H, Tao J, Li S. Expression of urocortin in rat lung and its effect on pulmonary vascular permeability. J Endocrinol. 2006;189:167–78. doi: 10.1677/joe.1.06607. [DOI] [PubMed] [Google Scholar]
- 6.Okosi A, et al. Expression and protective effects of urocortin in cardiac myocytes. Neuropeptides. 1998;32:167–71. doi: 10.1016/s0143-4179(98)90033-6. [DOI] [PubMed] [Google Scholar]
- 7.Imperatore A, et al. Urocortin 2 and urocortin 3 are expressed by the human placenta, deciduas, and fetal membranes. Am J Obstet Gynecol. 2006;195:288–95. doi: 10.1016/j.ajog.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 8.Buckinx R, Adriaensen D, Nassauw LV, Timmermans JP. Corticotrophin-releasing factor, related peptides, and receptors in the normal and inflamed gastrointestinal tract. Front Neurosci. 2011;5:54. doi: 10.3389/fnins.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang J, et al. Urocortin 1 modulates immunosignaling in a rat model of colitis via corticotropin-releasing factor receptor 2. Am J Physiol Gastrointest Liver Physiol. 2011;300:G884–94. doi: 10.1152/ajpgi.00319.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang J, et al. Urocortin 2 expression in the rat gastrointestinal tract under basal conditions and in chemical colitis. Peptides. 2007;28:1453–60. doi: 10.1016/j.peptides.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cureton EL, et al. Local secretion of urocortin 1 promotes microvascular permeability during lipopolysaccharide-induced inflammation. Endocrinology. 2009;150:5428–37. doi: 10.1210/en.2009-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Delgado M. Therapeutic effect of urocortin and adrenomedullin in a murine model of Crohn’s disease. Gut. 2006;55:824–32. doi: 10.1136/gut.2005.084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokkotou E, et al. Corticotropin-releasing hormone receptor 2-deficient mice have reduced intestinal inflammatory responses. J Immunol. 2006;177:3355–61. doi: 10.4049/jimmunol.177.5.3355. [DOI] [PubMed] [Google Scholar]
- 14.la Fleur SE, Wick EC, Idumalla PS, Grady EF, Bhargava A. Role of peripheral corticotropin-releasing factor and urocortin II in intestinal inflammation and motility in terminal ileum. Proc Natl Acad Sci U S A. 2005;102:7647–52. doi: 10.1073/pnas.0408531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaughan J, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–92. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 16.Chatzaki E, et al. Urocortin in human gastric mucosa: relationship to inflammatory activity. J Clin Endocrinol Metab. 2003;88:478–83. doi: 10.1210/jc.2002-020853. [DOI] [PubMed] [Google Scholar]
- 17.Kubo Y, et al. Urocortin prevents in-domethacin-induced small intestinal lesions in rats through activation of CRF2 receptors. Dig Dis Sci. 2009;55:1570–80. doi: 10.1007/s10620-009-0930-1. [DOI] [PubMed] [Google Scholar]
- 18.Im E, et al. Corticotropin-releasing hormone family of peptides regulates intestinal angiogenesis. Gastroenterology. 2010;138:2457–2467.e5. doi: 10.1053/j.gastro.2010.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitchumoni CS, Rubin A, Das K. Pancreatitis in inflammatory bowel diseases. J Clin Gastroenterol. 2010;44:246–53. doi: 10.1097/MCG.0b013e3181cadbe1. [DOI] [PubMed] [Google Scholar]
- 20.Bangasser DA, Valentino RJ. Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol. 2012;32:709–23. doi: 10.1007/s10571-012-9824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derks NM, Gaszner B, Roubos EW, Kozicz LT. Sex differences in urocortin 1 dynamics in the non-preganglionic Edinger-Westphal nucleus of the rat. Neurosci Res. 2010;66:117–23. doi: 10.1016/j.neures.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Florio P, et al. High fetal urocortin levels at term and preterm labor. J Clin Endocrinol Metab. 2005;90:5361–5. doi: 10.1210/jc.2005-0109. [DOI] [PubMed] [Google Scholar]
- 23.Perkins AV, et al. Corticotrophin-releasing hormone and corticotrophin-releasing hormone binding protein in normal and pre-eclamptic human pregnancies. Brit J Obstet Gynaecol. 1995;102:118–22. doi: 10.1111/j.1471-0528.1995.tb09063.x. [DOI] [PubMed] [Google Scholar]
- 24.Hubbard CS, et al. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. J Neurosci. 2011;31:12491–500. doi: 10.1523/JNEUROSCI.1860-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Künzel HE, et al. Treatment of depression with the CRH-1-receptor antagonist R121919: endocrine changes and side effects. J Psychiatr Res. 2003;37:525–33. doi: 10.1016/s0022-3956(03)00070-0. [DOI] [PubMed] [Google Scholar]
- 26.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–51. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 27.Sah RP, Garg P, Saluja AK. Pathogenic mechanisms of acute pancreatitis. Curr Opin Gastroenterol. 2012;28:507–15. doi: 10.1097/MOG.0b013e3283567f52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcea G, et al. Predictors of severity and survival in acute pancreatitis: validation of the efficacy of early warning scores. Pancreas. 2008;37:e54–61. doi: 10.1097/MPA.0b013e3181771451. [DOI] [PubMed] [Google Scholar]
- 29.Booth DM, et al. Reactive oxygen species induced by bile acid induce apoptosis and protect against necrosis in pancreatic acinar cells. Gastroenterology. 2011;140:2116–25. doi: 10.1053/j.gastro.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 30.Ceppa EP, et al. Serine proteases mediate inflammatory pain in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1033–42. doi: 10.1152/ajpgi.00305.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubisch CH, Logsdon CD. Secretagogues differentially activate endoplasmic reticulum stress responses in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1804–12. doi: 10.1152/ajpgi.00078.2007. [DOI] [PubMed] [Google Scholar]
- 32.Sah RP, Saluja A. Molecular mechanisms of pancreatic injury. Curr Opin Gastroenterol. 2011;27:444–51. doi: 10.1097/MOG.0b013e328349e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saluja AK, et al. Secretagogue-induced digestive enzyme activation and cell injury in rat pancreatic acini. Am. J. Physiol. 1999;276(4 Pt 1):G835–42. doi: 10.1152/ajpgi.1999.276.4.G835. [DOI] [PubMed] [Google Scholar]
- 34.Guzman EA, Zhang W, Lin TR, Mulholland MW. Stimulation of rat pancreatic exocrine secretion by urocortin and corticotropin releasing factor. Peptides. 2003;24:727–34. doi: 10.1016/s0196-9781(03)00125-6. [DOI] [PubMed] [Google Scholar]
- 35.Huising MO, et al. CRFR1 is expressed on pancreatic beta cells, promotes beta cell proliferation, and potentiates insulin secretion in a glucose-dependent manner. Proc Natl Acad Sci U S A. 2010;107:912–7. doi: 10.1073/pnas.0913610107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li C, Chen P, Vaughan J, Lee KF, Vale W. Urocortin 3 regulates glucose-stimulated insulin secretion and energy homeostasis. Proc Natl Acad Sci U S A. 2007;104:4206–11. doi: 10.1073/pnas.0611641104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coste SC, et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:403–9. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- 38.Hasdemir B, Mahajan S, Bunnett NW, Liao M, Bhargava A. Endothelin-converting enzyme-1 actions determine differential trafficking and signaling of corticotropin-releasing factor receptor 1 at high agonist concentrations. Mol Endocrinol. 2012;26:681–95. doi: 10.1210/me.2011-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ralston HJ, 3rd, Ohara PT, Meng XW, Wells J, Ralston DD. Transneuronal changes of the inhibitory circuitry in the macaque somatosensory thalamus following lesions of the dorsal column nuclei. J Comp Neurol. 1996;371:325–35. doi: 10.1002/(SICI)1096-9861(19960722)371:2<325::AID-CNE11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 40.Mashima H, et al. Interferon regulatory factor-2 regulates exocytosis mechanisms mediated by SNAREs in pancreatic acinar cells. Gastroenterology. 2011;141:1102–1113.e8. doi: 10.1053/j.gastro.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 41.Tsatsanis C, Androulidaki A, Dermitzaki E, Gravanis A, Margioris AN. Corticotropin releasing factor receptor 1 (CRF1) and CRF2 agonists exert an anti-inflammatory effect during the early phase of inflammation suppressing LPS-induced TNF-alpha release from macrophages via induction of COX-2 and PGE2. J Cell Physiol. 2007;210:774–83. doi: 10.1002/jcp.20900. [DOI] [PubMed] [Google Scholar]
- 42.Gaisano HY, Gorelick FS. New insights into the mechanisms of pancreatitis. Gastroenterology. 2009;136:2040–4. doi: 10.1053/j.gastro.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 43.Guerriero CJ, Brodsky JL. The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology. Physiol Rev. 2012;92:537–76. doi: 10.1152/physrev.00027.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 45.Chang L, Goldman RD. Intermediate filaments mediate cytoskeletal crosstalk. Nat Rev Mol Cell Biol. 2004;5:601–13. doi: 10.1038/nrm1438. [DOI] [PubMed] [Google Scholar]
- 46.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 47.Kholodenko BN. Untangling the signalling wires. Nat Cell Biol. 2007;9:247–9. doi: 10.1038/ncb0307-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Namkung W, Yoon JS, Kim KH, Lee MG. PAR2 exerts local protection against acute pancreatitis via modulation of MAP kinase and MAP kinase phosphatase signaling. Am J Physiol Gastrointest Liver Physiol. 2008;295:G886–94. doi: 10.1152/ajpgi.00053.2008. [DOI] [PubMed] [Google Scholar]
- 49.Scarabelli TM, et al. Urocortin promotes hemodynamic and bioenergetic recovery and improves cell survival in the isolated rat heart exposed to ischemia/reperfusion. J Am Coll Cardiol. 2002;40:155–61. doi: 10.1016/s0735-1097(02)01930-7. [DOI] [PubMed] [Google Scholar]
- 50.Huising MO, et al. Glucocorticoids differentially regulate the expression of CRFR1 and CRFR2alpha in MIN6 insulinoma cells and rodent islets. Endocrinology. 2011;152:138–50. doi: 10.1210/en.2010-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gukovsky I, et al. Impaired autophagy and organellar dysfunction in pancreatitis. J. Gastroenterol. Hepatol. 2012;27(Suppl 2):27–32. doi: 10.1111/j.1440-1746.2011.07004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lugea A, Waldron RT, French SW, Pandol SJ. Drinking and driving pancreatitis: links between endoplasmic reticulum stress and autophagy. Autophagy. 2011;7:783–5. doi: 10.4161/auto.7.7.15594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lugea A, et al. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology. 2011;140:987–97. doi: 10.1053/j.gastro.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 55.Barr CS, et al. Functional CRH variation increases stress-induced alcohol consumption in primates. Proc Natl Acad Sci U S A. 2009;106:14593–8. doi: 10.1073/pnas.0902863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakamura S, et al. Estradiol alleviates acinar cell apoptosis and chronic pancreatitis in male Wistar Bonn/Kobori rats. Pancreas. 2003;26:e59–66. doi: 10.1097/00006676-200304000-00024. [DOI] [PubMed] [Google Scholar]
- 57.Lee J, Goldberg IJ. Hypertriglyceridemia-induced pancreatitis created by oral estrogen and in vitro fertilization ovulation induction. J Clin Lipidol. 2008;2:63–6. doi: 10.1016/j.jacl.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trenque-Tessereau MG, Picot C, Herment N, Trenque TC. Combined estradiol/gestodene and acute pancreatitis. Ann Pharmacother. 2005;39:1953–4. doi: 10.1345/aph.1G292. [DOI] [PubMed] [Google Scholar]
- 59.Robles-Diaz G, Diaz-Sanchez V, Mendez JP, Altamirano A, Wolpert E. Low serum testosterone/dihydrotestosterone ratio in patients with pancreatic carcinoma. Pancreas. 1987;2:684–7. doi: 10.1097/00006676-198711000-00010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.