Abstract
Primary vitreoretinal lymphoma (PVRL), previously called primary intraocular lymphoma (PIOL), is a rare and fatal ocular malignancy. PVRL is a subset of primary central nervous system lymphoma (PCNSL), mostly a diffuse large B-cell lymphoma. The diagnosis of PVRL is often challenging as it often masquerades as chronic uveitis. PVRL requires invasive procedures for tissue diagnosis. Cytology/pathology, molecular pathology (immunoglobulin or T-cell receptor gene rearrangement), immunohistochemistry, biophysical technology (flow cytometry), and cytokine analysis (interleukine-10) are often required. The therapies that have been successful in systemic lymphomas have not been reliably effective in PVRL and PCNSL. Current management of PVRL involves aggressive chemotherapy (methotrexate and rituximab) and radiation therapy. PVRL normally responds well to initial treatment; however, relapse rate and CNS involvement are high, resulting in poor prognosis and limited survival. A professional team of medical experts in ophthalmology, oncology (particularly neuro-oncology), and pathology is essential for optimizing patient management.
Terminology
In a clinico-pathological survey of 618 lymphoma cases that was published in 1942, Gallo and Mallory documented a “stem cell lymphoma,” a histological subclass of “reticulum cell sarcoma” originated from the eyelid (Gallo and Mallory, 1942). They described the cells of this lymphoma with large nuclei and prominent densely staining nucleoli. In 1966, Rappaport classified lymphoma into three groups based on cellular morphology: Hodgkin’s disease, lymphosarcoma, and reticulum cell sarcoma (Rappaport, 1966). Although a few cases of intraocular “reticulum cell sarcoma” were reported in the 1950s and early 1960s, these handful of cases did not demonstrate lymphoma cells in the retina, vitreous, optic nerve, and/or central nerve system. In 1968, Vogel and associates presented 4 histological cases of “reticulum cell sarcoma” invading the retina (Vogel et al., 1968). Later, an unusual link was found between this intraocular lymphoma and primary central nervous system lymphoma (PCNSL) (Qualman et al., 1983; Char et al., 1981).
In the late 1970s and early 1980s the term “reticulum cell sarcoma” became a misnomer as researchers demonstrated the origin of the tumor cells to be transformed lymphoid cells (malignant B- and T- lymphocytes), not histiocytes. In 1982, the National Cancer Institute, National Institutes of Health sponsored a study of classification of non-Hodgkin’s lymphoma and replaced the Rappaport classification. With the advancement of immunology and genetics, the characterization and algorithm of lymphomas have been taken over by the World Health Organization (WHO) Lymphoma Classification. The “reticulum cell sarcoma in the eye” was changed to “primary intraocular lymphoma” (Char et al., 1988b). Primary intraocular lymphoma was defined as “isolated eye tumor” or “tumor that involves both the globe and the CNS” (Char et al., 1988b). The WHO classification emphasizes an approach whereby the clinical features are correlated with distinct morphology, immunophenotype, and genotype of each neoplasm. The term “primary intraocular lymphomas” should represent lymphomas originating from all ocular tissues. Since lymphomas originating from the retina/vitreous and the choroid are distinctive, “primary vitreoretinal lymphoma (PVRL)” is proposed and used (Coupland et al., 2009).
Diagnosis
PVRL is a rare extranodal non-Hodgkin’s lymphoma that originally invades intraocular tissues: the retina, vitreous, and/or optic nerve (Chan and Gonzalez, 2007; Chan et al., 2011). PVRL is the most common intraocular lymphoma, usually of the B-cell type as diffuse large B-cell lymphoma. Infrequently, there is PVRL of T-cell type. PVRL is closely related to PCNSL (Petersson et al., 1998). In the U.S., it is estimated that there are 300–380 new cases of PVRL per year (Chan et al., 2011). Approximately 80% of PVRL patients eventually develop PCNSL and approximately 20% of PCNSL patients present with PVRL (Coupland et al., 2004; Hochberg and Miller, 1988; Hong et al., 2011; Nasir and DeAngelis, 2000; Surawicz et al., 1999). Consequently, PVRL is often fatal because of ultimate CNS association (Chan et al., 2011; Hormigo et al., 2004; Peterson et al., 1993). When treated with conventional therapy, relapsing PCNSL had a poor prognosis with a one-year overall survival of 25–40% (Sierra del Rio et al., 2009). The mean survival of PVRL is 32 months and 5 year survival rate is 61% in Japan (Kimura et al., 2012). PVRL differs from the metastatic lymphoma in the eye, which often locates in the choroid of generally sick patients with known systemic lymphoma (Chan and Gonzalez, 2007). Bilateral ocular involvement is common.
PVRL is a masquerade syndrome that mimics chronic uveitis, which creates difficulty in diagnosis (Chan et al., 2011; Davis, 2013; Faia and Chan, 2009; Kinoshita et al., 2012; Mochizuki and Singh, 2009). The diagnosis of PVRL requires a clinical suspicion and more importantly, the tissue diagnosis and confirmation (Chan, 2003). Among the earliest true PVRL cases published in 1968 (Vogel et al., 1968), a 67-year-old male patient had clinical diagnosis of uveitis and the pathology showed lymphoma cells not only in his retina and uvea, but also in his brain. In another case, a 71-year-old female patient presented clinically with pericorneal injection of a steamy cornea and blindness in the right eye. Her enucleated right eye showed massive chronic inflammation in the anterior segments and vitreous; significantly, large atypical lymphoid cells were found in the thickened retina (Vogel et al., 1968). Since then, many similar cases that were first misdiagnosed as “uveitis” and later found to have lymphoma cells in the retina, vitreous, and brain have been reported in the literature (Davis, 2013; Sen et al., 2009).
Clinically, PVRL typically occurs in older patients with median age range of 60s (Chan et al., 2011; Mochizuki and Singh, 2009; Whitcup et al., 1993). In general PVRL patients do not have systemic symptoms such as cachexia, fever, and lymphadenopathy (Chan and Gonzalez, 2007; Hormigo and DeAngelis, 2003). If there is no CNS involvement, these patients usually complain of blurred vision and floaters (Chan et al., 2011; Davis, 2013; Mochizuki and Singh, 2009). Patients with CNS involvement can present with neurological symptoms depending on the tumor location in the brain. Since the frontal lobe is the most frequent location for PCNSL, the common presenting symptom is personality change (Mochizuki and Singh, 2009).
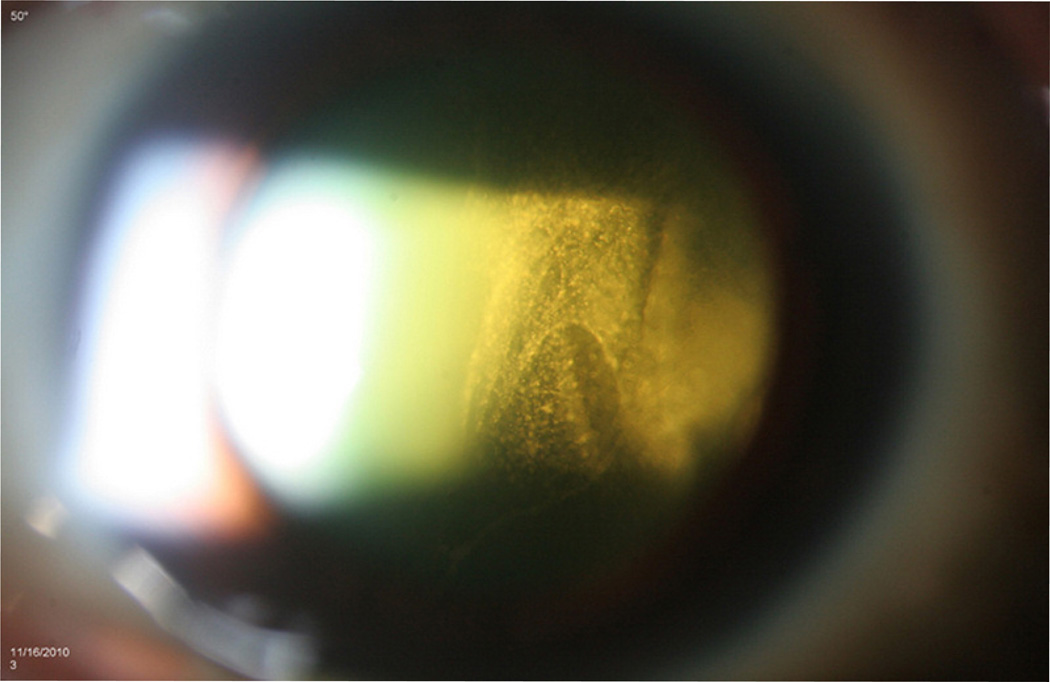
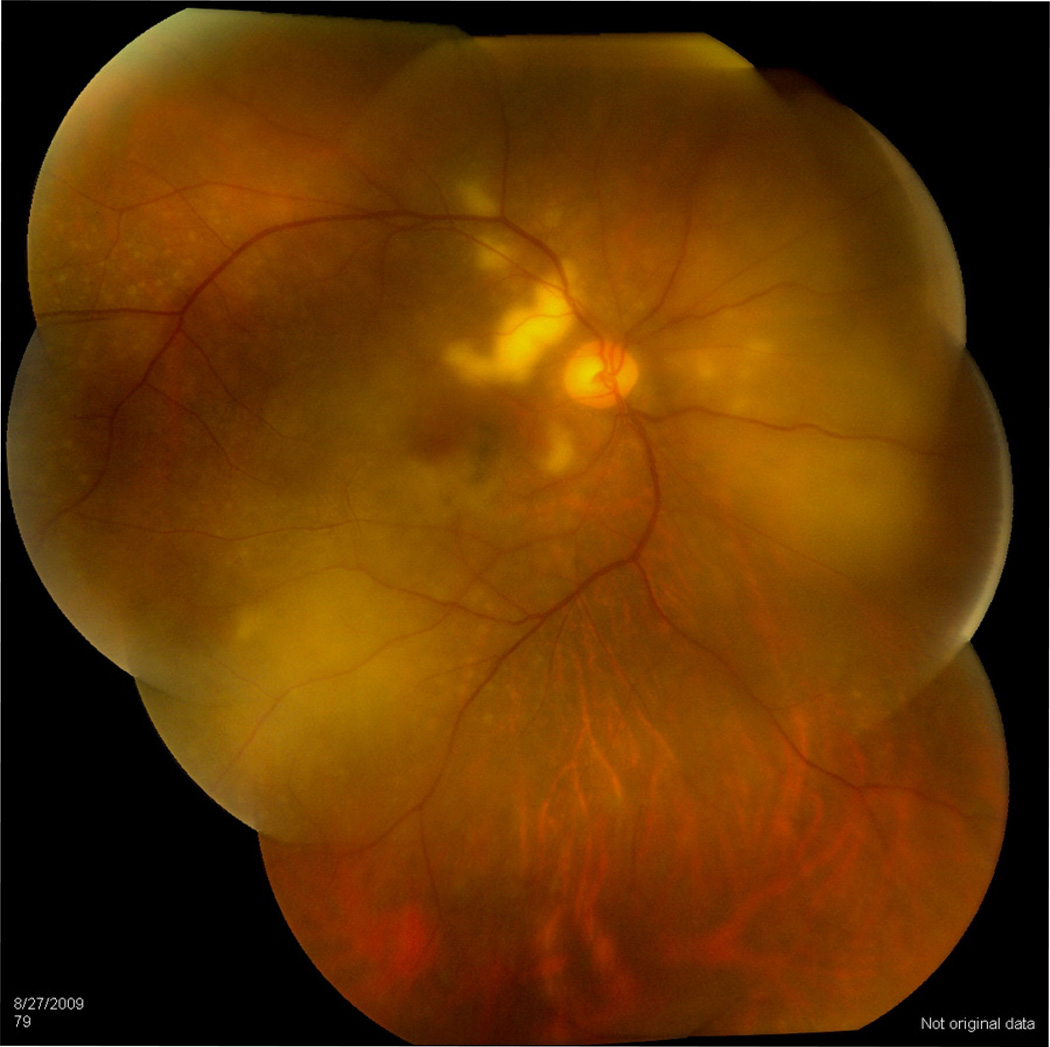
Most PVRL cases have little anterior segment inflammation and the eye is usually quiet and white (Chan et al., 2011). Slit lamp examination may show mild inflammation but it is non-specific. Dilated fundus examination is essential for the clinical diagnosis. Vitreous cells and haze are often striking (Faia and Chan, 2009). The cells can be in clumps or in sheets (Figure 1). In a series of 32 patients, Freeman et al. (1987) reported 50% presented with vitreous cells. Char et al. (1988a) reported 100% vitreous cells in their study of 20 patients. We and others also reported vitreous cells as the most common ocular finding (Chan, 2003; 2011; Hong et al., 2011; Levy-Clarke et al., 2005; Sen et al., 2009; Whitcup et al., 1993). Frequently, the cells (lymphoma cells mixed with reactive inflammatory cells) in the vitreous are abundant, but visual acuity can be unexpectedly good, or at least better than expected (Whitcup et al., 1993). Retinal and subretinal lesions are classically creamy, white to orange infiltrates (Figure 2). They may have feathery or distinct borders, may be single isolated or multiple confluents, and can be located in the sub-retinal pigment epithelial (RPE), sub-retinal, and/or intra-retinal regions.
Figure 1.
Slit lamp biomicroscopy shows intense vitreous cells seen in sheets of a 62 year-old female patient with PCNSL/PVRL.
Figure 2.
Fundoscopy shows elevated yellowish subretinal lesions along the superior arcade and diffuse yellowish infiltrate in the inferonasal retina of a 59 year-old female with PCNSL/PVRL who presented as panuveitis initially.
Since the PVRL cells tend to deposit in the sub-RPE, above the Bruch’s membrane first (Chan et al., 2003), various ocular images can illustrate different characteristic patterns (Chan et al., 2011; Chan and Sauer, 2009). Fundus autofluorescence in 5 eyes with PVRL showed bright hyperfluorescent spots corresponding to the sub-RPE infiltrates, and hypofluorescent areas corresponding to RPE atrophy where, presumably, tumor cells were previously resided (Ishida et al., 2010). Fundus fluorescein angiogram (FA) illustrates RPE disturbances as granular, mottling, and late staining patterns (Cassoux et al., 2000; Fardeau et al., 2009; Velez et al., 2002). Clusters of round, hyper- or hypo-fluorescent spots reflect RPE abnormalities. Ocular coherence tomography (OCT) shows nodular hyperreflective lesions at the RPE level. In an imaging study of 53 patients with PVRL, the positive predictive value of imaging with OCT, FA, or indocyanine green angiography (ICG) was 88.9% and the negative predictive value was 85%, the odds ratio was 45.22 (Fardeau et al., 2009).
Because PVRL is closely related to PCNSL, it is imperative to evaluate the CNS with contrast-enhanced magnetic resonance imaging (MRI) (Chan et al., 2011; Sen et al., 2009). Patients with PCNSL have either single or multiple lesions with discrete or diffuse borders. Cerebrospinal fluid (CSF) evaluation is highly recommended despite a low yield for lymphoma cells in the CSF (Chan et al., 2002). However, demonstration of lymphoma cells in the CSF supports the diagnosis of PVRL and spares the patient from further invasive diagnostic procedures such as diagnostic vitrectomy or retinal biopsy.
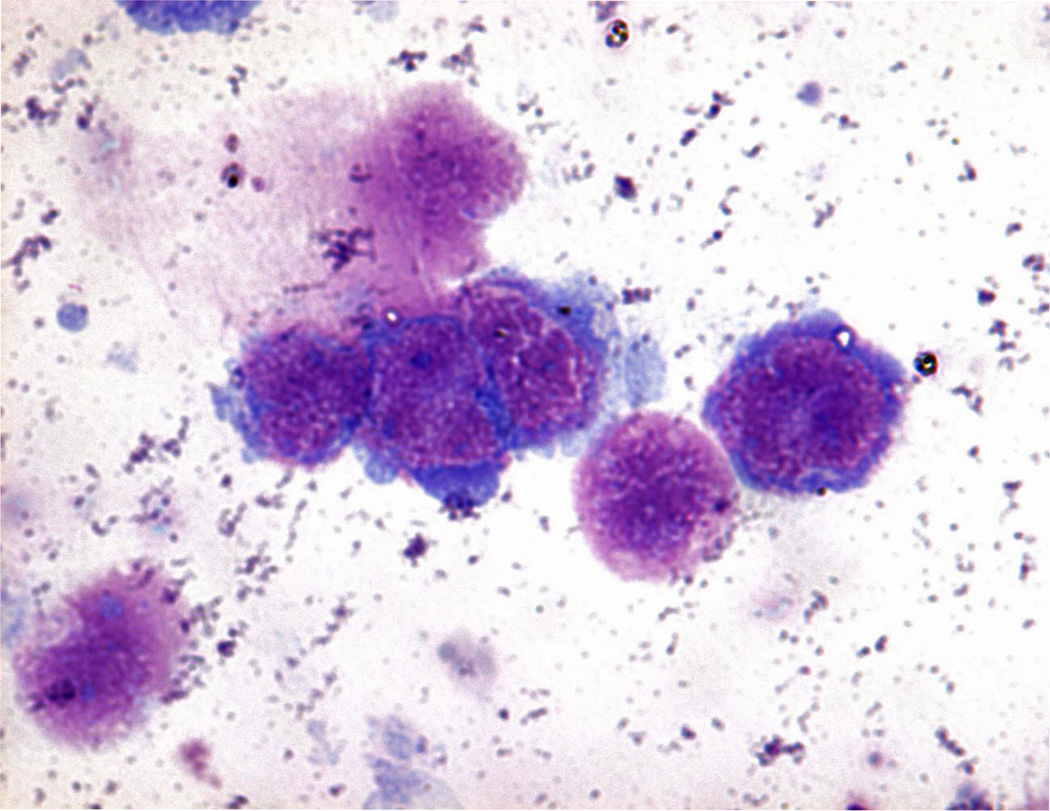
The gold standard for diagnosing PVRL requires the detection of malignant lymphoid cells in the retina, vitreous, and/or the optic nerve (Coupland et al., 2004; Rajagopal and Harbour, 2011; Sen et al., 2009; Zaldivar et al., 2004). Surgical removal of ocular fluids (aqueous aspiration and/or diagnostic vitrectomy), retinal or chorioretinal biopsy, and rarely diagnostic enucleation are performed. Since PVRL cells rapidly die once they leave the eye, it is critical to process the specimen promptly (Chan, 2003). Routine cytology and histopathology are used to identify PVRL cells, which are characterized with large irregular nuclei, prominent nucleoli, and scanty basophilic cytoplasm (Figure 3). Mitoses are variable. Reactive lymphocytes are often mixed with PVRL cells and the PVRL cells are easily necrotic and degenerated, which can create difficulty in recognition of the PVRL cells.
Figure 3.
Cytology of a vitreous specimen from a patient with PVRL showing large lymphoma cells with large irregular nuclei, prominent nucleoli and scanty basophilic cytoplasm. (Giemsa stain, original magnification ×400).
Immunohistochemistry or flow cytometry demonstrates the monoclonality, either B-cell (kappa or lambda light chain) or T-cell type for the lymphoma cells (Davis et al., 1992; 1997). Flow cytometry is an effective tool and can provide accurate information for the diagnosis (Davis et al., 1997). Molecular analysis of the PVRL cells detects either IgH gene rearrangements in B-cell lymphoma or T-cell receptor gene rearrangements in T-cell lymphoma. In a large series of 114 PVRL cases, the diagnostic efficiency of molecular techniques was 99.5% using microdissection by selecting a minimum of 15 atypical lymphoid cells from the specimen combined with polymerase chain reaction (Wang et al., 2011). Without microdissection, molecular testing may be less sensitive and specific (White et al., 1999).
B-cell lymphoma produces ample interleukin-10 (IL-10) and normal inflammatory cells (macrophages and lymphocytes) secrete more IL-6. In 1995, high levels of vitreous IL-10 were first reported in three B-cell PVRL patients (Chan et al., 1995); since then other publications have confirmed the elevation of IL-10 in ocular fluids of patients with PVRL (Cassoux et al., 2007; 2001; Whitcup et al., 1997). Currently, a high IL-10 and/or a ratio of IL-10:IL-6 greater than 1 in aqueous and/or vitreous have become adjunctive and supportive biomarkers for the diagnosis of PVRL, particularly the B-cell PVRL (Asencio-Duran et al., 2012; Chan et al., 2011; Davis, 2013; Kimura et al., 2012; Rajagopal and Harbour, 2011; Sen et al., 2009; Sugita et al., 2009). Additionally, IL-10 (−1082) G↔A polymorphism is recently found to be associated with PVRL and PCNSL (Ramkumar et al., 2012). This association is a risk factor for higher ocular IL-10 levels and correlates with more aggressive malignancy.
Clinical manifestation, imaging findings (eye and CNS), flow cytometry, ocular IL-10 levels, and molecular data are helpful adjuncts for correct diagnosis, although the diagnosis of PVRL relies on the identification of lymphoma cells in the retina, vitreous, and/or optic nerve.
Treatment
Currently there is no standard optimal therapy for PVRL due to its rarity. Even though PVRL cells are highly radiosensitive and chemosensitive (e.g., methotrexate), the overall survival rate is still quite low. In 2011, the International PCNSL Collaborative Group organized a symposium on PVRL and recommended the following therapeutic principles: systemic treatment if disease involves the CNS; local treatment (the eye) if the disease involves only the eye with close follow-up and ongoing collaboration between neurooncologists and ophthalmologists (Chan et al., 2011). It is crucial to have a team of ophthalmologist, oncologist (neuro-oncologist or hemato-oncologist), and pathologist for the management of each PVRL patient.
The recommendations of the therapeutic regimens for the PVRL without CNS involvement are: for PVRL that is limited to one eye, local ocular treatment with intravitreal methotrexate, intravitreal rituximab, or ocular radiation with 30–35 Gy external beam; for PVRL involving both eyes, the recommendations were mixed, with preferable local therapy combined with systemic treatment. In other words, systemic chemotherapy has been suggested in addition to intravitreal medications for bilateral PVRL (Chan et al., 2011; Pe’er et al., 2009). For patients with coexisting PVRL and PCNSL, a high-dose methotrexate based systemic therapy, possibly with systemic rituximab, was proposed in conjunction with local ocular therapy, especially given the limited penetration of systemic agents into the vitreous cavity. There was consideration of whole brain radiotherapy in conjunction with ocular radiotherapy in those who had failed systemic chemotherapy and were too debilitated or did not meet criteria for more aggressive therapy such as autologous stem cell transplantation (ASCT) (Chan et al., 2011). However, some neurooncologists might not include local treatment to the eyes if there was concurrent ocular and CNS lymphoma (Lisa DeAngelis, personal communication).
A recent review on current care of PCNSL discussed whole brain radiation with 20–50 Gy combined with or without chemotherapy (methotrexate, rituximab, or blood-brain barrier disruption) (Rosenfeld and Pruitt, 2012). Less than 5% of patients had associated systemic lymphoma. Whole brain radiation given with or without chemotherapy often induced a delayed neurotoxicity with decline in cognitive function, ataxia, urinary incontinence, dementia, and even death (Rosenfeld and Pruitt, 2012). Now more neuro-oncologists are avoiding initial radiation therapy and administering chemotherapy first, then consolidating with a lower dose of whole brain radiation (23 Gy) only after failure of systemic chemotherapy (Tracy Batchelor, personal communication). Another recent review of a comparison between chemotherapy alone or a combined modality therapy with high-dose methotrexate and whole brain radiotherapy for PCNSL in immunocompetent patients found that the combined modality had better response rates but higher neurotoxicity (Prica et al., 2012). The findings support that the preferred strategy is chemotherapy alone for older PCNSL patients.
For the relapsed or refractory PCNSL and PVRL, intense chemotherapy of thiotepa, busulfan, and cyclophosphamide, combined with hematopoietic stem cell rescue were used in 79 patients who did not respond to high-dose methotrexate (Soussain et al., 2012). The patients were followed for 56 months. The 5-year overall survival probability was 62% compared to the 51% probability in the general PCNSL population, and the 5-year event free survival probability was 43.7% compared to the 37.8% in the general PCNSL population. Although no firm conclusions can be made from this study, prospective multicenter randomized studies (NCT00863460 and NCT01011920) are underway.
Currently there are two National Cancer Institute (NCI) sponsored, open randomized clinical trials to address the following questions: (1) high dose chemotherapy/autotransplant versus standard chemotherapy for consolidation: “Combination chemotherapy with or without autologous stem cell transplant in treating patients with CNS B-cell lymphoma (CALGB 51101 - P.I.: Tracy Batchelor)” and (2) chemotherapy and low dose whole brain radiation therapy as consolidation: “Rituximab, methotrexate, vincristine sulfate, procarbazine hydrochloride, and cytarabine with or without radiation therapy in treating patients with PCNSL (GTOG 1114 - P.I.: Antonio Omuro) (http://clinicaltrials.gov/).
In summary, neuro-oncologists and ophthalmologists must consult one another and manage their PCNSL/PVRL patients with a team approach. Methotrexate-based polychemotherapy is recommended as the first line treatment. Combined radiation and chemotherapy exposes patients, especially elderly patients, to severe delayed neurotoxic effects. Intense chemotherapy with autologous stem-cell transplantation may become an effective salvage treatment for refractory and relapsed PCNSL (Chan et al., 2011; Ricard et al., 2012; Davis, 2013).
Conclusion
PVRL, a subset of PCNSL often masquerades as intraocular inflammation or uveitis; therefore the disease is easily misdiagnosed, resulting in inappropriate management and high morbidity and mortality. Ocular cytokine levels and molecular analyses can provide useful supplementary data for the diagnosis. Optimal therapy for PVRL becomes a great challenge to both the oncologist and ophthalmologist. Studying the cellular and molecular biology, epidemiology, pathology, physiology, immunology, and genetics of PVRL can make a considerable difference in the diagnosis, management, and prognosis of this devastating disease.
Acknowledgment
Lisa DeAngelis of Sloan-Kettering Institute and Tracy T. Batchelor of Harvard Medical School provided medical advice on treatment.
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- Asencio-Duran M, Vallejo-Garcia JL, Pastora-Salvador N, Fonseca-Sandomingo A, Romano MR. Vitreous diagnosis in neoplastic diseases. Mediators Inflamm. 2012;2012:930704. doi: 10.1155/2012/930704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassoux N, Giron A, Bodaghi B, Tran TH, Baudet S, Davy F, Chan CC, Lehoang P, Merle-Beral H. IL-10 measurement in aqueous humor for screening patients with suspicion of primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2007;48(7):3253–3259. doi: 10.1167/iovs.06-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassoux N, Merle-Beral H, Leblond V, Bodaghi B, Milea D, Gerber S, Fardeau C, Reux I, Xuan KH, Chan CC, Lehoang P. Ocular and central nervous system lymphoma: clinical features and diagnosis. Ocul Immunol Inflamm. 2000;8(4):243–250. doi: 10.1076/ocii.8.4.243.6463. [DOI] [PubMed] [Google Scholar]
- Cassoux N, Merle-Beral H, Lehoang P, Herbort C, Chan CC. Interleukin-10 and intraocular-central nervous system lymphoma. Ophthalmology. 2001;108(3):426–427. doi: 10.1016/s0161-6420(00)00401-2. [DOI] [PubMed] [Google Scholar]
- Chan CC. Molecular pathology of primary intraocular lymphoma. Trans Am Ophthalmol Soc. 2003;101:275–292. [PMC free article] [PubMed] [Google Scholar]
- Chan CC, Buggage RR, Nussenblatt RB. Intraocular lymphoma. Curr Opin Ophthalmol. 2002;13(6):411–418. doi: 10.1097/00055735-200212000-00012. [DOI] [PubMed] [Google Scholar]
- Chan CC, Gonzalez JA. Primary Intraocular Lymphoma. New Jersey, USA: World Scientific Publishing Co. Pte. Ltd.; 2007. [Google Scholar]
- Chan CC, Rubenstein JL, Coupland SE, Davis JL, Harbour JW, Johnston PB, Cassoux N, Touitou V, Smith JR, Batchelor TT, Pulido JS. Primary vitreoretinal lymphoma: a report from an International Primary Central Nervous System Lymphoma Collaborative Group symposium. Oncologist. 2011;16(11):1589–1599. doi: 10.1634/theoncologist.2011-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CC, Sauer TC. Ocular imaging in primary retinal lymphoma. Am J Ophthalmol. 2009;147(5):764–765. doi: 10.1016/j.ajo.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CC, Shen D, Hackett JJ, Buggage RR, Tuaillon N. Expression of chemokine receptors, CXCR4 and CXCR5, and chemokines, BLC and SDF-1, in the eyes of patients with primary intraocular lymphoma. Ophthalmology. 2003;110(2):421–426. doi: 10.1016/S0161-6420(02)01737-2. [DOI] [PubMed] [Google Scholar]
- Chan CC, Whitcup SM, Solomon D, Nussenblatt RB. Interleukin-10 in the vitreous of primary intraocular lymphoma. Am J Ophthalmol. 1995;120(5):671–673. doi: 10.1016/s0002-9394(14)72217-2. [DOI] [PubMed] [Google Scholar]
- Char DH, Ljung BM, Deschenes J, Miller TR. Intraocular lymphoma: immunological and cytological analysis. Br J Ophthalmol. 1988a;72(12):905–911. doi: 10.1136/bjo.72.12.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Char DH, Ljung BM, Miller T, Phillips T. Primary intraocular lymphoma (ocular reticulum cell sarcoma) diagnosis and management. Ophthalmology. 1988b;95:625–630. doi: 10.1016/s0161-6420(88)33145-3. [DOI] [PubMed] [Google Scholar]
- Char DH, Margolis L, Newman AB. Ocular reticulum cell sarcoma. Am J Ophthalmol. 1981;91(4):480–483. doi: 10.1016/0002-9394(81)90236-1. [DOI] [PubMed] [Google Scholar]
- Coupland SE, Chan CC, Smith J. Pathophysiology of retinal lymphoma. Ocul Immunol Inflamm. 2009;17(4):227–237. doi: 10.1080/09273940903168696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland SE, Heimann H, Bechrakis NE. Primary intraocular lymphoma: a review of the clinical, histopathological and molecular biological features. Graefes Arch Clin Exp Ophthalmol. 2004;242(11):901–913. doi: 10.1007/s00417-004-0973-0. [DOI] [PubMed] [Google Scholar]
- Davis JL. Intraocular lymphoma: a clinical perspective. Eye (Lond) 2013;27(2):153–162. doi: 10.1038/eye.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JL, Solomon D, Nussenblatt RB, Palestine AG, Chan CC. Immunocytochemical staining of vitreous cells. Indications, techniques, and results. Ophthalmology. 1992;99(2):250–256. doi: 10.1016/s0161-6420(92)31984-0. [DOI] [PubMed] [Google Scholar]
- Davis JL, Viciana AL, Ruiz P. Diagnosis of intraocular lymphoma by flow cytometry. Am J Ophthalmol. 1997;124(3):362–372. doi: 10.1016/s0002-9394(14)70828-1. [DOI] [PubMed] [Google Scholar]
- Faia LJ, Chan CC. Primary intraocular lymphoma. Arch Pathol Lab Med. 2009;133(8):1228–1232. doi: 10.5858/133.8.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardeau C, Lee CP, Merle-Béral H, Cassoux N, Bodaghi B, Davi F, Lehoang P. Retinal fluorescein, indocyanine green angiography, and optic coherence tomography in non-Hodgkin’s primary intraocular lymphoma. Am J Ophthalmol. 2009;147(5):886–894. 894, e881. doi: 10.1016/j.ajo.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Freeman LN, Schachat AP, Knox DL, Michels RG, Green WR. Clinical features, laboratory investigations, and survival in ocular reticulum cell sarcoma. Ophthalmology. 1987;94:1631–1639. doi: 10.1016/s0161-6420(87)33256-7. [DOI] [PubMed] [Google Scholar]
- Gallo EA, Mallory TB. Malignant lymphoma: a clinicopathological survey of 618 cases. Am J Pathol. 1942;18:381–429. [PMC free article] [PubMed] [Google Scholar]
- Hochberg FH, Miller DC. Primary central nervous system lymphoma. J Neurosurg. 1988;68(6):835–853. doi: 10.3171/jns.1988.68.6.0835. [DOI] [PubMed] [Google Scholar]
- Hong JT, Chae JB, Lee JY, Kim JG, Yoon YH. Ocular involvement in patients with primary CNS lymphoma. J Neurooncol. 2011;102(1):139–145. doi: 10.1007/s11060-010-0303-9. [DOI] [PubMed] [Google Scholar]
- Hormigo A, Abrey L, Heinemann MH, Deangelis LM. Ocular presentation of primary central nervous system lymphoma: diagnosis and treatment. Br J Haematol. 2004;126(2):202–208. doi: 10.1111/j.1365-2141.2004.05028.x. [DOI] [PubMed] [Google Scholar]
- Hormigo A, Deangelis LM. Primary intraocular lymphoma: Clinical features, diagnosis and treatment. Clin Lymphoma. 2003;4(1):22–29. doi: 10.3816/clm.2003.n.010. [DOI] [PubMed] [Google Scholar]
- Ishida T, Ohno-Matsui K, Kaneko Y, Tobita H, Shimada N, Takase H, Mochizuki M. Fundus autofluorescence patterns in eyes with primary intraocular lymphoma. Retina. 2010;30(1):23–32. doi: 10.1097/IAE.0b013e3181b408a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Usui Y, Goto H. Clinical features and diagnostic significance of the intraocular fluid of 217 patients with intraocular lymphoma. Jpn J Ophthalmol. 2012;56(4):383–389. doi: 10.1007/s10384-012-0150-7. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y, Takasu K, Adachi Y, Yuri T, Nagumo S, Shikata N. Retrospective cytological study of intraocular lymphoma using vitreous and intraocular perfusion fluid. Diagn Cytopathol. 2012;40(7):604–607. doi: 10.1002/dc.21596. [DOI] [PubMed] [Google Scholar]
- Levy-Clarke GA, Chan CC, Nussenblatt RB. Diagnosis and management of primary intraocular lymphoma. Hematol Oncol Clin North Am. 2005;19(4):739–749. doi: 10.1016/j.hoc.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Mochizuki M, Singh AD. Epidemiology and clinical features of intraocular lymphoma. Ocul Immunol Inflamm. 2009;17(2):69–72. doi: 10.1080/09273940902957305. [DOI] [PubMed] [Google Scholar]
- Nasir S, Deangelis LM. Update on the management of primary CNS lymphoma. Oncology (Huntingt) 2000;14(2):228–234. discussion 237–242,244. [PubMed] [Google Scholar]
- Pe’er J, Hochberg FH, Foster CS. Clinical review: treatment of vitreoretinal lymphoma. Ocul Immunol Inflamm. 2009;17(5):299–306. doi: 10.3109/09273940903370755. [DOI] [PubMed] [Google Scholar]
- Peterson K, Gordon KB, Heinemann MH, Deangelis LM. The clinical spectrum of ocular lymphoma. Cancer. 1993;72(3):843–849. doi: 10.1002/1097-0142(19930801)72:3<843::aid-cncr2820720333>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Petersson M, Charo J, Salazar-Onfray F, Noffz G, Mohaupt M, Qin Z, Klein G, Blankenstein T, Kiessling R. Constitutive IL-10 production accounts for the high NK sensitivity, low MHC class I expression, and poor transporter associated with antigen processing (TAP)-1/2 function in the prototype NK target YAC-1. J Immunol. 1998;161(5):2099–2105. [PubMed] [Google Scholar]
- Prica A, Chan K, Cheung MC. Combined modality therapy versus chemotherapy alone as an induction regimen for primary central nervous system lymphoma: a decision analysis. Br J Haematol. 2012;158(5):600–607. doi: 10.1111/j.1365-2141.2012.09208.x. [DOI] [PubMed] [Google Scholar]
- Qualman SL, Mendelsohn G, Mann RB, Green WR. Intraocular lymphomas. Natural history based on a clinicopathologic study of eight cases and review of the literature. Cancer. 1983;52:878–886. doi: 10.1002/1097-0142(19830901)52:5<878::aid-cncr2820520523>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Rajagopal R, Harbour JW. Diagnostic testing and treatment choices in primary vitreoretinal lymphoma. Retina. 2011;31(3):435–440. doi: 10.1097/IAE.0b013e31820a6743. [DOI] [PubMed] [Google Scholar]
- Ramkumar HL, Shen De F, Tuo J, Braziel RM, Coupland SE, Smith JR, Chan CC. IL-10 −1082 SNP and IL-10 in primary CNS and vitreoretinal lymphomas. Graefes Arch Clin Exp Ophthalmol. 2012;250(10):1541–1548. doi: 10.1007/s00417-012-2037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport H. Atlas of Tumor Pathology, Section III-Fascicle 8, Tumors of the Hematopoietic System. Washington, D.C., USA: Armed Forces Institute of Pathology; 1966. [Google Scholar]
- Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre JY. Primary brain tumours in adults. Lancet. 2012;379(9830):1984–1996. doi: 10.1016/S0140-6736(11)61346-9. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MR, Pruitt AA. Management of malignant gliomas and primary CNS lymphoma: standard of care and future directions. Continuum (Minneap Minn) 2012;18(2):406–415. doi: 10.1212/01.CON.0000413666.88539.0b. [DOI] [PubMed] [Google Scholar]
- Sen HN, Bodaghi B, Hoang PL, Nussenblatt R. Primary intraocular lymphoma: diagnosis and differential diagnosis. Ocul Immunol Inflamm. 2009;17(3):133–141. doi: 10.1080/09273940903108544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra Del Rio M, Rousseau A, Soussain C, Ricard D, Hoang-Xuan K. Primary CNS lymphoma in immunocompetent patients. Oncologist. 2009;14(5):526–539. doi: 10.1634/theoncologist.2008-0236. [DOI] [PubMed] [Google Scholar]
- Soussain C, Choquet S, Fourme E, Delgadillo D, Bouabdallah K, Ghesquieres H, Damaj G, Dupriez B, Vargaftig J, Gonzalez A, Houillier C, Taillandier L, Hoang-Xuan K, Leblond V. Intensive chemotherapy with thiotepa, busulfan and cyclophosphamide and hematopoietic stem cell rescue in relapsed or refractory primary central nervous system lymphoma and intraocular lymphoma: a retrospective study of 79 cases. Haematologica. 2012;97(11):1751–1756. doi: 10.3324/haematol.2011.060434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Takase H, Sugamoto Y, Arai A, Miura O, Mochizuki M. Diagnosis of intraocular lymphoma by polymerase chain reaction analysis and cytokine profiling of the vitreous fluid. Jpn J Ophthalmol. 2009;53(3):209–214. doi: 10.1007/s10384-009-0662-y. [DOI] [PubMed] [Google Scholar]
- Surawicz TS, Mccarthy BJ, Kupelian V, Jukich PJ, Bruner JM, Davis FG. Descriptive epidemiology of primary brain and CNS tumors: results from the Central Brain Tumor Registry of the United States, 1990–1994. Neuro Oncol. 1999;1(1):14–25. doi: 10.1093/neuonc/1.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez G, Chan CC, Csaky KG. Fluorescein angiographic findings in primary intraocular lymphoma. Retina. 2002;22(1):37–43. doi: 10.1097/00006982-200202000-00007. [DOI] [PubMed] [Google Scholar]
- Vogel MH, Font RL, Zimmerman LE, Levine RA. Reticulum cell sarcoma of the retina and uvea. Report of six cases and review of the literature. Am J Ophthalmol. 1968;66(2):205–215. doi: 10.1016/0002-9394(68)92065-5. [DOI] [PubMed] [Google Scholar]
- Whitcup SM, De Smet MD, Rubin BI, Palestine AG, Martin DF, Burnier MJ, Chan CC, Nussenblatt RB. Intraocular lymphoma. Clinical and histopathologic diagnosis. Ophthalmology. 1993;100(9):1399–1406. doi: 10.1016/s0161-6420(93)31469-7. [DOI] [PubMed] [Google Scholar]
- Whitcup SM, Stark-Vancs V, Wittes RE, Solomon D, Podgor MJ, Nussenblatt RB, Chan CC. Association of interleukin-10 in the vitreous and cerebral spinal fluid and primary central nervous system lymphoma. Arch Ophthalmol. 1997;115:1157–1160. doi: 10.1001/archopht.1997.01100160327010. [DOI] [PubMed] [Google Scholar]
- White VA, Gascoyne RD, Paton KE. Use of the polymerase chain reaction to detect B- and T-cell gene rearrangements in vitreous specimens from patients with intraocular lymphoma. Arch Ophthalmol. 1999;117(6):761–765. doi: 10.1001/archopht.117.6.761. [DOI] [PubMed] [Google Scholar]
- Zaldivar RA, Martin DF, Holden JT, Grossniklaus HE. Primary intraocular lymphoma: clinical, cytologic, and flow cytometric analysis. Ophthalmology. 2004;111(9):1762–1767. doi: 10.1016/j.ophtha.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shen D, Wang VM, Sen HN, Chan CC. Molecular biomarkers for the diagnosis of primary vitreoretinal lymphoma. Int J Mol Sci. 2011;12(9):5684–5697. doi: 10.3390/ijms12095684. [DOI] [PMC free article] [PubMed] [Google Scholar]