Abstract
Introduction and hypothesis
Mirabegron is a potent and selective β3-adrenoceptor agonist that may represent an alternative treatment option in place of antimuscarinics for patients with overactive bladder.
Methods
Patients completed a single-blinded, 2-week placebo run-in period followed by 12 weeks of randomized (n = 928) double-blinded treatment with mirabegron oral controlled absorption system (OCAS) 25, 50, 100, or 200 mg once-daily (QD), placebo or tolterodine extended release (ER) 4 mg QD. The primary endpoint was change from baseline to end-of-treatment in mean number of micturition episodes/24 h. Secondary endpoints included changes in mean volume voided per micturition; mean number of urinary incontinence, urgency urinary incontinence, and urgency episodes/24 h; severity of urgency; nocturia; and quality of life measures. Safety parameters included vital signs, adverse events, laboratory tests, electrocardiogram measurements and post-void residual volume.
Results
Mirabegron 25, 50, 100, and 200 mg resulted in dose-dependent reductions (improvements) from baseline to end-of-treatment in micturition frequency of 1.9, 2.1, 2.1, and 2.2 micturitions/24 h respectively, versus 1.4 micturitions/24 h with placebo (p ≤ 0.05 for the mirabegron 50-, 100-, and 200-mg comparisons). There was a statistically significant improvement with mirabegron compared with placebo for most secondary endpoints including quality of life variables. While there was a significant (p < 0.05) increase from baseline in pulse rate in the mirabegron 100-mg and 200-mg groups, this was not associated with an increased incidence of cardiovascular adverse events.
Conclusions
The favorable efficacy and tolerability of mirabegron in this phase II dose-finding study has led to its successful advancement into a phase III clinical development program.
Keywords: β3-adrenoceptor agonist, Mirabegron, Overactive, Urinary bladder
Introduction
Overactive bladder (OAB), estimated to occur in >50 million people worldwide [1], is characterized by symptoms of urinary urgency, frequency and nocturia, with/without urgency incontinence in the absence of lower urinary tract infection [2]. Antimuscarinic agents are used as first-line pharmacotherapy in the management of OAB; however, patients often discontinue antimuscarinics owing to side effects such as dry mouth, constipation and blurred vision [3], and because of an often insufficient response to treatment [4, 5].
The β-adrenoceptor is classified into β1, β2 and β3 subtypes. The largely post-synaptic β1-receptors are located primarily in the heart, but also in the salivary glands, platelets, and the gastrointestinal tract. β2-rceptors are also mainly post-synaptic and can be found in blood vessels, bronchi, skeletal muscle, liver and mast cells, as well as the gastrointestinal tract. The β3 subtype was first identified in adipose tissue, but has also been identified in bladder smooth muscle tissue (detrusor muscle). In the human bladder, the β3 subtype promotes detrusor relaxation and urine storage [6–8]. β3-adrenoceptor mRNA is predominantly expressed in the human urinary bladder, with 97 % of total β-adrenoceptor mRNA expressed by the β3 subtype and only 1.5 % and 1.4 % expressed by the β1 and β2 subtypes respectively [9]. Whereas antimuscarinic agents bind to muscarinic receptors in the bladder and inhibit involuntary bladder contractions, stimulation of the β3 receptors in the detrusor muscle of the bladder elicits relaxation of the bladder muscle during the storage phase of the micturition cycle [6–8]. Hence β3-adrenoceptor agonists improve the storage capacity of the bladder without inhibiting bladder voiding. These observations suggest that drugs which act at β3-adrenoceptors may have therapeutic potential in the treatment of the symptoms of OAB [9, 10].
Mirabegron is a β3-adrenoceptor agonist whose clinical efficacy was first evaluated in a phase II proof-of-concept study in which patients were randomized to receive mirabegron 100 mg or 150 mg twice-daily (BID), placebo, or tolterodine extended release (ER) 4 mg once-daily (QD) for 4 weeks. As no difference in efficacy was seen between the 100-mg and 150-mg BID doses, it was deduced that the maximum therapeutic dose of mirabegron was a total daily dose of 200 mg. The results of a subsequent phase II, randomized, double-blinded, placebo- and active-controlled clinical study (Clinicaltrials.gov number: NCT00337090) evaluating the efficacy and dose–response relationship of mirabegron utilizing a QD oral controlled absorption system (OCAS) formulation in patients with OAB are presented here. OCAS is a hydrophilic gel-forming matrix tablet that allows more steady absorption of mirabegron than seen with the original, immediate-release formulations, which were associated with high peak-to-trough fluctuations in plasma concentration and a considerable food effect. Tolterodine extended release (ER) is an antimuscarinic agent commonly used to treat the symptoms of OAB; it was included (at a daily dose of 4 mg) as the active control in order to validate the study and to enable the efficacy and safety of mirabegron to be placed in context against a commonly used therapy (although the study was not powered for a head-to-head comparison).
Materials and methods
Study patients
Enrolled patients were men and women ≥18 years of age experiencing symptoms of OAB for ≥3 months with frequency of micturition on average ≥8 times per 24 h and at least three episodes of urgency (grade 3 or 4) [11], with or without incontinence, during a 3-day micturition diary period at baseline.
Major exclusion criteria at study entry included clinically significant bladder outflow obstruction; significant post-void residual (PVR) volume (>200 ml); incontinence where stress was the predominant factor; indwelling catheters or intermittent self-catheterization; diabetic neuropathy; symptomatic urinary tract infection, interstitial cystitis, bladder stones, previous pelvic radiation therapy or previous or current malignant disease of the pelvic organs; contraindications for anticholinergics; nondrug treatment, including electro-stimulation therapy (although bladder training or pelvic floor exercise programs that had started more than 1 month prior to the start of the study could be continued); use of other urinary incontinence medications; known or suspected hypersensitivity to tolterodine, other anticholinergics, mirabegron, lactose, or any of the excipients; clinically significant cardiovascular (including ECG abnormalities) or cerebrovascular disease; or any other condition making the patient unsuitable for the study (as deemed by the investigator).
Study design and procedures
This was a multinational, multicenter, randomized, double-blind, double-dummy, parallel-group placebo- and active-controlled phase II study. Patients were enrolled into a single-blind, 2-week placebo run-in period followed by a 12-week double-blind treatment period in which patients were randomized to receive one of the following: an OCAS formulation of mirabegron QD at a dose of 25, 50, 100 or 200 mg, placebo or tolterodine ER 4 mg QD. There were six study visits: visit 1 (screening); visit 2 (baseline) after placebo run-in; and visits 3, 4, 5, and 6 after 1, 4, 8, and 12 weeks of treatment, respectively.
At visit 1, eligible patients received a micturition diary, used to evaluate mirabegron efficacy, which was to be completed during the 3 days preceding all visits after screening. For each micturition or incontinence episode, patients rated the degree of associated urgency on the five-point Patient’s Perception of Intensity of Urgency Scale (0, no urgency; 1, mild urgency; 2, moderate urgency; 3, severe urgency; and 4, urge incontinence) [11]. At visit 2, micturition diary scores were checked against inclusion criteria to confirm study eligibility.
Overactive bladder symptoms and quality of life (QOL) were assessed using the International Consultation on Incontinence Questionnaire-Overactive Bladder (ICIQ-OAB) [12, 13] and ICIQ-OAB QOL (ICIQ-OABqol) [14] questionnaires. Patients’ assessment of treatment benefit was also evaluated, starting at visit 2, with the question “has the treatment been of any benefit to you?” (possible responses: “no,” “yes, a little,” or “yes, very much”). These were assessed at all five study visits after screening.
Observed and spontaneously reported adverse events (AEs) were assessed at all visits, post-screening. Vital signs (heart rate, systolic and diastolic blood pressures) were measured by the investigator at visit 1 (screening) and by the patient in triplicate, twice-daily (BID), during the 3-day diary period preceding each visit in accordance with available guidelines [15]. 12-lead ECGs were performed at visits 1, 2, 3, 4 and 6. Blood and urine samples for safety assessments (hematology, biochemistry, urinalysis and urine culture) were collected by a central laboratory at visits 1, 3, 4, 5, and 6, and PVR was assessed by ultrasonography or bladder scan at visits 1 and 6.
The study was conducted in accordance with the Declaration of Helsinki (1996) and Good Clinical Practice (GCP) guidelines. Institutional Review Board and local Independent Ethics Committee approval for the protocol and amendments was obtained and patients provided written informed consent before any procedures.
Study endpoints
The primary efficacy endpoint was the change from baseline to end-of-treatment in the mean number of micturitions per 24 h. Secondary endpoints included changes in mean volume voided per micturition; mean number of urinary incontinence, urgency urinary incontinence, and urgency episodes per 24 h; severity of urgency; number of nocturia episodes; changes in ICIQ-OAB and ICIQ-OABqol symptom scores, and in patients’ perception of treatment benefit. Safety endpoints were incidence and severity of AEs, and changes from baseline to end-of-treatment in vital signs, laboratory tests, ECG parameters, and PVR.
Statistical analysis
A primary goal was to ascertain if there was a statistically significant improvement in the mean number of micturitions per 24 h in at least one active treatment group. Assuming a common standard deviation (SD) of 2.7 in the mean number of micturitions per 24 h, the ability to detect with 80 % power at an alpha level of 0.05 and a difference in means characterized by a variance of means of 0.126, it was necessary to recruit 140 patients to each of the five treatment arms (four mirabegron arms and a placebo arm). As tolterodine was included as an active control and was not included in the formal sample size calculation, a group of 70 patients in this arm was expected to be sufficient to establish assay sensitivity in evaluating the efficacy of mirabegron versus tolterodine. The study plan was to have at least 770 evaluable patients.
The primary analysis assessed changes from baseline to end-of-treatment in the mean number of micturitions per 24 h using an analysis of covariance (ANCOVA) model with treatment group (mirabegron dose) and country as fixed factors. The baseline value of the mean number of micturitions per 24 h was included in the models as a covariate. The null hypothesis, that the change in the mean number of micturitions per 24 h from baseline to end-of-treatment was the same for all mirabegron doses and for placebo, was evaluated using a two-sided test with a 0.05 significance level. Pairwise comparisons between each mirabegron group and placebo were performed; p values and 95 % confidence intervals (CIs) were obtained. The tolterodine group was not part of this primary analysis. The analysis described above was also performed for secondary efficacy variables. The percentage of patients showing a response in micturition frequency (defined as a mean of less than eight micturitions per 24 h), incontinence (patients who became dry), urgency, and nocturia episodes per 24 h (no episodes of either at a given visit) were compared between treatment groups using Mantel–Haenszel procedures.
In a secondary analysis, pairwise comparisons between tolterodine and placebo and between tolterodine and each mirabegron group were performed using the ANCOVA model described above.
The sum of scores for the ICIQ-OAB, ICIQ-OABqol, and mean changes from visit 2 to the end-of-treatment were derived for these variables and analyzed using the same ANCOVA model as for the primary variable. Frequencies and percentages for patients’ assessment of treatment benefit at end-of-treatment were reported. A responder for treatment benefit was defined by an improvement of at least one category at a visit relative to baseline (i.e., the response changing from “no” to “yes, a little”; from “no” to “yes, very much”; or from “yes, a little” to “yes, very much”). Efficacy data were analyzed using last observation carried forward (LOCF) methodology. Safety variables were analyzed descriptively.
Results
Study patients
A total of 1,108 patients were enrolled and 928 were randomized (Fig. 1); 927 patients received at least one dose of study medication (safety analysis set) and of these, 919 patients had primary efficacy data at baseline and at least one on-treatment visit (full analysis set [FAS]). At least 90 % of patients in each group completed the study. At week 12, data were obtained from a total of 854 subjects; thus, 65 subjects (7 %) without a week 12 measurement were analyzed at endpoint (LOCF). The study groups were well balanced with respect to demographic characteristics, type of OAB and prior drug therapy (Table 1). Approximately 30 % of patients had previously received nondrug therapy; this consisted mostly of exercises, electrical stimulation, behavioral training, and surgery. The mean duration of exposure to study medication ranged from 80 to 84.4 days across groups. Compliance was at least 98.5 % across treatment groups.
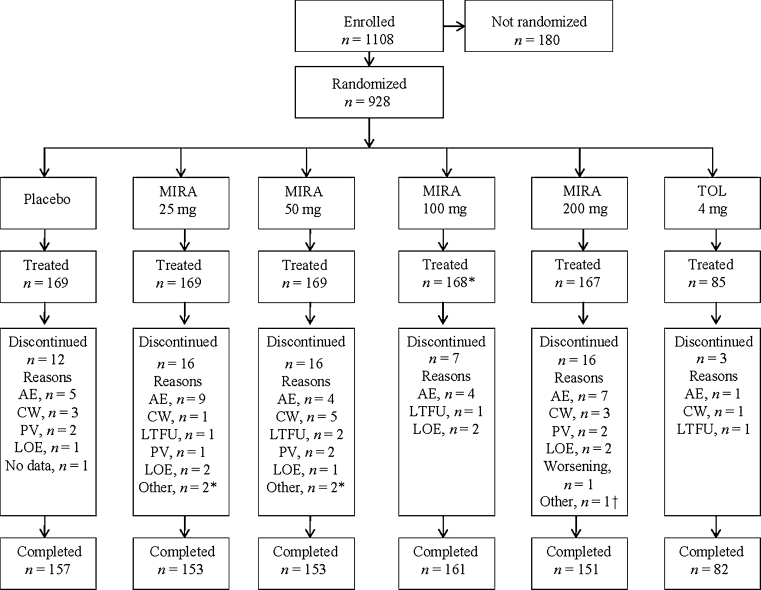
Fig. 1.
Disposition of subjects. MIRA mirabegron, TOL tolterodine, AE adverse event, CW consent withdrawn, PV protocol violation, LOE lack of efficacy, LTFU lost to follow-up. MIRA and TOL were administered once daily. Asterisks: In the mirabegron 100-mg group, 169 patients were randomized and 168 received treatment. Dagger: Other reasons for discontinuation included laboratory value out of range and medical advice to stop study drug (25-mg group); noncompliance and patient decision (50-mg group); and a patient stopped taking the study drug during hospitalization for arthroscopy of the knee (200-mg group)
Table 1.
Demographic and baseline characteristics (full analysis set)a
| Characteristic | Placebo | Mirabegron OCASc | Tolterodine ER | |||
|---|---|---|---|---|---|---|
| (n = 166) | 25 mg (n = 167) | 50 mg (n = 167) | 100 mg (n = 168) | 200 mg (n = 166) | 4 mgc (n = 85) | |
| Age (years) ± SD | 57.1 ± 12.9 | 57.2 ± 12.1 | 56.9 ± 12.5 | 57.1 ± 12.5 | 58.0 ± 13.7 | 56.6 ± 12.8 |
| Sex, n (%) | ||||||
| Male | 15 (9.0) | 20 (12.0) | 18 (10.8) | 17 (10.1) | 12 (7.2) | 16 (18.8) |
| Female | 151 (91.0) | 147 (88.0) | 149 (89.2) | 151 (89.9) | 154 (92.8) | 69 (81.2) |
| Race, n (%) | ||||||
| Asian | 0 | 1 (0.6) | 0 | 1 (0.6) | 0 | 2 (2.4) |
| Black | 0 | 2 (1.2) | 0 | 0 | 0 | 0 |
| White | 166 (100) | 162 (97.0) | 162 (97.0) | 167 (99.4) | 164 (98.8) | 81 (95.3) |
| Other | 0 | 1 (0.6) | 3 (1.8) | 0 | 0 | 1 (1.2) |
| Missing | 0 | 1 (0.6) | 2 (1.2) | 0 | 2 (1.2) | 1 (1.2) |
| Type of OAB, n (%) | ||||||
| Urge incontinence only | 74 (44.6) | 79 (47.3) | 67 (40.1) | 67 (39.9) | 63 (38.0) | 38 (44.7) |
| Mixed incontinenceb | 52 (31.3) | 41 (24.6) | 47 (28.1) | 54 (32.1) | 63 (38.0) | 24 (28.2) |
| Without incontinence | 40 (24.1) | 47 (28.1) | 53 (31.7) | 47 (28.0) | 40 (24.1) | 23 (27.1) |
| Prior drug therapy, n (%) | ||||||
| Yes, at least one effective | 41 (24.7) | 40 (24.0) | 39 (23.4) | 42 (25.0) | 34 (20.5) | 19 (22.4) |
| Yes, none effective | 30 (18.1) | 42 (25.1) | 38 (22.8) | 39 (23.2) | 38 (22.9) | 16 (18.8) |
| No | 95 (57.2) | 85 (50.9) | 90 (53.9) | 87 (51.8) | 94 (56.6) | 50 (58.8) |
| Non-drug therapy, n (%) | 51 (30.7) | 57 (34.1) | 49 (29.3) | 44 (26.2) | 40 (24.1) | 22 (25.9) |
OCAS oral controlled absorption system, ER extended release, OAB overactive bladder, SD standard deviation
aFull analysis set means all patients with primary efficacy data at baseline and at least one on-treatment visit
bUrge was the predominant factor
cMirabegron and tolterodine were administered once daily (QD)
Primary efficacy endpoint
The reduction (improvement) from baseline to end-of-treatment in the mean number of micturitions per 24 h increased with mirabegron dose and the improvement relative to placebo was statistically significant (p ≤ 0.05) for the mirabegron 50-, 100-, and 200-mg groups (Table 2); mirabegron 25, 50, 100, and 200 mg resulted in a mean baseline to end-of-treatment reduction of 1.9, 2.1, 2.1, and 2.2 micturitions per 24 h, respectively, compared with 1.4 micturitions per 24 h with placebo. No statistically significant differences in change in micturition frequency from baseline to end-of-treatment were noted between tolterodine and placebo or between tolterodine ER 4 mg and any mirabegron group.
Table 2.
Adjusted changes from baseline to endpoint and estimated differences versus placebo for efficacy variables by treatment group (full analysis set)
| Placebo | Mirabegron OCASb | Tolterodine ERc | ||||
|---|---|---|---|---|---|---|
| 25 mg | 50 mg | 100 mg | 200 mg | 4 mg | ||
| Micturitions/24 h | n = 166 | n = 167 | n = 167 | n = 168 | n = 166 | n = 85 |
| Adjusted mean CFB | −1.44 | −1.88 | −2.08 | −2.12 | −2.24 | −1.99 |
| Estimated difference from placebo | −0.45 | −0.64* | −0.68* | −0.80** | −0.52 | |
| 95 % CIs | −0.99, 0.10 | −1.19 , −0.10 | −1.22, −0.13 | −1.34, −0.25 | −1.18, 0.15 | |
| Mean volume voided/micturition (ml) | n = 165 | n = 167 | n = 167 | n = 168 | n = 166 | n = 85 |
| Adjusted mean CFB | 7.29 | 15.32 | 27.34 | 25.56 | 33.34 | 23.86 |
| Estimated difference from placebo | 8.03 | 20.05*** | 18.28*** | 26.06*** | 16.81* | |
| 95 % CIs | −1.54, 17.60 | 10.48, 29.63 | 8.66, 27.89 | 16.49, 35.62 | 5.09, 28.5 | |
| Incontinence episodes/24 h | n = 106 | n = 99 | n = 108 | n = 111 | n = 110 | n = 53 |
| Adjusted mean CFB | −0.53 | −1.36 | −1.15 | −1.06 | −1.10 | −0.81 |
| Estimated difference from placebo | −0.84** | −0.62* | −0.53 | −0.58 | −0.28 | |
| 95 % CIs | −1.45, −0.23 | −1.22, −0.02 | −1.12, 0.06 | −1.16, 0.01 | −1.01, 0.45 | |
| Nocturia episodes/24 h | n = 144 | n = 145 | n = 142 | n = 141 | n = 147 | n = 72 |
| Adjusted mean CFB | −0.38 | −0.52 | −0.60 | −0.42 | −0.59 | −0.59 |
| Estimated difference from placebo | −0.15 | −0.22* | −0.04 | −0.21 | −0.21 | |
| 95 % CIs | −0.36, 0.07 | −0.44, −0.01 | −0.26, 0.17 | −0.43, 0.00 | −0.47, 0.05 | |
| Urgency incontinence episodesa/24 h | n = 106 | n = 93 | n = 106 | n = 107 | n = 108 | n = 52 |
| Adjusted mean CFB | −0.44 | −1.31 | −1.13 | −1.18 | −1.24 | −0.76 |
| Estimated difference from placebo | −0.86** | −0.69** | −0.74** | −0.80** | −0.31 | |
| 95 % CIs | −1.38, −0.35 | −1.18, −0.19 | −1.23, −0.25 | −1.29, −0.31 | −0.92, 0.30 | |
| Urgency episodes/24 h (severity ≥3) | n = 165 | n = 167 | n = 166 | n = 168 | n = 165 | n = 85 |
| Adjusted mean CFB | −1.07 | −1.77 | −1.67 | −2.28 | −2.48 | −1.46 |
| Estimated difference from placebo | −0.70* | −0.60 | −1.21*** | −1.42*** | −0.37 | |
| 95 % CIs | −1.38, −0.01 | −1.29, 0.08 | −1.90, −0.52 | −2.10, −0.73 | −1.21, 0.47 | |
| Level of urgency/24 h | n = 166 | n = 166 | n = 166 | n = 168 | n = 166 | n = 85 |
| Adjusted mean CFB | −0.10 | −0.21 | −0.18 | −0.29 | −0.38 | −0.14 |
| Estimated difference from placebo | −0.12 | −0.08 | −0.19** | −0.28*** | −0.04 | |
| 95 % CIs | −0.25, 0.02 | −0.22, 0.05 | −0.33, −0.06 | −0.41, −0.15 | −0.21, 0.12 | |
| ICIQ-OAB | n = 166 | n = 167 | n = 166 | n = 168 | n = 166 | n = 85 |
| Adjusted mean CFB | −1.82 | −2.40 | −2.51 | −2.72 | −3.02 | −2.21 |
| Estimated difference from placebo | −0.58* | −0.69* | −0.90** | −1.20*** | −0.37 | |
| 95 % CIs | −1.13, −0.02 | −1.24, −0.13 | −1.45, −0.34 | −1.76, −0.65 | −1.05, 0.31 | |
| ICIQ-OABqol | n = 162 | n = 163 | n = 165 | n = 163 | n = 162 | n = 84 |
| Adjusted mean CFB | −16.11 | −17.09 | −20.36 | −20.57 | −22.19 | −17.42 |
| Estimated difference from placebo | −0.98 | −4.25 | −4.46 | −6.08* | −1.32 | |
| 95 % CIs | −5.88, 3.92 | −9.13, 0.62 | −9.37, 0.46 | −11.0, −1.19 | −7.32, 4.69 | |
OCAS oral controlled absorption system, CFB change from baseline, CI confidence interval, ICIQ International Consultation on Incontinence Questionnaire, OAB overactive bladder, qol quality of life
*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001
aUrgency incontinence (incontinence with urgency) is a subset of incontinence (with or without urgency)
bMirabegron was administered once daily (QD)
cThe statistics for values given in the placebo and mirabegron columns come from the primary analysis, in which treatment group was included as a fixed factor in the ANCOVA model; pairwise comparisons between each mirabegron group and placebo were performed; the tolterodine group was excluded. The statistics for values in the tolterodine column come from the secondary analysis in which tolterodine was also included as a fixed factor; in this analysis, pairwise comparisons between tolterodine and placebo and between tolterodine and each mirabegron group were performed
The percentage of patients classified as responders for micturition frequency at the endpoint (mean of less than eight micturitions per 24 h) were 19.3 % for placebo, 28.7 % for mirabegron 25 mg, and 30.1 % for the mirabegron 200-mg group (p ≤ 0.05 for all mirabegron dose groups versus placebo). For the tolterodine ER 4-mg group, 18.8 % of patients were classified as responders; the difference relative to placebo was not statistically significant (Table 3).
Table 3.
Number (%) of respondersa for selected efficacy variables at the study endpoint (full analysis set)
| Placebo | Mirabegron OCASb | Tolterodine ERb | ||||
|---|---|---|---|---|---|---|
| 25 mg | 50 mg | 100 mg | 200 mg | 4 mg | ||
| Patients, n (%)c | ||||||
| Micturitions/24 h* | n = 166 | n = 167 | n = 167 | n = 168 | n = 166 | n = 85 |
| 32 (19.3) | 48 (28.7) | 46 (27.5) | 55 (32.7) | 50 (30.1) | 16 (18.8) | |
| Incontinence episodes/24 h* | n = 106 | n = 99 | n = 108 | n = 111 | n = 110 | n = 53 |
| 39 (36.8) | 42 (42.4) | 45 (41.7) | 62 (55.9) | 53 (48.2) | 19 (35.8) | |
| Urgency episodes (grade ≥ 3)/24 h | n = 165 | n = 167 | n = 166 | n = 168 | n = 165 | n = 85 |
| 25 (15.2) | 27 (16.2) | 24 (14.5) | 33 (19.6) | 36 (21.8) | 13 (15.3) | |
| Nocturia episodes/24 h | n = 144 | n = 145 | n = 142 | n = 141 | n = 147 | n = 72 |
| 21 (14.6) | 34 (23.4) | 34 (23.9) | 20 (14.2) | 34 (23.1) | 13 (18.1) | |
OCAS oral controlled absorption system, ER extended release
*p ≤ 0.05 based on a Mantel–Haenszel test to compare all treatment groups, except tolterodine
aResponder definitions: micturitions, <8 micturitions/24 h; incontinence episodes, no episodes (patient became dry); urgency and nocturia episodes, no episodes of either
bMirabegron and tolterodine were administered once daily (QD)
cPercentages are based on the number of patients with available data at the endpoint
Secondary efficacy endpoints
Urinary symptoms
Mean baseline to end-of-treatment improvements relative to placebo were statistically significant for mirabegron for mean volume voided per micturition (at doses of 50, 100, and 200 mg); incontinence episodes (at doses of 25 and 50 mg); urgency incontinence episodes (at doses of 25, 50, 100 and 200 mg); urgency episodes (at doses of 25, 100, and 200 mg); level of urgency (at doses of 100 and 200 mg); and nocturia episodes (at a dose of 50 mg) (p < 0.05 for all comparisons; Table 2).
A statistically significant difference (p < 0.01) between tolterodine and placebo was noted for mean volume voided per micturition, but not for other secondary endpoints. Statistically significant differences (p ≤ 0.05) favoring the mirabegron 200-mg group relative to tolterodine were noted for grade at ≥3 urgency episodes (and level of urgency).
The percentage of patients classified as responders for incontinence episodes at the end of treatment (patients who became dry) was 36.8 % for placebo; 41.7–55.9 % across the four mirabegron groups (p ≤ 0.05 for all mirabegron groups versus placebo); and 35.8 % for tolterodine. At the end of treatment, more patients in the mirabegron than in the placebo group were responders with respect to urgency (grade ≥3) and nocturia episodes (no episodes of either at the end of treatment), although differences between groups were not statistically significant. The responder levels for urgency and nocturia episodes for the tolterodine ER 4-mg group were similar to those seen for placebo (Table 3).
Quality of life
The positive effect of mirabegron on QOL scores (a reduction in ICIQ-OAB score indicated improvement) increased with mirabegron dose (Table 2); moreover, the change from baseline to the end of treatment for all mirabegron groups was statistically significant versus placebo (p ≤ 0.05). Improvements from baseline to the end of treatment were also observed with the ICIQ-OABqol questionnaire, although only the comparison between the mirabegron 200-mg group and placebo was statistically significant (−22.19 versus −16.11, p ≤ 0.05; Table 2).
No statistically significant differences between tolterodine ER 4 mg and placebo were noted for the QOL scores obtained on both the ICIQ-OAB and ICIQ-OABqol. A statistically significant treatment effect favoring mirabegron 200 mg compared with tolterodine ER 4 mg (−3.05 versus −2.21, p < 0.05) was seen on the ICIQ-OAB.
The percentage of patients classified as “responders” in patient perception of treatment benefit (improvement of ≥1 category from baseline) at the end of treatment was 59.0 %, 65.0 %. 65.8 % and 70.8 % for the mirabegron 25-mg, 50-mg, 100-mg, and 200-mg groups, respectively, compared with 51 % for placebo. Approximately 55 % of the tolterodine ER 4-mg patients were classified as responders on this measure.
Safety
The incidence of treatment-related AEs was comparable for the mirabegron and placebo groups (Table 4). Dry mouth was more common with tolterodine ER 4 mg (3.5 %) than with mirabegron (1.8 % to 3.0 % depending on dose). Discontinuation owing to AEs was low at 3.0 % (placebo), 2.4–5.3 % (mirabegron groups), and 1.2 % (tolterodine). Serious adverse events were reported in <2 % of patients across treatment groups; two events, one of pneumonia and one of hypothyroidism, were considered to be possibly related to mirabegron.
Table 4.
Treatment-related AEs reported during treatment (safety populations)a
| System organ class preferred term | Placebo (n = 169) | Mirabegron | Mirabegron | Mirabegron | Mirabegron | Tolterodine |
|---|---|---|---|---|---|---|
| OCAS | OCAS | OCAS | OCAS | ER | ||
| 25 mgb | 50 mg | 100 mg | 200 mg | 4 mg | ||
| (n = 169) | (n = 169) | (n = 168) | (n = 167) | (n = 85) | ||
| Patients, n (%) | ||||||
| Overall | 26 (15.4) | 34 (20.1) | 38 (22.5) | 36 (21.4) | 37 (22.2) | 13 (15.3) |
| Cardiac disorders | 1 (0.6) | 4 (2.4) | 7 (4.1) | 2 (1.2) | 5 (3.0) | 1 (1.2) |
| Eye disorders | 3 (1.8) | 3 (1.8) | 2 (1.2) | 1 (0.6) | 6 (3.6) | 0 (0.0) |
| Gastrointestinal disorders | 9 (5.3) | 14 (8.3) | 14 (8.3) | 16 (9.5) | 12 (7.2) | 7 (8.2) |
| Constipation | 2 (1.2) | 2 (1.2) | 4 (2.4) | 3 (1.8) | 3 (1.8) | 1 (1.2) |
| Dry mouth | 3 (1.8) | 5 (3.0) | 3 (1.8) | 5 (3.0) | 3 (1.8) | 3 (3.5) |
| Dyspepsia | 1 (0.6) | 1 (0.6) | 1 (0.6) | 0 (0.0) | 4 (2.4) | 0 (0.0) |
| Nausea | 2 (1.2) | 2 (1.2) | 2 (1.2) | 7 (4.2) | 1 (0.6) | 1 (1.2) |
| General disorders and administration site conditions | 2 (1.2) | 3 (1.8) | 6 (3.6) | 3 (1.8) | 1 (0.6) | 1 (1.2) |
| Investigations | 9 (5.3) | 10 (5.9) | 4 (2.4) | 6 (3.6) | 7 (4.2) | 1 (1.2) |
| Gamma-glutamyl transferase increased | 2 (1.2) | 4 (2.4) | 0 (0.0) | 2 (1.2) | 2 (1.2) | 0 (0.0) |
| Nervous system disorders | 6 (3.6) | 7 (4.1) | 10 (5.9) | 7 (4.2) | 6 (3.6) | 1 (1.2) |
| Dizziness | 1 (0.6) | 0 (0.0) | 6 (3.6) | 2 (1.2) | 0 (0.0) | 0 (0.0) |
| Headache | 4 (2.4) | 6 (3.6) | 5 (3.0) | 4 (2.4) | 3 (1.8) | 1 (1.2) |
| Psychiatric disorders | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.6) | 2 (2.4) |
| Vascular disorders | 4 (2.4) | 4 (2.4) | 4 (2.4) | 6 (3.6) | 2 (1.2) | 0 (0.0) |
OCAS oral controlled absorption system, ER extended release
aThe AEs listed were reported in ≥2 % of the patients in either treatment group
bMirabegron and tolterodine were administered once daily (QD)
A statistically significant increase in pulse rate from baseline was seen with mirabegron 100 and 200 mg versus placebo. In addition, pulse rate was seen to increase in a dose-related manner. The adjusted mean change in morning pulse rate from baseline was 0.51 bpm for placebo, 2.15 bpm for mirabegron 100 mg (p ≤ 0.05), and 4.66 bpm for mirabegron 200 mg (p ≤ 0.001). Similarly, the adjusted mean change in afternoon pulse rate from baseline was –0.04 bpm for placebo, 2.71 bpm for mirabegron 100 mg (p ≤ 0.001), and 4.63 bpm for mirabegron 200 mg (p ≤ 0.001). However, this was not associated with a clinically significant increase in cardiovascular AEs (for example, atrial fibrillation or palpitations), the incidence of which was comparable to that seen with tolterodine. Morning and afternoon pulse rate increases from baseline for the mirabegron 25-mg (0.34 and 0.44 bpm, respectively) and 50-mg (1.64 and 1.12 bpm, respectively) groups were not statistically significant compared with placebo. By comparison, the adjusted mean changes from baseline in the morning and afternoon pulse rates seen with tolterodine were 1.50 and 2.50, respectively. No drug effect on systolic or diastolic blood pressure was observed; the net changes in morning and afternoon blood pressure from baseline in all mirabegron groups were <2 mm Hg and comparable to placebo. No differences between treatment groups were observed with respect to ECG parameters, including QTcF. Mean changes in PVR were small, at –4.6 ml for placebo; –4.7, –4.3, 0.6, and –3.0 mL for mirabegron 25, 50, 100, and 200 mg, respectively; and –1.3 for tolterodine ER 4 mg. There were no clinically relevant changes in laboratory parameters.
Discussion
In this study, mirabegron OCAS was effective and well tolerated with clear dose-dependent efficacy above 50 mg. The difference in response versus placebo was evident after 1 week of treatment and a maximum effect was achieved and sustained from 8 to 12 weeks, as seen in previous studies evaluating antimuscarinic therapy for OAB [16]. Approximately half of the incontinent patients in each mirabegron treatment group (from 41.7 % of the mirabegron 50-mg group to 55.9 % of the mirabegron 100-mg group) were dry at the end of treatment. Thus, mirabegron exhibited considerable clinical benefit as demonstrated by consistently improved symptoms of OAB and associated QOL measures.
The mirabegron doses of 25, 50, 100, and 200 mg QD used in this study were based on the results of a proof-of-concept study in which total daily doses of 200 and 300 mg (100 and 150 mg BID, respectively) were investigated. Notably, a QD OCAS formulation was utilized in this study. In the proof-of-concept study, no difference in efficacy was seen between the 100-mg and 150-mg BID doses, leading to the conclusion that a total daily dose of 200 mg provides maximum therapeutic efficacy. Hence, this was the highest dose investigated in the current study.
Tolterodine is a licensed antimuscarinic antagonist for OAB that was used as an active control for mirabegron in this study in order to give context to the efficacy and safety results seen with mirabegron. Hence, it is notable that the magnitude of improvements in efficacy outcomes in the mirabegron groups were within the same range as those of the tolterodine ER 4-mg group, further suggesting that mirabegron is a potentially effective treatment for the management of patients with OAB. However, one limitation of this study is that it was not powered to detect differences between mirabegron and tolterodine for which head-to-head comparison studies are required. In addition, the short duration of this study could also be perceived as a limitation, as it allows no insight into the long-term effects of mirabegron, notably whether its favorable efficacy and tolerability profile is maintained over a prolonged period and whether it leads to improved compliance. However, this was a phase II study, designed as a dose-finding study, and its design and conduct are usual and adequate for that purpose. It is worth noting too that between 24.6 % and 38.0 % of the patients across treatment groups had urge-predominant mixed incontinence at baseline.
Mirabegron was found to be well tolerated with a low incidence of AEs, between that seen with placebo and tolterodine. Dry mouth is the most common, and most bothersome side effect of antimuscarinic treatment [16–19]. Thus, the low incidence of dry mouth seen with mirabegron and the observation that its incidence was lower with mirabegron than with tolterodine may be an important benefit of mirabegron treatment. The small mean increase in pulse rate from baseline observed with mirabegron was less pronounced than that reported in other studies [20, 21] and was not associated with a clinically significant increase in cardiovascular AEs. There was no evidence of a dose–response relationship for these events and none of the patients discontinued the study because of symptoms associated with increased heart rate.
The favorable efficacy and tolerability profile of mirabegron, the first in this new class of compounds to be approved for the treatment of the symptoms of OAB, in once-daily doses ranging from 25 to 200 mg OCAS, has led to its successful advancement into a phase III clinical development program at doses of 25, 50, and 100 mg.
Conclusions
The satisfactory balance between efficacy and tolerability observed in this study of mirabegron in OAB patients may result in improved compliance compared with that seen with currently available antimuscarinics, whose use is hampered by bothersome side effects and insufficient efficacy.
Acknowledgements
Emad Siddiqui BSc, MBBS, MRCS, MD (Astellas Pharma Europe Ltd, Middlesex, UK) and Yolanda Cartwright, PhD (Ledell Inc.) provided assistance in the preparation of the manuscript.
Funding
This study and medical writing support were funded by Astellas.
Conflicts of interest
Christopher R. Chapple certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g., employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending) are the following: Christopher Chapple’s institution has received fees for his role as a consultant to Allergan, American Medical Systems, Astellas, Lilly, Pfizer, and Recordati. It has also received grants or has grants pending from Allergan, American Medical Systems, Astellas, Pfizer, and Recordati and has been reimbursed for lectures conducted by Christopher Chapple, including service on speakers’ bureaus, by Allergan, Astellas, Pfizer, and Recordati, and support for the trial with regard to document compilation and author liaison by Astellas. Vladimir Dvorak’s institution has received support for travel in regards to this study from Astellas. Pjotr Radziszewski has received fees for his role as a consultant to Astellas, Lilly, GSK, ONO, Pfizer, Studio PR, and OCI; has received grants or has grants pending (as does his institute) from NCBiR; and has received payment for lectures, including service on speakers’ bureaus, from Astellas, GSK, Lilly, G-Pharma, and Ipsen. Philip Van Kerrebroeck has received payment for lectures, including service with speakers’ bureaus, from Astellas, and serves on the board of Astellas; in addition, his institution has received grants or has grants pending from Astellas. Jean Jacques Wyndaele’s institution has received consulting fees or honoraria and support for travel from UZA and payment for lectures and consultancy from Astellas. Osamu Yamaguchi is a member of the Board of Astellas, is a consultant to Astellas, Pfizer, Hisamitsu, and Ferring, has received grants or has grants pending from Astellas, Kyorin, Ono, and Kissei, has been reimbursed for lectures by Astellas, Kyorin, Kissei, and Ono, and for the development of educational presentations by Astellas and Kyorin. Brigitte Bosman, Peter Boerrigter, Arwin Ridder, Ted Drogendijk, and Ingrid Van Der Putten-Slob are employees of the study sponsor.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Appendix
DRAGON Investigator Group
Prof. F. Keuppens, Universitair Ziekenhuis Brussel Laarbeeklaan 101, Brussels 1090, Belgium
Prof. Dirk De Ridder, Universitaire Ziekenhuizen, Leuven, Campus Gasthuisberg, Herestraat 49, Leuven 3000, Belgium
Dr. L. Dewilde, Academisch Ziekenhuis, Stuivenberg, Lange Beeldekenstraat 267, Antwerp 2060, Belgium
Prof. Dr. Jean-Jacques Wyndaele, Universitair Ziekenhuis, Antwerpen, Wilrijkstraat 10, Edegem 2650, Belgium
Dr. V. Vik, Faculty Thomayer´s Hospital, Vídeňská 800, Prague 4 140 59, Czech Republic
Dr. J. Zmrhal, Department of Gynecology and Obstetrics, Prazská 528, Melník 276 01, Czech Republic
MD Z. Adamik, Bata Hospital Zlín, Bata Hospital Zlín, Gynecology and Obstetrics Department, Havlíčkovo, nábřeží 600, Zlín 762 75, Czech Republic
Dr Vladimir Dvořák, Gynecology and Obstetrics Outpatient Centre Centrum ambulantní gynekologie a porodnictví, Orlí 18, Brno 602 00, Czech Republic
Dr. M. Halaska, Faculty Hospital Bulovka, Gynecology and Obstetrics Department, Budínova 2, Prague 180 81, Czech Republic
Dr. J. Krhut, University Hospital Ostrava 17. listopadu 1790, Ostrava 708 52, Czech Republic
Dr. J. Liehne, Ustecke Urocentrum Moskevská 42, Usti nad Labem 40001, Czech Republic
Prof. Alois Martan, General University Hospital Prague, Clinic of Gynecology and Obstetrics, Apolinářská 18, Prague 128 51, Czech Republic
Dr. M. Indig, Indig, Private Practice, Kutzbachstr. 7, Trier 54290, Germany
Dr. W. Hechelmann, Hechelmann Private Practice, Fabriciusstr. 9, Frankfurt 65933, Germany
Dr. M. Markov, Markov Private Practice Wilhelm-von-Klewiz-Strasse 11, Halle/Saale 06132, Germany
J. Knipphals, Knipphals Private Practice, Bahnhofstr. 1, Bad Ems 56130, Germany
Dr. J.Willgerodt, Willgerodt Private Practice, Käthe-Kollwitz-Str. 99, Leipzig 04109, Germany
Dr. Wolfgang Warnack, Warnack Private Practice, Poststrasse 25, Hagenow 19230, Germany
Mr. Detlef Müller, Müller Private Practice, Dr.-E.-Mucke-Str. 6, Bautzen 02625, Germany
H.P. Fischer, Fischer H.P. Private Practice, Löhrstr. 66c, Koblenz 56068, Germany
Dipl. med. Timo Liebald, Liebald Private Practice, Gostritzer Str. 2 A, Dresden 01217, Germany
Mr. Jens Schneider, Schneider Private Practice, Heinrich-Zille-Str. 13, Radebeul 01445, Germany
M.Voß, Voß Private Practice, Rathausstrasse 4, Uetersen 25436, Germany
Dr. W. Jörger, Jorger/Scholermann Private Practice, Hoheluftchaussee 56, Hamburg 20253, Germany
Dr. W. Grohmann, Grohmann Private Practice, Daphnestr. 4, Munich-Bogenhausen 81925, Germany
Dr. Elke Hessdörfer, Hessdörfer Private Practice, Reinickendorfer Str. 15, Berlin 13347, Germany
Dr. Torsten Sørensen, Kolding Sygehus Dept. of Gyn & Obs, Skovvangen 2-8, Kolding 6000, Denmark
Flemming Sørensen, Roskilde Amtssygehus, Køgevej 7-13, Roskilde 4000, Denmark
John Krogh, Holbaek Hospital Department of Urology, Smedelundsgade 60, Holbaek 4300, Denmark
L. Prieto, Hospital San Juan de Alicante Ctra. Alicante-Valencia N-332, s/n San Juan de Alicante 03550, Spain
Dr. Javier Cambronero Santos, Policlínico de Vigo, S.A.-POVISA, C/ Salamanca nº 5, Vigo 36211, Spain
Dr. P. Arañó Bertran, Fundacion Puigvert, C/ Cartagena 340, Barcelona 08025, Spain
Dr. Miguel A. Jimenez Cidre, Hospital Ramon y Cajal Servicio de Urología, Ctra. de Colmenar Viejo Km. 9.1, Madrid 28049, Spain
J. Rosa Arias, Hospital Comarcal Santiago Apostol, Ctra. de Orón, s/n, Miranda de Ebro 09200, Spain
Dr. Miguel Unda, Hospital de Basurto Avda. Montevideo, 18, Bilbao 48013, Spain
Dr. J. Benejam, Hospital de Manacor Servicio Urologia, Ctra. Manacor-Alcudia, s/n Manacor 07500, Spain
Dr. Montserrat Espuña, Hospital Clinic I Provencal, Calle Villarroel 170, Barcelona 08036, Spain
Prof. G. Amarenco, Hopital Rothschild, 33 boulevard de Picpus, Paris Cedex 12, 75571, France
Prof. F. Haab, Hopital Tenon, 4, Rue de la Chine, Paris Cedex 10, 75020, France
Dr. J-J. Labat, Centre medical Mauvoisin, 25 rue Mauvoisin, Nantes 44200, France
Prof. Christopher Chapple, Royal Hallamshire Hospital, Glossop Road, Sheffield S10 2JF, United Kingdom
Professor J. Malone Lee, Whittington Hospital Clerkenwell Building, Archway Campus, Highgate Hill, London N19 5LW, United Kingdom
P.M. Toozs-Hobson, Birmingham Womens Hospital, Metchley Park Road, Bimingham B15 2TG, United Kingdom
Mr. M. Lucas, Morriston, Swansea SA6 6NL, United Kingdom
R. Thakar, Mayday Hospital, 530 London Road, Croydon CR7 7YE, United Kingdom
S.J. Foley, The Royal Berkshire Hospital, London Road, Reading RG1 5AN, United Kingdom
S.R. Hill, Royal Blackburn Hospital, Haslingden Road, Blackburn BB2 3HH, United Kingdom
A. Monga, Princess Anne Hospital, Coxford Road, Southampton SO16 5YA, United Kingdom
Ms. S.Venn, St. Richards Hospital, Spitalfield Lane, Chichester PO19 4SE, United Kingdom
Dr. K. Erdei, Sopron Erzsébet Hospital, Győri út 15, Sopron 9400, Hungary
Dr. L. Farkas, University of Pécs Medical School Urology Clinic, Munkácsy str. 2, Pecs H-7621, Hungary
Dr. L. Kiss, Josa Andras Hospital, Szent István utca 68, Nyiregyhaza 4400, Hungary
Dr. G. Nagy, Szent Borbala County Hospital, Dózsa György út 77, Tatabánya 2800, Hungary
Prof. Dr. László Pajor, Szeged County Hospital, Kalvaria sgrt. 57, Szeged 6724, Hungary
Dr. József Pintér, Semmelweis Hospital, Csabai kapu 9-11, Miskolc 3529, Hungary
Prof. Giuseppe Vespasiani, Policlinico Universitario Università Di Roma Tor, Vergata Reparto di Urologia, V.le Oxford 71, Rome 00133, Italy
Dr. R. Damiano, Campus Universitario Germaneto, viale Europa, Catanzaro 88100, Italy
Prof. R.M. Scarpa, Azienda Ospedale San Luigi Gonzaga, Regione Gonzole, 10, Orbassano 10043, Italy
P. Bolis, Ospedale di Circolo e, Fondazione Macchi, Viale Borri, 57, Varese 21100, Italy
Prof. R. Milani, Presidio Ospedaliero e., Bassini, Via Massimo Gorki, 50, Cinisello Balsamo 20092 Italy
Prof. G. Morgia, Clinica Urologica, Viale San Pietro 43, Sassari 07100, Italy
Prof. P. Di Benedetto, Istituto di medicina fisica eriabilitazione, Via Gervasutta, 48, Udine 33100, Italy
Prof. P. Ferrari, Hesperia Hospital Urology Department, Via Arquà 80, Modena 41100, Italy
Prof. Antonio Carbone, U.O. Dip. di Neuro-Urologia; Univ. di Roma La Sapienza, Segreteria Facoltà Medicina e Chirurgia, Via Franco Faggiana 34, Latina 04100, Italy
Prof. Ph.E.V.A. van Kerrebroeck, Academisch Ziekenhuis, Maastricht, P. Debyelaan 25 - Wijk 29, Maastricht 6229 HX, Netherlands
P.H.M. Van de Weijer, 7314 ET Apeldoorn, Netherlands
Dr. E. Koldewijn, Catharina Ziekenhuis, Michelangelolaan 2, Eindhoven 5623 EJ, Netherlands
Dr. H. Franke, Medisch Spectrum Twente, Loc. Enschede, Netherlands
Ariënsplein 1, Enschede 7511 JX, Netherlands
Prof. B.L.H. Bemelmans, VU medisch centrum, De Boelelaan 1117, Amsterdam 1081 HV, Netherlands
Dr. M.R.van Balken, Ziekenhuis Rijnstate, Wagnerlaan 55, Arnhem 6815 AD, Netherlands
Vendil Vatne, Bergen 5021, Norway
Dr. H. Schiøtz, Hospital in Vestfold, Postboks 2168, Postterminalen, Tønsberg 3103, Norway
Trygve Talseth, Rikshospitalet, Department of Urology, 0027, Oslo, Norway
Prof. T.Rechberger, Samodzielny Publiczny Szpital Klinicznyul. Jaczewskiego 8, Lublin 20-954, Poland
Prof. A. Borkowski, Szpital Kliniczny Dzieciatka Jezus Ul. Lindleya 4, Warsaw 02-005, Poland
Prof. Mariusz Zimmer, Medical University Wroclaw II Department of Obstetrics &Fertility, ul. Dyrekcyjna 5/7, Wroclaw 50-528, Poland
Prof. Romuald Zdrojowy, Medical University Wroclaw ul. Borowska 213, Wroclaw 50-556, Poland
Prof. Barbara Darewicz, Medical University of Bialystok ul., Marii Sklodowskiej-Curie, 24A, Bialystok 15-276, Poland
Dr. W. Duda, Prof. Michalowski Urological Hospital, Ul. Strzelecka 9, Katowice 40-073, Poland
MD PhD Zenona Jablonska, Poland
Prof Wlodzimierz Baranowski, Military Institute of Medicine Department of Gynaecology and Gynaecological Oncology, ul. Szaserow, Warsaw 128, Poland
PhD Krzysztof Dynowski, Medical Centre of Postgraduate Education II Department of Obstetrics & Gynaecology, ul. Ceglowska, 80, Warsaw 01-809, Poland
Prof. M.F. Trapeznikova, Moscow Regional Scientific Research Institute, Schepkina str., 61/2, bld. 15, Moscow 129110, Russian Federation
Prof. V. Avdoshin, Peoples Friendship University of Russia City Hospital No. 29, City Hospital No. 29, Gospitalnaya square 2, Moscow 111020, Russian Federation
Dr. A. Kornev, Russian State Medical University, Veterans Hospital No 2, Volgogradsky prospect, 168, Moscow 109472, Russian Federation
V. Smetnik, Scientific Centre of Obstetrics, Department of gynaecological endocrinology, Academician Oparina str. 4, Moscow 117815, Russian Federation
Prof. N.A. Lopatkin, Moscow Research Institute of Urology, 3rd Parkovaya str., 51, Moscow 105425, Russian Federation
S.H. Al-Shukri, Pavlov St. Petersburg State Medical University, Prospect Parkhomenko 15, St. Petersburg 197022, Russian Federation
Prof. S. Petrov, Russian Medical and Military Academy, Embankment of Fontanka, river 106, St. Petersburg 198013, Russian Federation
Prof. B. Komyakov, City Multidisciplinary Hospital, No. 2, Uchebny per. 5, St. Petersburg 194354, Russian Federation
Prof. D.Pushkar, Moscow State Medical Stomatological University, City Hospital No 50, Vucheticha str., 21, Moscow 125206, Russian Federation
Prof. V.N. Surikov, Medical Centre of the Presidents Administration o, Out-patient Hospital No 1, Grokholsky per., 31, Moscow 129090, Russian Federation
Med. Dr. Aino Fianu-Jonasson, Karolinska University Hospital in Huddinge, Dept of Obs & Gyn, K63, Huddinge 14186, Sweden
Christian Falconer, Danderyds Hospital, Department of Obst & Gyn, Stockholm 18288, Sweden
Maud Ankardal, Sahlgrenska University Hospital, Kvinnokliniken, Department of Obs and Gyn, Bla Straket 6, Gothenburg 41685, Sweden
Footnotes
The members of the DRAGON Investigator Group are listed in the Appendix.
References
- 1.Sexton CC, Coyne KS, Kopp ZS, Irwin DE, Milsom I, Aiyer LP, et al. The overlap of storage, voiding and postmicturition symptoms and implications for treatment seeking in the USA, UK and Sweden: EpiLUTS. BJU Int. 2009;103(Suppl 3):12–23. doi: 10.1111/j.1464-410X.2009.08369.x. [DOI] [PubMed] [Google Scholar]
- 2.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Urol. 2003;61:37–49. doi: 10.1016/S0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 3.Abrams P, Andersson KE, Buccafusco JJ, Chapple C, de Groat WC, Fryer AD, et al. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol. 2006;148:565–578. doi: 10.1038/sj.bjp.0706780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Souza AO, Smith MJ, Miller LA, Doyle J, Ariely R. Persistence, adherence, and switch rates among extended-release and immediate-release overactive bladder medications in a regional managed care plan. J Manag Care Pharm. 2008;14:291–301. doi: 10.18553/jmcp.2008.14.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benner JS, Nichol MB, Rovner ES, Jumadilova Z, Alvir J, Hussein M, et al. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int. 2010;105:1276–1282. doi: 10.1111/j.1464-410X.2009.09036.x. [DOI] [PubMed] [Google Scholar]
- 6.Igawa Y, Yamazaki Y, Takeda H, Hayakawa K, Akahane M, Ajisawa Y, et al. Functional and molecular biological evidence for a possible beta3-adrenoceptor in the human detrusor muscle. Br J Pharmacol. 1999;126:819–825. doi: 10.1038/sj.bjp.0702358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeda M, Obara K, Mizusawa T, Tomita Y, Arai K, Tsutsui T, et al. Evidence for beta3-adrenoceptor subtypes in relaxation of the human urinary bladder detrusor: analysis by molecular biological and pharmacological methods. J Pharmacol Exp Ther. 1999;288:1367–1373. [PubMed] [Google Scholar]
- 8.Fujimura T, Tamura K, Tsutsumi T, Yamamoto T, Nakamura K, Koibuchi Y, et al. Expression and possible functional role of the beta3-adrenoceptor in human and rat detrusor muscle. J Urol. 1999;161:680–685. doi: 10.1016/S0022-5347(01)61994-3. [DOI] [PubMed] [Google Scholar]
- 9.Nomiya M, Yamaguchi O. A quantitative analysis of mRNA expression of alpha 1 and beta-adrenoceptor subtypes and their functional roles in human normal and obstructed bladders. J Urol. 2003;170:649–653. doi: 10.1097/01.ju.0000067621.62736.7c. [DOI] [PubMed] [Google Scholar]
- 10.Kumar V, Templeman L, Chapple CR, Chess-Williams R. Recent developments in the management of detrusor overactivity. Curr Opin Urol. 2003;13:285–291. doi: 10.1097/00042307-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Cartwright R, Srikrishna S, Cardozo L, Robinson D. Validity and reliability of the patient's perception of intensity of urgency scale in overactive bladder. BJU Int. 2011;107:1612–1617. doi: 10.1111/j.1464-410X.2010.09684.x. [DOI] [PubMed] [Google Scholar]
- 12.Donovan JL, Abrams P, Peters TJ, Kay HE, Reynard J, Chapple C, et al. The ICS-'BPH' Study: the psychometric validity and reliability of the ICSmale questionnaire. Br J Urol. 1996;77:554–562. doi: 10.1046/j.1464-410X.1996.93013.x. [DOI] [PubMed] [Google Scholar]
- 13.Jackson S, Donovan J, Brookes S, Eckford S, Swithinbank L, Abrams P. The Bristol Female Lower Urinary Tract Symptoms questionnaire: development and psychometric testing. Br J Urol. 1996;77:805–812. doi: 10.1046/j.1464-410X.1996.00186.x. [DOI] [PubMed] [Google Scholar]
- 14.Coyne K, Revicki D, Hunt T, Corey R, Stewart W, Bentkover J, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res. 2002;11:563–574. doi: 10.1023/A:1016370925601. [DOI] [PubMed] [Google Scholar]
- 15.Committee for Medicinal Products for Human Use (CHMP) guideline on the choice of the non-inferiority margin (2006) Stat Med 25:1628–1638 [DOI] [PubMed]
- 16.Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol. 2008;54:543–562. doi: 10.1016/j.eururo.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 17.Kessler TM, Bachmann LM, Minder C, Lohrer D, Umbehr M, Schunemann HJ, et al. Adverse event assessment of antimuscarinics for treating overactive bladder: a network meta-analytic approach. PLoS One. 2011;6:e16718. doi: 10.1371/journal.pone.0016718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novara G, Galfano A, Secco S, D'Elia C, Cavalleri S, Ficarra V, et al. A systematic review and meta-analysis of randomized controlled trials with antimuscarinic drugs for overactive bladder. Eur Urol. 2008;54:740–763. doi: 10.1016/j.eururo.2008.06.080. [DOI] [PubMed] [Google Scholar]
- 19.Nabi G, Cody JD, Ellis G, Herbison P, Hay-Smith J (2006) Anticholinergic drugs versus placebo for overactive bladder syndrome in adults. Cochrane Database Syst Rev CD003781 [DOI] [PMC free article] [PubMed]
- 20.Andersson KE, Sarawate C, Kahler KH, Stanley EL, Kulkarni AS. Cardiovascular morbidity, heart rates and use of antimuscarinics in patients with overactive bladder. BJU Int. 2010;106:268–274. doi: 10.1111/j.1464-410X.2009.09073.x. [DOI] [PubMed] [Google Scholar]
- 21.Olshansky B, Ebinger U, Brum J, Egermark M, Viegas A, Rekeda L. Differential pharmacological effects of antimuscarinic drugs on heart rate: a randomized, placebo-controlled, double-blind, crossover study with tolterodine and darifenacin in healthy participants > or = 50 years. J Cardiovasc Pharmacol Ther. 2008;13:241–251. doi: 10.1177/1074248408325404. [DOI] [PubMed] [Google Scholar]