Abstract
Migraine has been traditionally considered a non-progressive, paroxysmal disorder with no brain abnormalities between attacks. We used diffusion tensor imaging to examine interictal diffusion properties of the brains of migraineurs with aura, migraineurs without aura and matched healthy controls. Areas of lower fractional anisotropy (FA) were present in migraineurs along the thalamocortical tract. In addition, migraineurs with aura had lower FA in the ventral trigeminothalamic tract, and migraineurs without aura had lower FA in the ventrolateral periaqueductal gray matter. Our results indicate the presence of permanent interictal changes in migraineurs, pointing to an effect of migraine on the trigeminal somatosensory and modulatory pain systems.
Keywords: Migraine, DTI, trigeminal somatosensory system, periacqueductal grey, imaging, brain
Introduction
Migraine is a common neurological disorder affecting around 10–20% of the population worldwide [1], characterized by paroxysmal attacks of unilateral throbbing, severe headache, usually accompanied by nausea, vomiting, photo-and phonophobia. Attacks may be preceded by transitory focal neurological dysfunctions named aura, mostly of the visual nature (migraine with aura).
The pathophysiology of migraine is not fully understood. There is strong evidence indicating that genetically predisposed [2], abnormally hyperexcitable [3] areas of the central nervous system may be the source of an abnormal series of events ultimately leading to the migraine attack. Spreading depression, a self-propagating neurophysiologic phenomenon characterized by a spreading wave of depolarization associated with a reduction of the cortical activity, probably underlies migraine aura [4] and possibly also occurs in migraine without aura [5]. However, while spreading depression fits as the cause of aura, controversy remains as to how pain develops in migraine. Spreading depression induces trigeminal activation [6], and spreading vascular changes including a short hyperperfusion immediately followed by longer-lasting hypoperfusion. Trigeminal activation may in turn induce neurogenic inflammation, a reaction characterized by vasodilation and plasma extravasation. Pain is experienced from peripherally generated trigeminal noxious stimuli conveyed to the correspondent somatosensory cortex throughout the somatosensory pathway by successive synapses (Figure 1, Panel E).
figure 1.
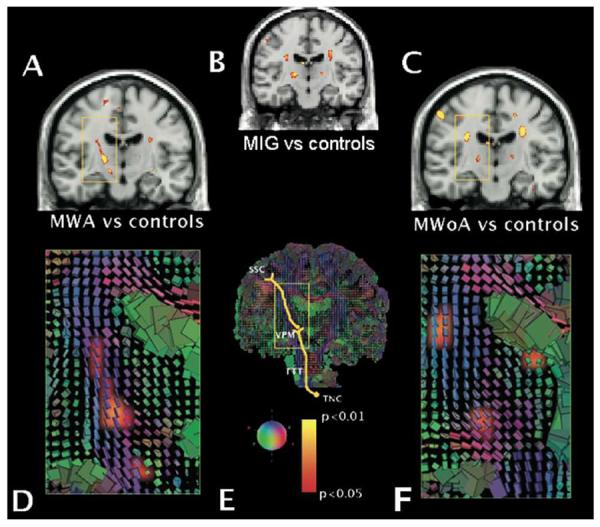
Panels A, B and C show FA average significance maps superimposed on a T1 coronal section comparing migraine with aura (MWA, panel A), all migraine patients (MIG, panel B) and migraine without aura (MWoA, panel C) vs. healthy controls. Panels D, E and F show cuboid maps depicting the diffusion tensor within each voxel. The axes of each cube are placed regarding the main fibres orientation and are coloured accordingly: green indicates antero-posterior fibres; blue indicates supero-inferior fibres; and red indicates medio-lateral fibres. In panels D and F, cuboid maps are superimposed to the FA maps shown in panels A and C, respectively. The trigeminal somatosensory pathway scheme is represented in panel E. The rectangular areas in all panels focus the same anatomical structures. Significant lower FA values were noticed at the thalamocortical tract (3rd order neurons) in both migraine subtypes. In addition, the trigeminothalamic tract (TTT) showed also significantly lower FA values in migraineurs with aura (panel A, lowest cluster inside the yellow square). In both migraine forms, FA clusters were located at the venteroposterior medial thalamic nucleus (VPM), extending upwards along the thalamocortical sensory pathway. The yellow-red shading code represents p values for lower FA changes. n=12 for MWA, MWoA and HC groups. SSC = somatosensory cortex. TNC = trigeminal nucleus caudalis.
Migraine is a paroxysmal disorder. The general idea that migraine does not induce pathology in the brain on a permanent basis and that migraineurs are indistinguishable from controls between attacks has been challenged by recent reports showing that migraine may be related to silent white mater lesions and stroke [7,8].
Diffusion tensor imaging (DTI) is a magnetic resonance imaging (MRI) tool used to visualize orientation and anisotropy of grey and white matter [9]. DTI is typically used to examine white matter affecting ailments such as multiple sclerosis, stroke, brain tumours, diffuse axonal injury, degenerative diseases and HIV infection[10]. DTI has also been used to address the effects of aging [11]. One of the DTI parameters, fractional anisotropy (FA), reflects cellular and subcellular tissue components and their preferable orientation, allowing identification of tracts separately.
In order to specifically test the hypothesis that white and/or organized grey matter diffusional changes along the trigeminal sensory system may be present between migraine attacks, DTI was used to examine migraineurs with and without aura and age- and gender-matched healthy controls. Data were correlated with clinical parameters including attack duration, frequency and the disease time span. The present results provide evidence of migraine interictal diffusional changes in brain areas involved with trigeminal pain processing.
Materials and methods
Subjects
Twenty-four migraine patients subdivided in two equal groups of 12 individuals (with and without aura) were recruited based to the guidelines of the International Headache Society ([12]). In addition, 12 age- and gender- matched healthy controls volunteered to participate in this study. The three groups were matched for age (migraine with aura: 33.8 ± 9.2; migraine without aura: 36.0 ± 7.7, healthy controls 31.0 ± 8.0, p=0.35). Females predominated in all groups (75%, 58.3% and 75%). Patients were considered as migraineurs without aura when no element suggestive of any type of aura was reported. Patients reporting comorbid headaches, chronic pain elsewhere, or other neurological condition (e.g. epilepsy) were excluded. Gross asymmetry or clear pathology on MRI anatomical scans prevented participation in the study. The study was conducted according to the Helsinki Declarations on human experimentation, and was approved by the Institutional Review Boards of the Massachusetts General Hospital (IRB #2002P-000652).
Clinical characteristics
The duration of the migraine history averaged 20 years in both migraine groups (migraine with aura: 20.3 ± 11.2; migraine without aura: 19.5 ± 8.5), with age-at-onset mostly during adolescence (migraine with aura: 13.6 ± 4.6; migraine without aura: 16.5 ± 7.1). Migraine attacks were generally described as moderate to severe by the patients. Patients referred a frequency of around 4 migraine attacks per month (migraine with aura: 4.1 ± 3.6; migraine without aura: 4.0 ± 3.6). Attack duration could not be estimated with precision as the vast majority of the subjects used acute medications for pain control. The predominant pain onset side (>50%) was left in 4, right in 2, and bilateral in 5 of the 12 migraineurs with aura. In migraineurs without aura, pain started predominantly in the right, left and bilateral sides in equal proportions, with 4 patients in each category. Aura occurred predominantly at the same side of the pain in 4 subjects, at the contralateral side in 2, alternating sides in 4. Two patients could not indicate the lateralization of their aura. All auras were visual in nature. In addition, 4 patients also described occasional sensory symptoms. Motor and language disturbances were not found. Other pre-headache symptoms in few individuals included olfactory, auditory, emotional and cognitive changes.
Six migraineurs without aura were under prophylactic treatment or were taking drugs for other reasons (Diazepam, Amitriptyline, Paroxetine, Metoprolol, Propranolol and Valproate). Four migraineurs with aura were taking prophylactic medications (Verapamil, Amitriptyline, Propranolol and Valproate).
Scanning protocol
Structural Imaging
3D reconstructions of the brain images were obtained using two high-resolution magnetization-prepared rapid acquisitions with gradient-echoes (MP-RAGE) on a 3.0 T Siemens Allegra Scanner (Erlangen, Germany). The two MP-RAGE sequences (1.0 × 1.0 × 1.25mm, 128 slices, 256×256 matrix, echo time (TE)=3.44 ms; repetition time (TR)=7.25 ms; flip=7°) were motion-corrected and averaged to create a single image volume.
Diffusion Tensor Imaging
The DTI scans used a single-shot, twice-refocused echo planar sequence [13]. Protocol parameters were: TR/TE: 9200/91ms, 2mm isotropic resolution, 64 slices, FOV 128×128 mm, 1 average, 60 directions of diffusion encoding with b=700s/mm2, and 10 encodings with b = 0 s/mm2. Head motion was reduced by the use of padded clamps tightly attached to the head coil. Patients were scanned during interictal periods (no headache), with at least 24 hours after the resolution of the last attack.
Analysis
Diffusion data, including diffusion tensor, associated eigenvectors, and FA metric were analyzed using multiple processes as described by Salat at al. [11] as follows: (1) motion and residual distortion correction; (2) calculation of FA maps from the previous volume normalized to MNI (Montréal Neurological Institute) space, with transformation of each subject's MP-RAGE volume to a MNI-152 T2 template in Talairach space; (3) resampling of the FA map using the transformation created by the previous step; (4) manual extraction of region-of-interest (ROI) FA values on the spatially normalized data of each subject after agreement by two observers; (5) t-test voxel-based calculation of group statistics on the spatially normalized data; (6) multiple comparison by Monte Carlo permutation analysis using 104 trials at threshold p<0.01. The group analysis was performed on the FA maps, comparing each migraine subtype group to controls. All data processing was performed using tools available as part of the Freesurfer and FSL processing streams. The localization of neuronal pathways and structures was defined by superposing the group comparison significance FA maps at p<0.05 onto the RGB and cuboid maps, which represent the main orientation vector of each voxel as displayed in figure 1, panels D, E and F.
Clinical correlations
Correlation of FA values with clinical data with disease duration, age-at-onset, frequency of the headache attacks, and disease time span were examined. Because aging has been correlated with FA decrease in healthy subjects [11,14], age was specified as a nuisance covariate or confound variable.
All data are shown as mean ± SD; p values lower than 0.05 were considered significant.
Results
DTI changes common to both types of migraine
Based on the multiple comparison by Monte Carlo permutation ranking analysis the lowest FA values were localized and common to all 24 migraineurs in two regions of the brain: the posterior limb of the internal capsule at the proximity of venteroposterior medial thalamus (right: p=0.004; left: p=0.001), and the corona radiata at the level of the lateral ventricle horn along the trigeminothalamic tract (right: p=0.001; left p=0.003) (Figure 1B).
DTI changes specific to migraine subgroups
Statistical differences in FA in the venteroposterior medial thalamus were found between migraineurs with aura and healthy controls (left: p=0.003; right p=0.007) and between migraineurs without aura and controls (left and right: p=0.01) (Figure 1A, C).
In addition, specific regions of reduced FA were found that were specific of the migraine subtype. In migraineurs with aura, lower FA values were present the ventral trigeminothalamic tract bilaterally (right: p=0.007; left p=0.02). In migraineurs without aura, lower FA values were observed in the ventrolateral periaqueductal gray matter (right: p=0.01; left: p=0.03).
Clinical correlations
We did not find any clear correlation between FA values and the clinical data (duration, age at onset, frequency and disease time-span).
Discussion
The present results show that prolonged migraine suffering is associated with changes in the diffusion properties of neuronal structures involved in trigeminal pain processing, the focus of this study. These findings represent changes that are not related to the migraine attack itself, because data were collected interictally.
Diffusion changes in the thalamocortical tract, corresponding to third order neurons, were present both types of migraineurs. The thalamocortical circuitry is based on mutual feedback loops that show marked plasticity [15]. We interpret these changes as the possible consequence of frequent migraine attacks, with the presence of induced axonal adaptative response in the thalamocortical tract.
FA changes do not necessarily indicate lesions, and can be influenced by different variables such as myelination, axon diameter and axon density [9]. In consequence, interpretation of the present data should also be considered from the functional perspective. Migraine patients present interictal functional abnormalities potentially related to trigeminal sensory system dysfunction, and clinicians have long been aware that migraine patients become abnormally sensitive outside attacks not just to light and sound, but also to cutaneous / mucosal stimuli [16]. Migraine patients appear to not habituate to repetitive nociceptive stimuli [17], and interictal thalamocortical somatosensory spike activity seems to be decreased in both migraine subtypes [18]. Ultimately, this indicates that the thalamo-cortical tract is an important component of migraine pathophysiology, regardless of the migraine subtype. Pain modulates brain function and repetitive noxious stimulation induces long-term potentiation and hyperalgesia. The central sensitization observed in migraineurs is most probably related to an abnormal activity of the trigeminal sensory pathway.
Chronic or repetitive activation may modify grey and/or white matter structure [19,20]. This has for example been shown in brains of individuals trained since childhood to play piano. The authors of this study [19] found a positive correlation between the number of training hours and FA in the internal capsule, which can be interpreted as a result of an increase in the density of myelinated axon. Paradoxically, intensive motor (piano) training results in lower FA values in the internal capsule of piano players compared to controls in two studies [19,21]. This observation can be interpreted as an increase in axonal diameter in the piano players. Training - or chronic use - may indeed have different results on white matter depending on whether it happens during brain maturation and myelination, or in the mature brain. Because repetitive, abnormally high frequency pain stimulation occurs in migraineurs, it is possible that low FA signals over the trigeminal sensory pathway do not reflect lesions in the brain, but rather an enlargement of axons as a response to over functioning.
One could argue that white matter changes could be related to cumulative lesions, from e.g. vascular origin. However, migraine-related lesions have a tendency to be located in posterior circulations areas [7], and not to be related to any particular functional tract as is the case in our findings. The arrangement along a functional tract makes a vascular origin unlikely, and we conclude that the differences observed in migraineurs are most probably related to changes in function at molecular level.
As a critical pain neuromodulating structure, the periacqueductal grey matter has been implicated with the activation of the nervous system in migraine. The ventrolateral periacqueductal grey is part of a descending pain inhibiting system from the hypothalamus and frontal cortex projecting to the medullary and spinal dorsal horns. Periacqueductal gray activation inhibits contralateral trigeminovascular nociception in cats [22]. Functional activation of the periacqueductal grey has been observed using PET during migraine attack [23], and the periacqueductal grey of migraineurs contain more iron, a marker of cellular function, with levels increasing with the duration of the disease [24]. We only found changes in the ventrolateral periacqueductal grey in migraineurs without aura, even if pain intensity and frequency was similar in both groups of migraineurs. These findings may indicate a possible dysfunction of the periacqueductal grey in migraineurs without aura, which could result in a lowering of the threshold for initiation of migraine attack through a lack of inhibition of the trigeminal sensory activation.
In conclusion, the data presented here show the presence of alterations in ascending (trigemino-cortical) and descending (periacqueductal gray) sensory pathways and structures in migraineurs, indicating an effect of migraine on the trigeminal sensory system at molecular level.
Further studies with larger amounts of patients are required for precise evaluation of possible pharmacologic influences; in addition, comparison with other headache disorders may indicate whether the findings reported in the present study are migraine-specific, or are the consequence of non-specific trigeminal sensory system chronic activation by corollary symptoms.
figure 2.
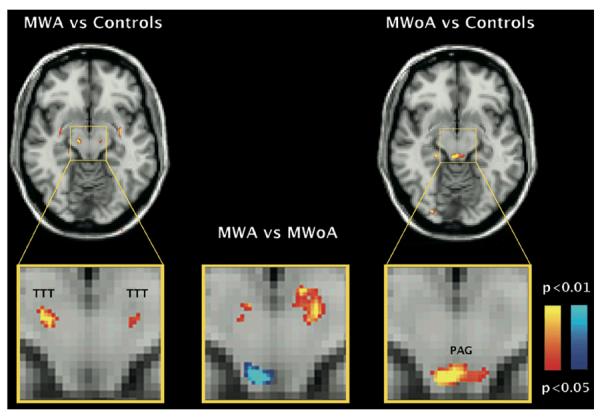
FA average significance maps comparing migraine with aura (MWA, left panel), and migraine without aura (MWoA, right panel) vs healthy controls (HC); and MWA vs. MWoA (middle panel). Significant changes were noticed in the trigeminothalamic tract (MWA vs. HC) and periaqueductal gray area (MWoA vs. HC). FA significant differences were also present between migraine subtypes. The yellow-red shading code represents p values for lower FA changes. The blue shading code represents p values for higher FA changes. n=12 for each group.
Acknowledgments
This work was supported by NIH grant # 5P01 NS 35611-09 (ADS, NH), the Harvard School of Dental Medicine Dean's Award (ADS), the Swiss Heart Foundation (CG), the CAPES Brazil (MV), the Fulbright Program (MV), and the National Alliance for Medical Image Computing (NIBIB U54 EB05149) (DT, JS), which is funded though the NIH Roadmap for Medical Research. We thank Dr. M. Moskowitz for his support and his valuable comments on the manuscript, as well as Dr. Elizabeth Loder and Dr. Egilius Spierings for their clinical support.
Footnotes
Disclosure: The authors have reported no conflicts of interest.
References
- 1.Lipton RB, Bigal ME. Migraine: epidemiology, impact, and risk factors for progression. Headache. 2005;45(Suppl 1):S3–S13. doi: 10.1111/j.1526-4610.2005.4501001.x. [DOI] [PubMed] [Google Scholar]
- 2.Kors E, Haan J, Ferrari M. Migraine genetics. Curr Pain Headache Rep. 2003;7:212–217. doi: 10.1007/s11916-003-0075-4. [DOI] [PubMed] [Google Scholar]
- 3.Welch KM. Brain hyperexcitability: the basis for antiepileptic drugs in migraine prevention. Headache. 2005;45(Suppl 1):S25–32. doi: 10.1111/j.1526-4610.2005.4501008.x. [DOI] [PubMed] [Google Scholar]
- 4.Lauritzen M. On the possible relation of spreading cortical depression to classical migraine. Cephalalgia. 1985;5:47–51. doi: 10.1177/03331024850050S208. [DOI] [PubMed] [Google Scholar]
- 5.Woods RP, Iacoboni M, Mazziotta JC. Brief report: bilateral spreading cerebral hypoperfusion during spontaneous migraine headache [see comments] N Engl J Med. 1994;331:1689–1692. doi: 10.1056/NEJM199412223312505. [DOI] [PubMed] [Google Scholar]
- 6.Moskowitz MA, Nozaki K, Kraig RP. Neocortical spreading depression provokes the expression of c-fos protein-like immunoreactivity within trigeminal nucleus caudalis via trigeminovascular mechanisms. J Neurosci. 1993;13:1167–1177. doi: 10.1523/JNEUROSCI.13-03-01167.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kruit MC, Launer LJ, Ferrari MD, van Buchem MA. Infarcts in the posterior circulation territory in migraine. The population-based MRI CAMERA study. Brain. 2005;128:2068–2077. doi: 10.1093/brain/awh542. [DOI] [PubMed] [Google Scholar]
- 8.Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. Jama. 2006;296:283–291. doi: 10.1001/jama.296.3.283. [DOI] [PubMed] [Google Scholar]
- 9.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 10.Sundgren PC, Dong Q, Gomez-Hassan D, Mukherji SK, Maly P, Welsh R. Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology. 2004;46:339–350. doi: 10.1007/s00234-003-1114-x. [DOI] [PubMed] [Google Scholar]
- 11.Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Silberstein SD, Olesen J, Bousser MG, Diener HC, Dodick D, First M, et al. The International Classification of Headache Disorders, 2nd Edition (ICHD-II)--revision of criteria for 8.2 Medication-overuse headache. Cephalalgia. 2005;25:460–465. doi: 10.1111/j.1468-2982.2005.00878.x. [DOI] [PubMed] [Google Scholar]
- 13.Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- 14.Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, et al. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Ann N Y Acad Sci. 2005;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- 15.Fox K, Glazewski S, Schulze S. Plasticity and stability of somatosensory maps in thalamus and cortex. Curr Opin Neurobiol. 2000;10:494–497. doi: 10.1016/s0959-4388(00)00112-4. [DOI] [PubMed] [Google Scholar]
- 16.Drummond PD. Scalp tenderness and sensitivity to pain in migraine and tension headache. Headache. 1987;27:45–50. doi: 10.1111/j.1526-4610.1987.hed2701045.x. [DOI] [PubMed] [Google Scholar]
- 17.de Tommaso M, Lo Sito L, Di Fruscolo O, Sardaro M, Pia Prudenzano M, Lamberti P, et al. Lack of habituation of nociceptive evoked responses and pain sensitivity during migraine attack. Clin Neurophysiol. 2005;116:1254–1264. doi: 10.1016/j.clinph.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Coppola G, Vandenheede M, Di Clemente L, Ambrosini A, Fumal A, De Pasqua V, et al. Somatosensory evoked high-frequency oscillations reflecting thalamo-cortical activity are decreased in migraine patients between attacks. Brain. 2005;128:98–103. doi: 10.1093/brain/awh334. [DOI] [PubMed] [Google Scholar]
- 19.Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- 20.Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmithorst VJ, Wilke M. Differences in white matter architecture between musicians and non-musicians: a diffusion tensor imaging study. Neurosci Lett. 2002;321:57–60. doi: 10.1016/s0304-3940(02)00054-x. [DOI] [PubMed] [Google Scholar]
- 22.Knight YE, Goadsby PJ. The periaqueductal grey matter modulates trigeminovascular input: a role in migraine? Neuroscience. 2001;106:793–800. doi: 10.1016/s0306-4522(01)00303-7. [DOI] [PubMed] [Google Scholar]
- 23.Weiller C, May A, Limmroth V, Juptner M, Kaube H, Schayck RV, et al. Brain stem activation in spontaneous human migraine attacks. Nat Med. 1995;1:658–660. doi: 10.1038/nm0795-658. [DOI] [PubMed] [Google Scholar]
- 24.Welch KM, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache. 2001;41:629–637. doi: 10.1046/j.1526-4610.2001.041007629.x. [DOI] [PubMed] [Google Scholar]


