Abstract
Background
: There is limited information on antiretroviral (ARV) regimens and outcomes in perinatally HIV (PHIV) -infected youth. Substantial drug resistance after long-term ARV use and non-adherence hinder efforts to design suppressive regimens for PHIV-infected youth. This study compares clinical outcomes by expected activity of the prescribed ARV regimens.
Methods
A retrospective cohort study of 13-24 year-old PHIV-infected youth on stable ARV regimens for ≥ 6 months was conducted at a pediatric HIV clinic. ARV regimens were retrospectively categorized as optimal or suboptimal based on accumulated genotypic resistance prior to study regimen initiation.
Results
Fifty-two patients with similar baseline characteristics met inclusion criteria (21 optimal and 31 suboptimal regimens). Patients on optimal regimens had significantly higher increases in CD4 than those on suboptimal regimens by week 48 of treatment (+62 vs. +8 cells/mm3, respectively; p = 0.04) and by the end of study period (+93 vs. –1 cells/mm3, respectively; p = 0.03). There were no significant differences between the groups in decline of viral load, frequency of opportunistic infections or hospitalizations, or accumulation of resistance mutations. Overall, 60% of the optimal and 45% of the suboptimal groups had non-adherence during the study regimen (p = 0.3).
Conclusions
PHIV-infected youth receiving optimal regimens had greater CD4 improvements but no difference in virologic outcomes compared to those on suboptimal regimens. In a patient population with significant non-adherence, providers must weigh the immunologic benefits of initiating an optimal regimen vs. the potential risks of further resistance accumulation limiting future treatment options.
Keywords: human immunodeficiency virus, antiretroviral, perinatally HIV-infected youth
Introduction
Advances in human immunodeficiency virus (HIV) treatment, especially with the introduction of highly active antiretroviral therapy (HAART) in 1996, have led to a marked increase in the survival of perinatally HIV (PHIV) -infected youth.1,2 However, after a lifetime of treatment, frequently with intermittent adherence and non-suppressive ARV regimens, PHIV-infected youth commonly approach and enter adulthood with substantial drug resistance that leaves them with few or even no effective HAART options. Many of these patients may go on to have uncontrolled viremia for many years along with inflammatory conditions and other co-morbidities.3 They enter adolescent developmental stages that may be influenced by cognitive factors (e.g., concrete thinking) and psychosocial factors such as lack of family support leading to risk-taking and non-adherence behaviors, often resulting in lowered adherence and virologic failure.3,4,5 Providers are challenged by the composition of optimal regimens (i.e., balancing minimizing pill burden and side effects with potency) and timing to switch to an optimal regimen for these youth who continue to struggle with adherence and who have many potential years of life and treatment ahead of them. Initiating an optimal regimen in the setting of continued non-adherence would likely result in failure to improve immunologic response and accumulation of resistance mutations.
Children who remain on suboptimal regimens, which are unlikely to lead to complete virologic suppression due to pre-existing resistance, even in the setting of good adherence, may accumulate resistance and limit future treatment options. Alternatively, treatment with optimal regimens in the setting of non-adherence may also lead to similar negative clinical outcomes and drug resistance.6,7 However, when patients need treatment but have persistent non-adherence, it is unclear which management approach is more beneficial: to strive for better clinical and immunologic outcomes but risk losing future treatment options by switching to an optimal regimen early despite continued non-adherence; or to limit additional resistance and preserve future treatment options but risk clinical and immunologic deterioration by employing a suboptimal regimen while making intensive efforts to improve adherence.
Our objective was to characterize the ARV regimens prescribed for PHIV-infected youth in terms of expected activity level and compare the clinical, immunologic, and virologic outcomes among patients treated with optimal vs. suboptimal therapies.
Methods
Study design and patient selection
This was a retrospective cohort study of PHIV-infected youth aged 13-24 years being followed at the outpatient pediatric HIV clinic at The Johns Hopkins Hospital (JHH). Patients were screened for study inclusion on 6/30/10, and patients were eligible if they had been receiving a stable ARV regimen continuously for a minimum of 6 months with < 60 days of treatment interruption prior to 6/30/10. Exclusion criteria included pregnancy at the time of ARV initiation, absence of a baseline genotype, and loss to follow-up after initiation of ARV regimen. Loss to follow-up was defined as < 2 follow-up patient contacts (visits with the HIV provider, case manager, social worker, or for laboratory tests) within a 12-month period. This study was reviewed and approved by the Institutional Review Board at JHH with waiver of informed consent.
Data collection
Data collected from electronic and paper medical records included baseline demographics (age, gender, race) and clinical characteristics (comorbid conditions, history of opportunistic infections [OIs] / hospitalizations, HIV provider visits, adherence, CDC classification, and ARV drug history); drug resistance mutations at baseline (including results of all genotype testing performed at or before current study ARV regimen initiation) and during follow-up; and clinical outcomes of interest (CD4 count, VL, emergence of new OIs, hospitalizations, and/or resistance mutations) during follow-up. Adherence was assessed from provider documentation in the clinic notes, and patients were categorized as “non-adherent” if providers documented anything more than occasional missed doses prior to or at any time during the study period. Rationales for regimen selection and barriers to initiation of optimal regimens were abstracted from the medical records when available. HIV provider visits were defined as visits excluding those with case managers, social workers, or visits for laboratory tests.
Classification of ARV regimens
Each subject's ARV regimen was categorized as optimal or suboptimal independently by a HIV pediatric HIV/Infectious Diseases (ID) attending physician (A.L.A) and a pediatric HIV/ID clinical pharmacist (A.J.H.), based on evaluating the subject's accumulated genotypic resistance testing results according to the International AIDS Society algorithm.8 In the event of discordance between the categorization of regimens, an adult HIV/ID clinical pharmacist (P.A.P.) evaluated the ARV regimens to determine categorization. Individual antiretroviral agents were classified as either fully, partially, or not active, based on predicted phenotypic activity given the pattern of resistance mutations. Optimal therapy was defined as a regimen with overall summative activity equivalent to at least two fully active agents (including regimens with multiple partially active agents that, taken together, were the equivalent of a regimen of at least two active agents). For example, in a patient on lopinavir/ritonavir, tenofovir and emtricitabine, with only an M184V mutation and one minor lopinavir mutation (L63P), the regimen would be classified as an optimal regimen because although emtricitabine would be inactive, lopinavir/ritonavir would be active, and tenofovir would be hypersusceptible, making at least 2 active agents. Suboptimal therapy was defined as a regimen for which the overall summative activity was less than two fully active agents.
Outcomes
The primary outcome included change in CD4 count, proportion achieving an increase in CD4 count by at least 50 cells/mm3, change in VL log10 values, and proportion of patients achieving a VL < 400 copies/mL. Primary outcomes were assessed at week 24, week 48, and end of study period (6/30/10). Of note, changes in VL were calculated with actual measured VL values, except for VL < 50, < 400, and > 100,000 copies/mL that were substituted with VL 49, 399, and 100,001 copies/mL; respectively. This method was used to include patients who would otherwise be excluded due to limitation in calculating the VL difference. Secondary outcomes included the development of new OIs or hospitalization, and accumulation of additional resistance mutations.
Statistical analysis
Patient demographic and clinical characteristics were summarized by ARV group (optimal vs. suboptimal) using descriptive statistics (median and interquartile range (IQR), frequencies, and percentages). The groups were compared using the χ2 test for categorical data and the Mann-Whitney U test or Wilcoxon rank sum test for continuous data. Univariate and multivariable logistic regression analyses were used to estimate odds ratios (OR) and 95% confidence intervals (CIs) for the outcome of increase in CD4 count by at least 50 cells/mm3. The multivariable models were determined a priori and included age at start of study regimen, baseline CD4 count, optimal vs. suboptimal regimen, adherent vs. non-adherent to the regimen, and VL change (increase vs. decrease from baseline). All analyses were performed using STATA® statistical software version 11 (StataCorp LP., College Station, TX); and a two-sided p < 0.05 was used to determine statistical significance.
Results
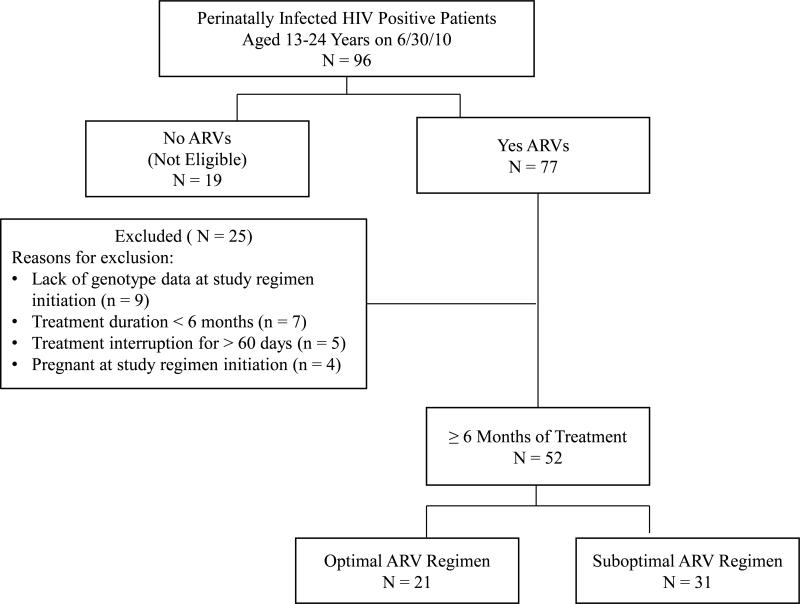
As of June 30, 2010, 77 of the 96 PHIV-infected youth, who were 13-24 years of age receiving ARVs (Fig. 1), were reviewed for study inclusion. Twenty-five patients were excluded due to lack of baseline genotype, ARV treatment duration less than 6 months or treatment interruption for more than 60 days, or pregnancy. Of the 52 patients included for analysis, 21 were receiving optimal and 31 were receiving suboptimal ARV regimens. All ARV regimens met conventional definition of HAART. As shown in Table 1, there was a similar proportion of patients in each group on protease inhibitor (PI) based HAART (29% optimal vs. 26% suboptimal, p = 0.83) but a higher proportion of patients on non-nucleoside reverse transcriptase inhibitor (NNRTI) based HAART in the suboptimal group than in the optimal group (61% vs. 33%, respectively; p = 0.09). Twenty-nine percent of the patients in the optimal group were on a regimen consisted of darunavir/ritonavir, raltegravir, and etravirine; no suboptimal group patients were on this regimen (p = 0.01).
Figure 1. Description of study population selection.
Note: ARV = antiretroviral
Table 1.
Baseline characteristics prior to or by the time of study ARV regimen initiation
| Optimal N = 21 | Suboptimal N = 31 | P value | |
|---|---|---|---|
| Age at start of study ARV regimen (years), median [IQR] | 17 [15-18.7] | 16 [13.9-18.3] | 0.34 |
| Females, n (%) | 11 (52) | 18 (58) | 0.69 |
| Race, n (%) | 0.66 | ||
| African-American | 19 (90) | 28 (90) | |
| White | 2 (10) | 2 (7) | |
| Other (Interracial) | 0 (0) | 1 (3) | |
| # of Co-morbidities, median [IQR] | 3 [2-4] | 3 [2-4] | 0.31 |
| Presence of prior OIs, n (%) | 16 (76) | 20 (65) | 0.37 |
| Presence of prior hospitalizations related to HIVa, n (%) | 4 (19) | 3 (10) | 0.33 |
| # HIV provider visits, mediana, b [IQR] | 3 [2-5] | 3 [2-4] | 0.42 |
| Documented non-adherence prior to the initiation of study ARV regimen, n (%) | 11/21 (52) | 17/29 (59) | 0.66 |
| CD4 (cells/mm3), median [IQR] | 471 [208-676] | 588 [403-1077] | 0.09 |
| CD4 nadir (cells/mm3), median [IQR] | 130 [26-304] | 266 [37-351] | 0.53 |
| VL log10c (copies/mL), median [IQR] | 3.07 [1.69-3.80] | 2.56 [1.69-3.23] | 0.23 |
| Patients with VL < 400 copies/mL, n (%) | 8 (38) | 18 (58) | 0.26 |
| CDC Classification, n (%) | 0.72 | ||
| A | 4 (19) | 5 (16) | |
| B | 4 (19) | 9 (29) | |
| C | 13 (62) | 17 (55) | |
| Study regimen rationale documented, n (%) | 0.57 | ||
| Resistanced | 7 (34) | 5 (16) | |
| Regimen simplificatione | 5 (23) | 5 (16) | |
| Adverse drug effects | 2 (10) | 4 (13) | |
| Pill burden reduction | 1 (5) | 5 (16) | |
| Unknown | 5 (23) | 10 (32) | |
| Otherf | 1 (5) | 2 (7) | |
| Patients with presence of RT resistance mutations, n (%) | 21 (100) | 31 (100) | N/A |
| Patients with presence of PR resistance mutations, n (%) | 19 (90) | 29 (94) | 0.68 |
| ARV class(es) of resistance, n (%) | 0.37 | ||
| 1 | 5 (24) | 6 (19) | |
| 2 | 5 (24) | 12 (39) | |
| 3 | 11 (52) | 11 (36) | |
| ≥ 4 | 0 (0) | 2 (6) | |
| #ARV class(es) exposure, median [IQR] | 3 [2-3] | 3 [3-3] | 0.28 |
| #ARV agent(s) exposure, median [IQR] | 8 [5-8] | 8 [7-9] | 0.25 |
| NNRTI based study ARV regimeng, n (%) | 7 (33) | 19 (61) | 0.09 |
| PI based study ARV regimenh, n (%) | 6 (29) | 8 (26) | 0.83 |
| DRV/r + RAL + ETR regimen, n (%) | 6 (29) | 0 (0) | 0.01 |
| Otheri, n (%) | 2 (9) | 4 (13) | 0.71 |
Preceding 12 months
Excluding visits with case manager, social worker, or for laboratory work
Actual VL values were used except patients with VL < 50, < 400, and > 100,000 copies/mL (VL 49, 399, and 100,001 copies/mL were substituted respectively).
Based on genotype testing interpreted by HIV provider documented in the medical records
Including changing regimen to reduced daily dosing frequency
Optimal arm: 1 patient due to drug-drug interaction and drug-disease interaction; suboptimal arm: 1 patient due to adverse drug effect and adherence problem, and 1 patient due to taking the medication incorrectly.
NNRTI based regimen excludes any PI agents
PI based regimen excludes any NNRTI agents
Optimal arm: 1 RAL + PI + NRTI, 1 PI + NNRTI + NRTI; suboptimal arm: 1 RAL + NNRTI + 3NRTIs, 1 RAL + PI + 2NRTIs, 2 PIs + NNRTI + NRTI.
ARV = antiretroviral, DRV/r = darunavir/ritonavir, ETR = etravirine, IQR = interquartile range, N/A = not applicable, NNRTI = non-nucleoside reverse transcriptase inhibitor, NRTI = nucleoside/nucleotide reverse transcriptase inhibitor, OI = opportunistic infection, PI = protease inhibitor, PR = protease gene, RAL = raltegravir, RT = reverse transcriptase, VL = viral load
Note: bolded value represents significant finding.
In two patients on suboptimal regimens, a more optimal regimen was not possible at the time of regimen initiation. The remainder 29 patients on suboptimal regimens (94%) were able to have an optimal regimen constructed at time of regimen initiation. Most of the optimal regimens were initiated during or after 2008 (62%), whereas the majority of suboptimal regimens were started prior to 2008 (71%). In the 13 patients receiving suboptimal therapy with documented barriers to the initiation of optimal therapy, the most frequently documented barrier was non-adherence (n = 10, 77%). Other barriers included psychological/psychosocial factors in seven patients (54%, including stressors from life or school, lack of family assistance and oppositional behaviors), adverse drug effect in one patient, and accumulation of resistance mutations in one patient. Multiple barriers were found in five patients (38%) mostly due to a combination of adherence and psychological/psychosocial issues.
Baseline demographic and clinical characteristics were similar between the optimal and suboptimal groups with respect to age, gender, race, and co-morbidities (Table 1). The most frequently documented co-morbidities included vitamin D deficiency, asthma, and psychiatric or neurodevelopmental conditions, such as attention deficit hyperactivity disorder, developmental delay, and depression. Two patients in each group were co-infected with hepatitis C virus, and one patient in the suboptimal group with hepatitis B virus. There was a trend towards lower median (IQR) baseline CD4 count in the optimal vs. suboptimal group (471 (208-676) vs. 588 (403-1077) cells/mm3, respectively; p = 0.09). More than half of patients from both groups had a history of medication non-adherence prior to the initiation of study regimens. Patients in both groups were previously exposed to a median of three ARV classes and eight agents; and there were no significant differences in the numbers of baseline reverse transcriptase (RT) and protease (PR) mutations (Table 1).
Outcomes
By the end of the study period (6/30/10), all patients received treatment with the study ARV regimen for a median (IQR) of 35.4 (14.5-51.8) months. Patients in the optimal group completed a median (IQR) of 92 (56-139) weeks of therapy vs. 192 (111-227) weeks in the suboptimal group (p = 0.04). Non-adherence to the study ARV regimen was noted in 12 (60%) optimal group patients vs. 14 (45%) suboptimal group patients (p = 0.3).
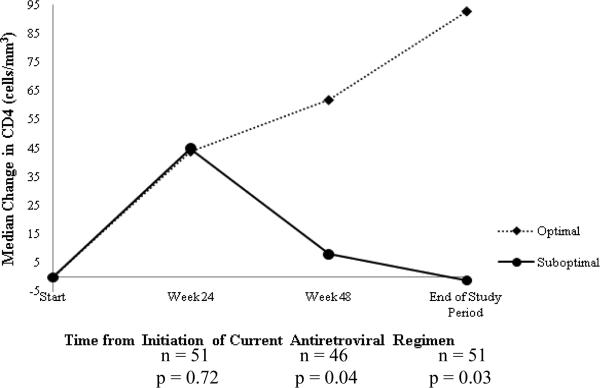
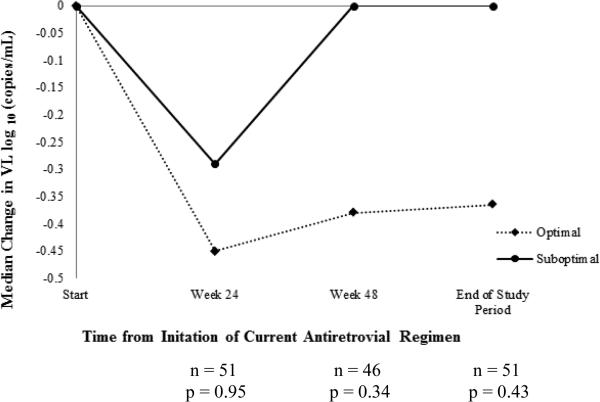
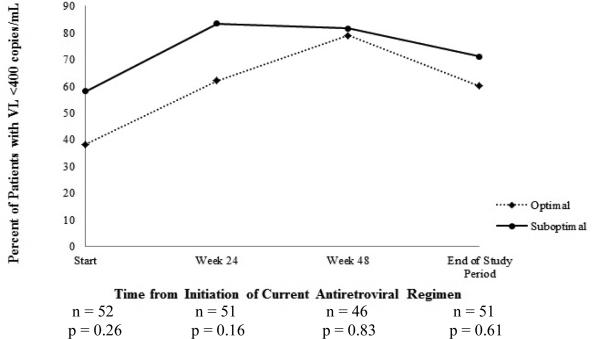
At week 24, there was no difference in change in CD4 count between the two groups (Figure 2A). However, by week 48, the median (IQR) CD4 count increase of +62 (+25 to +200) cells/mm3 in optimal group was significantly greater than the increase of +8 (–98 to +68) cells/mm3 in suboptimal group (p = 0.04). This difference persisted through the end of study period: median (IQR) change in CD4 count of +93 (+9 to +205) vs. –1 (–93 to +54) cells/mm3, respectively, (p = 0.03). There were no significant differences in median (IQR) changes in VL between the optimal and suboptimal groups at weeks 24 [–0.45 (–1.26 to 0) vs. –0.29 (–0.93 to 0) log10 copies/mL, p = 0.95], 48 [–0.38 (–1.50 to 0) vs. 0 (–0.92 to 0) log10 copies/mL, p = 0.34] or end of study period [–0.37 (–1.45 to +0.07) vs. 0 (–0.76 to +0.06) log10 copies/mL, p = 0.43] (Figure 2B). Overall, no significant differences were found in the proportion of patients with VL < 400 copies/mL between optimal and suboptimal groups at any of the study time points (Figure 2C).
Figure 2A. Comparison of changes in CD4.
Median change in CD4 over the course of the antiretroviral regimen (optimal vs. suboptimal)
Figure 2B. Comparison of changes in viral load (VL).
Median change in HIV-1 viral load (VL) during the course of the antiretroviral regimen (optimal vs. suboptimal)
All calculated median changes at each time point are changes from baseline.
Figure 2C. Comparison of proportion of patients achieving viral load (VL) < 400 copies/mL.
Proportion of patients achieving HIV-1 viral load (VL) < 400 copies/mL during the course of the antiretroviral regimen (optimal vs. suboptimal)
Logistic regression analysis for the primary outcome of increase in CD4 count by at least 50 cells/mm3 is summarized in Table 2. On univariate analysis, being on an optimal regimen was associated with a higher likelihood of having a CD4 count increase of at least 50 cells/mm3 by the end of study period, and having a VL decline from baseline was also associated with a higher likelihood of having a CD4 count increase of at least 50 cells/mm3 by week 24 and week 48. Adherence was not associated with having a CD4 count increase of at least 50 cells/mm3 at any of the time points on univariate analysis. On multivariable logistic regression, after controlling for age at start of study regimen, baseline CD4 count, adherent vs. non-adherent to the regimen, and VL change from baseline, patients receiving optimal therapy were more likely to have a CD4 count gain of at least 50 cells/mm3 by week 48 of therapy (adjusted odds ratio (AOR) 4.40, 95% CI 1.03-18.8, p = 0.046) and by the end of study period (AOR 5.39, 95% CI 1.06-27.2, p = 0.042) as compared to those receiving suboptimal therapy. On multivariable analysis, decrease in VL from baseline was associated with a higher likelihood of a CD4 count gain of at least 50 cells/mm3 at week 24 only (AOR 8.01, 95% CI 2.04-31.5, p = 0.003).
Table 2.
Logistic regression analysis for the primary outcome of increase in CD4 count by at least 50 cells/mm3
| Variables | Univariate | Multivariate | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 Wks | 48 Wks | End | 24 Wksa | 48 Wksa | Endb | |||||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | Adj OR (95% CI) | P | Adj OR (95% CI) | P | Adj OR (95% CI) | P | |
| Optimal regimen | 0.97 (0.32-2.94) | 0.96 | 3.17 (0.99-10.1) | 0.051 | 3.26 (1.02-10.4) | 0.046 | 0.92 (0.24-3.48) | 0.90 | 4.40 (1.03-18.8) | 0.046 | 5.39 (1.06-27.2) | 0.042 |
| Suboptimal regimen | ref | ref | ref | ref | ref | ref | ||||||
| Adherent | 1.00 (0.36-2.77) | 0.99 | 0.67 (0.24-1.86) | 0.44 | 1.36 (0.48-3.81) | 0.57 | 1.09 (0.31-3.81) | 0.89 | 0.83 (0.23-2.99) | 0.78 | 2.87 (0.70-11.8) | 0.14 |
| Non-adherent | ref | ref | ref | ref | ref | ref | ||||||
| Viral load < baseline | 6.46 (1.84-22.7) | 0.004 | 3.51 (1.03-11.9) | .044 | 2.37 (0.75-7.50) | 0.14 | 8.01 (2.04-31.5) | 0.003 | 3.04 (0.77-12.1) | 0.11 | 3.04 (0.70-13.1) | 0.14 |
| Viral load ≥ baseline | ref | ref | ref | ref | ref | ref | ||||||
Multivariable regression model included age at start of study regimen, baseline CD4 count, optimal vs. suboptimal regimen, adherent vs. nonadherent to the regimen, and viral load change
Multivariable regression model included age at start of study regimen, duration of regimen, baseline CD4 count, optimal vs. suboptimal regimen, adherent vs. nonadherent to the regimen, and viral load change
Adj OR = adjusted odds ratio, ARV = antiretroviral, CI = confidence interval, end = end of study period, P = p-value, OR = odds ratio, ref = reference, wks = weeks
Note: variables not presented were not significant in the model. Bolded values represent significant findings.
No significant differences were seen in accumulation of new OIs or hospitalizations related to HIV complications between the two groups at the end of study period (Table 3). The most frequently observed OI was recurrent pneumonia in both groups (3 patients in the suboptimal vs. 2 patients in the optimal group). The most common reasons for hospitalization were disseminated Mycobacterium avium complex infection (3 hospitalizations), recurrent pneumonia (3 hospitalizations), and influenza (2 hospitalizations) in the suboptimal group vs. recurrent pneumonia (3 hospitalizations) and Cryptosporidium diarrhea (2 hospitalizations) in the optimal group.
Table 3.
Comparison of secondary outcomes between optimal and suboptimal regimens
| Week 24 | Week 48 | End of Study Period | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Optimal n = 21 | Suboptimal n = 31 | p valuea | Optimal n = 20 | Suboptimal n = 27 | P valuea | Optimal n = 21 | Suboptimal n =31 | P valuea | |
| New OIsb | 5 | 2 | 0.34 | 7 | 4 | 0.64 | 8 | 10 | 0.91 |
| New hospitalizations related to HIVb | 3 | 2 | 0.73 | 7 | 4 | 0.7 | 10 | 20 | 0.77 |
| # HIV provider visits,b, c median [IQR1 | 2 [1-2] | 2 [1-2] | 0.87 | 4 [3-4] | 3 [2-4] | 0.31 | 7 [4-10] | 11 [9-15] | 0.04 |
Wilcoxon rank sum test
Accumulated from baseline at initiation of study ARV regimens
Excluding visits with case manager, social worker, or for laboratory work
ARV = antiretroviral, IQR = interquartile range, VL = viral load.
Note: bolded value represents significant finding.
Accumulation of additional resistance mutations was evaluated in patients who had repeat genotypes during the study period. The median (IQR) time to first resistance testing after baseline was 105 (42-163) weeks, and 142 (74-203) weeks for a second resistance testing. Five patients in the optimal group and eight patients in the suboptimal group had one repeat genotype; three patients in the optimal group and two patients in the suboptimal group had a second repeat genotype while on the study ARV regimen. No significant differences were found between the two groups in terms of the number of new resistance mutations. In the optimal group, new RT mutations that emerged on therapy included K101E, K103N, P225H, A98G, M41L, 69insSSC, and L210W. No new PR mutations developed. In the suboptimal group, new RT mutations that emerged on therapy included K103N, V108I, M184V, L210W and T215Y; and new PR mutations that emerged included M46I.
Discussion
In this study, we found that heavily pretreated PHIV-infected youth maintained on suboptimal ARV regimens are less likely to have a CD4 count gain of at least 50 cells/mm3 by 48 weeks on the study regimen and by the end of study period. Patients maintained on optimal regimens for 48 weeks were four times as likely to have an increase in CD4 count by at least 50 cells/mm3. Almost all of the patients included were highly treatment experienced with exposure to a median of eight ARV agents, similar to the previously reported median of seven different ARVs in PHIV-infected youth.9 Overall, at baseline, 100% of the study patients harbored RT mutations and ≥ 90% harbored PR mutations. More than half of all study patients had documented non-adherence at baseline as well as with the current study ARV regimen, which is more than what was previously reported in adolescents.4,10 This is likely due to the different definitions of adherence used in studies.
Sixty percent of patients were initiated on suboptimal regimens based on existing genotypic resistance. The choice of not initiating the most optimal regimen was mainly influenced by patients’ history of non-adherence potentially along with providers’ fear of further accumulation of resistance limiting future treatment options.
Immunologic outcomes, as measured by change in CD4 count from baseline, were superior in the optimal group compared to the suboptimal group. This difference was observed as early as week 48 of treatment and remained significant until the end of study period. Previous studies have also demonstrated declines in CD4 count from baseline at a rate of –6 cells/mm3 per year in adolescents who continued on failing regimens.7 Our study showed an initial improvement in CD4 count in the suboptimal group, but the CD4 count eventually returned to baseline by the end of study period. However, unlike the previous study,7 our study did not show a significant difference in VL reduction between the two groups as one would expect. The lack of significant difference in VL reduction may be explained by the partial virologic suppression from suboptimal regimens. Previously shown, partially stopping the PI agent while continuing suboptimal regimens composed of NRTI and NNRTI agents in PHIV-infected viremic children for a median of one year (range 0.41-3.35 years) resulted in no significant changes in mean VL before and during PI interruption (3.87 vs. 4.00 log10 copies/mL, respectively; p = 0.17).11 Similar to our study, despite the lack of change in VL, the CD4 count declined slowly during this treatment interruption (before interruption mean CD4 was 681 cells/mm3 vs. after interruption mean CD4 was 501 cells/mm3, p = 0.002). Lastly, there were no statistical significant differences in terms of other clinical outcomes including new OIs, hospitalizations related to HIV complications, or accumulation of new resistance mutations. The non-significant difference in new resistance development can be attributed to the already high level of resistance in this group of patients before the initiation of study ARV regimens.
While guidelines recommend that initiation of the most optimal regimen is preferred in patients having virologic failure,12 our study did not find that optimal regimens are better than suboptimal regimens at achieving VL < 400 copies/mL in this population of patients with > 50% non-adherence and > 60% with resistance to two or more ARV classes. Currently, there is no clear consensus for managing virologic failure in patients who need HAART but are non-adherent. Based on our study, in the setting of high resistance and barriers to the initiation of an optimal regimen, the use of suboptimal regimens while awaiting for improvements in adherence or resolution of barriers may potentially be considered for a duration of up to 24 weeks; however, continuing for ≥ 48 weeks will significantly increase the risk of poorer immunologic outcomes.
There are limitations to our study, notably the small sample size and retrospective study design. Our data collection was limited to the documentation available in the medical records. We were unable to capture all rationales for suboptimal therapy selection. For patients transferred into the clinic after their diagnosis of PHIV infection, we may not have had complete documentation of previous ARV regimens and genotypes performed elsewhere. More than half of our study population exhibited non-adherence to their study regimen, therefore, our findings may not be generalizable to populations with different adherence patterns. Additionally, our definition of non-adherence was based on provider documentation and description in the clinic notes, and not objectively measured in a quantitative systematic way. There was a significant difference in total treatment duration between the optimal and suboptimal groups, which can potentially be a confounding variable although we controlled for treatment duration in multivariable analyses at the end of study time point. With the high number of baseline mutations, we may be limited in our ability to fully elucidate the potential accumulation of resistance mutations. Finally, due to the heavy pretreatment status of our cohort of PHIV-infected youth, our findings may not be generalizable to youth with behaviorally acquired HIV, or other PHIV-infected populations.
In summary, PHIV-infected youth represent a behaviorally, developmentally, and psychosocially unique group of patients who are often heavily treatment-experienced with resistance to three or more ARV classes. Many of these patients are non-adherent to their medications. The choice of initiating optimal vs. suboptimal regimens is potentially influenced by multiple factors including adherence, adverse drug effects, pill burden, and fear of accumulation of resistance mutations. However, providers struggle with the decision about the right time to initiate optimal regimens, particularly in the setting of persistent non-adherence. In these patients providers must weigh the benefits of initiating an optimal regimen (e.g., increase in CD4 count) against the potential risk that poor adherence will lead to further accumulation of resistance, which may limit future treatment options. Further studies are needed to determine the best treatment in this challenging population of PHIV-infected youth.
Acknowledgements
The authors would like to thank Jennifer Chang, BS for her assistance with data extraction from the clinic database.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institutes of Health or the Department of Health and Human Services.
This study was presented at the 30th Eastern States Conference, May 2011, in Hershey, PA.
Conflicts of Interest and Source of Funding: A.L.A. is supported by The Johns Hopkins Ross Clinician Scientist Award and the National Institutes of Allergy and Infectious Diseases (1K23 AI084549). No conflicts of interest or disclosures reported by the remaining authors.
References
- 1.Brady MT, Oleske JM, Williams PL, et al. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr. 2010;53(1):86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel K, Hernan MA, Williams PL, et al. Long-term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: a 10-year follow-up study. Clin Infect Dis. 2008;46(4):507–515. doi: 10.1086/526524. [DOI] [PubMed] [Google Scholar]
- 3.Hazra R, Siberry GK, Mofenson LM. Growing up with HIV: children, adolescents, and young adults with perinatally acquired HIV infection. Annu Rev Med. 2010;61:169–185. doi: 10.1146/annurev.med.050108.151127. [DOI] [PubMed] [Google Scholar]
- 4.Rudy BJ, Murphy DA, Harris DR, Muenz L, Ellen J. Adolescent Trials Network for HIV/AIDS Interventions. Prevalence and interactions of patient-related risks for nonadherence to antiretroviral therapy among perinatally infected youth in the United States. AIDS Patient Care STDS. 2010;24(2):97–104. doi: 10.1089/apc.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson DC, Farley JJ. Efficacy of and adherence to highly active antiretroviral therapy in children infected with human immunodeficiency virus type 1. Pediatr Infect Dis J. 1999;18(8):682–689. doi: 10.1097/00006454-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Cozzi-Lepri A, Phillips AN, Ruiz L, et al. Evolution of drug resistance in HIV-infected patients remaining on a virologically failing combination antiretroviral therapy regimen. AIDS. 2007;21(6):721–732. doi: 10.1097/QAD.0b013e3280141fdf. [DOI] [PubMed] [Google Scholar]
- 7.Agwu A, Lindsey JC, Ferguson K, et al. Analyses of HIV-1 drug-resistance profiles among infected adolescents experiencing delayed antiretroviral treatment switch after initial nonsuppressive highly active antiretroviral therapy. AIDS Patient Care STDS. 2008;22(7):545–552. doi: 10.1089/apc.2007.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: December 2009. Top HIV Med. 2009;17(5):138–145. [PubMed] [Google Scholar]
- 9.Van Dyke RB, Patel K, Siberry GK, et al. Antiretroviral Treatment of U.S. Children with Perinatally-Acquired HIV Infection: Temporal Changes in Therapy between 1991 and 2009 and Predictors of Immunologic and Virologic Outcomes. J Acquir Immune Defic Syndr. 2011 Mar 9; doi: 10.1097/QAI.0b013e318215c7b1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy DA, Belzer M, Durako SJ, et al. Longitudinal antiretroviral adherence among adolescents infected with human immunodeficiency virus. Arch Pediatr Adolesc Med. 2005;159(8):764–770. doi: 10.1001/archpedi.159.8.764. [DOI] [PubMed] [Google Scholar]
- 11.Legrand FA, Abadi J, Jordan KA, et al. Partial treatment interruption of protease inhibitors augments HIV-specific immune responses in vertically infected pediatric patients. AIDS. 2005;19:1575–1585. doi: 10.1097/01.aids.0000186816.99993.8e. [DOI] [PubMed] [Google Scholar]
- 12.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children [April 3, 2011];Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. 2010 Aug;16:1–219. Available at http://aidsinfo.nih.gov/ContentFiles/PediatricGuidelines.pdf. [Google Scholar]