SUMMARY
The vitamin A metabolite retinoic acid (RA) provides patterning information during vertebrate embryogenesis, but the mechanism through which RA influences limb development is unclear. During patterning of the limb proximodistal axis (upper limb to digits), avian studies suggest that a proximal RA signal generated in the trunk antagonizes a distal fibroblast growth factor (FGF) signal. However, mouse and zebrafish genetic studies suggest that loss of RA suppresses forelimb initiation. Here, using genetic and pharmacological approaches, we demonstrate that limb proximodistal patterning is not RA dependent, thus indicating that RA-FGF antagonism does not occur along the proximodistal axis of the limb. Instead, our studies show that RA-FGF antagonism acts prior to limb budding along the anteroposterior axis of the trunk lateral plate mesoderm to provide a patterning cue that guides formation of the forelimb field. These findings reconcile disparate ideas regarding RA-FGF antagonism and provide insight into how endogenous RA programs the early embryo.
INTRODUCTION
Investigation of the signaling mechanisms underlying limb development has served as a paradigm for understanding general principles that govern embryogenesis. Many different signaling molecules converge to generate new tissues during growth and patterning of the limb (Rabinowitz and Vokes, 2012). Retinoic acid (RA), the active metabolite of vitamin A (retinol), is generated in specific tissues during embryogenesis and regulates many developmental processes, including limb formation, by serving as a ligand for nuclear RA receptors (RARs) (Duester, 2008). The mechanism through which RA controls limb development has been a topic of considerable debate ever since it was first reported that RA treatment of chick limbs could alter limb anteroposterior patterning (Tickle et al., 1982). In this case, RA treatment was found to merely mimic the action of sonic hedgehog (Shh) and did not provide the endogenous cue for anteroposterior patterning as originally suggested (Noji et al., 1991; Wanek et al., 1991; Litingtung et al., 2002).
Thinking has evolved over the past few years to a model in which RA antagonism of fibroblast growth factor (FGF) signaling has been proposed to control limb proximodistal patterning (Tabin and Wolpert, 2007). Patterning along the proximodistal axis of the vertebrate limb has been suggested to be controlled by opposing diffusible signals, with RA functioning as a proximal signal and FGF acting as a distal signal (Mercader et al., 2000). Chick forelimbs or hindlimbs ectopically exposed to RA or FGF8, or to antagonists of RAR or FGF receptor, display proximodistal limb patterning fate changes that either expand or contract expression of the proximal limb markers Meis1 and Meis2 (Mercader et al., 2000). Although Meis genes have not yet been demonstrated to be necessary for proximodistal patterning of vertebrate limbs, the Drosophila homolog is required for proximodistal patterning of fly limbs (Mercader et al., 1999). During normal limb proximodistal patterning, mouse genetic loss-of-function studies have verified a requirement for FGF8 or other distal FGF signals from the apical ectodermal ridge (AER) to control cell fate specification and survival to drive normal limb outgrowth and to restrict Meis1 and Meis2 expression to the proximal limb (Mariani et al., 2008). In contrast, mouse Rdh10 mutants (Sandell et al., 2007) and Raldh2 (Aldh1a2) mutants (Zhao et al., 2009) lacking RA synthesis have been unable to verify that endogenous RA functions as a proximal limb signal. However, mouse and zebrafish RA loss-of-function mutations disrupt initiation of forelimb development, although hindlimb development is not affected (Begemann et al., 2001;Grandel et al., 2002; Sandell et al., 2007; Zhao et al., 2009;Cunningham et al., 2011a).
Recent studies using recombinant heterotopic chick limb transplantations provided a further indication that RA may be needed for proximodistal patterning of both forelimbs and hindlimbs (Cooper et al., 2011; Roselló-Díez et al., 2011). While these studies report the ability of RA treatment to reprogram distal limb mesenchyme within a short time window to a proximal fate (Roselló-Díez et al., 2011) and to maintain early limb mesenchyme in a Meis1+ proximal fate alongside Wnt and FGF treatment (Cooper et al., 2011), they do not address a requirement for endogenous RA in proximal limb mesenchyme. RA gain-of-function effects on Meis1/2 could represent disruption to specific FGF signaling functions that are already known to restrict Meis1/2 expression in the distal limb from mouse genetic studies (Mariani et al., 2008), a conclusion not previously accounted for by the chick studies (Cooper et al., 2011; Roselló-Díez et al., 2011).
Here, we take a comprehensive genetic and complementary pharmacological approach to analyze limb development through initiation and patterning phases to consolidate conflicting data concerning the function of RA during limb development. We show that RA signaling is not required for limb proximodistal patterning, thus calling into question a role for RA-FGF antagonism during limb development. However, we provide genetic evidence that RA-FGF antagonism does occur during limb development but only along the trunk lateral plate mesoderm prior to forelimb budding to permit correct spatiotemporal induction of Tbx5 needed for forelimb initiation. Thus, our studies show that RA controls limb development in a manner much different than that originally envisioned, and we provide insights into the biological function of RA-FGF antagonism.
RESULTS
Limb Proximal Identity Is Maintained following Combined Genetic and Pharmacologic Abrogation of RA Signaling
Our genetic analysis incorporates use of two key RA-signaling mouse mutants. The Raldh2−/− knockout lacks RA activity in both mesoderm and neuroectoderm (based upon the RARE-lacZ RA-reporter transgene), resulting in a failure to develop beyond embryonic day 8.75 (E8.75), but this mutant can be used to examine axial patterning of lateral plate mesoderm that gives rise to the forelimb field at E8.5 (Zhao et al., 2009). The ENU-induced Rdh10trex/trex mutant survives through limb-patterning stages from E10.5 to E14.5. trex contains a point mutation that generates a form of RDH10 lacking detectable enzyme activity, and trex embryos lack detectable RA activity (RARE-lacZ expression) in limb mesoderm although RA activity remains in posterior neuroectoderm, likely due to another retinol-metabolizing enzyme; thus, trex is essentially a null mutant (Sandell et al., 2007; Cunningham et al., 2011a). The Rdh10−/− knockout mouse phenotype was suggested be more severe than trex with less survival beyond E10.5 (Rhinn et al., 2011; Sandell et al., 2012), but this may be explained by strain differences (Rhinn et al., 2011). Here, we used trex mutants referred to as Rdh10 mutants.
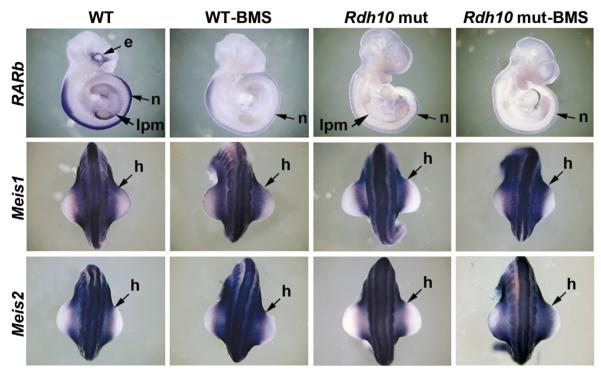
We analyzed expression of the proximal limb markers Meis1 and Meis2 in Rdh10 mutants lacking RARE-lacZ expression in limb mesoderm and found no deficit in proximal limb expression at E10.5 for either gene (Figure S1). To eliminate the possibility that Meis1/2 genes are induced by residual RA signaling that may still persist in limb field mesoderm of Rdh10 mutants, we cultured E10.25 wild-type and Rdh10 mutant embryos for 6 hr with the pan-RAR antagonist BMS493 at 10 μM (Germain et al., 2002). Previous studies in chick embryos treated with RAR antagonist-loaded beads (including BMS493) were performed at an equivalent stage (HH20) and culture time, although at much higher concentration (6 mM), leading to the conclusion that RA is actively needed at this stage for proximal limb expression of Meis1/2 (Mercader et al., 2000; Roselló-Díez et al., 2011). We utilized BMS493 at 10 μM as this has previously been shown to downregulate RA signaling in mouse embryos (Wendling et al., 2000; Chen et al., 2007), and this concentration was nontoxic compared to 100 μM BMS493, which caused tissue necrosis during culture (data not shown). RARb, an established RA target gene (Mendelsohn et al., 1991), was already strongly downregulated in control Rdh10 mutants versus control wild-type embryos (n = 2/2), but Meis1/2 expression was unchanged (n = 3/3 for Meis1; n = 3/3 for Meis2) (Figure 1). BMS493 treatment of wild-type embryos resulted in strong RARb downregulation in lateral plate mesoderm, neural tube, and eye (n = 3/3), but Meis1/2 expression remained normal in lateral plate mesoderm and proximal limb (n = 2/2 for Meis1; n = 5/5 for Meis2). Without BMS493 treatment, Rdh10 mutant embryos displayed residual RARb expression in the interlimb lateral plate mesoderm (n = 2/2), but treatment with BMS493 eliminated this residual expression (n = 3/3). In contrast, Rdh10 mutants treated with BMS493 maintained normal expression of Meis1/2 in the proximal limb (n = 2/2 for Meis1; n = 3/3 for Meis2) (Figure 1). Thus, BMS493 nullification of RAR nuclear receptors in Rdh10 mutants that already have a severe loss of RA synthesis provides conclusive evidence that RA signaling is unnecessary to control Meis1/2 limb expression.
Figure 1. Meis1/2 Expression Is Maintained During Limb Proximodistal Patterning following Loss of RA Signaling.
Shown are in situ hybridization results for Meis1, Meis2, and RARb expression in E10.25 wild-type (WT) and Rdh10 mutant (Rdh10 mut) embryos cultured for 6 hr in DMEM/F-12 medium ± 10 μM BMS493; eye (e), neural (n), lateral plate mesoderm (lpm), and proximal hindlimb bud (h) expression are indicated. See also Figure S1.
RA Signaling Is Not Required to Establish Meis1/2 Expression during Limb Field Prepatterning
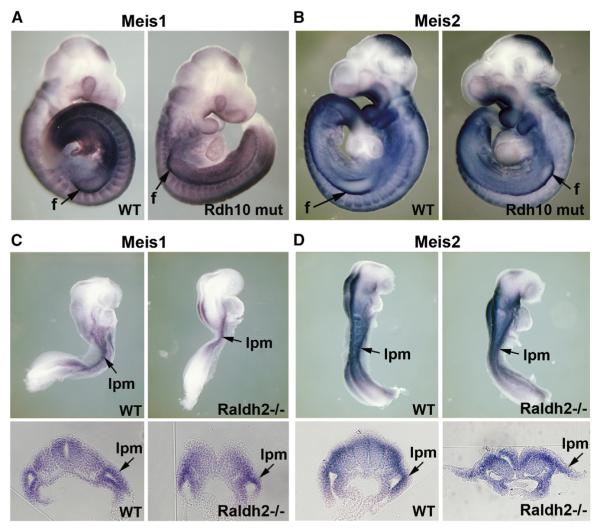
RA signaling is first observed in lateral plate mesoderm at E7.5 when RARE-lacZ expression is first detected (Rossant et al., 1991). As Rdh10 mutants lack RA activity (RARE-lacZ expression) in lateral plate mesoderm at early stages prior to limb budding (Cunningham et al., 2011a), we also analyzed Meis1/2 at E9.5 to look for a potential role in RA controlling the earliest stages of limb proximodistal patterning. Meis1/2 expression was maintained similar to wild-type in E9.5 Rdh10 mutant proximal forelimbs shortly after budding (n = 9/9) (Figures 2A and 2B) and in lateral plate mesoderm of the prebudding hindlimb field (n = 9/9) (Figure S2). In order to examine Meis1/2 expression at E8.5, we utilized Raldh2−/− embryos lacking RARE-lacZ expression in all trunk and caudal tissues; Meis1/2 expression was expressed robustly throughout the lateral plate mesoderm of Raldh2−/− embryos similar to wild-type (n = 13/13) (Figures 2C and 2D). Thus, in the absence of regulation by RA, we postulate that Meis1/2 expression in lateral plate mesoderm from its onset prior to limb development until its appearance in the proximal limb represents a default proximal limb state.
Figure 2. Meis1/2 Expression Is Established during Limb Field Prepatterning in the Absence of RA Signaling.
(A–D) In situ hybridization for Meis1 and Meis2 prior to and during limb development.
(A and B) Meis1 and Meis2 expression at E9.5 in wild-type and Rdh10 mutant embryos; forelimb bud (f) expression is indicated.
(C and D) Meis1 and Meis2 expression prior to budding at E8.5 in wild-type and Raldh2−/− embryos. Lower panels represent transverse sections through the presumptive forelimb field revealing the lateral plate mesoderm (lpm).
See also Figure S2.
Demonstration of RARE-lacZ Sensitivity to Subnanomolar RA Levels
Previous studies on Rdh10 and Raldh2 mutants indicated a lack of RA activity in lateral plate mesoderm based upon the absence of RARE-lacZ RA-reporter transgene expression (Sandell et al., 2007; Zhao et al., 2009). However, the sensitivity of RARE-lacZ has been questioned. To quantify the sensitivity of RARE-lacZ, we cultured wild-type and Rdh10 E8.5 embryos in a series of low RA concentrations. Treatment of Rdh10 mutants with as little as 0.25 nM induced RARE-lacZ in lateral plate mesoderm, intermediate mesoderm, somites, and neural tube (n = 2/2), whereas treatment with 1 nM or 2.5 nM showed additional RARE-lacZ induction in the head (n = 3/3) (Figure 3; Figure S3). The RA activity we detect at 0.25 nM is at the level of the dissociation constant (kD) of RA with the three RA receptors (0.2–0.7 nM) (Allenby et al., 1993), demonstrating very high sensitivity of the transgene. In comparison, the endogenous RA concentration in mouse E10.5 proximal hindlimbs is reported to be 30 nM (Horton and Maden, 1995). Control Rdh10 mutant embryos displayed no RARE-lacZ activity in the lateral plate mesoderm, typical of uncultured mutants (Figure 3; Figure S3). This demonstration of substantial (if not complete) loss of limb field RA signaling in Rdh10 mutants, which we additionally nullified with an RAR antagonist (Figure 1), would be expected to yield significant observable reductions of Meis1/2 expression if RA was an endogenous proximal signal in limb proximodistal patterning.
Figure 3. The RARE-lacZ Reporter Transgene Is Sensitive to RA Signaling Down to 0.25 nM RA.
Wild-type (WT) and Rdh10 mutant (Rdh10 mut) E8.5 embryos stained with X-gal to detect RARE-lacZ RA signaling activity following culture for 12 hr in DMEM/F-12 medium (control) or DMEM/F-12 plus 0.25 nM, 1 nM, or 2.5 nM RA. Neural (n) and lateral plate mesoderm (lpm) expression is indicated. See also Figure S3.
Defective Forelimb Initiation in Rdh10 Mutants Relative to Loss of RA-FGF Antagonism in the Adjacent Second Heart Field
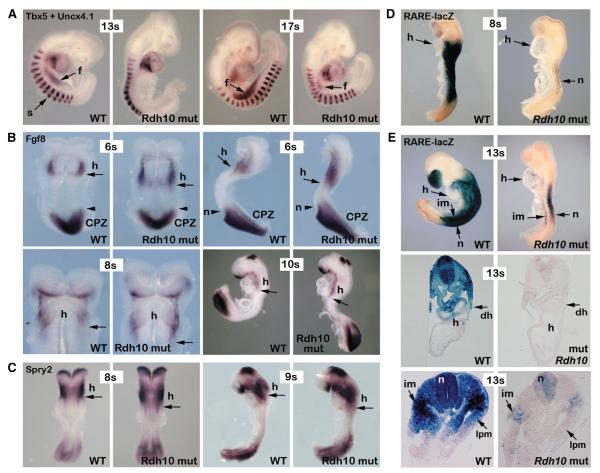
Next, we turned to the investigation of forelimb hypoplasticity reported for Rdh10 mutants (Sandell et al., 2007). Tbx5 expression in lateral plate mesoderm of the forelimb field, normally induced at the eight-somite stage and essential for forelimb specification (Agarwal et al., 2003), totally fails in Raldh2−/− mutants that display no sign of forelimb initiation (Zhao et al., 2009). Here, Rdh10 mutants at 10–15 somites similarly displayed no forelimb Tbx5 expression (Figure 4A; n = 5/5). However, at 16–19 somites we did observe Tbx5 induction (Figure 4A; n = 4/4). Furthermore, in Rdh10 mutants, the anterior-posterior length of the Tbx5 domain was greatly reduced relative to somite position (Figure 4A). Since Tbx5 demarcates the domain destined for Fgf10 upregulation and forelimb budding (Agarwal et al., 2003), the observed reduction in Tbx5 expression correlates with the consequent stunted forelimb, which is reduced in size in terms of both its anteroposterior and proximodistal length. We suggest this reduction in anteroposterior length precludes and impedes sufficient proximodistal growth and patterning due to the inherently smaller AER. Similarly, TBX5 haploinsufficiency in humans (Holt-Oram syndrome) causes both forearm shortening and subsequent patterning defects, and left-right forelimb asymmetry (left smaller than right) is observed in Holt-Oram individuals (Bruneau et al., 2001) and here in mouse Rdh10 mutants (n = 17/20; also see Figure S1). Therefore, the observed Tbx5 deficiency in Rdh10 mutants is a sufficient and likely explanation for the stunted forelimb and consequential patterning defects.
Figure 4. Spatiotemporal Tbx5 Deficiency Is Concomitant with Expanded Heart FGF Signaling and Reduced Heart RA Signaling.
(A–C) In situ hybridization at E8.5 in wild-type (WT) and Rdh10 mutant (Rdh10 mut) embryos (lateral view unless stated).
(A) Shown is Tbx5 expression in the presumptive forelimb field (f) in 13-somite (13s) and 17-somite (17s) embryos; embryos were costained for Uncx4.1 expression, which labels somites as previously described (Zhao et al., 2009).
(B) Fgf8 expression preceding and during normal Tbx5 initiation at six-somite (6s; dorsal and lateral views), eight-somite (8s; ventral view), and ten-somite (10s) stages. Arrows represent the posterior boundary of the heart (h); arrowheads represent the anterior boundary of the caudal progenitor zone (CPZ) and node (n).
(C) Spry2 expression during normal Tbx5 initiation at 8s (dorsal view) and nine-somite (9s) stages. Arrows represent the posterior boundary of the heart (h).
(D and E) Wild-type (WT) and Rdh10 mutant embryos stained with X-gal to detect RARE-lacZ RA signaling activity preceding and immediately following normal Tbx5 initiation at 6s and 13s stages. (E) Transverse sections through the heart (middle panels) and presumptive forelimb field (lower panels) are shown. Activity in heart (h), neural tube (n), intermediate mesoderm (im), dorsal heart (dh), and lateral plate mesoderm (lpm) is indicated.
We next examined FGF8 signaling in Rdh10 mutant embryos, since Raldh2−/− embryos that completely lack RA activity display two fronts of ectopic Fgf8 expression that encroach into the forelimb field, one emanating from the heart (Ryckebusch et al., 2008;Sirbu et al., 2008) and another from the caudal progenitor zone (CPZ) (Zhao et al., 2009). Immediately prior to Tbx5 induction and during early lateral plate Tbx5 expression (six-, eight-, and ten-somite stages), Rdh10 mutants displayed only a single front of ectopic Fgf8 expression, from the heart alone, which moved closer to the anterior portion of the forelimb domain (Figure 4B; n = 7/7). Rdh10 mutants from six somites onward retained a normal CPZ Fgf8 expression domain and anterior boundary relative to the node (Figure 4B; n = 7/7). Simultaneously, Spry2, a negative regulator of FGF signaling induced by FGF8-ERK activity (Minowada et al., 1999), was found to be expressed ectopically in the trunk and forelimb field posterior to the heart in eight-to nine-somite Rdh10 mutants (Figure 4C; n = 3/3). This intriguing difference in ectopic Fgf8 expansion between Raldh2−/− and Rdh10 mutants led us to more closely examine the distribution of RA activity at this time. Rdh10 mutant RARE-lacZ expression was initially restricted to the posterior neural tube at six- to seven-somite stages (and intermediate mesoderm at 10- to 15-somite stages) reaching down to the CPZ Fgf8 expression domain, but RARE-lacZ was absent from the dorsal heart where it is normally present (Figures 4D and 4E; n = 10/10). Thus, compared to Raldh2−/− embryos, Rdh10 mutants display only one front of ectopic Fgf8 expression (emanating from the heart) and concomitantly display a less severe disruption to forelimb Tbx5 expression (delayed and reduced versus absent).
RA Controls Forelimb Initiation and Second Heart Field Expansion Independently
Isl1, which is required for proliferation of cardiac progenitors, is a highly conserved marker of second heart field progenitors (Cai et al., 2003; Stolfi et al., 2010). FGF8 is required for Isl1 expression and second heart field expansion in mammals (Ilagan et al., 2006; Park et al., 2006), and FGF signaling also induces Isl1 in primitive chordates (Davidson et al., 2006). Rdh10 mutants, which exhibit ectopic cardiac Fgf8 expression as shown above, displayed ectopic posterior expansion of Isl1 extending from cardiac lateral plate mesoderm into the forelimb lateral plate domain (Figure 5A; n = 6/6). These results are similar to previously analyzed Raldh2−/− embryos that display ectopic cardiac Isl1 expression and a distended heart phenotype (Ryckebusch et al., 2008; Sirbu et al., 2008). To address the possibility that an enlarged cardiac domain caused by loss of RA signaling may influence the balance between cardiac and forelimb progenitors in the lateral plate mesoderm, as first suggested in studies on zebrafish raldh2 mutants (Keegan et al., 2005), we crossed Raldh2+/− mice with Isl1-nlacZ+/− knockin mice (Sun et al., 2007) to generate embryos that have lost both RA activity and Isl1. Raldh2−/− embryos were used in this experiment due to their total absence of Tbx5 expression in the forelimb field lateral plate mesoderm (Zhao et al., 2009) and the appearance of a distended heart phenotype (Ryckebusch et al., 2008; Sirbu et al., 2008). In Raldh2−/−;Isl1-nlacZ+/− embryos, we observed ectopic posterior expansion of lacZ expression (Figure 5B; Figure S4; n = 8/8) indicating that the Isl1-nlacZ knockin transgene recapitulates the ectopic expansion of Isl1 messenger RNA (mRNA) following loss of RA activity. Raldh2−/−;Isl1-nlacZ−/− embryos (that lack Isl1 function) displayed a loss of the distended heart phenotype observed in Raldh2−/− embryos (n = 5/5), but they did not display forelimb Tbx5 expression (n = 0/5), which was absent in Raldh2−/− embryos (n = 0/8) but maintained in Isl1-nlacZ−/− embryos (n = 6/6) (Figure 5C; Figure S5). Thus, while we provide evidence that the distended heart phenotype of Raldh2−/− embryos is dependent upon ectopic Isl1 expression downstream of ectopic Fgf8 expression, ectopic Fgf8 expression disrupts forelimb initiation through a pathway that does not require Isl1. As restriction of the heart field is insufficient to rescue forelimb initiation, our results genetically uncouple RA loss-of-function forelimb defects from second heart field expansion due to ectopic heart Fgf8 expression.
Figure 5. RA Controls Limb Tbx5 Independently of Heart Isl1.
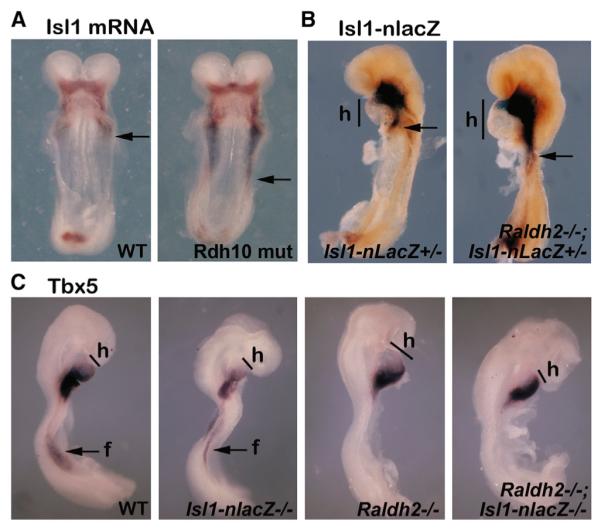
(A–C) E8.5 mouse embryos analyzed by in situ hybridization (A and C) or stained with X-gal to detect Isl1-nlacZ expression (B).
(A) Isl1 mRNA in Rdh10 mutant (Rdh10 mut) versus wild-type (WT) embryos (ventral view). Arrows represent the posterior boundary of the heart field.
(B) Isl1-nlacZ expression in Raldh2−/−;Isl1-nlacZ+/− versus Isl1-nlacZ+/− embryos. Arrows represent the posterior boundary of the heart field (h).
(C) Assessment of heart expansion (h) and forelimb field Tbx5 expression (f) in wild-type, Isl1-nlacZ−/−, Raldh2−/−, and Raldh2−/−;Isl1-nlacZ−/− embryos.
See also Figures S4 and S5.
RA-FGF Antagonism Is Required to Program the Forelimb Field
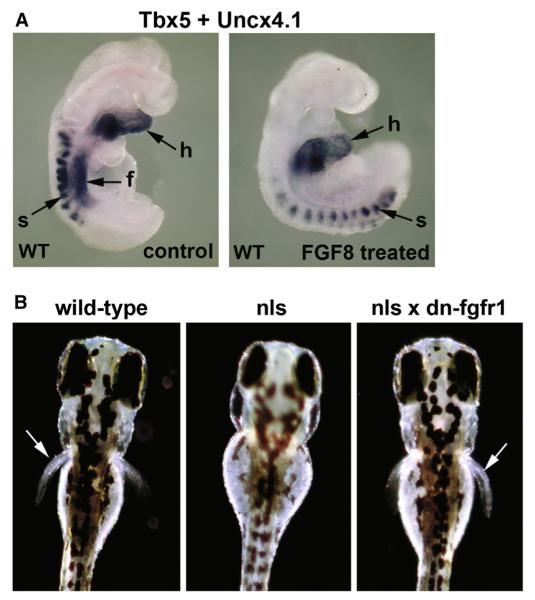
To test the sufficiency of FGF8 to inhibit Tbx5 induction in mouse, we cultured wild-type embryos in recombinant FGF8 protein during the period when Tbx5 expression normally initiates in the forelimb field (i.e., seven-somite embryos were cultured for 12 hr to generate 12-somite embryos). Control cultured embryos yielded normal Tbx5 induction in the forelimb field (n = 4/4), while those cultured with FGF8 (10 μg/ml) had no sign of a forelimb Tbx5 domain (n = 0/5), although heart Tbx5 expression was unaffected (n = 5/5) (Figure 6A). Culturing embryos in recombinant FGF10 protein (10 μg/ml) did not noticeably change limb Tbx5 expression (n = 6/6), revealing a distinction between FGF8 and FGF10 function (Figure S6). We postulate that in the absence of RA signaling, ectopic Fgf8 expression negatively impacts axial patterning of the forelimb field, resulting in loss of Tbx5 induction. Therefore, axial RA-FGF antagonism near the early heart has two distinct functions: one to restrict the second heart field via Isl1 repression, and another to permit correct axial patterning of forelimb field lateral plate mesoderm through a mechanism independent of Isl1.
Figure 6. RA-FGF Antagonism Is Required to Generate the Forelimb Field.
(A) Assessment of forelimb field Tbx5 expression (f) in control versus FGF8-treated wild-type (WT) embryos cultured in vitro for 12 hr. Heart Tbx5 mRNA (h) and somite Uncx4.1 mRNA (s) are also indicated.
(B) Rescue of missing pectoral fins in raldh2 (nls) mutant zebrafish by crossing to heat-shock-inducible dn-fgfr1 transgenic fish (heat shock at 37°C; 8–10 hpf). Arrows indicate pectoral fins.
See also Figure S6.
To more rigorously test the hypothesis that forelimb initiation requires axial RA-FGF antagonism, we performed genetic rescue studies on zebrafish raldh2 (nls) mutants lacking pectoral fins (forelimbs) (Begemann et al., 2001) crossed with hsp70:dn-frgr1-EGFP fish carrying a heat-shock-inducible dominant-negative FGF receptor transgene (Lee et al., 2005). Genetic abrogation of FGF signaling by heat shock resulted in pectoral fin rescue in raldh2 mutants (Figure 6B; n = 10/39). These findings complement our mouse data, underpinning a requirement for RA-FGF antagonism to allow normal forelimb initiation.
DISCUSSION
Antagonism between RA and FGF was postulated as a mechanism controlling limb proximodistal patterning based upon a pharmacological analysis of chick embryos. Those studies suggested that proximal RA signaling induces Meis1/2 in the proximal limb, thus opposing distal FGF signaling from the AER that represses Meis1/2 in the distal limb, establishing the border between the stylopod and zeugopod (Mercader et al., 2000). Using genetic RA loss-of-function studies, we provide convincing evidence that RA is not required for limb patterning and RA-FGF antagonism does not occur along the limb proximodistal axis as originally proposed. Our findings instead demonstrate that RA-FGF antagonism along the anteroposterior axis of the trunk lateral plate mesoderm prior to budding is required to permit correct spatiotemporal induction of Tbx5 for normal forelimb initiation (Figure 7). Thus, the gain-of-function studies performed in chick identified RA and FGF as important signaling agents for limb development, but the genetic loss-of-function studies reported here show that RA controls limb development in a much different manner than originally envisioned.
Figure 7. Model for Axial RA-FGF Antagonism prior to Limb Budding.

Previous genetic studies support FGF8 repression of Meis1/2 in distal limb elements, but our genetic findings show that RA signaling is not required for Meis1/2 expression in the proximal limb. Thus, RA-FGF antagonism is not required along the limb proximodistal axis to provide Meis1/2 expression proximally in the stylopod (blue) and prevent distal expression in the zeugopod (orange) and autopod (green). Instead, our studies support a model in which RA is required earlier for axial antagonization of Fgf8 expression (in both the second heart field and caudal progenitor zone -CPZ) prior to forelimb budding for correct induction and positioning of Tbx5 expression in the forelimb field.
The early pharmacological studies suggesting antagonism between RA and FGF along the proximodistal axis of the limb (Mercader et al., 2000) were followed by studies employing heterotopic transplantation of intact or recombinant chick limbs to further support a role for endogenous RA as a proximalizing signal for limb patterning (Cooper et al., 2011; Roselló-Díezet al., 2011). However, the ability of RA treatment to increase Meis1/2 could represent abnormal disruption to FGF signaling that is known to repress Meis1/2 in the distal limb (Mariani et al., 2008). Also, the apparent link between RA and limb pattern reprogramming in chick but not mouse may be explained by different experimental methodology in the use of RAR antagonists to eliminate RA signaling. In chick, high concentrations of RAR antagonists are typically used to soak beads prior to limb implantation. Beads soaked in very high millimolar concentrations of BMS493 (2 mg/ml = 6 mM) were used to demonstrate negative effects on Meis1 (Roselló-Díez et al., 2011), contrasting with our results from BMS493-treated mouse embryos. Local concentrations at the implantation site in chick were likely to be far higher than the 10 μM BMS493 sufficient to suppress RA signaling used in our study and others (Germain et al., 2002; Chen et al., 2007). BMS493 functions as an inverse agonist and actively recruits corepressors to RARs bound at retinoic acid response elements (RAREs), thus silencing nearby genes below basal levels (Germain et al., 2002). Recently, chromatin immunoprecipitation analysis using a pan-RAR antibody revealed 13,385 RAREs in the mouse genome (Moutier et al., 2012), but most are unlikely to be associated with endogenous RA signaling. It is possible that very high RAR-antagonist concentrations silence numerous genes in the vicinity of these RAREs, including genes not normally regulated by RA.
Our findings demonstrate that both the initial expression of Meis1 and Meis2 in lateral plate mesoderm and their subsequent expression in the proximal limb bud do not require RA signaling. This indicates that some other mechanism is responsible for induction of trunk and proximal limb Meis1/2 expression. Downregulation of Meis1/2 expression in the distal limb requires FGF8 signaling derived from the AER, driving more distal fates (Mariani et al., 2008). We suggest that since AER Fgf8 expression appears after limb bud formation (Crossley et al., 1996), the proximal-most limb domain is out of range of early AER FGF signals, leading to maintenance of proximal Meis1/2 while AER FGF activity restricts Meis1/2 expression as distal elements are formed. Autonomous mechanisms may also play a part in specifying Meis1/2-negative distal domains. Autonomous collinear expression along the Hoxd cluster has been shown to be evident in the developing limb via progressive shifting of individual Hox genes from association with transcriptionally silent to transcriptionally active cis-regulatory elements (Noordermeer et al., 2011). Considering the evidence against RA as a proximal signal in mouse and the general conservation of RA signaling components among vertebrate embryos (i.e., expression of synthetic enzymes, degradative enzymes, receptors, binding proteins), we favor a single diffusible signal model for proximodistal patterning of vertebrate embryos with distal Fgf8 expression guiding autonomous mechanisms, much like how posterior Shh expression acts across the anteroposterior axis (Litingtung et al., 2002).
A key hypothesis made from chick studies speculates that departure from an RA signal and exposure to an FGF signal provide the patterning cue for distal limb fate specification (Cooper et al., 2011; Roselló-Díez et al., 2011). While freedom from RA is indeed necessary for normal distalization of limb mesenchyme, we show here that RA cannot provide an endogenous proximal cue since Meis1/2 and limb patterning are not RA dependent. The presence of RA in the proximal limb is thus an unnecessary consequence of diffusion from the lateral plate mesoderm where RA action is needed, but RA is prevented from entering the distal limb bud by RA degradation controlled by distal limb expression of Cyp26b1 (Yashiro et al., 2004). Another study utilizing RA-treated chick limbs displaying loss of AER Fgf8 expression suggested that clearance of RA in the distal limb by Cyp26b1 (which is induced by Fgf8) may be needed to achieve normal distal Fgf8 expression in the AER (Probst et al., 2011). However, our previous studies on Rdh10 mutants demonstrated that loss of limb RA activity does not expand distal limb Fgf8 expression (Cunningham et al., 2011a), indicating that RA-FGF antagonism is not required along the limb proximodistal axis to control AER Fgf8 expression. Our data are consistent with the idea that Cyp26b1 functions to prevent RA from interfering with FGF-driven proximodistal patterning and growth (downstream effects of Fgf8) rather than instructively driving a proximal fate via RA compartmentalization to control either Meis1/2 or Fgf8 limb expression.
Instead of RA-FGF antagonism occurring along the limb proximodistal axis after budding has occurred, our findings demonstrate that axial RA-FGF antagonism in the trunk prior to budding helps program the forelimb field to permit induction of Tbx5. This action of RA prevents the appearance of FGF8 signaling in the region fated for the forelimb field and sets the stage for proper proximodistal patterning at a later point when distal limb FGF8 signaling initiates in the AER. Our studies demonstrate that models for limb patterning do not need to invoke proximodistal RA-FGF antagonism, but that models for forelimb initiation need to incorporate axial RA-FGF antagonism to more fully understand how lateral plate mesoderm progenitors exiting the primitive streak achieve a forelimb fate. The lack of an RA requirement for hindlimb initiation is likely related to the observation that the RA requirement for caudal repression of Fgf8 during body axis extension ends between E8.5 and E9.5 when the primitive streak has regressed (Cunningham et al., 2011b), thus prior to hindlimb initiation, which occurs at E9.5. Therefore, the role of RA in forelimb development is intimately intertwined with its role in controlling the late aspects of gastrulation (Duester, 2008). These findings advance our understanding of the regulatory logic that drives limb development, providing a basis to understand how limb deformities arise and how they may be treated or prevented.
EXPERIMENTAL PROCEDURES
Mutant Models
Raldh2−/− mice (germ-line null) have been described previously (Zhao et al., 2009). Genotyping was performed by PCR analysis of yolk sac DNA. Rdh10trex/trex mice (Rdh10 mutants) generated via ethylnitrosourea mutagenesis have also been reported previously (Sandell et al., 2007). Mutants were identified by DNA sequencing of a PCR product overlapping the mutation from analysis of yolk sac DNA. Mice carrying the RARE-lacZ RA-reporter transgene (Rossant et al., 1991) were crossed with Raldh2−/− and Rdh10 mutants. Isl1-nlacZ knockin mice, which express lacZ under control of the Isl1 promoter and exhibit a homozygous null phenotype, were genotyped by PCR analysis of yolk sac DNA (Sun et al., 2007). Zebrafish raldh2 mutants (nls) have been reported previously (Begemann et al., 2001), and zebrafish carrying the hsp70:dn-frgr1-EGFP transgene expressing a heat-shock-inducible dominant-negative FGF receptor have been described elsewhere (Lee et al., 2005). All mouse and fish studies conformed to the regulatory standards adopted by the Animal Research Committee at the Sanford-Burnham Medical Research Institute.
Mouse Embryo In Vitro Culture and Treatment
Wild-type, Rdh10 mutant, and Raldh2−/− embryos at the six- to nine-somite stages or at E10.5 were cultured for 12 hr in serum-free (retinoid-free) Dulbecco’s modified Eagle’s medium with nutrient mixture F-12 (DMEM/F-12) culture media (GIBCO-Life Technologies) in Millicell culture plate inserts (Millipore) at 37°C in 5% CO2. Retinoid treatments included all-trans-retinoic acid or BMS493 (both from Sigma Chemical) dissolved in DMSO vehicle, which was administered as a control. FGF treatments included FGF8 (eBio-science) or FGF10 (R&D Systems). After culture, embryos were processed for whole-mount in situ hybridization or stained with X-gal as described below.
Detection of RA and mRNA
RA activity was detected using mouse embryos carrying the RARE-lacZ transgene in which β-galactosidase, encoded by lacZ, is under the transcriptional control of a RARE; β-galactosidase activity was detected in embryos by staining 18 hr with X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) as previously described (Rossant et al., 1991). Detection of mRNA was performed by whole-mount in situ hybridization as previously described (Cunningham et al., 2011a). For tissue sectioning, stained embryos were incubated in a gelatin-BSA solution (0.5% gelatin, 30% BSA, 20% sucrose in 1 × PBS; Sigma) for 1 hr and then embedded in fresh gelatin-BSA solution polymerized with 1.75% glutaraldehyde and sectioned at 30 μm with a vibratome.
Zebrafish Studies
Homozygous raldh2 mutant zebrafish do not develop pectoral fins (Begemann et al., 2001). Heterozygous raldh2 mutants were crossed with fish carrying the hsp70:dn-frgr1-EGFP transgene, which after heat shock expresses a dominant-negative form of the FGFR1 receptor (Lee et al., 2005). Crosses of heterozygous raldh2;hsp70:dn-frgr1-EGFP fish to heterozygous raldh2 mutants generated embryos that were either heat shocked at 37°C for 2 hr prior to pectoral fin development or not subject to heat shock. Heat shock for 2 hr from 8–10 hr postfertilization resulted in 180 embryos carrying hsp70:dn-frgr1-EGFP (identified by enhanced GFP [EGFP] fluorescence) that were then examined at 3 days old. PCR genotyping identified 39 homozygous raldh2 mutants and 141 fish that were either wild-type or heterozygous for the raldh2 mutation. Among the 39 homozygous raldh2 mutants carrying hsp70:dn-frgr1-EGFP, we found that about 25% displayed pectoral fins (n = 10/39). Similar analysis of control embryos (those not heat shocked or those heat shocked but not carrying hsp70:dn-frgr1-EGFP) demonstrated that all homozygous raldh2 mutants failed to develop pectoral fins.
Supplementary Material
ACKNOWLEDGMENTS
We thank V. Prince for providing zebrafish raldh2 mutants, K. Poss and P.D.S. Dong for providing zebrafish carrying the hsp70:dn-frgr1-EGFP transgene, and J. Rossant for providing RARE-lacZ mice. We also thank the following for mouse complementary DNAs used to prepare in situ hybridization probes: V. Giguere (RARb), P. Gruss (Meis2), J.C. Izpisua-Belmonte (Meis1), A. Mansouri (Uncx4.1), G. Martin (Fgf8, Spry2), P. Ruiz-Lozano (Isl1), and V. Papaioannou (Tbx5). We gratefully acknowledge the SBMRI Animal Facility and Zebrafish Facility for timed matings and animal care. This work was funded by National Institutes of Health grants GM062848 (G.D.) and DE016082 (P.T.) and by the Stowers Institute for Medical Research (P.T). T.J.C., X.Z., and G.D. designed the study, analyzed the data, and wrote the paper. T.J.C. and X.Z. performed all the experiments. L.L.S., S.M.E., and P.A.T. provided valuable reagents for the study. All authors discussed the results and commented on the manuscript.
Footnotes
Supplemental Information includes six figures and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2013.03.036.
REFERENCES
- Agarwal P, Wylie JN, Galceran J, Arkhitko O, Li C, Deng C, Grosschedl R, Bruneau BG. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development. 2003;130:623–633. doi: 10.1242/dev.00191. [DOI] [PubMed] [Google Scholar]
- Allenby G, Bocquel M-T, Saunders M, Kazmer S, Speck J, Rosenberger M, Lovey A, Kastner P, Grippo JF, Chambon P, et al. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc. Natl. Acad. Sci. USA. 1993;90:30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begemann G, Schilling TF, Rauch GJ, Geisler R, Ingham PW. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development. 2001;128:3081–3094. doi: 10.1242/dev.128.16.3081. [DOI] [PubMed] [Google Scholar]
- Bruneau BG, Nemer G, Schmitt JP, Charron F, Robitaille L, Caron S, Conner DA, Gessler M, Nemer M, Seidman CE, Seidman JG. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106:709–721. doi: 10.1016/s0092-8674(01)00493-7. [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Desai TJ, Qian J, Niederreither K, Lü J, Cardoso WV. Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development. 2007;134:2969–2979. doi: 10.1242/dev.006221. [DOI] [PubMed] [Google Scholar]
- Cooper KL, Hu JK, ten Berge D, Fernandez-Teran M, Ros MA, Tabin CJ. Initiation of proximal-distal patterning in the vertebrate limb by signals and growth. Science. 2011;332:1083–1086. doi: 10.1126/science.1199499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley PH, Minowada G, MacArthur CA, Martin GR. Roles for FGF8 in the induction, initiation, and maintenance of chick limb development. Cell. 1996;84:127–136. doi: 10.1016/s0092-8674(00)80999-x. [DOI] [PubMed] [Google Scholar]
- Cunningham TJ, Chatzi C, Sandell LL, Trainor PA, Duester G. Rdh10 mutants deficient in limb field retinoic acid signaling exhibit normal limb patterning but display interdigital webbing. Dev. Dyn. 2011a;240:1142–1150. doi: 10.1002/dvdy.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham TJ, Zhao X, Duester G. Uncoupling of retinoic acid signaling from tailbud development before termination of body axis extension. Genesis. 2011b;49:776–783. doi: 10.1002/dvg.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B, Shi W, Beh J, Christiaen L, Levine M. FGF signaling delineates the cardiac progenitor field in the simple chordate, Ciona intestinalis. Genes Dev. 2006;20:2728–2738. doi: 10.1101/gad.1467706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain P, Iyer J, Zechel C, Gronemeyer H. Co-regulator recruitment and the mechanism of retinoic acid receptor synergy. Nature. 2002;415:187–192. doi: 10.1038/415187a. [DOI] [PubMed] [Google Scholar]
- Grandel H, Lun K, Rauch GJ, Rhinn M, Piotrowski T, Houart C, Sordino P, Küchler AM, Schulte-Merker S, Geisler R, et al. Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development. 2002;129:2851–2865. doi: 10.1242/dev.129.12.2851. [DOI] [PubMed] [Google Scholar]
- Horton C, Maden M. Endogenous distribution of retinoids during normal development and teratogenesis in the mouse embryo. Dev. Dyn. 1995;202:312–323. doi: 10.1002/aja.1002020310. [DOI] [PubMed] [Google Scholar]
- Ilagan R, Abu-Issa R, Brown D, Yang YP, Jiao K, Schwartz RJ, Klingensmith J, Meyers EN. Fgf8 is required for anterior heart field development. Development. 2006;133:2435–2445. doi: 10.1242/dev.02408. [DOI] [PubMed] [Google Scholar]
- Keegan BR, Feldman JL, Begemann G, Ingham PW, Yelon D. Retinoic acid signaling restricts the cardiac progenitor pool. Science. 2005;307:247–249. doi: 10.1126/science.1101573. [DOI] [PubMed] [Google Scholar]
- Lee Y, Grill S, Sanchez A, Murphy-Ryan M, Poss KD. Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development. 2005;132:5173–5183. doi: 10.1242/dev.02101. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Dahn RD, Li Y, Fallon JF, Chiang C. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature. 2002;418:979–983. doi: 10.1038/nature01033. [DOI] [PubMed] [Google Scholar]
- Mariani FV, Ahn CP, Martin GR. Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature. 2008;453:401–405. doi: 10.1038/nature06876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn C, Ruberte E, LeMeur M, Morriss-Kay G, Chambon P. Developmental analysis of the retinoic acid-inducible RAR-β 2 promoter in transgenic animals. Development. 1991;113:723–734. doi: 10.1242/dev.113.3.723. [DOI] [PubMed] [Google Scholar]
- Mercader N, Leonardo E, Piedra ME, Martínez-A C, Ros MA, Torres M. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development. 2000;127:3961–3970. doi: 10.1242/dev.127.18.3961. [DOI] [PubMed] [Google Scholar]
- Mercader N, Leonardo E, Azpiazu N, Serrano A, Morata G, Martínez C, Torres M. Conserved regulation of proximodistal limb axis development by Meis1/Hth. Nature. 1999;402:425–429. doi: 10.1038/46580. [DOI] [PubMed] [Google Scholar]
- Minowada G, Jarvis LA, Chi CL, Neubüser A, Sun X, Hacohen N, Krasnow MA, Martin GR. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–4475. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- Moutier E, Ye T, Choukrallah MA, Urban S, Osz J, Chatagnon A, Delacroix L, Langer D, Rochel N, Moras D, et al. Retinoic acid receptors recognize the mouse genome through binding elements with diverse spacing and topology. J. Biol. Chem. 2012;287:26328–26341. doi: 10.1074/jbc.M112.361790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noji S, Nohno T, Koyama E, Muto K, Ohyama K, Aoki Y, Tamura K, Ohsugi K, Ide H, Taniguchi S, et al. Retinoic acid induces polarizing activity but is unlikely to be a morphogen in the chick limb bud. Nature. 1991;350:83–86. doi: 10.1038/350083a0. [DOI] [PubMed] [Google Scholar]
- Noordermeer D, Leleu M, Splinter E, Rougemont J, De Laat W, Duboule D. The dynamic architecture of Hox gene clusters. Science. 2011;334:222–225. doi: 10.1126/science.1207194. [DOI] [PubMed] [Google Scholar]
- Park EJ, Ogden LA, Talbot A, Evans S, Cai CL, Black BL, Frank DU, Moon AM. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development. 2006;133:2419–2433. doi: 10.1242/dev.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst S, Kraemer C, Demougin P, Sheth R, Martin GR, Shiratori H, Hamada H, Iber D, Zeller R, Zuniga A. SHH propagates distal limb bud development by enhancing CYP26B1-mediated retinoic acid clearance via AER-FGF signalling. Development. 2011;138:1913–1923. doi: 10.1242/dev.063966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz AH, Vokes SA. Integration of the transcriptional networks regulating limb morphogenesis. Dev. Biol. 2012;368:165–180. doi: 10.1016/j.ydbio.2012.05.035. [DOI] [PubMed] [Google Scholar]
- Rhinn M, Schuhbaur B, Niederreither K, Dollé P. Involvement of retinol dehydrogenase 10 in embryonic patterning and rescue of its loss of function by maternal retinaldehyde treatment. Proc. Natl. Acad. Sci. USA. 2011;108:16687–16692. doi: 10.1073/pnas.1103877108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselló-Díez A, Ros MA, Torres M. Diffusible signals, not autonomous mechanisms, determine the main proximodistal limb subdivision. Science. 2011;332:1086–1088. doi: 10.1126/science.1199489. [DOI] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Ryckebusch L, Wang Z, Bertrand N, Lin S-C, Chi X, Schwartz R, Zaffran S, Niederreither K. Retinoic acid deficiency alters second heart field formation. Proc. Natl. Acad. Sci. USA. 2008;105:2913–2918. doi: 10.1073/pnas.0712344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell LL, Sanderson BW, Moiseyev G, Johnson T, Mushegian A, Young K, Rey JP, Ma JX, Staehling-Hampton K, Trainor PA. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 2007;21:1113–1124. doi: 10.1101/gad.1533407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell LL, Lynn ML, Inman KE, McDowell W, Trainor PA. RDH10 oxidation of Vitamin A is a critical control step in synthesis of retinoic acid during mouse embryogenesis. PLoS ONE. 2012;7:e30698. doi: 10.1371/journal.pone.0030698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu IO, Zhao X, Duester G. Retinoic acid controls heart anteroposterior patterning by down-regulating Isl1 through the Fgf8 pathway. Dev. Dyn. 2008;237:1627–1635. doi: 10.1002/dvdy.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolfi A, Gainous TB, Young JJ, Mori A, Levine M, Christiaen L. Early chordate origins of the vertebrate second heart field. Science. 2010;329:565–568. doi: 10.1126/science.1190181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Liang X, Najafi N, Cass M, Lin L, Cai CL, Chen J, Evans SM. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev. Biol. 2007;304:286–296. doi: 10.1016/j.ydbio.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin C, Wolpert L. Rethinking the proximodistal axis of the vertebrate limb in the molecular era. Genes Dev. 2007;21:1433–1442. doi: 10.1101/gad.1547407. [DOI] [PubMed] [Google Scholar]
- Tickle C, Alberts BM, Wolpert L, Lee J. Local application of retinoic acid to the limb bond mimics the action of the polarizing region. Nature. 1982;296:564–566. doi: 10.1038/296564a0. [DOI] [PubMed] [Google Scholar]
- Wanek N, Gardiner DM, Muneoka K, Bryant SV. Conversion by retinoic acid of anterior cells into ZPA cells in the chick wing bud. Nature. 1991;350:81–83. doi: 10.1038/350081a0. [DOI] [PubMed] [Google Scholar]
- Wendling O, Dennefeld C, Chambon P, Mark M. Retinoid signaling is essential for patterning the endoderm of the third and fourth pharyngeal arches. Development. 2000;127:1553–1562. doi: 10.1242/dev.127.8.1553. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Zhao X, Uehara M, Yamashita K, Nishijima M, Nishino J, Saijoh Y, Sakai Y, Hamada H. Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing mouse limb. Dev. Cell. 2004;6:411–422. doi: 10.1016/s1534-5807(04)00062-0. [DOI] [PubMed] [Google Scholar]
- Zhao X, Sirbu IO, Mic FA, Molotkova N, Molotkov A, Kumar S, Duester G. Retinoic acid promotes limb induction through effects on body axis extension but is unnecessary for limb patterning. Curr. Biol. 2009;19:1050–1057. doi: 10.1016/j.cub.2009.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.