Abstract
Background/Aim:
Bleeding from Gastric Varices (GV) is not only life threatening, but also leads to many hospitalizations, contributes to morbidity and is resource intensive. GV are difficult to diagnose and their treatment can be challenging due to their location and complex structure. To assess the safety and efficacy of endoscopic gastric fundal variceal gluing using periodic endoscopic injections of N-butyl-2-cyanoacylate (NBCA) and to assess the utility of endoscopic ultrasound (EUS) in assessing for the eradication of GV post-NBCA treatment.
Materials and Methods:
Analysis of prospectively collected data of a cohort of patients with GV who underwent periodic endoscopic variceal gluing from 2005 to 2011. Outcomes included success of GV obliteration, incidence of rebleeding, complications from the procedure, and analysis of factors that might predict GV rebleeding. The success of GV eradication was assessed by both EUS and direct endoscopy.
Results:
The cohort consisted of 29 consecutive patients that had undergone NBCA injection for GV. The mean age was 60.8 years standard deviations (SD 13.3, range 20-81). The average follow-up was 28 months (SD 19.61, range 1-64) and the most common cause for GV was alcoholic liver cirrhosis (34.48%). A total of 91 sessions of NBCA injections were carried out for 29 patients (average of 3.14 sessions/patient, SD 1.79, range 1-8) with a total of 124 injections applied (average of 4.28 injections/patient, SD 3.09, range 1-13). 24 patients were treated for previously documented GV bleeding while five were treated for primary prevention. Overall, 79% of patients were free of rebleeding once three sessions of histoacryl® injection were completed. None of the patients treated for primary prevention developed bleeding during follow-up. 11 of the 24 patients (46%) with previous bleeding however had rebleeding. 4/11 (36%) patients had GV rebleeding while awaiting scheduled additional NBCA sessions. 19/29 (60%) patients had complete eradication of GV, 11/19 (58%) documented by endoscopic assessment alone, 4/19 (21%) by EUS alone and 4/19 (21%) by both techniques. Two of the 11 (18%) patients that had rebleeding had recurrence of GV bleeding after documented eradication by EUS compared to 5/11 (45%) patients documented eradication by endoscopic assessment and 2/11 (18%) patients that had rebleeding after documented eradication by both modalities. Twenty five patients in total had documented residual GV by EUS (14, 56%), direct endoscopic assessment (18, 72%) or both modalities (9, 36%), two of which developed recurrent bleeding (13%). No immediate or long-term complications of NBCA injection occurred, nor any related endoscopic complications were reported in any of these cases during the time of follow-up.
Conclusion:
NBCA injection of GV is a safe and successful therapeutic intervention. A minimum of three endoscopic sessions is required to significantly decrease the risk of bleeding/rebleeding. In this small sample of patients, neither EUS nor direct endoscopic assessment was reliable in predicting the recurrence of GV bleeding.
Keywords: Cyanoacrylate injection, gastric varices, rebleeding
Gastric fundal varices (GV) are seen in 16-70% of patients with portal hypertension and are responsible for up to 10-15% of all variceal bleeding.[1] The outcome of treating esophageal varices (EV) has improved dramatically due to therapeutic advances, such as endoscopic injection sclerotherapy (EIS) and more recently, endoscopic variceal ligation (EVL). Undiluted N-Butyl-2-cyanoacylate (NBCA) injection has also been used to treat EV with varying results.[2] In contrast, different treatment methods are still being considered for the management of GV and the modality chosen differs between primary prevention and secondary prevention. Therapeutic options are mainly dependent on the individual endoscopist and differ between institutions.[3,4] Endoscopic injection of NBCA is thought to be relatively safe and is the treatment of choice for the control of active bleeding of GV[5] and prevention of rebleeding,[6,7] but its use has not been studied in a randomized clinical setting for primary prevention and therefore, is not currently recommended for primary prevention of GV bleeding.[8] As the 1-year risk of bleeding of GV in a patient with Child C cirrhosis with red marks visualized endoscopically on a large fundal varix is estimated at 65%, treatment of GV with such high-risk features is logical. As such, prophylactic glue therapy is widely practiced for high-risk GV i.e., varices > 1 cm with either red signs or advanced liver disease (Child B or C).[9] The recommended treatment of choice for primary prevention of GV bleeding remains medical treatment using beta-adrenergic blockers.[10]
No clear definition exists that describes GV obliteration following endoscopic treatment. Clinicians and endoscopists seldom rely on rebleeding and the endoscopic appearance of GV post-glue injection to decide whether or not to proceed with further sessions of gluing. Over the past decade, endoscopic ultrasound (EUS) has been used for many diagnostic and therapeutic interventions.[11,12] Its use has expanded to include management of EV and GV.[13,14,15,16,17] Furthermore, EUS has been used to predict rebleeding in EV but to this date, there are limited data that examine the use of EUS in assessing eradication and predicting re/bleeding of GV, particularly after treatment with NBCA injection.[18] The aim of this study is to examine the long-term safety and efficacy of gastric fundal variceal gluing in both the primary and secondary prevention setting using periodic endoscopic injections of NBCA as well as to assess the use of EUS in assessing GV post-NBCA treatment to help determine the need for further injection therapy and determine the risk of GV rebleeding.
MATERIALS AND METHODS
All consecutive patients who underwent periodic endoscopic injection of undiluted NBCA for either primary or secondary prevention of GV bleeding at the London Health Sciences Centre between January 2005 and November 2011 were prospectively included in this study. All out-patient clinic notes, endoscopic reports and emergency room records were retrieved at the time of data collection and analyzed retrospectively. Laboratory and radiological investigations were similarly reviewed and analyzed. A standard data collection sheet was used and followed by data entry into a computerized database for further statistical analysis. Statistical analysis was subsequently performed to identify predictors of GV bleeding/rebleeding. All patients provided informed consent to undergo the endoscopic procedures and for clinical data collection.
Procedure
As the best way to titrate injection volume to the size of GV during routine NBCA injection remains unclear, the following technique has been developed and adopted as a standard method based on our institute's own clinical experience.[19] All patients provided informed consent for endoscopic injection of NBCA. Specific complications of the procedure and for NBCA specifically were detailed. After topical oro-pharyngeal xylocaine spray, patients received conscious sedation with a combination of Midazolam and Fentanyl. Blood pressure, heart rate, and oxygenation were carefully monitored. A single channel gastroscope was then inserted. GV were visualized directly and typically in the retroflexed position. Prior to histoacryl® NBCA injection, the single channel gastroscope was primed with 3 mL of Lipiodol separately. A hemostasis catheter 200 cm in length with a 23-gauge needle was also primed with 3 cc of Lipiodol. The catheter was inserted through the primed working channel of the gastroscope until its tip is visualized approximately 4 cm from the working channel exit. A mixture of 1 cc of histoacryl® and 0.5 cc Lipiodol was then introduced into the hemostasis catheter. The hemostasis catheter was then advanced into the target gastric varix. Following this, the needle was deployed and a 3 cc Lipiodol push resulting in a single intra-variceal injection of 3 cc volume, comprised of a 33% concentration of histoacryl® [Figure 1]. The catheter needle was then withdrawn from the varix and any bleeding (flashback) was visualized and timed. If visualization allowed, a second and/or a third injection of a similar concentration and volume were applied to adjacent GV. Throughout the procedure, endoscopic suction is disabled. After completion, the catheter was kept visible several centimeters beyond the exit of the gastroscope-working channel. The gastroscope was then anti-flexed and removed with subsequent careful removal of the catheter via the exit channel. Depending on the number and size of GV as well as the measured time of bleeding or flash back from the GV puncture site, individuals would be scheduled for repeat GV histoacryl® injection typically in 4-6 weeks’ time intervals.
Figure 1.
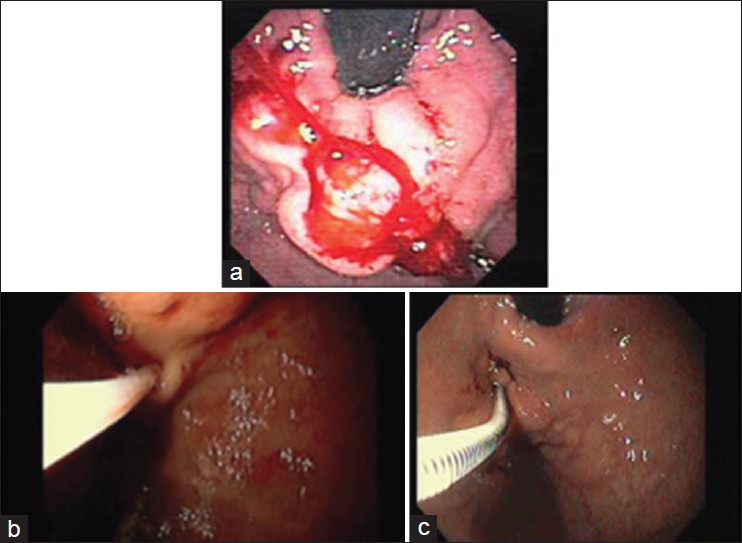
(a) Gastric fundal varices seen in retroflex view (b,c) Endoscopic view of cyanoacrylate injection
Follow-up
All patients were seen and assessed during scheduled endoscopy unit visits for treatment. Symptoms of gastrointestinal (GI) bleeding, adverse effects of the procedure or specific effects from NBCA injection were recorded at each visit. Patients were subsequently referred for EUS assessment after either documented eradication (defined as absence or minimal flashback of blood following injection of NBCA into the main varices) or completion of a total of three gluing sessions to confirm eradication and decide whether or not to proceed with further sessions of NBCA injection. This was mainly decided by the treating endoscopist. During the EUS assessment, a radial echo endoscope with 7.5 MHz frequency was inserted into an empty stomach. The balloon was partially inflated and care was taken not to fully inflate the balloon as this might result in compressing a small varix. The areas of the stomach where GV were observed endoscopically were then carefully examined. Special attention was also given to the fundus and body of the stomach. GV were identified as round or tubular anechoic structures arising from the lamina propria or from the sub-mucosa and color Doppler was used to detect blood flow and confirm vascularity. Persistent blood flow detected by color Doppler in sub-mucosal feeding vessels with an intraluminal clot present with no further blood flow superficially into the gastric wall was considered an obliterated GV. Persistent flow in sub-mucosal feeding vessels with no clot or evidence of blood flow on color Doppler in the superficial vessel at the gastric wall was considered as patent GV. Consequently, patients with persistent blood flow on EUS received additional sessions of NBCA injection.
Outcome measures
The primary outcomes were the rates of bleeding/rebleeding for both primary and secondary prevention and the rates of both immediate and long-term adverse events of NBCA injections. The secondary outcomes were to identify, which endoscopic method could accurately determine GV obliteration as well as the need for either a shunt procedure (trans-jugular intrahepatic porto-systemic shunt [TIPS]) or surgical shunt or orthotopic liver transplantation (LT) to control recurrent bleeding.
Statistical analysis
Descriptive statistics were computed for continuous variables, means, standard deviations (SD) and minimum and maximum values were used; for categorical variables frequencies were used. Univariable logistic regression was used to examine the association between independent variables and bleeding/rebleeding. Odds ratios (OR) and 95% confidence intervals (CI) were estimated. Multivariable logistic regression would be performed if the number of variables available permits. STATA 11.2 (Stata Corp, Texas, USA) was used in our analysis. A P value of < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
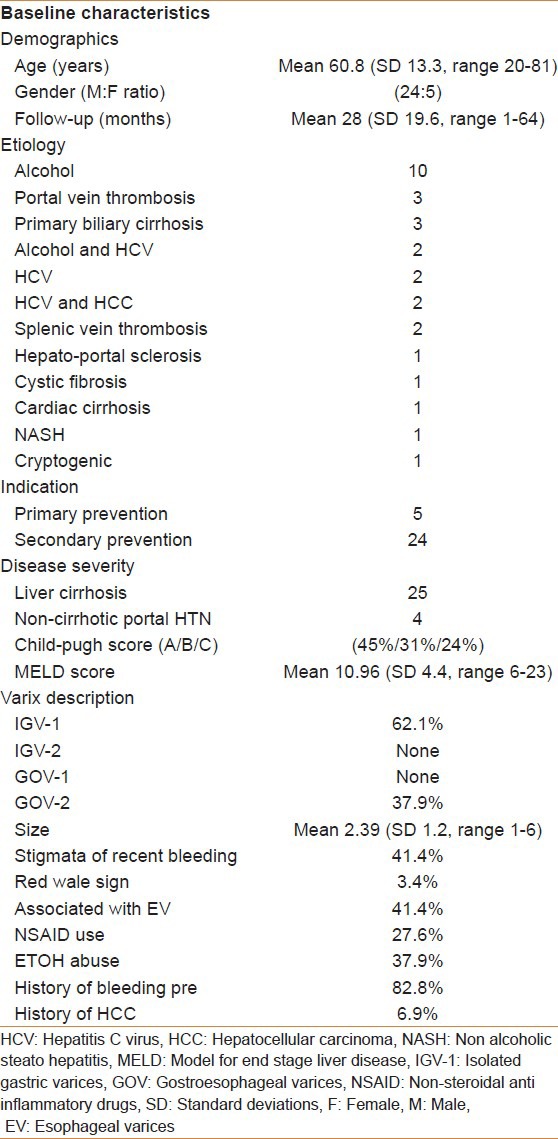
A total of 29 patients were included in the study with a mean age of 60.8 years (SD 13.3, range 20-81). The majority of patients were males 79.3% (95% CI, 63.6-95%). The average follow-up was 28 months (SD 19.61, range 1-64). Portal hypertension was secondary to liver cirrhosis in 25 (86%) and the remaining had non-cirrhotic portal hypertension. The most common cause for GV was alcoholic liver cirrhosis (34.48%) followed by portal vein thrombosis (10%) and primary biliary cirrhosis (10%). A total of 8 (28%) patients had a history of regular non steroidal anti-inflammatory drug (NSAID) use, two of which developed bleeding and 11 (38%) patients had history of active alcohol intake at the time of their first presentation, two of which developed bleeding. Two (7%) patients had documented hepatocellular carcinoma (HCC) on imaging.
Five (17%) patients were referred for NBCA injection as a form of primary prevention after GV were diagnosed incidentally on upper GI surveillance for EV, the remaining 24 (83%) patients had previous history of upper GI bleeding secondary to GV. The majority of patients with liver cirrhosis were found to be child-pugh-turcott (CPT) class A (45%), the remainder were CPT class B (31%) and C (24%), respectively. The average model for end stage liver disease (MELD) score at the time of presentation was 11 (SD 4.4, range 6-23). Baseline characteristics are further shown in Table 1.
Table 1.
Baseline characteristics of patients included
Intervention
A total of 91 sessions of NBCA injections were carried out in the 29 patients (average of 3.14 sessions/patient, SD 1.79, range 1-8) with a total of 124 injections applied (average of 4.28 injections/patient, SD 3.09, range 1-13). The most common type of GV based on Sarin's classification was isolated GV type-1 (62%) followed by gostroesophageal varices type-2 (38%). 12 (50%) patients had stigmata of recent bleeding with one patient documented to have a red wale sign. Only 18 out of the 29 (62%) patients underwent a post-eradication evaluation by EUS to assess the need for further NBCA injection sessions based on the presence or absence of blood flow.
Outcome
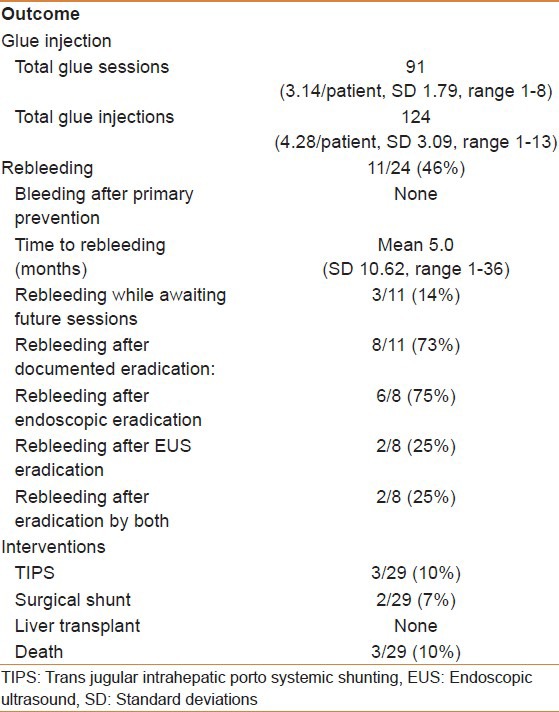
In total, 62% of included patients were free of bleeding after over 2 years of follow-up. 79% of patients were free of rebleeding once three sessions of histoacryl® injections were completed. Four patients (14%) had GV rebleeding while being scheduled for additional future sessions [Figure 2]. Five (45%) patients had eradication of their GV by direct endoscopic assessment “defined as absent or minimal flashback of blood after histoacryl® injection,” 2 (18%) were documented by EUS “defined as absent blood flow in the GV on EUS,” and 2 (18%) by both. 25 (86%) patients had documented residual GV by either EUS (14, 56%), direct endoscopic assessment (18, 72%) or both (9, 36%). 2/11 (18%) patients had recurrence of GV bleeding after documented eradication by EUS compared to 5/11 (45%) patients documented eradication by endoscopic assessment (50% vs. 55%, P = 1). 2/11 (18%) patients had rebleeding after documented eradication by both modalities. One patient failed to respond to NBCA injections and subsequently went on to have TIPS with trans-TIPS embolization of GV. Two patients needed further NBCA injections after failing TIPS. Two patients were referred for a surgical shunt due to anatomic reasons and three patients died during follow-up, none of which were thought to be directly related to GV bleeding. All patients that underwent NBCA injections for primary prevention were free of bleeding during 23 months of follow-up. No patients required liver LT during follow-up [Table 2].
Figure 2.
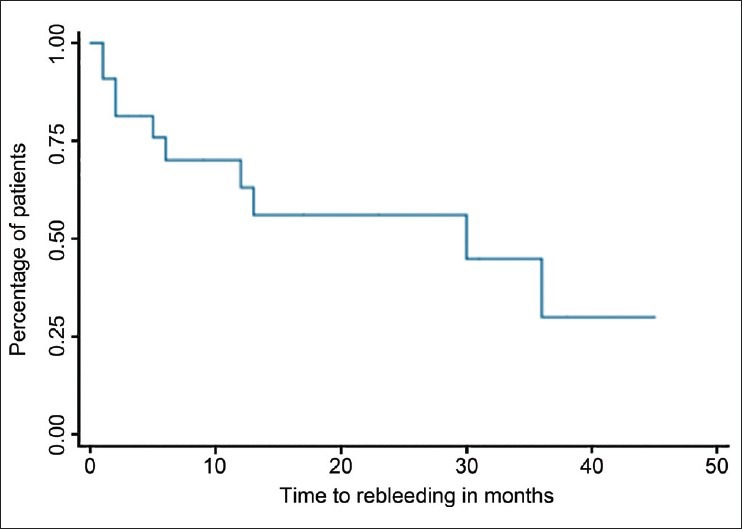
Kaplan-Meier survival curve outlining time to rebleeding in months
Table 2.
Outcome measured over a mean follow-up of 28 months
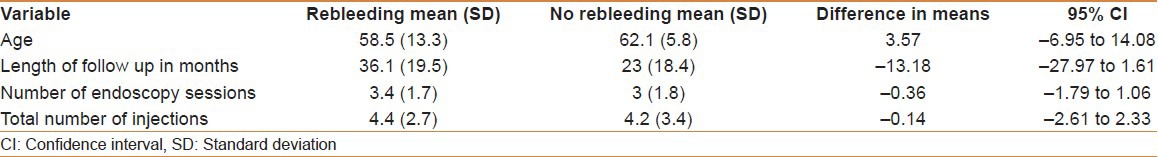
On further statistical analysis, a comparison between responders and non-responders to NBCA treatment showed that there was no difference between patients who re-bled and those who did not in the mean age (3.57 years, 95% CI;−6.95-14.08), length of follow-up (−13.18 months, 95% CI;−27.97-1.16), mean number of endoscopy sessions (−0.36, 95% CI;−1.79-1.06), or number of injections (−0.14, 95% CI;−2.61-2.33) [Table 3].
Table 3.
Comparison between patients who had rebleeding and those free of bleeding/rebleeding
Univariate analysis
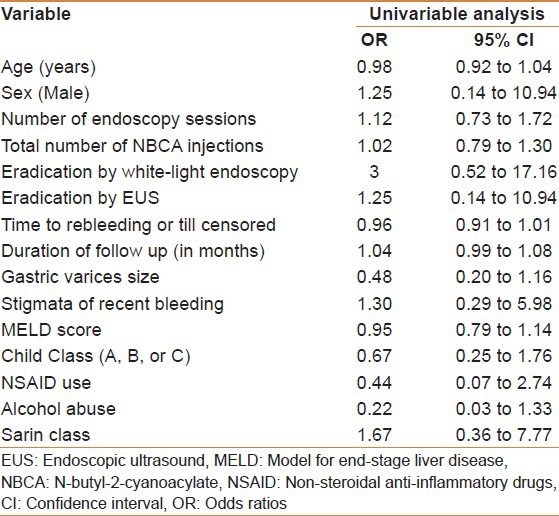
On univariable analysis [Table 4], rebleeding could not be predicted by age OR = 0.98 (95% CI, 0.92-1.04), sex OR = 1.25 (95% CI, 0.14-10.94), number of endoscopy sessions OR = 1.12 (95% CI, 0.73-1.72), total number of NBCA injections OR = 1.02 (95% CI, 0.79-1.30), eradication by white-light endoscopy OR = 1.12 (95% CI, 0.73-1.72), eradication by EUS OR = 1.25 (95% CI, 0.14-10.94), time to rebleeding or till censored OR = 0.96 (95% CI, 0.91-1.01), duration of follow-up OR = 1.04 (95% CI, 0.99-1.08), GV size OR = 0.48 (95% CI, 0.20-1.16), stigmata of recent bleeding OR = 1.30 (95% CI, 0.29-5.98), MELD score OR = 0.95 (95% CI, 0.79-1.14), CPT Class (A, B, or C) OR = 0.67 (95% CI, 0.25-1.76), NSAID use OR = 0.44 (95% CI, 0.07-2.74), alcohol abuse OR = 0.22 (95% CI, 0.03-1.33), or Sarin class OR = 1.67 (95% CI, 0.36-7.77). As the number of events/observations was low, a multi-variable logistic regression was not feasible.
Table 4.
Univariable analysis of predictors of rebleeding in patients undergoing N-butyl-2-cyanoacylate injection for gastric varices
Safety
No immediate (specifically, no post-injection bleeding, fever, splenic infarction, endoscopy related complications or embolic phenomena) or long-term complications of this procedure were reported in any of these cases during the time of follow-up. One case of instrument damage requiring repair was reported.
DISCUSSION
GV bleeding is associated with a significant mortality risk approaching 60% in the setting of liver failure.[20] In this study, we report results that support the safety and efficacy of GV NBCA injections. The treatment options available for GV bleeding are not well established compared to other modalities available for treating bleeding EV.[21,22,23] However, endoscopic NBCA injection of GV remains the recommended option according to guidelines.[24] Soehendra et al., first reported GV histoacryl® injection in 1986.[25] There are a small number of studies assessing the safety and efficacy of GV injection with NBCA compared to EVL or ethanol injection.[26] In a small-randomized study of 37 patients with GV bleeding endoscopic injection of NBCA was more effective in achieving hemostasis (78% vs. 38%, P < 0.05) and variceal obliteration (100% vs. 44%) when compared to EIS with alcohol.[27] In another randomized study, 36 patients with CPT class C cirrhosis and variceal bleeding were randomized to either receive EIS with a 3% ethanolamine oleate solution or injection of NBCA. NBCA was found to be superior in achieving hemostasis compared to sclerotherapy.[28] Furthermore, in a retrospective study of 131 patients with GV who underwent obliteration with NBCA; the cumulative rebleeding-free rate at 1, 3, and 5 years was 94.5%, 89.3%, and 82.9% respectively. In this study, endoscopic injection of NBCA was more effective in achieving hemostasis compared to EVL (87% vs. 45%, P = 0.03) and a lower rebleeding rate (31% vs. 54%, P = 0.0005).[29] The rebleeding rate after gastric NBCA injection of this study was found to be consistent with previously reported rebleeding rates (22-59%).[30,31,32] In our study, the rebleeding-free rate was 62% after more than 2 years of follow-up, which is within the range of previously reported rebleeding rates. We however, based on our results, believe that the needed number of sessions to effectively reduce rebleeding rates is three. Endoscopic NBCA injection of GV is currently also recommended for controlling actively bleeding GV and prevention of rebleeding. Historically, re-bleeding after NBCA treatment of GV has been mainly related to incomplete obliteration, early extrusion of the glue plug or presence of infections. Glue extrusion is considered one of the major causes of rebleeding. In a study by Wang et al., early extrusion of glue within 1 week of treatment was noted in 12.1% of cases and during serial follow-up, in 42.8% after 2 weeks, in 27.9% after 3 months and in 28.9% after 6 months through late re-bleeding.[33] Our study did not report any cases of glue extrusion.
The majority of our studied patient population (83%) underwent treatment for secondary prevention of GV rebleeding. However, 5 patients (17%) had GV NBCA injection done for primary prevention. This group had a 100% bleeding-free rate during follow-up of more than 2-years. Few guidelines recommend the use of non-selective B-blockers among patients with fundal GV.[10] Conversely, GV NBCA injection is considered the standard of care in some centers for both patient populations. Alternatively, non-endoscopic methods such as TIPS and surgical porto-systemic shunts are also effective methods in treating patients with GV but carry a higher rate of complications (10-30%)[34,35,36] and is not as effective in the setting of GV secondary to causes other than portal hypertension.
The safety of GV gluing has been repeatedly addressed in the past with some conflicting reports. Large cohort studies, have reported an overall rate of injection related complications ranging between 5.2% and 57%.[1,30,37] There have been a few reports of serious adverse events such as histoacryl-embolization leading to portal vein thrombosis, pulmonary involvement or widespread tissue necrosis,[38,39,40] which we did not observe in the follow-up of our study population. We attribute the absence of such complications in our study to our small sample size, the low volume of glue/lipiodol mixture and the low concentration of injection slurry as well as the awareness that most of these complications are technical-related.
As EUS is thought to be a reliable tool in detecting vascular blood flow, it can be utilized to search for persistent blood flow at the site of GV post-NBCA treatment. Assessment of obliteration of GV by EUS is however, a technically demanding procedure that might require filling the entire stomach with water, which is practically difficult. Furthermore, results can be different depending on the type of echo endoscope used (linear vs. radial), the position of the instrument during the exam and the length of time spent by the operator trying to detect findings suggestive of superficial, deep varices or perforators. The interpretation of the findings can also be difficult as its translation into clinical relevance is challenging.[41] The documentation of the presence and the duration of any ‘flashback’ of blood from a GV after injection is a novel way and we describe in this study to determine the adequacy of GV eradication. We believe it is potentially a more accurate method to determine thrombosis; this method however, needs future studies to determine its validity. Further, we acknowledge that there may be bias by indication as not all patients were systematically referred for evaluation EUS.
There are several predictors of EV rebleeding. Most importantly, the mere presence of GV, and a measured hepatic vein pressure gradient (HVPG) exceeding 16 mmHg.[22,42,43] In patients with GV, a presentation of active bleeding, carrying an associated diagnosis of HCC and a MELD score greater than 15 are poor prognostic factors found to be associated with a higher risk of GV rebleeding and both short and long-term mortality.[44] Two patients with GV on the background of having HCC were included in our cohort and responded well to NBCA treatment with no post-treatment bleeding observed till the time of this report. HVPG however, was not measured as an outcome in our study. Our statistical analysis on the other hand, could not identify any significant predictors of bleeding/rebleeding.
CONCLUSION
NBCA injection of GV is a safe and successful therapeutic intervention. A minimum of three endoscopic sessions is required to decrease the risk of bleeding/rebleeding. Adopting more accurate methods to determine GV eradication post-NBCA injection is needed. We describe a novel method of visualizing post-injection flashback, which needs further studying and validation. When compared, neither EUS nor direct endoscopic assessment was reliable in predicting the recurrence of GV bleeding. However, this study might have been underpowered and a larger sample size may be required to confirm our findings.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Cheng LF, Wang ZQ, Li CZ, Cai FC, Huang QY, Linghu EQ, et al. Treatment of gastric varices by endoscopic sclerotherapy using butyl cyanoacrylate: 10 years’ experience of 635 cases. Chin Med J (Engl) 2007;120:2081–5. [PubMed] [Google Scholar]
- 2.Cipolletta L, Zambelli A, Bianco MA, De Grazia F, Meucci C, Lupinacci G, et al. Acrylate glue injection for acutely bleeding oesophageal varices: A prospective cohort study. Dig Liver Dis. 2009;41:729–34. doi: 10.1016/j.dld.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Dhiman RK, Chowdhry N, Chawla YK. Has the time come for cyanoacrylate injection to become the standard-of-care for gastric varices? Trop Gastroenterol. 2010;31:141–4. [PubMed] [Google Scholar]
- 4.Ogawa K, Ishikawa S, Naritaka Y, Shimakawa T, Wagatsuma Y, Katsube A, et al. Clinical evaluation of endoscopic injection sclerotherapy using n-butyl-2-cyanoacrylate for gastric variceal bleeding. J Gastroenterol Hepatol. 1999;14:245–50. doi: 10.1046/j.1440-1746.1999.01842.x. [DOI] [PubMed] [Google Scholar]
- 5.Marques P, Maluf-Filho F, Kumar A, Matuguma SE, Sakai P, Ishioka S. Long-term outcomes of acute gastric variceal bleeding in 48 patients following treatment with cyanoacrylate. Dig Dis Sci. 2008;53:544–50. doi: 10.1007/s10620-007-9882-5. [DOI] [PubMed] [Google Scholar]
- 6.Sato T, Yamazaki K. Evaluation of therapeutic effects and serious complications following endoscopic obliterative therapy with Histoacryl. Clin Exp Gastroenterol. 2010;3:91–5. doi: 10.2147/ceg.s12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choudhuri G, Chetri K, Bhat G, Alexander G, Das K, Ghoshal UC, et al. Long-term efficacy and safety of N-butylcyanoacrylate in endoscopic treatment of gastric varices. Trop Gastroenterol. 2010;31:155–64. [PubMed] [Google Scholar]
- 8.Sarin SK, Mishra SR. Endoscopic therapy for gastric varices. Clin Liver Dis. 2010;14:263–79. doi: 10.1016/j.cld.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Binmoeller KF. Glue for gastric varices: Some sticky issues. Gastrointest Endosc. 2000;52:298–301. doi: 10.1067/mge.2000.108042. [DOI] [PubMed] [Google Scholar]
- 10.de Franchis R, Baveno V Faculty. Revising consensus in portal hypertension: Report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762–8. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh JS, Jan CM, Lu CY, Chen FM, Wang JY, Huang TJ. Preoperative evaluation of endoscopic ultrasonography and portography in selecting devascularization surgery for esophagogastric varices. Am Surg. 2005;71:439–44. [PubMed] [Google Scholar]
- 12.Krstić M, Pesko P, Pavlović A, Tomić D, Micev M, Krstić S, et al. The role of endoscopic ultrasound (EUS) in differential diagnosis of subepithelial oesophago-gastric lesions. Acta Chir Iugosl. 2005;52:101–8. doi: 10.2298/aci0501101k. [DOI] [PubMed] [Google Scholar]
- 13.Bastid C, Sahel J. Use of the curved linear-array echo endoscope to identify gastrorenal shunts in patients with gastric fundal varices. Endoscopy. 2005;37:398. doi: 10.1055/s-2005-861097. [DOI] [PubMed] [Google Scholar]
- 14.Battaglia G, Bocus P, Morbin T, Ancona E. Endoscopic Doppler US-guided injection therapy for gastric varices: Case report. Gastrointest Endosc. 2003;57:608–11. doi: 10.1067/mge.2003.164. [DOI] [PubMed] [Google Scholar]
- 15.Bocus P, Ceolin M, Battaglia G. Endoscopic ultrasonography (EUS) in portal hypertension. Minerva Med. 2007;98:431–6. [PubMed] [Google Scholar]
- 16.Chien CH, Chien RN, Yen CL, Fang KM, Liu CJ, Lin CL, et al. The role of endoscopic ultrasonography examination for evaluation and surveillance of gastric subepithelial masses. Chang Gung Med J. 2010;33:73–81. [PubMed] [Google Scholar]
- 17.de Paulo GA, Ardengh JC, Nakao FS, Ferrari AP. Treatment of esophageal varices: A randomized controlled trial comparing endoscopic sclerotherapy and EUS-guided sclerotherapy of esophageal collateral veins. Gastrointest Endosc. 2006;63:396–402. doi: 10.1016/j.gie.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 18.Kakutani H, Hino S, Ikeda K, Mashiko T, Sumiyama K, Uchiyama Y, et al. Use of the curved linear-array echo endoscope to identify gastrorenal shunts in patients with gastric fundal varices. Endoscopy. 2004;36:710–4. doi: 10.1055/s-2004-825658. [DOI] [PubMed] [Google Scholar]
- 19.Seewald S, Ang TL, Imazu H, Naga M, Omar S, Groth S, et al. A standardized injection technique and regimen ensures success and safety of N-butyl-2-cyanoacrylate injection for the treatment of gastric fundal varices (with videos) Gastrointest Endosc. 2008;68:447–54. doi: 10.1016/j.gie.2008.02.050. [DOI] [PubMed] [Google Scholar]
- 20.Monsanto P, Almeida N, Rosa A, Maçôas F, Lérias C, Portela F, et al. Endoscopic treatment of bleeding gastric varices with histoacryl (N-butyl-2-cyanoacrylate): A south European single center experience. Indian J Gastroenterol. 2012 doi: 10.1007/s12664-012-0191-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Carbonell N, Pauwels A, Serfaty L, Fourdan O, Lévy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;40:652–9. doi: 10.1002/hep.20339. [DOI] [PubMed] [Google Scholar]
- 22.D’Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: An evidence-based approach. Semin Liver Dis. 1999;19:475–505. doi: 10.1055/s-2007-1007133. [DOI] [PubMed] [Google Scholar]
- 23.Luca A, D’Amico G, La Galla R, Midiri M, Morabito A, Pagliaro L. TIPS for prevention of recurrent bleeding in patients with cirrhosis: Meta-analysis of randomized clinical trials. Radiology. 1999;212:411–21. doi: 10.1148/radiology.212.2.r99au46411. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W Practice guidelines committee of the American association for the study of liver diseases, practice parameters committee of the American college of gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–38. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 25.Soehendra N, Nam VC, Grimm H, Kempeneers I. Endoscopic obliteration of large esophagogastric varices with bucrylate. Endoscopy. 1986;18:25–6. doi: 10.1055/s-2007-1013014. [DOI] [PubMed] [Google Scholar]
- 26.Rajoriya N, Forrest EH, Gray J, Stuart RC, Carter RC, McKay CJ, et al. Long-term follow-up of endoscopic histoacryl glue injection for the management of gastric variceal bleeding. QJM. 2011;104:41–7. doi: 10.1093/qjmed/hcq161. [DOI] [PubMed] [Google Scholar]
- 27.Sarin SK, Jain AK, Jain M, Gupta R. A randomized controlled trial of cyanoacrylate versus alcohol injection in patients with isolated fundic varices. Am J Gastroenterol. 2002;97:1010–5. doi: 10.1111/j.1572-0241.2002.05622.x. [DOI] [PubMed] [Google Scholar]
- 28.Maluf-Filho F, Sakai P, Ishioka S, Matuguma SE. Endoscopic sclerosis versus cyanoacrylate endoscopic injection for the first episode of variceal bleeding: A prospective, controlled, and randomized study in Child-Pugh class C patients. Endoscopy. 2001;33:421–7. doi: 10.1055/s-2001-14257. [DOI] [PubMed] [Google Scholar]
- 29.Lo GH, Lai KH, Cheng JS, Chen MH, Chiang HT. A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology. 2001;33:1060–4. doi: 10.1053/jhep.2001.24116. [DOI] [PubMed] [Google Scholar]
- 30.Kang EJ, Jeong SW, Jang JY, Cho JY, Lee SH, Kim HG, et al. Long-term result of endoscopic histoacryl (N-butyl-2-cyanoacrylate) injection for treatment of gastric varices. World J Gastroenterol. 2011;17:1494–500. doi: 10.3748/wjg.v17.i11.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo GH, Liang HL, Chen WC, Chen MH, Lai KH, Hsu PI, et al. A prospective, randomized controlled trial of transjugular intrahepatic portosystemic shunt versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy. 2007;39:679–85. doi: 10.1055/s-2007-966591. [DOI] [PubMed] [Google Scholar]
- 32.Tan PC, Hou MC, Lin HC, Liu TT, Lee FY, Chang FY, et al. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage: N-butyl-2-cyanoacrylate injection versus band ligation. Hepatology. 2006;43:690–7. doi: 10.1002/hep.21145. [DOI] [PubMed] [Google Scholar]
- 33.Wang YM, Cheng LF, Li N, Wu K, Zhai JS, Wang YW. Study of glue extrusion after endoscopic n-butyl-2-cyanoacrylate injection on gastric variceal bleeding. World J Gastroenterol. 2009;15:4945–51. doi: 10.3748/wjg.15.4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barange K, Péron JM, Imani K, Otal P, Payen JL, Rousseau H, et al. Transjugular intrahepatic portosystemic shunt in the treatment of refractory bleeding from ruptured gastric varices. Hepatology. 1999;30:1139–43. doi: 10.1002/hep.510300523. [DOI] [PubMed] [Google Scholar]
- 35.Spina GP, Henderson JM, Rikkers LF, Teres J, Burroughs AK, Conn HO, et al. Distal spleno-renal shunt versus endoscopic sclerotherapy in the prevention of variceal rebleeding. A meta-analysis of 4 randomized clinical trials. J Hepatol. 1992;16:338–45. doi: 10.1016/s0168-8278(05)80666-x. [DOI] [PubMed] [Google Scholar]
- 36.Thomas PG, D’Cruz AJ. Distal splenorenal shunting for bleeding gastric varices. Br J Surg. 1994;81:241–4. doi: 10.1002/bjs.1800810227. [DOI] [PubMed] [Google Scholar]
- 37.Al-Ali J, Pawlowska M, Coss A, Svarta S, Byrne M, Enns R. Endoscopic management of gastric variceal bleeding with cyanoacrylate glue injection: Safety and efficacy in a Canadian population. Can J Gastroenterol. 2010;24:593–6. doi: 10.1155/2010/276273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou MC, Lin HC, Lee HS, Liao WC, Lee FY, Lee SD. A randomized trial of endoscopic cyanoacrylate injection for acute gastric variceal bleeding: 0.5 mL versus 1.0 mL. Gastrointest Endosc. 2009;70:668–75. doi: 10.1016/j.gie.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Saracco G, Giordanino C, Roberto N, Ezio D, Luca T, Caronna S, et al. Fatal multiple systemic embolisms after injection of cyanoacrylate in bleeding gastric varices of a patient who was noncirrhotic but with idiopathic portal hypertension. Gastrointest Endosc. 2007;65:345–7. doi: 10.1016/j.gie.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 40.van Beek AP, van Erpecum KJ. Fatal N-butyl-2-cyanoacrylate pulmonary embolism after sclerotherapy for variceal bleeding. Endoscopy. 2005;37:687. doi: 10.1055/s-2005-861427. [DOI] [PubMed] [Google Scholar]
- 41.Sharma M, Goyal A. Bleeding after glue injection in gastric varices. Rebleeding from a glue ulcer. Gastroenterology. 2012;142:e1–2. doi: 10.1053/j.gastro.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 42.D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: A meta-analytic review. Hepatology. 1995;22:332–54. doi: 10.1002/hep.1840220145. [DOI] [PubMed] [Google Scholar]
- 43.Goulis J, Armonis A, Patch D, Sabin C, Greenslade L, Burroughs AK. Bacterial infection is independently associated with failure to control bleeding in cirrhotic patients with gastrointestinal hemorrhage. Hepatology. 1998;27:1207–12. doi: 10.1002/hep.510270504. [DOI] [PubMed] [Google Scholar]
- 44.Chang CJ, Hou MC, Liao WC, Lee FY, Lin HC, Lee SD. Risk factors of early re-bleeding and mortality in patients with ruptured gastric varices and concomitant hepatocellular carcinoma. J Gastroenterol. 2012;47:531–9. doi: 10.1007/s00535-011-0518-3. [DOI] [PubMed] [Google Scholar]


