Abstract
Background/Aim:
The purpose of this study was to evaluate the clinical significance of visualized area percentage assessment of cleansing score (AAC) and computed assessment of cleansing score (CAC) of these two small bowel cleanliness scores systems for capsule endoscopy (CE).
Materials and Methods:
The reliability and consistency of the AAC and CAC scores were evaluated by comparing the scores by two examiners (one expert, one without any training in CE). Reliability was determined using the intraclass correlation coefficient (ICC) and consistency was determined using the kappa statistic.
Results:
The inter-observer agreement was excellent for both the AAC and CAC scores. For AAC, the ICC was 0.791 (95% confidence interval: 0.677-0.868), and for CAC the ICC was 1.000. Using 1.5 as the cutoff, there was no significant difference between AAC and CAC results by the expert examiner (kappa = 0.756, P = 0.000) or the non-expert examiner (kappa = 0.831, P = 0.000). Evaluation of small bowel cleanliness using AAC took 15-30 min, and evaluation using CAC took about 2-3 min. The overall adequacy assessment (OAA) using the AAC was not significantly different between the two examiners (χ2 = 0.586, P = 0.444). There were also no significant differences between the OAA using the AAC and the OAA using the CAC by the expert examiner (χ2 = 1.730, P = 0.188) or the non-expert examiner (χ2 = 1.124, P = 0.289).
Conclusion:
Both of these scores for assessment of small bowel cleanliness can be useful in clinical practice, but the CAC is simpler to use.
Keywords: Capsule endoscopy, computed assessment of cleansing score, small bowel cleaning score system, visualized area percentage assessment of cleansing score
Capsule endoscopy (CE) cannot be controlled by the physician after the capsule has been swallowed by the patient. It is, therefore, not possible to control the focus or the direction of observation as in conventional endoscopy, and it is not possible to collect tissue biopsy samples. CE also cannot deliver water or air into the intestine and cannot suction, inflate or wash the intestinal lumen.[1] The cost of the procedure is very high, and the capsule cannot be reused. For these reasons, adequate bowel preparation before CE is particularly important. The manufacturer recommends 12 h of fasting prior to CE,[2] but many studies have reported that this may be insufficient for optimal visibility.[3,4,5,6,7,8] Food debris, bile, bubbles, and opaque mucus have been reported as the primary causes of poor visibility.[9,10] As a moderate or severe amount of intestinal debris is an independent risk factor for inability to adequately inspect the small intestine, clinicians using CE try to ensure adequate bowel preparation. However, there is no agreement regarding the criteria for adequate small bowel preparation.[11,12] It is important to evaluate small bowel cleanliness in patients undergoing CE and assess the degree to which small bowel cleanliness affects the diagnostic accuracy of CE.[9,13,14] The aim of this study was to evaluate the clinical value of the visualized area percentage assessment of cleansing score (AAC) and computed assessment of cleansing score (CAC), which are the currently available methods of assessing small bowel cleanliness.[15]
MATERIALS AND METHODS
A retrospective study was performed on 63 patients who had undergone CE at Sanming First Affiliated Hospital of Fujian Medical University between January 2010 and July 2012. Patients with impaired intestinal motility were excluded, considering that this could affect enteric cleansing. In that sense, history of gastrointestinal or abdominal surgery, use of antispasmodic, analgesic or prokinetic drugs, diarrhea, thyroid disease, diabetes mellitus, known or suspected small bowel obstruction and strictures, chronic renal or hepatic or cardiac failure were defined as exclusion criteria. Bowel preparation with 250 mL 20% mannitol and 1 L 0.9% saline were taken orally at 20:00 h on the day before the procedure and at 05:00 h on the next day. In addition, 20 mL oral simethicone (Espumisan; Berlin-Chemie, Germany, containing 40 mg simethicone in 1 mL emulsion) and 200 mL water were drunk 30 min before capsule ingestion. All patients abstained from solid food the day before the procedure. After swallowing the capsule, patients were not allowed to drink clear fluids for 2 h and were permitted a light meal after 4 h. Written informed consent was obtained in all cases. One senior gastroenterologists with image-reviewing experience of more than 200 cases and one non-CE reading experience of physicians who separately evaluated 63 CE cases of small bowel cleansing by using the two cleansing grading system. All of them were unaware of the patient's medical history. The recorder data were analyzed and scored by using Chongqing Jinshan Image Processing Software, version 4.64. The study was approved by the local ethics committee.
Scoring system
AAC
Our previously published cleansing grading system simply described as follows.[16] First the whole visibility of the small bowel mucosa in a single frame of the video was evaluated by an image processing software, image-pro plus version 6.0 (Media Cybernetics). After the selected single frame was open in the window, the area of part of the invisible mucosa was outlined, calculated and summed, irrespective of brightness or obstructing elements defined by Brotz[17] including fluid, debris, bubbles and bile/chyme staining, followed by a total area of a single frame counted in the same way. Finally, a ratio was arrived at of the area of unmasked mucosa, divided by the total area of a single frame scored using a modified 4-grade scale based on criteria set by Dai[4] (3 points were given if the ratio was 76-100%, 2, 1 and 0 point mean 51-75%, 26-50% and 0-25%, respectively.) with a maximum possible score of 3. Based on the score, the view quality of a single frame was graded as excellent (scoring 3 points), good (scoring 2 points), fair (scoring 1 point) and poor (scoring 0 point).
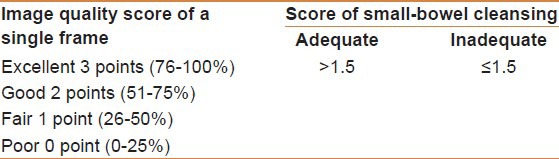
Serial images of the small bowel were manually selected at 5-min intervals using the Jinshan Image Processing Software system. A scale plate was placed under the tissue color bar. Images were selected at 0.25 cm intervals, which was approximately equal to an image every 5 min. If the capsule became stuck or remained in the same place for more than 5 min, the relevant images were scored only once. Overall small bowel cleanliness was scored by dividing the sum of the individual image scores by the total number of images. In view, an overall adequacy assessment (OAA) of small-bowel cleanliness, the score of small-bowel cleansing > 1.5 was considered “adequate” and “inadequate” if the score of small-bowel cleansing ≤ 1.5 [Table 1].
Table 1.
Image quality score of a single frame and the score of small-bowel cleansing
CAC
According to the description by Van Weyenberg SJB, etc.,[18] the Rapid software of the reading station (Given Imaging, Yoqneam, Israel) shows a tissue color bar for identifying anatomical landmarks and maintaining perspective on the location of images (Jinshan OMOM Imaging Station 4.64 also contents this tissue color bar). The tissue color bar comprises a computed summary of color representations of individual CE images. They noticed that the tissue color bar often contains pronounced greenish segments, corresponding to individual CE images of greenish luminal content. They postulated that if the tissue color bar was transformed to red–green–blue color mode, the intensity in the green channel in relation to the intensity of the red channel could be used as a measure of small-bowel contamination. Good mucosal visibility could be associated with high values of red intensity and low values of green intensity, whereas fecal contamination could be associated with low values of red intensity and high values of green intensity.
In order to be able to perform measurements of color intensity, an electronic high-resolution image of the Rapid user interface was captured using commercially available software (Screen Print and Capture 32 3.5, Provtech Ltd., West Kilbride, UK). The image was imported into commercially available photo-editing software (Photoshop CS2, version 9.0.2, Adobe Systems Inc., San Jose, California, USA), and cropped to contain only the tissue color bar. Using the histogram option, the mean intensity value of the green and the red channel could be measured within selections of the bar. The red–green ratio was calculated by dividing the intensity of the red channel by the intensity of the green channel.
For OAA of small-bowel cleanliness, a red-green ratio of > 1.5 was considered adequate, and a ratio of ≤ 1.5 was considered inadequate.
Efficacy evaluation
Inter-observer agreement on AAC and CAC scores was evaluated using two examiners: One expert, and the other without any training in CE. Reliability was determined using the ICC, and consistency was determined using the kappa statistic.
To assess inter-observer agreement on the AAC score, the examiners each scored the same selected frames from the first duodenal frame to the ileocecal valve at 5-min intervals.[14] Comparisons were made between the scores for single frames, and the OAA of small bowel cleanliness.
To assess inter-observer agreement on CAC, the examiners used software (Screen Print and Capture 32 3.5) to capture the tissue color bars from the first duodenal frame to the ileocecal valve. The tissue color bar was imported into photo-editing software (Photoshop CS2), and was scored using the histogram option.
Statistical analysis
Reliability between the quantitative variables was assessed by intraclass correlation coefficient (ICC). An ICC value less than 0.40 was considered poor, between 0.40 and 0.75 was considered fair to good, and greater than 0.75 was considered excellent. Consistency was determined by using the Kappa statistic. A Kappa value greater than 0.75 was considered excellent and lesser than 0.40 was considered poor. Differences between groups were evaluated by t test for categorical variables. Differences in means were assessed by analysis of variance for normally distributed variables and Kruskal-Wallis test for non-normally distributed variables. Differences for categorical variables were assessed by the χ2 test or Fisher exact test (when expected count was < 5) and Pearson χ2 test. Differences in constituent proportions were evaluated by the one-sample goodness-of-fit test. A two-tailed P < 0.05 was considered statistically significant. SPSS (version 19.0) was used for statistical analysis.
RESULTS
A total of 63 patients who were referred for CE because of suspected small bowel disease were enrolled in the study. All examinations were complete from the duodenum to the ileocecal valve. The patients were 35 women (55.6%) and 28 men (44.4%), of which 47 were outpatients and 16 were inpatients. The mean overall age was 51.6 ± 12.3 years (range 13-86 years). The indications for CE were obscure gastrointestinal bleeding (32/63, 50.8%), unexplained abdominal pain (18/63, 28.6%), and chronic diarrhea (13/63, 20.6%). The color intensity in the red-green channel could be measured in all studies. The mean red intensity ranged from 124.85 to 236.00 (mean 173.20 ± 23.91), and the mean green intensity ranged from 102.09 to 167.11 (mean 123.06 ± 10.16). The red-green ratio ranged from 1.12 to 1.78 (mean 1.52 ± 0.14). All color measurements were separately scored by the two examiners, who obtained exactly the same results. The average length of the captured tissue color bar ranged from 10.2 cm to 22.6 cm (mean 16.3 ± 5.6 cm). Evaluation using AAC involved analysis of 41to 90 images (mean 70.3 ± 18.7 images) and took 15-30 min (mean 25.2 ± 8.6 min). Evaluation using CAC only took about 2-3 min.
Assessment of the AAC and CAC scores
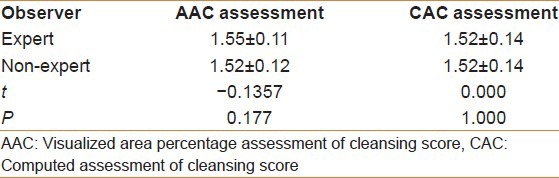
The AAC scores by the expert and the non-expert examiners were 1.23-1.82 (mean 1.55 ± 0.11) and 1.21-1.79 (mean 1.52 ± 0.12), respectively. This was not a significant difference between the two examiners (t = −0.1357, P = 0.177). The CAC scores by the expert and non-expert examiners were exactly the same, ranging from 1.12 to 1.78 (mean 1.52 ± 0.14) (t = 0.000, P = 1.000; Table 2).
Table 2.
The score of AAC assessment and CAC assessment
Assessment of reliability and consistency
Reliability assessment showed that AAC scores by the expert and non-expert examiners had an ICC of 0.791 (95% confidence interval: 0.677-0.868), and CAC scores by the expert and non-expert examiners had an ICC of 1.0. Using 1.5 as the cutoff, there was no significant difference between AAC and CAC results by the expert examiner (kappa = 0.756, P = 0.000) or the non-expert examiner (kappa = 0.831, P = 0.000).
Reliability of OAA
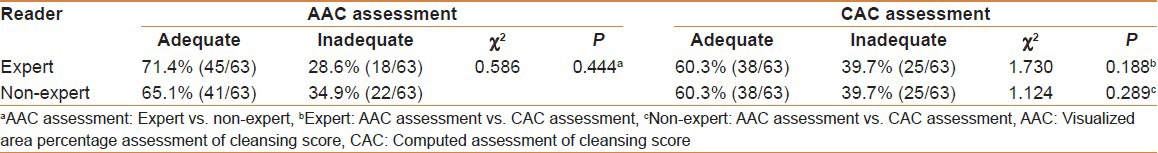
Using 1.5 as the cut-off, the expert and non-expert examiners rated the OAA as “adequate” using the AAC in 71.4% of cases (45/63) and 65.1% of cases (41/63), respectively, and using the CAC in 60.3% of cases (38/63) and 60.3% of cases (38/63), respectively. The expert and non-expert examiners rated the OAA as “inadequate” using the AAC in 28.6% of cases (18/63) and 34.9% of cases (22/63), respectively, and using the CAC in 39.7% of cases (25/63) and 39.7% of cases (25/63), respectively. The OAA using the AAC was not significantly different between the two examiners (χ2 = 0.586, P = 0.444; Table 3). There were also no significant differences between the OAA using the AAC and the OAA using the CAC by the expert examiner (χ2 = 1.730, P = 0.188) or the non-expert examiner (χ2 = 1.124, P = 0.289; Table 3).
Table 3.
Overall adequacy assessment by using AAC and CAC
DISCUSSION
Bowel preparation methods for CE have been assessed by relevant associations in the United States and Europe,[19,20,21] however, controversies about optimal methods persist, and there are no criteria for qualitative or quantitative assessment of the intestinal tract cleanliness. This has led to confusion among gastroenterologists using CE regarding optimal methods of assessing small bowel cleanliness. The average time for passage of the capsule through the small intestine is 4-6 h, yielding 30,000-40,000 images at 2 frames/s. Some of these images are clear and some are very poor. Accurate assessment of such a large number of images is time consuming and difficult in clinical settings. This study used a quality grading standard to assess the image quality of a single frame every 5 min, which only required assessment of 41-90 (mean 70.3 ± 18.7) images to evaluate small bowel cleanliness. This method was relatively quick and easy to perform in clinical settings.
The advantages of using the AAC are that it can provide intuitive and quantitative evaluation of small bowel cleanliness [Figures 1 and 2], and that it is the method generally used in published papers.[4,6,7,11,16,17,22,23,24,25,26] The disadvantages of using the AAC are that the results are influenced by the quality of the samples when using interval sampling, and that calculation of the visualized area is time-consuming and influenced by human variability. The advantages of using the CAC are that it can evaluate a single image or a number of images in any part of the small intestine [Figures 3 and 4], it is less time-consuming, and the results are not influenced by human variability. The disadvantage of using the CAC is that it uses a visual analog scale (color intensity ratio of red to green) to evaluate the images. The visual analog scale cannot differentiate between bubbles, food residue, fecal material, opaque secretions, and bile. The usefulness of the CAC has not been validated in large-scale studies.
Figure 1.
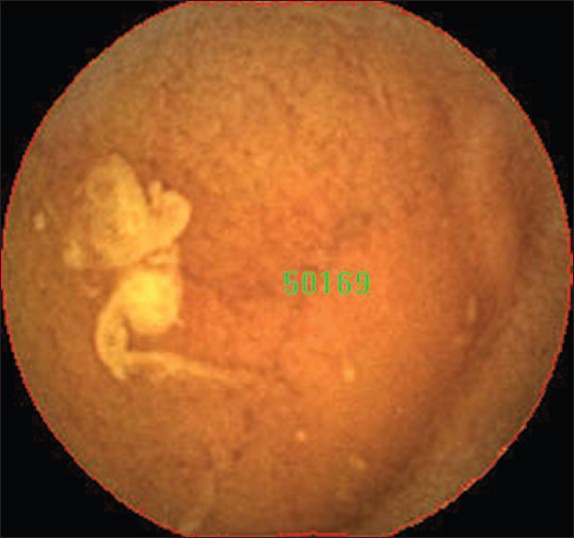
Visualized area percentage assessment of cleansing score scores: The image was covered with bile. The total area of this picture is 50169
Figure 2.
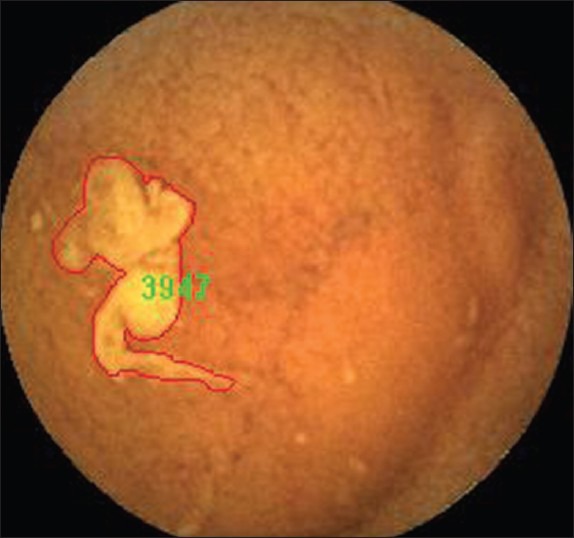
Visualized area percentage assessment of cleansing score scores: The image was covered with bile. The area covered by bile of this picture is 3947. Thus 3947/50168 = 0.0787 (7.87%); 1 - 0.0787 = 0.921 (92.1%). It is scored 3 points
Figure 3.
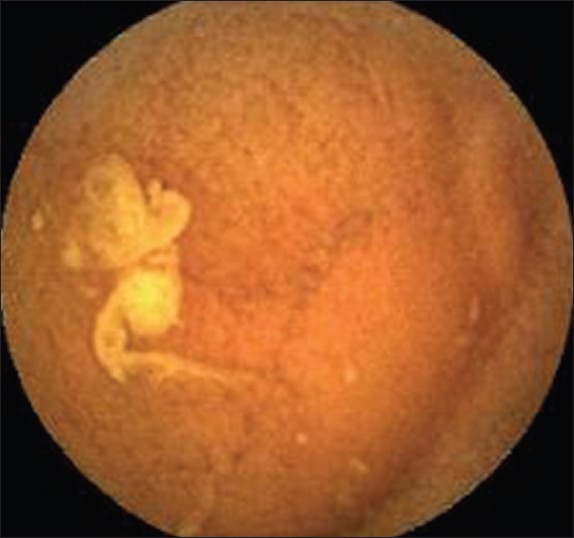
Computed assessment of cleansing score: The mean intensity value of the red channel was 150.88 and the green channel was 87.64. The red–green ratio = 150.88/87.64 = 1.72
Figure 4.

Computed assessment of cleansing scores: The mean intensity value of the red channel was 173.15 and the green channel was 114.32. The red–green ratio = 173.15/114.32 = 1.51
In conclusion, assessment of inter-observer reliability of these two scores between two examiners showed a high ICC and no significant difference using the kappa statistic. There were no significant differences in OAA of small bowel preparation using the AAC or CAC. We propose that a cutoff value of 1.5 may be used to indicate adequate small bowel preparation for CE. Both the AAC and CAC scores can be obtained in clinical settings, but the CAC score is easier to obtain and therefore, may be more useful.
ACKNOWLEDGMENT
We wish to thank our colleagues, technicians and nurses for their great help and cooperation during performing this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Koornstra JJ. Bowel preparation before small bowel capsule endoscopy: What is the optimal approach? Eur J Gastroenterol Hepatol. 2009;21:1107–9. doi: 10.1097/MEG.0b013e32832b8d2f. [DOI] [PubMed] [Google Scholar]
- 2. [Last accessed on 2004 Aug 13]. Available from: http://www.givenimaging.com .
- 3.Pons Beltrán V, Carretero C, Gonzalez-Suárez B, Fernandez-Urien I, Muñoz-Navas M. Intestinal preparation prior to capsule endoscopy administration. World J Gastroenterol. 2008;14:5773–5. doi: 10.3748/wjg.14.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai N, Gubler C, Hengstler P, Meyenberger C, Bauerfeind P. Improved capsule endoscopy after bowel preparation. Gastrointest Endosc. 2005;61:28–31. doi: 10.1016/s0016-5107(04)02444-7. [DOI] [PubMed] [Google Scholar]
- 5.Van Tuyl SA, den Ouden H, Stolk MF, Kuipers EJ. Optimal preparation for video capsule endoscopy: A prospective, randomized, single-blind study. Endoscopy. 2007;39:1037–40. doi: 10.1055/s-2007-966988. [DOI] [PubMed] [Google Scholar]
- 6.Lapalus MG, Ben Soussan E, Saurin JC, Favre O, D’Halluin PN, Coumaros D, et al. Capsule endoscopy and bowel preparati on with oral sodium phosphate: A prospective randomized controlled trial. Gastrointest Endosc. 2008;67:1091–6. doi: 10.1016/j.gie.2007.11.053. [DOI] [PubMed] [Google Scholar]
- 7.Wei W, Ge ZZ, Lu H, Gao YJ, Hu YB, Xiao SD. Purgative bowel cleansing combined with simethicone improves capsule endoscopy imaging. Am J Gastroenterol. 2008;103:77–82. doi: 10.1111/j.1572-0241.2007.01633.x. [DOI] [PubMed] [Google Scholar]
- 8.Spada C, Riccioni ME, Familiari P, Spera G, Pirozzi GA, Marchese M, et al. Polyethylene glycol plus simethicone in small-bowel preparation for capsule endoscopy. Dig Liver Dis. 2010;42:365–70. doi: 10.1016/j.dld.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Villa F, Signorelli C, Rondonotti E, de Franchis R. Preparations and prokinetics. Gastrointest Endosc Clin N Am. 2006;16:211–20. doi: 10.1016/j.giec.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Niv Y. Efficiency of bowel preparation for capsule endoscopy examination: A meta-analysis. World J Gastroenterol. 2008;14:1313–7. doi: 10.3748/wjg.14.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SC, Keum B, Seo YS, Kim YS, Jeen YT, Chun HJ, et al. Effect of bowel preparation with polyethylene glycol on quality of capsule endoscopy. Dig Dis Sci. 2011;56:1769–75. doi: 10.1007/s10620-010-1500-2. [DOI] [PubMed] [Google Scholar]
- 12.Mergener K, Ponchon T, Gralnek I, Pennazio M, Gay G, Selby W, et al. Literature review and recommendations for clinical application of small-bowel capsule endoscopy, based on a panel discussion by international experts. Consensus statements for small-bowel capsule endoscopy, 2006/2007. Endoscopy. 2007;39:895–909. doi: 10.1055/s-2007-966930. [DOI] [PubMed] [Google Scholar]
- 13.Chen HB, Huang Y, Chen SY, Deng DY, Gao LH, Xie JT, et al. A comparative study of two kinds of small bowel cleaning score system for capsule endoscopy. Acta Gastroenterol Belg. 2012;75:342–8. [PubMed] [Google Scholar]
- 14.Park SC, Keum B, Hyun JJ, Seo YS, Kim YS, Jeen YT, et al. A novel cleansing score system for capsule endoscopy. World J Gastroenterol. 2010;16:875–80. doi: 10.3748/wjg.v16.i7.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher LR, Hasler WL. New vision in video capsule endoscopy: Current status and future directions. Nat Rev Gastroenterol Hepatol. 2012;9:392–405. doi: 10.1038/nrgastro.2012.88. [DOI] [PubMed] [Google Scholar]
- 16.Chen HB, Huang Y, Chen SY, Song HW, Li XL, Dai DL, et al. Small bowel preparations for capsule endoscopy with mannitol and simethicone: A prospective, randomized, clinical trial. J Clin Gastroenterol. 2011;45:337–41. doi: 10.1097/MCG.0b013e3181f0f3a3. [DOI] [PubMed] [Google Scholar]
- 17.Brotz C, Nandi N, Conn M, Daskalakis C, DiMarino M, Infantolino A, et al. A validation study of 3 grading systems to evaluate small-bowel cleansing for wireless capsule endoscopy: A quantitative index, a qualitative evaluation, and an overall adequacy assessment. Gastrointest Endosc. 2009;69:262–70. doi: 10.1016/j.gie.2008.04.016. 270.e1. [DOI] [PubMed] [Google Scholar]
- 18.Van Weyenberg SJ, De Leest HT, Mulder CJ. Description of a novel grading system to assess the quality of bowel preparation in video capsule endoscopy. Endoscopy. 2011;43:406–11. doi: 10.1055/s-0030-1256228. [DOI] [PubMed] [Google Scholar]
- 19.Mishkin DS, Chuttani R, Croffie J, Disario J, Liu J, Shah R, et al. ASGE Technology Status Evaluation Report: Wireless capsule endoscopy. Gastrointest Endosc. 2006;63:539–45. doi: 10.1016/j.gie.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Rey JF, Gay G, Kruse A, Lambert R ESGE Guidelines Committee. European Society of Gastrointestinal Endoscopy guideline for video capsule endoscopy. Endoscopy. 2004;36:656–8. doi: 10.1055/s-2004-814557. [DOI] [PubMed] [Google Scholar]
- 21.Rey JF, Ladas S, Alhassani A, Kuznetsov K ESGE Guidelines Committee. European Society of Gastrointestinal Endoscopy (ESGE). Video capsule endoscopy: Update to guidelines (May 2006) Endoscopy. 2006;38:1047–53. doi: 10.1055/s-2006-944874. [DOI] [PubMed] [Google Scholar]
- 22.Kantianis A, Karagiannis S, Liatsos C, Galanis P, Psilopoulos D, Tenta R, et al. Comparison of two schemes of small bowel preparation for capsule endoscopy with polyethylene glycol: A prospective, randomized single-blind study. Eur J Gastroenterol Hepatol. 2009;21:1140–4. doi: 10.1097/meg.0b013e32832b2107. [DOI] [PubMed] [Google Scholar]
- 23.Fang YH, Chen CX, Zhang BL. Effect of small bowel preparation with simethicone on capsule endoscopy. J Zhejiang Univ Sci B. 2009;10:46–51. doi: 10.1631/jzus.B0820148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pons Beltrán V, González Suárez B, González Asanza C, Pérez-Cuadrado E, Fernández Diez S, Fernández-Urién I, et al. Evaluation of different bowel preparations for small bowel capsule endoscopy: A prospective, randomized, controlled study. Dig Dis Sci. 2011;56:2900–5. doi: 10.1007/s10620-011-1693-z. [DOI] [PubMed] [Google Scholar]
- 25.Ito T, Ohata K, Ono A, Chiba H, Tsuji Y, Sato H, et al. Prospective controlled study on the effects of polyethylene glycol in capsule endoscopy. World J Gastroenterol. 2012;18:1789–92. doi: 10.3748/wjg.v18.i15.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosono K, Endo H, Sakai E, Sekino Y, Uchiyama T, Watanabe S, et al. Optimal approach for small bowel capsule endoscopy using polyethylene glycol and metoclopramide with the assistance of a real-time viewer. Digestion. 2011;84:119–25. doi: 10.1159/000323225. [DOI] [PubMed] [Google Scholar]


