Abstract
Blastocystis is a highly controversial protozoan parasite. It has been variably regarded as a commensal and pathogen. Scientists have for decades wondered whether it is truly an enteropathogen and if it is observed in symptomatic patients whether treatment is required because patient recovery and improvement has been noted even without any treatment. Though associated with self-limiting infection, treatment is warranted in many patients due to persistence of symptoms. This particularly holds true for children and adults who are immuno compromised. Several drugs have been used to treat Blastocystis but each one of them has produced widely variable rates of clinical cure and eradication of the parasite from the feces. Based on the studies carried out in vitro and clinical responses obtained in patients, metronidazole appears to be the most effective drug for Blastocystis infection. However, the therapy is complicated due to different dosages and regimens adopted and the unresponsiveness to treatment observed in several sections of the population studied. Recently, the finding of different subsets of Blastocystis exhibiting resistance to metronidazole and associated with variable degrees of symptoms has underscored the importance of typing the subsets of the parasite in order to foretell the clinical response and the need to treat. Till date, the mode of action of the drugs used and the mechanism of resistance is not entirely known and is a topic of speculation. Other drugs with anti Blastocystis activity and used in therapy includes trimethoprim sulfamethoxazole and nitazoxanide. Several other compounds have also been evaluated for the treatment either alone or in combination with the first or second line drugs. A lot of interest has also been generated on the role of probiotics particularly Saccharomyces boularrdii and other natural food compounds on eradication of the parasite. This review provides a comprehensive overview of antimicrobials used to target Blastocystis and discusses the issues pertaining to drug resistance, treatment failure, reinfection, and the current views on treatment modalities.
KEY WORDS: Blastocystis hominis, metronidazole, nitazoxanide, trimethoprim-sulfamethoxazole
INTRODUCTION
Blastocystis presents a great challenge to biologists seeking to describe its taxonomy and for parasitologists and clinicians who have struggled for many decades to determine whether it is truly an enteropathogen.[1] Despite being one of the most common human intestinal protozoans in the developing countries, current knowledge about this parasite is incomplete and contradictory. Several issues are still unresolved and much debated regarding this parasite such as the clinical relevance, pathogenicity and the need for treatment.[2] In 1916, a study described blastocystosis as “an infection that is difficult to get rid of” although treatment has been available and used for several decades.[3,4] Patients infected with Blastocystis frequently present with gastrointestinal complaints and are treated with the intention to eradicate the parasite. However, an accumulating body of data on treatment modalities indicates that successful antimicrobial eradication of Blastocystis is far from straight forward.[5] In a study from Taiwan on 100 patients who had Blastocystis in the feces, all patients improved without specific treatment for Blastocystis. Further, on stool examination within 1 year of follow-up, the parasite was undetectable in 91.2% of patients despite their having no specific antiprotozoal treatment.[6] Little is known about the organism's metabolic processes, and conclusive animal models are lacking for cause- and-effect relationship.[3] As a result of this uncertainty surrounding its pathogenic role, large scale treatment trials are also lacking. Treatment of symptomatic patients is often warranted despite the self-limiting nature of the infection.[7] Recent data also suggest that Blastocystis causes symptoms frequently. Therefore, therapy should be limited to patients with persistent symptoms subsequent to a complete work up for alternative etiologies.[8] Further, studies reporting therapeutic improvement concomitant with parasite clearance in symptomatic patients substantiates the pathogenic role of the organism and hence, the need for treatment.[9]
This review provides a comprehensive overview of antimicrobials used to target Blastocystis and discusses issues pertaining to drug resistance, treatment failure, re-infection and the current views on treatment modalities.
THERAPEUTIC OPTIONS
To date a number of antimicrobial agents have been used to treat Blastocystis infection. This includes metronidazole, nitazoxanide, trimethoprim-sulfamethoxazole (TMP-SMX), paramomycin, iodoquinol, ketoconazole, secnidazole, emetine, tinidazole, and the probiotic Saccharomyces boulardii. Table 1 depicts the common drugs used and the treatment regimens.[10]
Table 1.
Drugs and dosage schedules for treatment of B. hominis
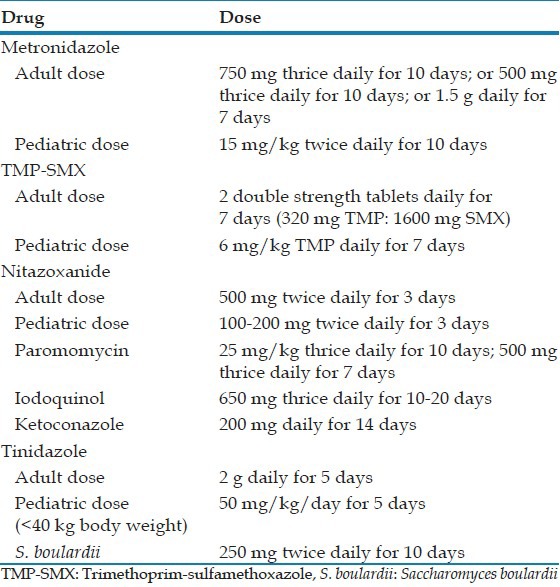
In instances where treatment is warranted, metronidazole is the most frequently prescribed antibiotic.[9] With metronidazole, a high proportion of patients achieve clinical remission (88%) and a 6 month fecal clearance (80%).[11] Though considered for first line treatment, the success of eradicating the parasite with this drug has been reported to be anywhere between 0% and 100%.[5,10] Metronidazole is prescribed in various dosage regimens ranging from 250 mg to 750 mg 3 times a day for 10 days, to 1.5 g/day for 10 days. It has been used alone or in combination with other drugs such as paramomycin or cotrimoxazole.[9] There is extensive variation in treatment response to metronidazole.[12] Accurate prediction of a response is difficult in individual patients.[1] In a placebo controlled treatment trial of Blastocystis infection with metronidazole administered at a dosage of 1.5 g/day for 10 consecutive days, it was observed that diarrhea improved, and there was parasitic clearance from the stools at 1 month following therapy. However, an extension of follow-up at 6 months resulted in an elevated occurrence of parasitological relapses in the metronidazole treated group, both symptomatic, and asymptomatic. Therefore, it is suggested that metronidazole may be ineffective in achieving complete eradication possibly due to resistance. Surprisingly, in the placebo treated group there was a sporadic spontaneous clearance of the parasite from stools and this was more evident at 6 months evaluation.[7]
In all placebo controlled studies, successful treatment is indicated by the resolution of symptoms in association with the partial or complete eradication of the organisms. However, with metronidazole, microbiological resolution is found only in 80% of treated patients.[7,10] There are many reasons attributed to this. Treatment confers an enhancement in the reproductive potential of the parasite and thus, the treated parasite reverts to becoming granular with the release of reproductive granules that account for the increased number of parasites in microscopy and cultures encountered sometimes in the post treatment phase.[13] The cysts forms in addition to being genetically heterogeneous are resistant to the cytotoxic effect of the drug.[9,14]
Resistance to metronidazole was first reported as early as 1991, thus, undermining its value as a first line treatment.[3,13] It has been noted that this drug is effective for certain patients but does not provide complete eradication for others, particularly those with severe infection. This variation in treatment response suggests the existence of resistant subtypes of the parasite. Currently no in vitro and in vivo data unequivocally supports this.[12] The unresponsive patients are perhaps infected with the resistant subtypes.[10,13] In a study by Constantine Vassalos et al., it was concluded that Blastocystis species subtype 3 is the most predominant and intra subtype differences in subtype 3 morphotype exists in relation to their pathogenic potential. Transition from vacuolar to amoeboid form reflects the progression from an asymptomatic to symptomatic state. Hence, searching for amoeboid forms may be helpful before antiprotozoal treatment is attempted.[15] It is also possible that the apparent failure of treatment is due to patient non-compliance, differing pharmacokinetic properties of drugs and inactivation of the drug by the normal bacterial flora.[13,16] Recent studies have indicated that the human isolates of Blastocystis from different geographical locations possess varying degrees of resistance to metronidazole. This implies that an efficient drug or drug combinations at a proper dosage must be instituted in order to eliminate the parasite.[3,13] One clinic has reported successful treatment with a combination of secnidazole, nitazoxanide, and furazolidone. However, it is to be noted that all three drugs are not currently available in many countries.[3] A 2006 text described an USA patient returning from Nepal with chronic Blastocystosis who was treated without success over a period of 3 years with iodoquinol, paramomycin, doxycycline, albendazole, tinidazole, ornidazole, quinacrine, nitazoxanide, rifaximin, furazolidone, cotrimoxazole, itraconazole, ketoconazole, and various combinations of these drugs.[17] One recent study on metronidazole resistance and subtype dependent variation in drug susceptibilities of Blastocystis revealed that subtype 7 and subtype 4 are resistant to metronidazole and also exhibit cross resistance to tinidazole, indicating that new unknown mechanisms of activation and/or resistance may be involved.[12] Nasirudeen et al., reported that metronidazole induces programed cell death in Blastocystis and apoptosis like features are observable in growing axenic cultures. The central vacuole of Blastocystis acts as a repository where apoptotic bodies are stored before being released into the extracellular space. Little is known about the organism's metabolic processes, although one study has identified the role of mitochondrion like organelles in the reduction of ferrodoxins in subtype 7 that plays a role in the conversion of metronidazole into its active state.[18,19,20]
Association between Blastocystis and irritable bowel syndrome (IBS) has been suggested in the recent literature. One study on IBS patients, revealed 60% resistance to 0.1 mg/ml of metronidazole while another Indonesian study observed resistance at 1.0 mg/ml metronidazole.[9,13,21] In at least 10% of IBS patients, Blastocystis cannot be successfully treated. This can be either due to resistance or defective clearance secondary to decreased humoral and cellular immune functions.[22]
TMP-SMX has been shown to have good effects on the cure rate and the clinical symptoms in patients with Blastocystis infection. It is considered to be superior to metronidazole in the treatment without the side-effects.[23] This drug is suggested as an alternative particularly if symptoms persist after treatment with metronidazole.[24] TMP-SMX is safe to use in pregnant women and is cheaper in terms of cost.[23] Whether the drug has a direct effect on the parasite itself or kills the essential intestinal bacteria for the survival of Blastocystis is not clear.[23] In a study carried out in Turkey by Ok et al., it was demonstrated that patients treated with TMP-SMX experienced 100% recovery.[25] In another study, treatment with TMP-SMX for 7 days, only 97.3% of cases showed clinical improvement.[26] More recently, it has been opined that there is no advantage of TMP-SMX over placebo in the treatment of recurrent abdominal pain caused by Blastocystisin children. The eradication rate in children is 35% compared to 44% with metronidazole as second line treatment. It is assumed that TMP/SMX leads only to a reduction in the number of Blastocystis and not to permanent eradication.[27] Research on subtype dependent variation in susceptibility revealed higher susceptibility of Blastocystis to TMP-SMX ratio of 1:2 than to a ratio of 1:5. At this ratio, it was observed that there is no subtype dependent variation in susceptibility and 95-100% of cases achieved eradication[12] In contrast Moghaddam et al., reported only 22% eradication of the parasite on treatment with TMP-SMX.[28] In Human immunodeficiency virus infected patients the prophylactic use of TMP-SMX leads to a decreased detection rate of Blastocystis.[29]
Nitazoxanide, a 5-nitro thiazole, broad spectrum antiparasitic agent is found to have potent activity against Blastocystis.[10,30] In children, the achievable clearance rates are 97-100% on treatment with this drug. It is well-tolerated with no serious adverse effects.[30] Metronidazole treatment failures in Blastocystis may well respond to nitazoxanide.[12] In vitro, Blastocystis subtype 7 has been found to be significantly more susceptible to nitazoxanide than subtype 4, though both are considered to be nonhuman subtypes. A placebo controlled trial of nitazoxanide 500 mg twice daily for 3 days reported a clinical and parasitological cure of 86%.[31] Paramomycin, a broad spectrum aminoglycoside antibiotic was useful to successfully treat Blastocystis associated cutaneous lesions particularly urticaria.[32] In some studies, it has exhibited superior performance in comparison to metronidazole. With an eradication rate of 77%, treatment with paramomycin appears significantly more effective than treatment with metronidazole (38%) or no treatment (22%).[2] Treatment failure was suspected in one out of three patients in a study, and this patient was subsequently successfully treated with metronidazole.[9,33] Curiously, an early in vitro study revealed that paramomycin was not inhibitory to the parasite. Since, it is bactericidal it might act by the destruction of the gut bacterial flora essential for Blastocystis survival.[9] Mirza et al., have reported that paramomycin has no activity against the nonhuman subtypes of Blastocystis.[12]
Other drugs that are found to have variable efficacy on Blastocystis are tinidazole, ornidazole, secnidazole, ketoconazole, pentamidine, furazolidone, quinine, iodoquinol, iodochlorhydroxyquin, and emetine.[10,12] The activity of emetine against Blastocystis has been evaluated in 3 previous in vitro studies. While two studies reported its effectiveness, Zierdt et al., reported strain-to-strain variation in the susceptibility of the parasite to the drug.[34] It has also been observed that emetine resistance occurs along with metronidazole resistance thereby suggesting that multidrug resistant phenotypes might be present in the parasite. Clinically, however, emetine has limited use because of its severe side effects. Blastocystis subtype 4 is resistant to emetine and variably susceptible to quinacrine and mefloquine. Furazolidone is another drug with anti Blastocystis activity and to date no subtype dependent variation has been observed for this drug.[12]
Cysteine proteases play an important role in the cell cycle and pathophysiology of Blastocystis and induce up regulation of pro-inflammatory cytokines. This finding suggests a potential role for cysteine proteases inhibitors as a therapeutic option for Blastocystis isolates resistant to conventional agents.[12,35]
S. boulardii is a non-pathogenic yeast and has proven effective in gastrointestinal diseases with predominant inflammatory component, indicating its role in interference with cellular signaling pathways and it is marketed as a dietary supplement. It regulates the intestinal microbial homeostasis, interferes with the ability of pathogens to colonize and infect the mucosa, modulates local and systemic immune responses, stabilizes gastrointestinal barrier functions and induces enzymatic activity favoring absorption and nutrition.[36] In a randomized single blinded clinical trial in symptomatic children who had Blastocystis positive stools, both clinical and parasitological cure rates were 94.4% in comparison with 73.3% achieved in the metronidazole treated group. These findings challenge the existing guidelines for treatment.[8]
A few traditional Chinese medicinal herbs have also been examined for in vitro activity against Blastocystis (Brucea javanica and Coptis chinensis). Their inhibitory activity was not as great as with similar concentrations of metronidazole. As an adjunct, dietary management strategies like introduction of high fiber and lactose free diet is shown to improve clinical signs and symptoms.[24]
There have been several studies examining the use of alternative agents for the treatment of Blastocystis infection. Blastocystis isolates from IBS patients mostly genotype-1 have demonstrated increased susceptibility to garlic at 0.01 mg/ml. Other investigational agents such as ginger, black pepper, and white cumin did not have significant inhibitory effect in drug susceptibility assays.[37]
In summary, a variety of drug treatment options are available for Blastocystis infections [Table 1]. Metronidazole appears to be the most effective drug for Blastocystis chemotherapy despite some evidence for treatment failure. In such circumstances, TMP-SMX and nitazoxanide may be considered as second choice drugs. Treatment should be instituted if the diarrhea is persistent and no other causative pathogen is identified in fecal specimens. Future studies should investigate the association between genotypes and variations in drug susceptibility. The mechanisms of action and the mode of resistance to each of the drug has to be evaluated further.[9] Genome analysis of the species and application of modern techniques, such as microsatellites, microarrays and differential display coupled with proteonomics and bioinformatics analysis are more likely to elucidate the differences in pathogenicity, virulence and response to treatment.[15] The development of new therapeutic options to counter antimicrobial resistance requires the use of high through put screening tools.[12]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Farthing MJ. Treatment options for the eradication of intestinal protozoa. Nat Clin Pract Gastroenterol Hepatol. 2006;3:436–45. doi: 10.1038/ncpgasthep0557. [DOI] [PubMed] [Google Scholar]
- 2.van Hellemond JJ, Molhoek N, Koelewijn R, Wismans PJ, van Genderen PJ. Is paromomycin the drug of choice for eradication of Blastocystis in adults? J Infect Chemother. 2012 Oct; doi: 10.1007/s10156-012-0496-2. PMID: 23053509. [DOI] [PubMed] [Google Scholar]
- 3.Boorom KF, Smith H, Nimri L, Viscogliosi E, Spanakos G, Parkar U, et al. Oh my aching gut: Irritable bowel syndrome, Blastocystis, and asymptomatic infection. Parasit Vectors. 2008;1:40. doi: 10.1186/1756-3305-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Low G. Two chronic amoebic dysentery carriers treated by emetine, with some remarks on the treatment of Lamblia, Blastocystis and E. coli infections. J Trop Med Hyg. 1916;19:29–34. [Google Scholar]
- 5.Stensvold CR, Smith HV, Nagel R, Olsen KE, Traub RJ. Eradication of Blastocystis carriage with antimicrobials: Reality or delusion? J Clin Gastroenterol. 2010;44:85–90. doi: 10.1097/MCG.0b013e3181bb86ba. [DOI] [PubMed] [Google Scholar]
- 6.Kuo HY, Chiang DH, Wang CC, Chen TL, Fung CP, Lin CP, et al. Clinical significance of Blastocystis hominis: Experience from a medical center in northern Taiwan. J Microbiol Immunol Infect. 2008;41:222–6. [PubMed] [Google Scholar]
- 7.Nigro L, Larocca L, Massarelli L, Patamia I, Minniti S, Palermo F, et al. A placebo-controlled treatment trial of Blastocystis hominis infection with metronidazole. J Travel Med. 2003;10:128–30. doi: 10.2310/7060.2003.31714. [DOI] [PubMed] [Google Scholar]
- 8.Dinleyici EC, Eren M, Dogan N, Reyhanioglu S, Yargic ZA, Vandenplas Y. Clinical efficacy of Saccharomyces boulardii or metronidazole in symptomatic children with Blastocystis hominis infection. Parasitol Res. 2011;108:541–5. doi: 10.1007/s00436-010-2095-4. [DOI] [PubMed] [Google Scholar]
- 9.Tan KS. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin Microbiol Rev. 2008;21:639–65. doi: 10.1128/CMR.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coyle CM, Varughese J, Weiss LM, Tanowitz HB. Blastocystis: To treat or not to treat. Clin Infect Dis. 2012;54:105–10. doi: 10.1093/cid/cir810. [DOI] [PubMed] [Google Scholar]
- 11.Aguilar C, Lucia JF. An overview of Blastocystis hominis infection and published experience in hemophilic population. J Coagul Disord. 2010;2:1–4. [Google Scholar]
- 12.Mirza H, Teo JD, Upcroft J, Tan KS. A rapid, high-throughput viability assay for Blastocystis spp. reveals metronidazole resistance and extensive subtype-dependent variations in drug susceptibilities. Antimicrob Agents Chemother. 2011;55:637–48. doi: 10.1128/AAC.00900-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haresh K, Suresh K, Khairul Anus A, Saminathan S. Isolate resistance of Blastocystis hominis to metronidazole. Trop Med Int Health. 1999;4:274–7. doi: 10.1046/j.1365-3156.1999.00398.x. [DOI] [PubMed] [Google Scholar]
- 14.Zaman V, Zaki M. Resistance of Blastocystis hominis cysts to metronidazole. Trop Med Int Health. 1996;1:677–8. doi: 10.1111/j.1365-3156.1996.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 15.Vassalos CM, Spanakos G, Vassalou E, Papadopoulou C, Vakalis N. Differences in clinical significance and morphologic features of Blastocystis sp subtype 3. Am J Clin Pathol. 2010;133:251–8. doi: 10.1309/AJCPDOWQSL6E8DMN. [DOI] [PubMed] [Google Scholar]
- 16.Dunn LA, Boreham PF. The in-vitro activity of drugs against Blastocystis hominis. J Antimicrob Chemother. 1991;27:507–16. doi: 10.1093/jac/27.4.507. [DOI] [PubMed] [Google Scholar]
- 17.Boorom K. Corvallis: FBH Press; 2006. Commensal and Pathogenic Blastocystis with Case Studies from Oregon's Willamette Valley. [Google Scholar]
- 18.Nasirudeen AM, Hian YE, Singh M, Tan KS. Metronidazole induces programmed cell death in the protozoan parasite Blastocystis hominis. Microbiology. 2004;150:33–4. doi: 10.1099/mic.0.26496-0. [DOI] [PubMed] [Google Scholar]
- 19.Lantsman Y, Tan KS, Morada M, Yarlett N. Biochemical characterization of a mitochondrial-like organelle from Blastocystis sp. subtype 7. Microbiology. 2008;154:2757–66. doi: 10.1099/mic.0.2008/017897-0. [DOI] [PubMed] [Google Scholar]
- 20.Upcroft P, Upcroft JA. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev. 2001;14:150–64. doi: 10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yakoob J, Jafri W, Jafri N, Islam M, Asim Beg M. In vitro susceptibility of Blastocystis hominis isolated from patients with irritable bowel syndrome. Br J Biomed Sci. 2004;61:75–7. doi: 10.1080/09674845.2004.11732647. [DOI] [PubMed] [Google Scholar]
- 22.Tungtrongchitr A, Manatsathit S, Kositchaiwat C, Ongrotchanakun J, Munkong N, Chinabutr P, et al. Blastocystis hominis infection in irritable bowel syndrome patients. Southeast Asian J Trop Med Public Health. 2004;35:705–10. [PubMed] [Google Scholar]
- 23.Mahdi NK, Strak SK. The effectiveness of metronidazole, praziquantel and cotrimoxazole on Blastocystis hominis. Int J Glob Educ. 2005;1:5. [Google Scholar]
- 24.Stenzel DJ, Boreham RE. Blastocystis. In: Gillespie S, Pearson RD, editors. Principles and Practice of Clinical parasitology. Hoboken, New Jersey: John Wiley and Sons; 2001. pp. 355–67. [Google Scholar]
- 25.Ok UZ, Girginkardeşler N, Balcioğlu C, Ertan P, Pirildar T, Kilimcioğlu AA. Effect of trimethoprim-sulfamethaxazole in Blastocystis hominis infection. Am J Gastroenterol. 1999;94:3245–7. doi: 10.1111/j.1572-0241.1999.01529.x. [DOI] [PubMed] [Google Scholar]
- 26.Ertuğ S, Dost T, Ertabaklar H, Gültekin B. The effect of trimethoprim-sulfamethoxazole in Blastocystis hominis infection. Turkiye Parazitol Derg. 2009;33:270–2. [PubMed] [Google Scholar]
- 27.Heyland K, Friedt M, Buehr P, Braegger CP. No advantage for antibiotic treatment over placebo in Blastocystis hominis-positive children with recurrent abdominal pain. J Pediatr Gastroenterol Nutr. 2012;54:677–9. doi: 10.1097/MPG.0b013e31823a29a7. [DOI] [PubMed] [Google Scholar]
- 28.Moghaddam DD, Ghadirian E, Azami M. Blastocystis hominis and the evaluation of efficacy of metronidazole and trimethoprim/sulfamethoxazole. Parasitol Res. 2005;96:273–5. doi: 10.1007/s00436-005-1363-1. [DOI] [PubMed] [Google Scholar]
- 29.Idris NS, Dwipoerwantoro PG, Kurniawan A, Said M. Intestinal parasitic infection of immunocompromised children with diarrhoea: Clinical profile and therapeutic response. J Infect Dev Ctries. 2010;4:309–17. doi: 10.3855/jidc.275. [DOI] [PubMed] [Google Scholar]
- 30.Diaz E, Mondragon J, Ramirez E, Bernal R. Epidemiology and control of intestinal parasites with nitazoxanide in children in Mexico. Am J Trop Med Hyg. 2003;68:384–5. [PubMed] [Google Scholar]
- 31.Rossignol JF, Kabil SM, Said M, Samir H, Younis AM. Effect of nitazoxanide in persistent diarrhea and enteritis associated with Blastocystis hominis. Clin Gastroenterol Hepatol. 2005;3:987–91. doi: 10.1016/s1542-3565(05)00427-1. [DOI] [PubMed] [Google Scholar]
- 32.Kick G, Rueff F, Przybilla B. Palmoplantar pruritus subsiding after Blastocystis hominis eradication. Acta Derm Venereol. 2002;82:60. doi: 10.1080/000155502753600948. [DOI] [PubMed] [Google Scholar]
- 33.Valsecchi R, Leghissa P, Greco V. Cutaneous lesions in Blastocystis hominis infection. Acta Derm Venereol. 2004;84:322–3. doi: 10.1080/00015550410025949. [DOI] [PubMed] [Google Scholar]
- 34.Zierdt CH, Swan JC, Hosseini J. In vitro response of Blastocystis hominis to antiprotozoal drugs. J Protozool. 1983;30:332–4. doi: 10.1111/j.1550-7408.1983.tb02925.x. [DOI] [PubMed] [Google Scholar]
- 35.Tan KS, Mirza H, Joshua DW, Wu B, MacAry Pa. Current views on the clinical relevance of Blastocystis spp. Curr Infect Dis Rep. 2010;12:28–35. doi: 10.1007/s11908-009-0073-8. [DOI] [PubMed] [Google Scholar]
- 36.Kelesidis T, Pothoulakis C. Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Therap Adv Gastroenterol. 2012;5:111–25. doi: 10.1177/1756283X11428502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yakoob J, Abbas Z, Beg MA, Naz S, Awan S, Hamid S, et al. In vitro sensitivity of Blastocystis hominis to garlic, ginger, white cumin, and black pepper used in diet. Parasitol Res. 2011;109:379–85. doi: 10.1007/s00436-011-2265-z. [DOI] [PubMed] [Google Scholar]


