Abstract
Diarrhea is a major cause of morbidity and mortality in human immunodeficiency virus (HIV)-infected individuals. Opportunistic enteric parasitic infections are encountered in 30-60% of HIV seropositive patients in developed countries and in 90% of patients in developing countries. Once the CD4+ cell count drops below 200 cells/μl, patients are considered to have developed acquired immunodeficiency syndrome (AIDS), with the risk of an AIDS-defining illness or opportunistic infection significantly increasing. Opportunistic enteric parasites encountered in these patients are Cryptosporidium, Isospora, Cyclospora, and microsporidia; as well as those more commonly associated with gastrointestinal disease, for example, Giardia intestinalis, Entamoeba histolytica, Strongyloides stercoralis, and also rarely Balantidium coli. In view of AIDS explosion in India, opportunistic enteric parasites are becoming increasingly important and it has to be identified properly. Apart from wet mounts, concentration methods for stool samples and special staining techniques for identification of these parasites, commercially available fecal immunoassays are widely available for the majority of enteric protozoa. Molecular methods such as polymerase chain reaction (PCR), PCR-restriction fragment length polymorphism, flow cytometry, and sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), have also come in the pipeline for early diagnosis of these infections. Proper disposal of the feces to prevent contamination of the soil and water, boiling/filtering drinking water along with improved personal hygiene might go a long way in preventing these enteric parasitic infections.
KEY WORDS: HIV-infected patients, laboratory diagnosis, opportunistic enteric parasites
INTRODUCTION
Diarrhea is a major cause of morbidity and mortality in human immunodeficiency virus (HIV)-infected individuals. In the coming years there is likely to be an increase in the number of HIV/acquired immunodeficiency syndrome (AIDS) deaths, with worrying projections of 6.5 million deaths in 2030 and HIV/AIDS being the main burden of disease in some developing countries by 2015. Globally, the number of immunosuppressed (IS) people increases each year, with the HIV pandemic continuing to spread unabated in many parts of the world, with an estimated 14,000 new infections occurring daily.[1] HIV infection is the most common immunodeficiency state worldwide, with the hallmark of infection being depletion of CD4+ T lymphocytes. Once the CD4+ cell count drops below 200 cells/μl, patients are considered to have developed AIDS, with the risk of an AIDS-defining illness or opportunistic infection significantly increasing.
IS hosts are more likely to acquire infection after exposure, have more severe disease once the infection is established, have disseminated infection rather than localized infection, and be unable to clear parasites with chronic carriage states. These all lead to greater morbidity and mortality in these patients.
All individuals affected by immunosuppression are at risk of infection by opportunistic enteric parasites such as Cryptosporidium, Isospora, Cyclospora, and microsporidia; as well as those more commonly associated with gastrointestinal disease, for example, Giardia intestinalis, Entamoeba histolytica, Strongyloides stercoralis, and also rarely Balantidium coli.[2] Opportunistic enteric parasitic infections are encountered in 30-60% of HIV seropositive patients in developed countries and in 90% of patients in developing countries.[3] The outcome of infection by enteric protozoan parasites is dependent on absolute CD4+ cell counts, with lower counts being associated with more severe disease, more atypical disease, and a greater risk of disseminated disease. CD4 counts of <100 cells/μL poses greatest risk.[4]
CLINICO-EPIDEMIOLOGY OF THE OPPORTUNISTIC ENTERIC PARASITES
Cryptosporidium parvum, a eucoccidian parasite, was first observed in the gastric mucosa of laboratory mouse by Tyzzer in 1907.[5] First case was diagnosed in a homosexual man with AIDS in New York in August 1981.[6] C. parvum and Cryptosporidium hominis are the species most commonly associated with human cryptosporidiosis. Although their genomes are 97% identical, their host range is strikingly different. C. parvum infects humans and animals and is primarily a zoonotic infection, whereas C. hominis is typically not detected in animals.[7]
Isospora belli was first described by Virchow in 1860 villi of intestinal mucosa at autopsy.[8] It is an AIDS-defining illness if infection persists >4 weeks.[9]
Cyclospora cayatenensis was 1st noted in the intestine of moles by Eimer in 1870[8] but 1st described in 1977 in Papua, New Guinea. It was first found in stool samples of patients with AIDS and chronic diarrhea at Haiti in 1983.[10] For C. cayatenensis, humans are the only known host. With progressive immune suppression (CD4+ T cell counts of < 200 cells/μl in HIV-infected individuals), frequent severe relapses occur which may last from 4 to 7 weeks, resulting in severe malnutrition and significant morbidity and mortality.[10,11]
Microsporidia is of historical importance, being reported in silkworms as early as 1857. First case of microsporidiosis was reported by Hisakichi Matsubayashi in 1959, in a Japanese boy exposed to farm animals.[8,12] First case in HIV-infected patients was reported in 1985 due to Enterocytozoon bieneusi.[13] First case reported from India was in 2001 in a patient with diarrhea.[12] The term microsporidia is used as general nomenclature for the obligate intracellular parasites belonging to the phylum microsporidia. Over 1300 species of microsporidia belonging to 160 genera and infecting a wide range of vertebrate and invertebrate hosts have been described. Fourteen microsporidian species have been identified as human pathogens, which include Anncalia algerae, Anncalia connori, Anncalia vesicularum, Encephalitozoon cuniculi, Encephalitozoon hellem, Encephalitozoon intestinalis (syn. Septata intestinalis), E. bieneusi, Microsporidium ceylonensis, Microsporidium africanum, Nosema ocularum, Pleistophora ronneafiei, Trachipleistophora hominis, Trachipleistophora anthropophthera, and Vittaforma corneae. E. bieneusi is the most common microsporidian in humans and the second most prevalent cause of diarrhea in IS patients, after Cryptosporidium.[13]
Human microsporidiosis occurs mainly but not exclusively in severely immunocompromised patients with AIDS. Dissemination to the hepatobiliary system with cholangitis, to the maxillary sinus with invasive sinusitis and to the respiratory system with pulmonary infections has all been reported for E. bieneusi/HIV co-infection. Encephalitozoon species is the second most common microsporidia in humans and the most common cause of disseminated microsporidiosis. There are three species within this genus that are known to cause human disease – E. cuniculi, E. hellem, and E. intestinalis, with E. intestinalis being usually associated with enteric disease.[14]
E. histolytica is an amoeboid protozoan parasite. The genus Entamoeba includes six species (E. histolytica, Entamoeba dispar, Entamoeba moshkovskii, Entamoeba polecki, Entamoeba coli, and Entamoeba hartmanni). E. histolytica, E. dispar, and E. moshkovskii are capable of infecting the intestinal lumen of humans. All the three are morphologically identical, but genetically different.[5] Recent studies have reported E. moshkovskii as an enteropathogen in patients presenting with gastrointestinal symptoms. There have also been no adequate studies examining the pathogenic potential of this organism in IS groups and thus further study is needed to assess the true pathogenicity of this organism.[15] Complications include various extraintestinal manifestations. G. intestinalis is a common and ubiquitous flagellated protozoan parasite with a worldwide distribution. Humans become infected by ingestion of cysts, which develop into trophozoites after excystation. In developing countries the burden of disease remains high in IC individuals, especially in children less than 10 years of age.[5] Symptoms of giardiasis in HIV-infected individuals appear to be similar to, and not more severe than those of giardiasis in HIV-negative individuals, with asymptomatic infection occurring commonly in the presence of HIV. With progressive immunosuppression following reduced CD4+ counts, the risk of symptomatic Giardia infection is increased.[16] Complications of giardiasis include steatorrhea leading to malabsorption and weight loss.
Almost 80% of sexually active gay men currently carry Entamoeba dispar, Entamoeba coli, Entamoeba hartmanni, Iodamoeba butschlii, Dientamoeba fragilis, and G. intestinalis among other protozoa, as compared to its incidence in the general population of 1-3%.
S. stercoralis, the ovoviviparous nematode, was first demonstrated in stool samples of French soldiers suffering from diarrhea in Cochin war.[17] It causes intractable diarrhea with blood and mucus. This intestinal nematode has also been frequently observed in association with HIV-1. Complications of strongyloidiasis include hyperinfection syndrome (massive larval invasion of lungs, central nervous system, heart, liver, and other organs); and respiratory distress, which is a life threatening condition, if not treated.
Infective form, mode of infection, infections caused, and complications of common opportunistic enteric parasites are shown in Table 1.
Table 1.
Infective form, mode of infection, infections caused, complications and treatment of common opportunistic enteric parasites
Overall prevalence of opportunistic enteric parasites in India varies from 7.5% to 73.3%. The prevalence of pathogenic enteric protozoa in HIV-infected persons in India[18,19,20,21,22,23,24] and worldwide[13,17,25,26,27,28,29,30,31] are shown in Table 2.
Table 2.
Prevalence of opportunistic enteric parasites in human immunodeficiency virus.infected patients-Indian and world scenario
LABORATORY DIAGNOSIS OF OPPORTUNISTIC ENTERIC PARASITES
Microscopy and its utility
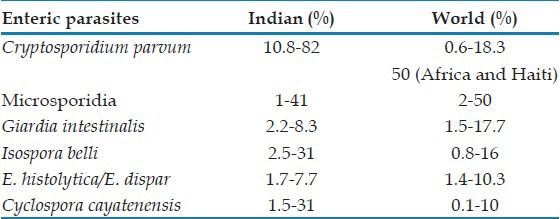
Laboratory diagnosis of cryptosporidiosis traditionally relies on special staining techniques, such as modified Ziehl-Neelsen (Z-N) stain (using 10% H2SO4), Kinyoun's acid fast stain (cold method) [Figure 1], Safranin methylene blue, and Giemsa stains. The oocysts may be confused with yeast cells which are pear-shaped and show evidence of budding. Negative staining with methenamine silver and nigrosin can also be done for demonstration of Cryptosporidial oocysts. Concentration of stool can be done by Sheather's sucrose floatation technique (using saturated solution of sucrose).[32]
Figure 1.
Stool specimen showing acid fast oocysts of Cryptosporidium, Isospora, and Cyclospora stained with (a, b, & c) modified Kinyoun's acid fast stain and spores of microsporidia stained (d) with Ryan Blue modification of chromotrope 2R stain
Fluorescent staining methods using acridine orange and auramine-rhodamine can also be employed for screening.
Diagnosis of I. belli is by direct visualization of elongated pink oocyst in feces by modified Z-N stain [Figure 1]. Concentration of stool by formol ether sedimentation can be done in asymptomatic or partially treated oocyst passers. A mucosal biopsy may be required sometimes for a definitive diagnosis.[32]
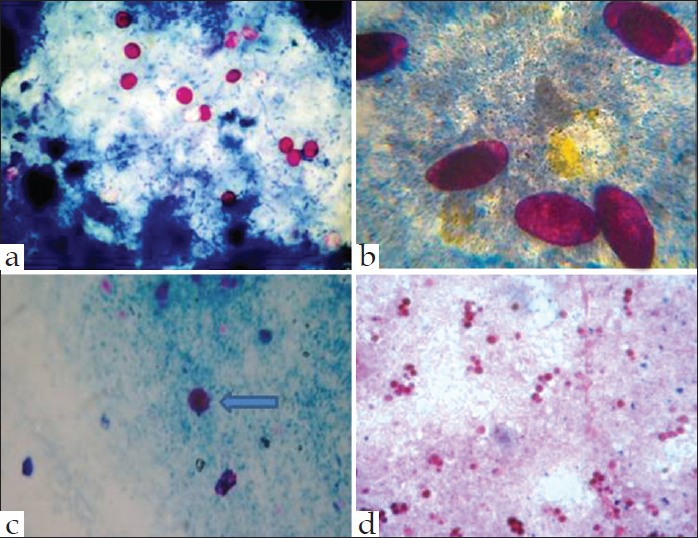
In direct immunofluorescence using polyclonal and mouse monoclonal antibodies, an apple green fluorescence is produced, having a sensitivity of 99% and a specificity of 100% for Cryptosporidium, Isospora, Cyclospora, microsporidia, and Giardia [Figure 2].[33]
Figure 2.
Apple green immunofluorescence of Cyclospora, Cryptosporidium, Giardia intestinalis, and Microsporidia
Diagnosis of Cyclospora oocysts may be problematic, as most laboratories fail to recognize them in direct fecal smears by modified Z-N stain [Figure 1]. In Safranin methylene blue stain, oocysts are reddish orange in colour. Special stains such as modified acid-fast auramine or modified iron-hematoxylin are usually required for definitive diagnosis. Autofluorescence under UV epifluorescence under 340-380 bp filter also can be done to visualise the oocysts of C. cayatenensis. Formol ether sedimentation is the preferred concentration method for cyclosora.[32]
Traditionally, light microscopy based on modified trichrome stains (e.g., Weber Green and Ryan Blue) has been used by most laboratories for the diagnosis of enteric microsporidiosis. Spores appear bright red with a green background in Weber Green[34] and a blue background in Ryan Blue[35] [Figure 1] staining methods. The former method has 60-90% sensitivity and 95-100% specificity, whereas the latter method has 100% sensitivity and 82.8% specificity.[36,37]
Modification of chromotrope stain by staining at a temperature of 50°C for 10 min, instead of room temperature as is conventional, was performed by Kokoskin et al. Advantage of this method – the background contains less debris and the spores stain intensely. In Quick-Hot Gram chromotrope stain, spores stain deep violet, belt-like stripe is enhanced and is a rapid method.[36]
Immunofluorescence tests using the chitin binding fluorochromes – Calcofluor white and Uvitex 2B are available for microsporidia. Chemofluorescent agents like Calcofluor white (bluish white) has a sensitivity of 83.3% and a specificity of 96%. Uvitex 2B (light green) has a sensitivity of 100% and a specificity of 77.4%. Fungifluor and Fungiqual A are also used for detecting microsporidial spores in stool, intestinal fluid, biopsy, and tissue specimens.[38]
Cysts and trophozoites of Entamoeba species can be readily identified in saline and iodine mounts. E. dispar, E. moshkovskii, and E. histolytica are morphologically identical, though genetic differences have confirmed the separation of these three as independent species. Therefore, staining of fixed fecal smears with iron-hematoxylin or Z-N stain can determine the presence of the E. histolytica/E. dispar/E. moshkovskii complex within a stool specimen, while other techniques such as polymerase chain reaction (PCR) or enzyme-linked immunosorbent assay (ELISA) must be employed for differentiation of the three species.[5,32]
G. intestinalis infection can be easily diagnosed microscopically by identification of cysts and trophozoites, as also larvae of S. stercoralis in stained or unstained fecal smears[5,32] [Figure 3].
Figure 3.
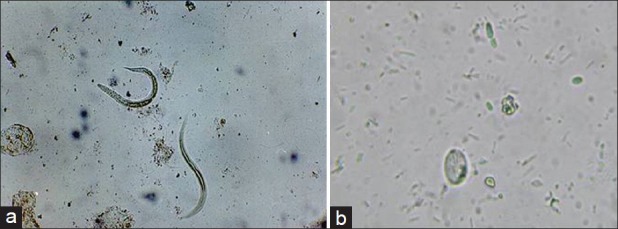
Saline mount of stool sample showing larvae of Strongyloides stercoralis and cyst of Giardia lamblia
Electron microscopy is highly specific but less sensitive. By scanning electron microscopy, microsporidia is seen as ovoid structures 1.5 × 0.9 μm and Cyclospora as a spherical ball 8-10 μm in size. By transmission electron microscopy, microsporidia appears as electron dense outer layer (exospore) and electron lucent inner layer (endospore) and Cyclospora with outer fibrillar coat and cell wall.[39]
Detection of antigen directly from fecal specimens
Several commercial companies have developed rapid diagnostic tests that are simple to perform, for example, lateral-flow immunoassays, immunochromatograhic assays [Figure 4] and direct fluorescent-antibody tests. These are less time-consuming and easier to perform.
Figure 4.
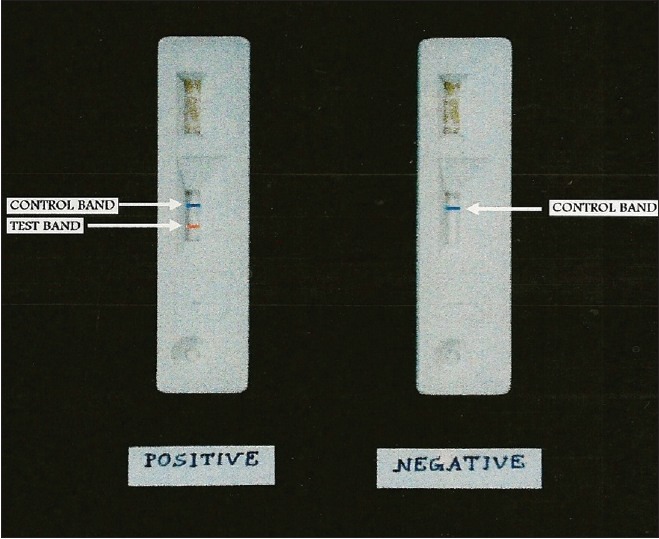
Rapid antigen detection of Cryptosporidium species
The use of ELISA for the detection of Cryptosporidium antigen in stools (Ridascreen®-Biopharm) has sensitivity of 66-100% and specificity of 93-100%[40] [Figure 5].
Figure 5.

Detection of Cryptosporidium antigen from stool by enzyme-linked immunosorbent assay
Enzyme immunoassays, immunochromatographic assays, and direct-fluorescence assays for detection of G. intestinalis in stool have been available in the form of commercial kits for several years. These kits are commonly used in diagnostic laboratories. A monoclonal antibody based capture ELISA and immunofluorescence assay can detect giardia specific antigens in stool samples. Compared to microscopy, the coproantigen assays are less time-consuming and easier to perform. However, conflicting data are there in regard to the performance of these rapid assays. Some researchers have reported excellent sensitivity and specificity, while others have reported the rapid tests to be generally less sensitive than conventional microscopic methods.[41]
Detection of G. intestinalis and C. parvum antigens in human fecal specimens using the ColorPAC combination rapid solid-phase qualitative immunochromatographic assay has been widely used,[42] as also the detection of G. lamblia, E. histolytica/E. dispar, and C. parvum antigens in human fecal specimens using the triage parasite panel enzyme immunoassay.[43]
Commercial antigen capture and rapid lateral-flow cartridge tests are available for Entamoeba species but the above tests cannot differentiate the three species.[44]
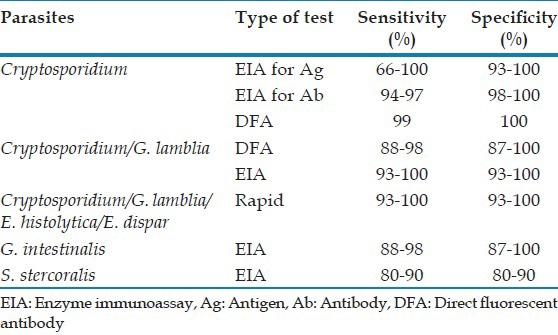
Table 3 shows the sensitivity and specificity of the various serological tests.[40,41,42,43,44,45,47]
Table 3.
Comparison of the serological tests for opportunistic enteric parasites
Antibody detection
Antibody detection for cryptosporidium is done to study prevalence of infection in a community. It cannot diagnose acute infection. Commercial kits available are ProSpecT ELISA, IDEIA, Color VUE, having a sensitivity of 95-97% and specificity of 98-100%.[45]
An immunofluorescent-antibody test (IFAT) for the detection of E. bieneusi and E. intestinalis is also available which is cheaper and more rapid, but currently there are no commercially available or FDA-approved IFAT kits.[46]
For E. histolytica/E. dispar, serological tests like indirect hemagglutination assays, latex agglutination assays, complement fixation assays, indirect immunofluorescence assays, and antibody-detecting ELISA kits to detect 170 KDa lectin of E. histolytica have all been developed. However, serological testing in patients with intestinal disease is normally not recommended. The sensitivity of serological tests is 82% in those with invasive intestinal disease.[44]
For S. stercoralis, ELISA to detect antibodies using larvae as antigen has a sensitivity of 80-90% and is specific. Indirect haemagglutination assays and indirect fluorescent antibody assays are also available, though not commonly used for diagnosis.[47]
Cultural methods
Monkey and rabbit kidney cell lines (Vero and RK-13), human fetal lung fibroblast cell lines (MRC-5) and Madine–Darby canine kidney canine kidney cell lines (MDCK) are employed for microsporidia.[13,48] For S. stercoralis, Harada–Mori filter paper method and Filter paper slant culture can be used.[5]
Molecular methods
While being more expensive and time-consuming, PCR and ELISA have shown superior sensitivity for the detection of Cryptosporidium species as compared to conventional staining and microscopy.
Agarose gel (2%) analyzing a PCR diagnostic test for detection of C. parvum DNA has been widely used with primers CPBDIAGF and CPBDIAGR. This detects <10 oocysts in crude samples. Molecular base pair standard is a 100-bp ladder and C. parvum positive fecal specimen shows a diagnostic band of 435 bp.[49]
A TaqMan PCR assay targeting the 18S ribosomal DNA (rDNA) has allowed sensitive detection of Cryptosporidium species in stools. Nested PCR and Real Time RT-PCR is employed to assess oocyst viability. PCR-restricted fragment length polymorphism (RFLP) analysis can also be done directly from stool samples and the prevalent genotypes of a particular region can be identified.[50] Cryptosporidial DNA can be extracted from stool samples and genotyped by small subunit ribosomal ribonucleic acid-based nested PCR-RFLP.[51] This has an excellent sensitivity of PCR coupled with accuracy of RFLP for species identification. Nested PCR combined with immuno-magnetic separation (IMS) can detect polymorphism (SSCP) for detecting a single oocyst in water sample. Single stranded conformation variation with C. parvum and C. hominis and flow cytometry for relative size of the oocysts are other molecular methods for the diagnosis of cryptosporidium.[49]
Molecular tools not only detect but can also differentiate cryptosporidium at the species/genotype and subtype levels. Based on these studies, geographic, seasonal and socioeconomic differences in the distribution of Cryptosporidium species in humans have been identified. Apart from the common species like C. parvum and C. hominis, other species include C. felis, C. meleagridis, C. muris, C. bailey, etc.[52]
Detection of I. belli by PCR has been used as an additional diagnostic tool in clinical laboratories. Conventional PCR using primers based on 18S rDNA sequences shows excellent sensitivity and specificity. Recently, a real-time PCR targeting the internal transcribed spacer 2 region of the rRNA gene for the detection of I. belli DNA in fecal samples has been developed with 100% specificity and sensitivity.[53]
Cyclospora DNA amplification by PCR also has its value in diagnosis. Conventional PCR and nested PCR targeting small subunit ribosomal RNA for Cyclospora has a sensitivity of 62%. False positivity with some Eimeria species have been noted.[54]
Several PCR based methods to amplify different regions of small subunits and large subunits ribosomal RNA gene are available for diagnosis of E. bieneusi and E. intestinalis infections but PCR inhibitors interfere with the test. In-situ hybridization can be done for E. bieneusi in humans. Multiplex nested PCR for microsporidia is also available. The predominant species in a recent Indian study was E. intestinalis, in contrast to other parts of the world where E. bieneusi is more common.[55]
Other molecular methods used for the diagnosis of microsporidia are Random amplified polymorphic DNA obtained by PCR, PCR-RFLP and SDS-PAGE. A 75 kDa band on 1D Western Blot of E. cuniculi and E. intestinalis spore protein can be done using purified C1 Hsp 70 protein.[48,56] PCR amplification and species determination of microsporidia in formalin-fixed feces can also be done after immunomagnetic separation.[57]
A number of PCR assays are available for detection and/or differentiation of Entamoeba species. The species-specific product size for E. histolytica, E. moshkovskii and E. dispar is 439, 553 and 174 bp, respectively. PCR is more useful than antigen capture ELISA for detection of E. histolytica in stools due to higher sensitivities observed in PCR and the reduced chance of cross-reactivity with other Entamoeba species.[44,58] Specific ribosomal DNA sequences help in the differentiation of pathogenic and non-pathogenic isolates of E. histolytica.[59]
A number of PCR assays, RT-PCR and PCR-RFLP are also available for the detection of G. intestinalis in stool specimens.[60] PCR detection of G. intestinalis in stool can be performed by targeting intergenic spacer region of multicopy rRNA gene.[61]
Histopathological examination
Histopathological examination of jejunal biopsy specimens from infected individuals with Cryptosporidium, Isospora and Cyclospora shows mild to moderate acute inflammation of the lamina propria and surface epithelial disarray.
Staining of tissue sections by the Warthin-Starry staining method is an effective diagnostic tool for the microscopic detection of microsporidia and has better diagnostic capabilities than the hematoxylin and eosin stain. Plastic embedded tissue sections by Toluidine blue stain is also a suitable alternative.[13]
Zymodeme pattern of E. histolytica
Pathogenic and non-pathogenic strains can be distinguished on the basis of differences in the electrophoretic patterns of the isoenzymes (zymodeme) such as glucose phosphate isomerase and phosphoglucomutase.
A non-cloned and a cloned isolate of non-pathogenic (zymodeme I) E. histolytica incubated in Diamonds’ TP-S-1 medium in the presence of Crithidia for 52 d with a single addition of Escherichia coli irradiated at 1000 Gy rendered the culture axenic. Analysis of zymodeme patterns established that both isolates had transformed to zymodeme II. The re-establishment of cultures in the presence of microflora from the original cultures resulted in a return to zymodeme I, whereas incubation with microflora from an amoebic isolate exhibiting a zymodeme II pattern maintained the pathogenic pattern.[62]
Treatment of opportunistic enteric parasitic infections
Table 1 shows the various drugs recommended in common opportunistic enteric parasitic infections.
Management of microsporidial infection most often includes oral treatment with the drug albendazole. The drug nitazoxanide has demonstrated activity against E. bieneusi. The drug fumagillin has also demonstrated good anti-E. bieneusi activity, but has adverse side effects. Furazolidone has also demonstrated a degree of anti-Enterocytozoon activity. As with most opportunistic infections, HAART plays a key role in eradicating microsporidia in HIV-infected patients and effective HAART is likely to reduce the incidence of microsporidial infections in the future.[63,64]
Giardiasis is treated by metronidazole 15 mg/kg/day in three divided doses for 5 days or 2 g daily for 3 days; furazolidone, metronidazole, tinidazole, ornidazole, albendazole, nitazoxanide, quinacrine, paromomycin.[5,65,66] Intestinal amoebiasis is treated by paromomycin, diloxanide furoate, iodoquinol; Metronidazole for 5 days in combination with diloxanide furoate for 10 days.[65,66,67] Strongyloidiasis is treated by ivermectine, albendazole, and thiabendazole.[5]
Prevention and control
Proper disposal of the feces to prevent contamination of the soil and water, boiling/filtering drinking water along with improved personal hygiene might go a long way in preventing the enteric parasitic infections.
CONCLUSION
In view of AIDS explosion in India, opportunistic enteric parasites are becoming increasingly important and it has to be identified properly. Advances in the diagnosis of infectious diseases occur regularly, although the first form of diagnosis of parasitic infections is still by light microscopy of stool by an experienced microscopist. Commercially available fecal immunoassays now not only are widely available for the majority of enteric protozoa but also provide a cost-effective alternative to traditional diagnosis of protozoan enteric parasites. These kits are rapid, easy to use and are far more practical for most laboratories than PCR assays. A confirmatory test such as PCR may be run subsequently. Even in IC individuals, the presence of parasites is normally overlooked in cases of diarrhea, as the provision of appropriate technology in diagnostic laboratories is not yet available.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollok RC, Farthing MJ. Enteric viruses in HIV-related diarrhoea. Mol Med Today. 2000;6:483–7. doi: 10.1016/S1357-4310(00)01816-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cegielski JP, Ortega YR, McKee S, Madden JF, Gaido L, Schwartz DA, et al. Cryptosporidium, enterocytozoon and cyclospora infections in pediatric and adult patients with diarrhea in Tanzania. Clin Infect Dis. 1999;28:314–21. doi: 10.1086/515131. [DOI] [PubMed] [Google Scholar]
- 4.Stark D, Barratt JL, van Hal S, Marriott D, Harkness J, Ellis JT. Clinical significance of enteric protozoa in the immunosuppressed human population. Clin Microbiol Rev. 2009;22:634–50. doi: 10.1128/CMR.00017-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parija SC. 4th ed. New Delhi: All India Publishers and Distributors; 2013. In Textbook of Medical Parasitology: Protozoology and Helminthology. [Google Scholar]
- 6.Ma P, Soave R. Three-step stool examination for cryptosporidiosis in 10 homosexual men with protracted watery diarrhea. J Infect Dis. 1983;147:824–8. doi: 10.1093/infdis/147.5.824. [DOI] [PubMed] [Google Scholar]
- 7.Joachim A. Human cryptosporidiosis: An update with special emphasis on the situation in Europe. J Vet Med B Infect Dis Vet Public Health. 2004;51:251–9. doi: 10.1111/j.1439-0450.2004.00765.x. [DOI] [PubMed] [Google Scholar]
- 8.Cox FE. History of human parasitology. Clin Microbiol Rev. 2002;15:595–612. doi: 10.1128/CMR.15.4.595-612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeHovitz JA, Pape JW, Boncy M, Johnson WD., Jr Clinical manifestations and therapy of Isospora belli infection in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1986;315:87–90. doi: 10.1056/NEJM198607103150203. [DOI] [PubMed] [Google Scholar]
- 10.Wurtz R. Cyclospora: A newly identified intestinal pathogen of humans. Clin Infect Dis. 1994;18:620–3. doi: 10.1093/clinids/18.4.620. [DOI] [PubMed] [Google Scholar]
- 11.Santana Añé M, Núñez Fernández FA, Pérez Avila J, Barrero Bringuez M, Velázquez Viamontes B. Emergence of a new pathogen: Cyclospora cayetanensis in patients infected with human immunodeficiency virus. Rev Cubana Med Trop. 2000;52:66–9. [PubMed] [Google Scholar]
- 12.Joseph J, Vemuganti GK, Sharma S. Microsporidia: Emerging ocular pathogens. Indian J Med Microbiol. 2005;23:80–91. doi: 10.4103/0255-0857.16045. [DOI] [PubMed] [Google Scholar]
- 13.Weber R, Bryan RT, Schwartz DA, Owen RL. Human microsporidial infections. Clin Microbiol Rev. 1994;7:426–61. doi: 10.1128/cmr.7.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asmuth DM. Microsporidia and diarrhea in AIDS patients. Clin Microbiol Newsl. 1994;16:179–86. [Google Scholar]
- 15.Fotedar R, Stark D, Beebe N, Marriott D, Ellis J, Harkness J. Laboratory diagnostic techniques for Entamoeba species. Clin Microbiol Rev. 2007;20:511–32. doi: 10.1128/CMR.00004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gazzard B. AIDS and the gastrointestinal tract. Medicine. 2009;37:357–60. [Google Scholar]
- 17.Grove DI. Human strongyloidiasis. Adv Parasitol. 1996;38:251–309. doi: 10.1016/s0065-308x(08)60036-6. [DOI] [PubMed] [Google Scholar]
- 18.Kumar SS, Ananthan S, Lakshmi P. Intestinal parasitic infection in HIV infected patients with diarrhoea in Chennai. Indian J Med Microbiol. 2002;20:88–91. [PubMed] [Google Scholar]
- 19.Mohandas, Sehgal R, Sud A, Malla N. Prevalence of intestinal parasitic pathogens in HIV-seropositive individuals in Northern India. Jpn J Infect Dis. 2002;55:83–4. [PubMed] [Google Scholar]
- 20.Anand L, Brajachand NG, Dhanachand CH. Cryptosporidiosis in HIV infection. J Commun Dis. 1996;28:241–4. [PubMed] [Google Scholar]
- 21.Agarwal A, Ningthouja S, Sharma D, Mohen Y, Singh NB. Cryptosporidium and HIV. J Indian Med Assoc. 1998;96:276–7. [PubMed] [Google Scholar]
- 22.Prasad KN, Nag VL, Dhole TN, Ayyagari A. Identification of enteric pathogens in HIV-positive patients with diarrhoea in northern India. J Health Popul Nutr. 2000;18:23–6. [PubMed] [Google Scholar]
- 23.Mirdha BR, Kabra SK, Samantray JC. Isosporiasis in children. Indian Pediatr. 2002;39:941–4. [PubMed] [Google Scholar]
- 24.De A, Patil K, Mathur M. Detection of enteric parasites in HIV positive patients with diarrhea. Indian J Sex Transm Dis. 2009;30:55–6. doi: 10.4103/2589-0557.55483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber R, Ledergerber B, Zbinden R, Altwegg M, Pfyffer GE, Spycher MA, et al. Enteric infections and diarrhea in human immunodeficiency virus-infected persons: Prospective community-based cohort study. Swiss HIV Cohort Study. Arch Intern Med. 1999;159:1473–80. doi: 10.1001/archinte.159.13.1473. [DOI] [PubMed] [Google Scholar]
- 26.Soave R. Cyclospora: An overview. Clin Infect Dis. 1996;23:429–35. doi: 10.1093/clinids/23.3.429. [DOI] [PubMed] [Google Scholar]
- 27.Griffiths JK. Human cryptosporidiosis: Epidemiology, transmission, clinical disease, treatment, and diagnosis. Adv Parasitol. 1998;40:37–85. doi: 10.1016/s0065-308x(08)60117-7. [DOI] [PubMed] [Google Scholar]
- 28.Mukhopadhyay C, Wilson G, Pradhan D, Shivananda PG. Intestinal protozoal infestation profile in persistent diarrhea in children below age 5 years in western Nepal. Southeast Asian J Trop Med Public Health. 2007;38:13–9. [PubMed] [Google Scholar]
- 29.Didier ES, Snowden KF, Shadduck JA. Biology of microsporidian species infecting mammals. Adv Parasitol. 1998;40:283–320. doi: 10.1016/s0065-308x(08)60125-6. [DOI] [PubMed] [Google Scholar]
- 30.Hunter PR, Nichols G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin Microbiol Rev. 2002;15:145–54. doi: 10.1128/CMR.15.1.145-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorvillo FJ, Lieb LE, Seidel J, Kerndt P, Turner J, Ash LR. Epidemiology of isosporiasis among persons with acquired immunodeficiency syndrome in Los Angeles County. Am J Trop Med Hyg. 1995;53:656–9. doi: 10.4269/ajtmh.1995.53.656. [DOI] [PubMed] [Google Scholar]
- 32.Garcia LS. 5th ed. Washington, DC: ASM Press; 2007. Diagnostic Medical Parasitology-FAQs; pp. 4–11. [Google Scholar]
- 33.Alles AJ, Waldron MA, Sierra LS, Mattia AR. Prospective comparison of direct immunofluorescence and conventional staining methods for detection of Giardia and Cryptosporidium spp. in human fecal specimens. J Clin Microbiol. 1995;33:1632–4. doi: 10.1128/jcm.33.6.1632-1634.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber R, Bryan RT, Owen RL, Wilcox CM, Gorelkin L, Visvesvara GS. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. The Enteric Opportunistic Infections Working Group. N Engl J Med. 1992;326:161–6. doi: 10.1056/NEJM199201163260304. [DOI] [PubMed] [Google Scholar]
- 35.Ryan NJ, Sutherland G, Coughlan K, Globan M, Doultree J, Marshall J, et al. A new trichrome-blue stain for detection of microsporidial species in urine, stool, and nasopharyngeal specimens. J Clin Microbiol. 1993;31:3264–9. doi: 10.1128/jcm.31.12.3264-3269.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Didier ES, Orenstein JM, Aldras A, Bertucci D, Rogers LB, Janney FA. Comparison of three staining methods for detecting microsporidia in fluids. J Clin Microbiol. 1995;33:3138–45. doi: 10.1128/jcm.33.12.3138-3145.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patil K, De A, Mathur M. Comparison of Weber Green and Ryan Blue modified trichrome staining for the diagnosis of microsporidial spores from stool samples of HIV-positive patients with diarrhoea. Indian J Med Microbiol. 2008;26:407. doi: 10.4103/0255-0857.43554. [DOI] [PubMed] [Google Scholar]
- 38.Franzen C, Müller A, Salzberger B, Fätkenheuer G, Eidt S, Mahrle G, et al. Tissue diagnosis of intestinal microsporidiosis using a fluorescent stain with Uvitex2B. J Clin Pathol. 1995;48:1009–10. doi: 10.1136/jcp.48.11.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satheeshkumar S, Ananthan S. Electron microscopy identification of microsporidia (Enterocytozoon bieneusi) and Cyclospora cayetanensis from stool samples of HIV infected patients. Indian J Med Microbiol. 2004;22:119–22. [PubMed] [Google Scholar]
- 40.Jayalakshmi J, Appalaraju B, Mahadevan K. Evaluation of an enzyme-linked immunoassay for the detection of Cryptosporidium antigen in fecal specimens of HIV/AIDS patients. Indian J Pathol Microbiol. 2008;51:137–8. doi: 10.4103/0377-4929.40427. [DOI] [PubMed] [Google Scholar]
- 41.Johnston SP, Ballard MM, Beach MJ, Causer L, Wilkins PP. Evaluation of three commercial assays for detection of Giardia and Cryptosporidium organisms in fecal specimens. J Clin Microbiol. 2003;41:623–6. doi: 10.1128/JCM.41.2.623-626.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia LS, Shimizu RY. Detection of Giardia lamblia and Cryptosporidium parvum antigens in human fecal specimens using the ColorPAC combination rapid solid-phase qualitative immunochromatographic assay. J Clin Microbiol. 2000;38:1267–8. doi: 10.1128/jcm.38.3.1267-1268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia LS, Shimizu RY, Bernard CN. Detection of Giardia lamblia, Entamoeba histolytica/Entamoeba dispar, and Cryptosporidium parvum antigens in human fecal specimens using the triage parasite panel enzyme immunoassay. J Clin Microbiol. 2000;38:3337–40. doi: 10.1128/jcm.38.9.3337-3340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stark D, van Hal S, Fotedar R, Butcher A, Marriott D, Ellis J, et al. Comparison of stool antigen detection kits to PCR for diagnosis of amebiasis. J Clin Microbiol. 2008;46:1678–81. doi: 10.1128/JCM.02261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minak J, Kabir M, Mahmud I, Liu Y, Liu L, Haque R, et al. Evaluation of rapid antigen point-of-care tests for detection of Giardia and Cryptosporidium species in human fecal specimens. J Clin Microbiol. 2012;50:154–6. doi: 10.1128/JCM.01194-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alfa Cisse O, Ouattara A, Thellier M, Accoceberry I, Biligui S, Minta D, et al. Evaluation of an immunofluorescent-antibody test using monoclonal antibodies directed against Enterocytozoon bieneusi and Encephalitozoon intestinalis for diagnosis of intestinal microsporidiosis in Bamako (Mali) J Clin Microbiol. 2002;40:1715–8. doi: 10.1128/JCM.40.5.1715-1718.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040–7. doi: 10.1086/322707. [DOI] [PubMed] [Google Scholar]
- 48.Garcia LS. Laboratory identification of the microsporidia. J Clin Microbiol. 2002;40:1892–901. doi: 10.1128/JCM.40.6.1892-1901.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laxer MA, Timblin BK, Patel RJ. DNA sequences for the specific detection of Cryptosporidium parvum by the polymerase chain reaction. Am J Trop Med Hyg. 1991;45:688–94. doi: 10.4269/ajtmh.1991.45.688. [DOI] [PubMed] [Google Scholar]
- 50.Das P, Roy SS, MitraDhar K, Dutta P, Bhattacharya MK, Sen A, et al. Molecular characterization of Cryptosporidium spp. from children in Kolkata, India. J Clin Microbiol. 2006;44:4246–9. doi: 10.1128/JCM.00091-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma P, Sharma A, Sehgal R, Malla N, Khurana S. Genetic diversity of Cryptosporidium isolates from patients in North India. Int J Infect Dis. 2013 doi: 10.1016/j.ijid.2012.12.003. In press. [DOI] [PubMed] [Google Scholar]
- 52.Xiao L. Molecular epidemiology of cryptosporidiosis: An update. Exp Parasitol. 2010;124:80–9. doi: 10.1016/j.exppara.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 53.Ten Hove RJ, van Lieshout L, Brienen EA, Perez MA, Verweij JJ. Real-time polymerase chain reaction for detection of Isospora belli in stool samples. Diagn Microbiol Infect Dis. 2008;61:280–3. doi: 10.1016/j.diagmicrobio.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Lalonde LF, Gajadhar AA. Highly sensitive and specific PCR assay for reliable detection of Cyclospora cayatanensis oocysts. Appl Environ Microbiol. 2008;74:4354–8. doi: 10.1128/AEM.00032-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saigal K, Sharma A, Sehgal R, Sharma P, Malla N, Khurana S. Intestinal microsporidiosis in India: A two year study. Parasitol Int. 2013;62:53–6. doi: 10.1016/j.parint.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 56.Weiss LM, Vossbrinck CR. Microsporidiosis: Molecular and diagnostic aspects. Adv Parasitol. 1998;40:351–95. doi: 10.1016/s0065-308x(08)60127-x. [DOI] [PubMed] [Google Scholar]
- 57.Dowd SE, Gerba CP, Enriquez FJ, Pepper IL. PCR amplification and species determination of microsporidia in formalin-fixed feces after immunomagnetic separation. Appl Environ Microbiol. 1998;64:333–6. doi: 10.1128/aem.64.1.333-336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fotedar R, Stark D, Beebe N, Marriott D, Ellis J, Harkness J. PCR detection of Entamoeba histolytica, Entamoeba dispar, and Entamoeba moshkovskii in stool samples from Sydney, Australia. J Clin Microbiol. 2007;45:1035–7. doi: 10.1128/JCM.02144-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cruz-Reyes JA, Spice WM, Rehman T, Gisborne E, Ackers JP. Ribosomal DNA sequences in the differentiation of pathogenic and non-pathogenic isolates of Entamoeba histolytica. Parasitol. 1992;104:239–46. doi: 10.1017/s0031182000061679. [DOI] [PubMed] [Google Scholar]
- 60.Amar CF, Dear PH, McLauchlin J. Detection and genotyping by real-time PCR/RFLP analyses of Giardia duodenalis from human faeces. J Med Microbiol. 2003;52:681–3. doi: 10.1099/jmm.0.05193-0. [DOI] [PubMed] [Google Scholar]
- 61.Ghosh S, Debnath A, Sil A, De S, Chattopadhyay DJ, Das P. PCR detection of Giardias in stool: Targeting intergenic spacer region of multicopy rRNA gene. Mol Cell Probes. 2000;14:181–9. doi: 10.1006/mcpr.2000.0302. [DOI] [PubMed] [Google Scholar]
- 62.Andrews BJ, Mentzoni L, Bjorvatn B. Zymodeme conversion of isolates of Entamoeba histolytica. Trans R Soc Trop Med Hyg. 1990;84:63–5. doi: 10.1016/0035-9203(90)90385-r. [DOI] [PubMed] [Google Scholar]
- 63.Gross U. Treatment of microsporidiosis including albendazole. Parasitol Res. 2003;90:S14–8. doi: 10.1007/s00436-002-0753-x. [DOI] [PubMed] [Google Scholar]
- 64.Conteas CN, Berlin OG, Ash LR, Pruthi JS. Therapy for human gastrointestinal microsporidiosis. Am J Trop Med Hyg. 2000;63:121–7. doi: 10.4269/ajtmh.2000.63.121. [DOI] [PubMed] [Google Scholar]
- 65.Farthing MJ. Treatment options for the eradication of intestinal protozoa. Nat Clin Pract Gastroenterol Hepatol. 2006;3:436–45. doi: 10.1038/ncpgasthep0557. [DOI] [PubMed] [Google Scholar]
- 66.Gupta YK, Gupta M, Aneja S, Kohli K. Current drug therapy of protozoal diarrhoea. Indian J Pediatr. 2004;71:55–8. doi: 10.1007/BF02725657. [DOI] [PubMed] [Google Scholar]
- 67.Katiyar SK, Gordon VR, McLaughlin GL, Edlind TD. Antiprotozoal activities of benzimidazoles and correlations with beta-tubulin sequence. Antimicrob Agents Chemother. 1994;38:2086–90. doi: 10.1128/aac.38.9.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]


