Abstract
Intraocular infestation by live Gnathostoma spinigerum is a rare occurrence in humans. Most of the published reports are from South-East Asia. We report a case of ocular gnathostomiasis from Western Maharashtra, where the worm was removed live from the anterior chamber of the left eye. Identification of the worm was confirmed by light microscopy.
KEY WORDS: Gnathostoma spinigerum, gnathostomiasis, intraocular infestation
Human ocular infestation by live Gnathostoma spinigerum is a rare occurrence and mostly reported from South-East Asia. It involves the eyelids, conjunctiva, cornea, anterior chamber, uvea and vitreous cavity. The first case of ocular gnathostomiasis was reported from India in 1945 by Sen et al.[1] A Medline search found that among 18 intraocular gnathostomiasis cases reported so far intra-vitreal live parasite was found only in four cases.[2] Here, we report a case presenting a live worm in the anterior chamber of the eye.
THE CASE
A 55-year-old man came to a private ophthalmologist at Malegaon with complains of redness, itching and pain in the left eye since 1½ month. The onset was insidious and progressive. The ophthalmologist on examination noticed diminished vision, secondary glaucoma, and hypopyon (1+). The right eye was normal. There were no systemic signs observed. The man has not given any history of diabetic or hypertension. He was given a course of broad-spectrum antibiotics and oral prednisolone (60 mg/day for 5 days). The inflammation almost subsided with the prednisolone and antibiotic and full ocular examination was then possible. On the 3rd day, on slit lamp examination the ophthalmologist observed a moving white object in the anterior chamber [Figure 1] along with fresh retinochoroidal hemorrhage and two chorioretinal scars. Ophthalmologist diagnosed it as iridocyclitis with raised intra-ocular pressure. Hemogram was normal and no eosinophilia or worm was detected. Enzyme immune-assay for antibodies to cysticercosis and echinococcosis was negative. No parasitic form was demonstrated in stool examination.
Figure 1.
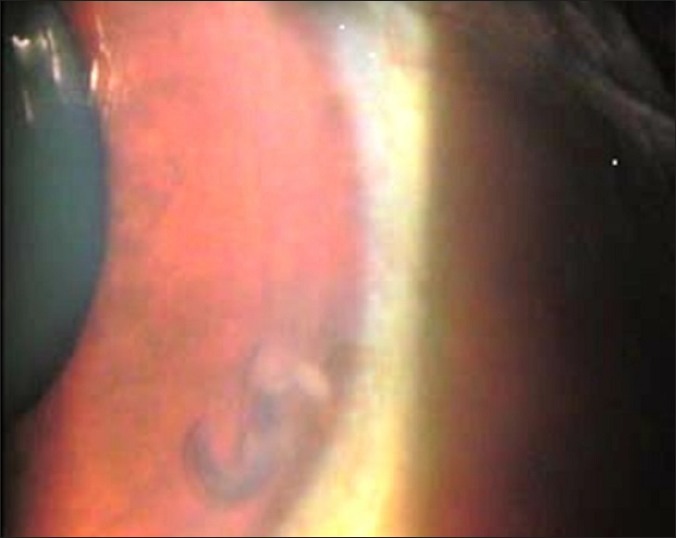
White object in anterior chamber under slit lamp
The ophthalmologist planned on 4th day for pars planar vitrectomy a live worm was removed and sent to Microbiology for identification. The worm was short and stout, reddish brown colored measuring about 3 mm in length and 0.5 mm in diameter [Figure 2]. On light microscopy, the worm was observed to be slightly transparent, with a cephalic bulb and a tapering body, separated by a cervical constriction. Cephalic bulb had a cup shaped mouth with two lips and four circumferential rows of hook lets. Spiny cuticle noticed on the anterior part of the body. A bulbar esophagus seen with two salivary glands was observed. It was identified as a third stage larva of G. spinigerum [Figures 3 and 4] on the basis of
Figure 2.

Reddish brown colored third stage larva of Gnathostoma spinigerum
Figure 3.
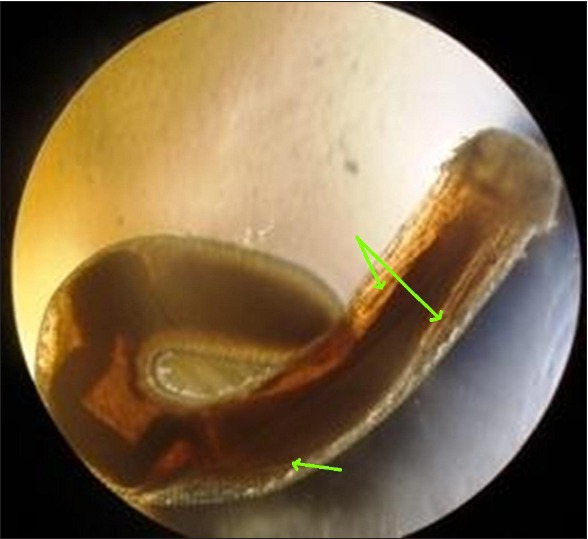
Two salivary glands and bulbar esophagus in the body cavity of Gnathostoma
Figure 4.
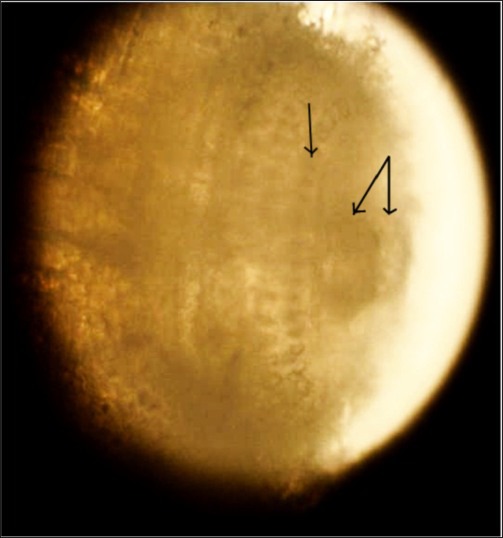
Cephalic bulb of Gnathostoma larva with a pair of lip and rows of hook-lets
Reddish brown colored transparent, short and stout larva.
Cephalic bulb with four rows of circumferential hook-lets and a pair of lip.
Cuticular spines on body.
At the time of dispatching the report to the patient, we had discussed the consumption of any non-vegetarian food in the past days, for which he had mentioned about his recent visit to Goa and consumption of fish.
CONCLUSIONS
Intraocular parasitic infections are uncommon, with most cases reported from countries in endemic areas and the nematodes are the commonest causative agent. However, the intraocular Gnathostomiasis is very rare. The genus Gnathostoma contains 12 species. Out of these, four (G. spinigerum, Gnathostoma hispidum, Gnathostoma doloresi and Gnathostoma nipponicum) are zoonotic in South-East Asia and Far-East. Among these, G. spinigerum is well studies species and was discovered in 1889 in Thailand. It less frequently reported to cause zoonosis in man. G. spinigerum has been reported from Thailand, Japan, Malaysia, China, India, Java, Israel, and Vietnam and Philippines.[3] The commonest definitive hosts are dogs, cats, and feline groups of animals, in which adult worm resides in the stomach wall. These hosts release eggs into the water and develop further into the first stage larva. The fresh water living Copepods acts as first intermediate host where the second stage of larva present in the gut. These are eaten by second intermediate hosts like freshwater fish, snakes, snails, frogs, and paratenic hosts (birds, pigs etc.) where the second stage larva converts into the third stage larva (A L3).[2,3]
Man is an accidental host and represents the dead end for the parasite. Human infection occurs due to consumption of third-stage larva in raw fish or other intermediate hosts such as shellfish snakes, frogs, and chickens. The other route of entry is penetration through the skin during food handling and rarely transplacental transmission.[4,5] However, the larva cannot mature into the adult form in humans, the third-stage larva can only wander within the body of the host; clinical symptoms of gnathostomiasis are due to the inflammatory reaction provoked by these migrating larvae. Clinically, it may present as painful cutaneous larva migrans, gastrointestinal, genitourinary, and central nervous system infections, with or without peripheral blood eosinophilia (eosinophil count >0.4 × 109/L), or undiagnosed eosinophilia with nonspecific symptoms.[6] Once the disease is diagnosed, management is of gnathostomiasis is straightforward. In non-endemic area its unusual symptoms or absence of physical symptoms the diagnosis is being overlooked. It may prevent due to consideration of possible parasitic causes. Hence, eosinophilia cannot be considered as a screening tool. However, David et al. have commenced that it could be used as a marker of treatment response in those with eosinophilia at baseline.[7]
In the present case, there was no cutaneous manifestation. A female preponderance has been reported in the literature, the ratio was 1:0.4.[1,2,4,8,9,10,11,12,13] Most of the ocular gnathostomiasis reported cases including our case have shown no eosinophilia and which were localized.[7] The patient was treated with albendazole 400 mg/day for 21 days. Residued damage to the eye was not noticed. There was no further systemic or localized manifestation observed.
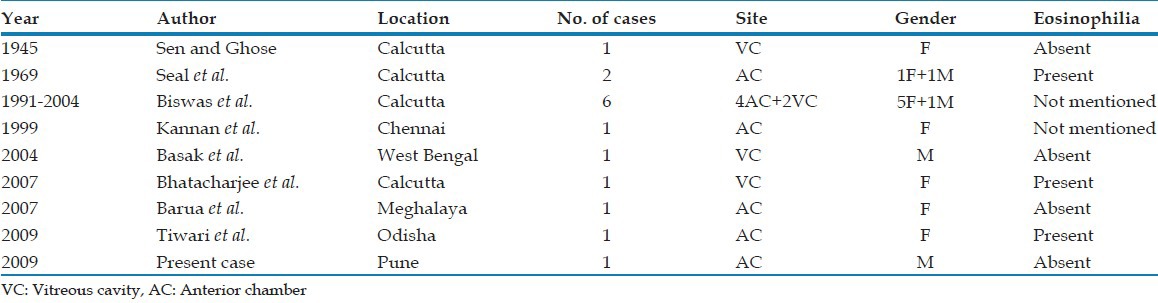
Up till now 14 intra-ocular gnathostomiasis cases have been documented from India, 10 were from Calcutta and one each from Chennai, West Bengal, Meghalaya and Odisha [Table 1].[1,2,4,8,9,10,11,12,13] The case is being presented as no such case has been reported previously from Maharashtra due to consumption of extensive fish. [Table 1]
Table 1.
Intra-ocular gnathostomiasis reported cases from India
Ocular larva migrans syndrome due to advanced third stage larva (A L3) of Gnathostoma species seems to be emerging public health problem. This could be due to two factors; the custom of eating raw freshwater fish in the form of a traditional dish and tiny pieces of raw fillet is common in the coastal part of India.
ACKNOWLEDGEMENTS
The authors are thankful to Dr. Bhambre, an Ophthalmologist at Nashik and the patient himself for providing the clinical history.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Sen K, Ghose N. Ocular gnathostomiasis. Br J Ophthalmol. 1945;29:618–26. [PMC free article] [PubMed] [Google Scholar]
- 2.Basak SK, Sinha TK, Bhattacharya D, Hazra TK, Parikh S. Intravitreal live Gnathostoma spinigerum. Indian J Ophthalmol. 2004;52:57–8. [PubMed] [Google Scholar]
- 3.Herman JS, Chiodini PL. Gnathostomiasis, another emerging imported disease. Clin Microbiol Rev. 2009;22:484–92. doi: 10.1128/CMR.00003-09. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barua P, Hazarika NK, Barua N, Barua CK, Choudhury B. Gnathostomiasis of the anterior chamber. Indian J Med Microbiol. 2007;25:276–8. doi: 10.4103/0255-0857.34775. [DOI] [PubMed] [Google Scholar]
- 5.Houston S. Gnathostomiasis: Report of a case and brief review. Can J Infect Dis. 1994;5:125–9. doi: 10.1155/1994/739818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kraivichian K, Nuchprayoon S, Sitichalernchai P, Chaicumpa W, Yentakam S. Treatment of cutaneous gnathostomiasis with ivermectin. Am J Trop Med Hyg. 2004;71:623–8. 6. [PubMed] [Google Scholar]
- 7.Moore DA, McCroddan J, Dekumyoy P, Chiodini PL. Gnathostomiasis: An emerging imported disease. Emerg Infect Dis. 2003;9:647–50. doi: 10.3201/eid0906.020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baquera-Heredia J, Cruz-Reyes A, Flores-Gaxiola A, López-Pulido G, Díaz-Simental E, Valderrama-Valenzuela L. Case report: Ocular gnathostomiasis in northwestern Mexico. Am J Trop Med Hyg. 2002;66:572–4. doi: 10.4269/ajtmh.2002.66.572. [DOI] [PubMed] [Google Scholar]
- 9.Seal GN, Gupta AK, Das MK. Intra-ocular gnathostomiasis. J All India Ophthalmol Soc. 1969;17:109–13. [PubMed] [Google Scholar]
- 10.Kannan KA, Vasantha K, Venugopal M. Intraocular gnathostomiasis. Indian J Ophthalmol. 1999;47:252–3. [PubMed] [Google Scholar]
- 11.Biswas RK, Biswas J, Gopal L, Sharma T, Bhende MP. Intraocular gnathostomiasis a series of six cases with review of literature published in AIOC proceeding. (516-8).2005:11. [Google Scholar]
- 12.Bhattacharjee H, Das D, Medhi J. Intravitreal gnathostomiasis and review of literature. Retina. 2007;27:67–73. doi: 10.1097/01.iae.0000224943.98423.e3. [DOI] [PubMed] [Google Scholar]
- 13.Tiwari S, Chayani N, Rautaraya B. Intraocular Gnathostoma spinigerum: A case report. Cases J. 2009;2:9370. doi: 10.1186/1757-1626-2-9370. [DOI] [PMC free article] [PubMed] [Google Scholar]


