Highlights
-
•
Galvanic vestibular stimulation is used to study vestibulospinal asymmetry.
-
•
Responses to monaural stimulation of left and right healthy ears are not different.
-
•
In schwannoma the unaffected-ear response is larger than the affected-ear response.
-
•
The asymmetry ratio of right and left-ear responses is elevated in schwannoma.
-
•
The method provides a lateralising test of vestibulospinal pathways for balance.
Keywords: Galvanic vestibular stimulation, Vestibular schwannoma, Balance, Vestibular system, Vestibulospinal
Abstract
Objective
We investigated the potential of galvanic vestibular stimulation (GVS) to quantify lateralised asymmetry of the vestibulospinal pathways by measuring balance responses to monaural GVS in 10 subjects with vestibular schwannoma and 22 healthy control subjects.
Methods
Subjects standing without vision were stimulated with 3 s, 1 mA direct current stimuli delivered monaurally. The mean magnitude and direction of the evoked balance responses in the horizontal plane were measured from ground-reaction forces and from displacement and velocity of the trunk. Vestibular-evoked myogenic potentials (VEMPs) to 500 Hz air and bone-conducted tones were also recorded.
Results
In healthy subjects, the magnitudes of the force, velocity and displacement responses were not significantly different for left compared to right ear stimulation. Their individual asymmetry ratios were always <30%. Subjects with vestibular schwannoma had significantly smaller force, velocity and displacement responses to stimulation of the affected compared with non-affected ear. Their mean asymmetry ratios were significantly elevated for all three measures (41.2 ± 10.3%, 40.3 ± 15.1% and 21.9 ± 14.6%).
Conclusions
Asymmetry ratios of balance responses to monaural GVS provide a quantitative and clinically applicable lateralising test of the vestibulospinal pathways.
Significance
This method offers a more clinically relevant measure of standing balance than existing vestibular function tests which assess only vestibuloocular and vestibulocollic pathways.
1. Introduction
The vestibular system helps maintain stability of the head and body during a variety of movements. The neural pathways begin at the vestibular primary afferents and end at spinal motoneurones that project to muscles of the neck, trunk and limbs. We shall refer to these as vestibulospinal pathways (Baloh and Honrubia, 2001). These pathways can be compromised in peripheral and central vestibular disorders as well as from ablative procedures resulting in vestibular loss. To date, there is no clinically applicable measure of lateralised asymmetry of the vestibulospinal pathways, the assessment of which is likely to be valuable in subjects with gait or stance instability from vestibular disorders. Currently available measures of vestibular lateralised asymmetry focus either on the oculomotor system using caloric vestibular stimulation, or the neck musculature using bone or air-conducted sound vestibular stimulation.
Galvanic Vestibular Stimulation (GVS) offers a non-physiological means of stimulating vestibular afferents (Goldberg et al., 1984). Although the stimulus is non-physiological it evokes a functional balance response involving the legs, trunk and neck (Day et al., 1997). This occurs because the net semicircular canal afferent input evoked by GVS is analogous to a real rotation of the head about an axis in the mid-sagittal plane of the head, close to the roll axis (Fitzpatrick and Day, 2004; Day and Fitzpatrick, 2005). With GVS it is possible to stimulate afferents from each labyrinth separately using monaural stimuli via electrodes placed on one side of the head and to measure the magnitude and direction of the GVS-evoked body sway in three dimensions (Day et al., 2010). Monaural cathodal stimulation produces an increase in the firing rate of the ipsilateral vestibular afferents to evoke a compensatory body sway away from the stimulus, while anodal stimulation decreases the afferent fring rate and evokes oppositely directed sway towards the stimulus (Day et al., 2010). Since the first description of postural responses to GVS (Coates and Stoltz 1969) the attributes of the responses have been extensively described in the literature, yet the technique has not been utilized in clinical settings thus far.
Vestibular schwannomas are slow-growing neoplasms that may present with tinnitus or altered hearing as well as disequilibrium (Asthagiri et al., 2007). They may arise from the superior or inferior division of the vestibular nerve. Diagnosis is made on magnetic resonance imaging. Auditory brainstem responses show interaural differences in wave V latency and wave I–V interpeak intervals in 90–100% subjects (Asthagiri et al., 2007). Caloric asymmetries have been found in up to 85% of pre-surgical subjects (Tringali et al., 2010) while asymmetrical or absent sound-evoked VEMPs are observed in 78% of subjects at diagnosis (Murofushi et al., 1998). In the present study, we examine the direction, magnitude and symmetry of whole-body balance responses to monaural GVS in healthy control subjects and subjects with schwannoma. Our primary aim was to compare the range of asymmetries produced by the lesions to assess the technique’s potential for quantifying lateralised asymmetry in vestibulospinal pathways, analogous to the caloric test or the VEMP used for vestibuloocular and vestibulocollic pathways respectively.
2. Methods
Subjects were recruited from the Neuro-otology Clinics of the National Hospital for Neurology and Neurosurgery Queen Square, London and the Royal Prince Alfred Hospital, Sydney. They gave written informed consent and were studied according to the guidelines of the Helsinki Declaration, with local ethics committee approval. The presence of a vestibular schwannoma was diagnosed and quantified on magnetic resonance imaging using the maximum extrameatal tumour diameter (Kanzaki et al., 2003). Twenty-two healthy controls aged 41.3 ± 5.9 years were studied for purposes of collecting laboratory normative data.
2.1. Screening tests
All patients underwent audiometry, magnetic resonance imaging and a clinical examination inclusive of neurological assessment of cranial nerves and limbs, oculomotor examination and head impulse testing. Caloric function tests or auditory brainstem responses were not performed.
2.2. Screening vestibular evoked myogenic potentials (VEMP)
Subjects lay semi-recumbent at 45° on a couch with an adjustable backrest. For sound evoked VEMPs, they were delivered 256 monaural 120 dB SPL/500 Hz/6 ms tones at 5/s. For vibration evoked VEMPs, 136 dB FL vibration pulses of 500 Hz/6 ms were delivered via a B71 bone conduction vibrator. Rectified and unrectified EMG were sampled at 10 kHz, bandpass filtered 20–2000 Hz and averaged for 256 trials. A standard recording montage was used, with the active electrode over the middle third of the sternocleidomastoid muscle and the reference electrode over the sternoclavicular joint. The VEMP was measured peak-to-peak and divided by the mean rectified EMG over a 20 ms prestimulus period.
2.3. Galvanic vestibular stimulation
One milliampere monaural cathodal or anodal galvanic stimulation was delivered using 2.5 cm diameter circular neurostimulation electrodes (PALs plus, Nidd Valley Medical Ltd., Knaresborough, North Yorkshire, UK). The active electrode was placed over the mastoid process and the reference over the T2 spinous process
Ground reaction force data were measured in 3 dimensions using a fixed force plate (Kistler type 9281B). The 3 dimensional position and velocity of an infrared marker attached to the prominence overlying the C7 spinous process was measured using a CODA motion analysis system (hybrid MPX30 and CX1 units: Charnwood Dynamics, Rothley, Leicestershire, UK). Force and kinematic data were collected at 200 Hz.
Before commencement of each trial, subjects stood quietly on the force plate with their feet together and eyes shut. Data collection was initiated with a key press and commenced after a random delay of 50–500 ms. Each trial consisted of a 2 s prestimulus period, a 3 s stimulus and a 2 s post stimulus period (7 s in total). The side of the active electrode (left or right) and the polarity (cathode or anode) was randomized across trials. For every condition tested (2 sides × 2 polarities), 20 trials were time-locked to stimulus onset and averaged offline.
2.4. Analyses
To increase the signal-to-noise ratio of the responses the averages to the two stimulation polarities were combined separately for the x-direction and the y-direction. Because the two polarities evoked responses in opposite directions the signs of the response to one polarity was inverted (multiplied by −1) before the two polarities were averaged. For the left ear the response to anodal stimulation was inverted, whereas for the right ear the response to cathodal stimulation was inverted. This ensured that the lateral component of the averaged response, when present, was directed towards the positive x-axis i.e. towards the right. To increase response reliability further, the mean response to turning the stimulus off was averaged with the mean response to turning the stimulus on. Again, the on and off responses were oppositely directed and so the off-response was inverted before averaging with the on–response.
Response measurements were made on the x and y-components of these net averaged responses using the three variables of force, velocity and displacement. The force response was quantified as the mean horizontal force level between 0.2 and 1.0 s after stimulus onset/offset. The velocity response was quantified as the difference in horizontal velocity of the C7 marker between 0.2 and 1.0 s after stimulus onset/offset. The displacement response was quantified as the difference in horizontal position of the C7 marker between 0.2 and 2.0 s after stimulus onset/offset. For each of these variables the response magnitude was calculated as square root (x2 + y2), and the response direction was calculated as arctan (y/x), where x and y represent the x-components and y-components respectively of the force, velocity and displacement responses. The response direction convention was such that the positive x-axis (rightward) was set at 0° and the positive y-axis (forward) was at +90°. A response that was directed perfectly towards the anode or away from the cathode would register a response direction of 0°.
The lateralised asymmetry from stimulation of each ear was expressed using an asymmetry ratio (AR) of the responses such that AR = absolute (R − L)/(R + L), where R and L represent the response magnitudes obtained from stimulation of the right and left ears respectively. An AR equal to zero denotes perfect symmetry. The AR was calculated separately for the VEMP response and each measure of GVS response for each subject.
2.5. Statistical analysis
SPSS v16 was used for statistical analyses (SPSS Inc., Chicago, Ill). All descriptive data are given as mean ± SD. Response magnitudes to stimulation of each ear were compared using two-tailed paired t-tests. Response asymmetry ratios were compared between controls and patients using two-tailed unpaired t-tests. Hotelling’s test with 1-tailed probability for paired angular data was used to compare response directions evoked by stimulation of each ear (Zar, 2010). The significance level was set at p < 0.05.
3. Results
3.1. Clinical assessment
All subjects presented with an auditory symptom (muffled hearing: n = 8, tinnitus n = 7). One presented with imbalance and none reported vertigo. Clinical symptoms, examination findings and screening VEMP results are summarized in Table 1.
Table 1.
Presenting symptoms, examination findings, VEMP asymmetry, maximum tumour diameter and asymmetry of the 3 sway parameters in 10 subjects with unilateral vestibular schwannoma.
| HL | TIN | HIT | HSN | ROMB | UNT | AC VEMP%AR | BC VEMP%AR | DIAM (mm) | (%) AR (Force) | (%) AR (Velocity) | (%) AR (Displacement) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | + | + | + | + | − | + | 100 | 100 | 40 | 51.8 | 55.9 | 40.8 |
| S2 | + | + | − | − | − | 100 | 100 | 30 | 44.1 | 51.7 | 38.3 | |
| S3 | + | + | − | − | − | 100 | 100 | 35 | 32.1 | 40.0 | 38.4 | |
| S4 | + | − | − | − | − | 100 | 100 | 23 | 32.8 | 34.4 | 25.1 | |
| S5 | − | + | − | − | − | 46.6 | 3.9 | 17 | 58.6 | 62.3 | 15.8 | |
| S6 | + | + | − | − | − | 15.4 | 11.9 | 25 | NR | 19.4 | 3.6 | |
| S7 | + | − | − | − | − | 100 | 100 | 25 | NR | 54.4 | 29.3 | |
| S8 | + | − | − | − | − | 100 | 17.4 | 18 | 44.7 | 33.9 | 6.6 | |
| S9 | + | − | − | − | − | 100 | 100 | 20 | 34.4 | 20.7 | 3.9 | |
| S10 | + | + | + | + | − | + | 100 | 100 | 40 | 30.9 | 30.4 | 16.9 |
HL = hearing loss; TIN = tinnitus; HIT = head impulse test; HSN = head shaking nystagmus; UNT = unterberger test; AC = air conducted; BC = bone conducted; AR = asymmetry ratio.
3.2. Vestibular-evoked myogenic potentials (VEMP)
Seven of the 10 subjects tested had absent vestibular-evoked myogenic potentials to both air and bone conducted pure tones upon stimulation of the affected side. Two subjects with 18 and 17 mm schwannomas had asymmetrical VEMPs evoked by air-conducted sound (AR = 100% and 46.6%) but not bone-conducted vibration (AR = 17.4% and 3.9%). One subject with a 25 mm schwannoma had symmetrical VEMPs to both stimuli (AR of 15.4% and 11.9%).
3.3. GVS-evoked balance responses
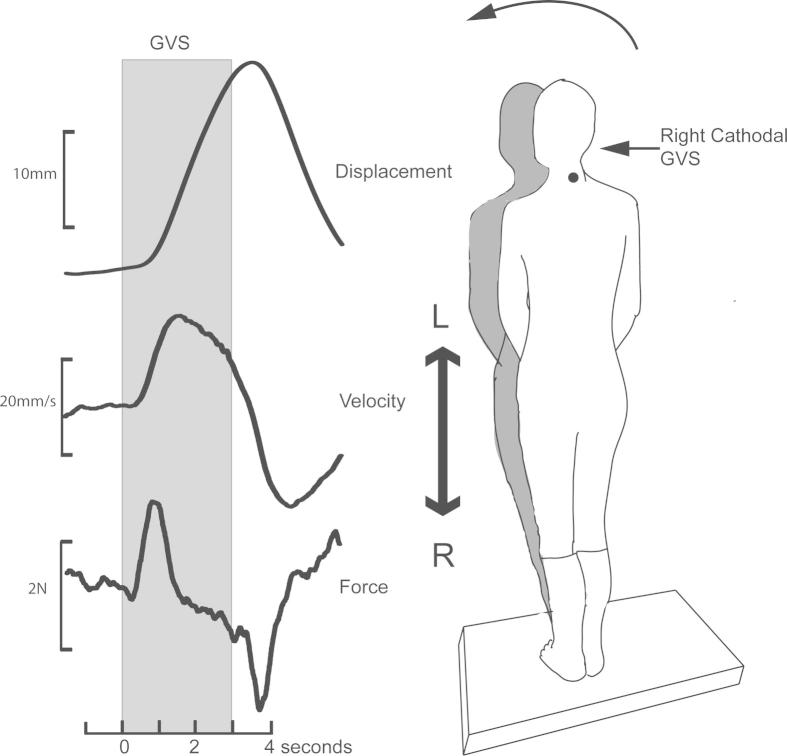
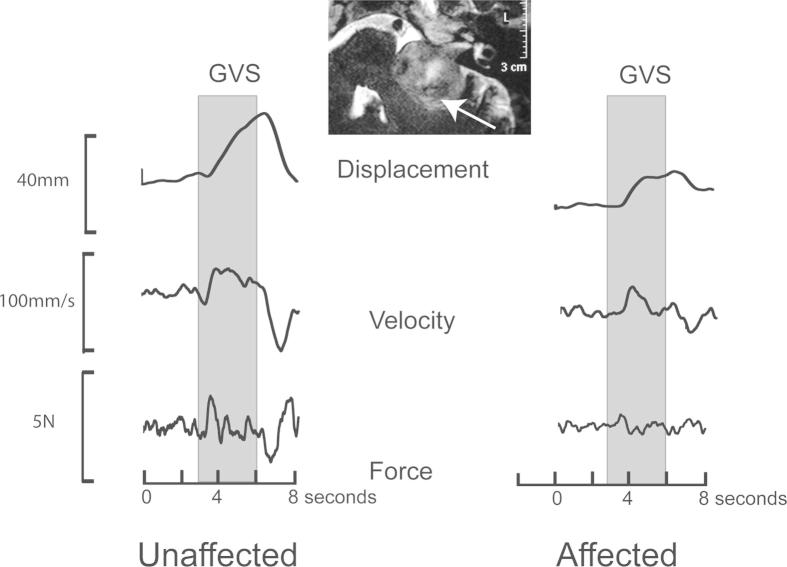
In response to a 1 mA monaural galvanic stimulus, healthy controls swayed laterally away from the cathodal ear or towards the anodal ear. The earliest response to GVS was a change in the ground reaction force, which began about 200 ms after stimulus onset and peaked at 450 ms (Fig. 1). Following stimulus offset, an oppositely directed force response was recorded at a similar latency (“off response”). About 250 ms from stimulus onset, the velocity of the body (measured at C7) began to increase, peaking at 1 s and decreasing towards baseline levels thereafter. Displacement, also measured at C7, began at ∼450 ms after stimulus onset and kept increasing for the stimulus duration. At stimulus offset, an oppositely directed “off response” returned the body approximately to its starting position. Fig. 2 shows traces from one patient with schwannoma. The amplitudes and directions of the force, velocity and displacement responses were measured in all subjects except for 2 subjects with schwannoma in whom the force records were not available due to equipment failure (Table 1).
Fig. 1.
Horizontal displacement, velocity and shear force responses during and after a 3 s pulse of 1 mA GVS. A right cathodal stimulus evokes leftward sway after stimulus onset “On response” and a return to the baseline at stimulus offset “Off response”.
Fig. 2.
Horizontal displacement, velocity and shear force responses in a subject with a large left sided schwannoma affecting the left side and causing brainstem compression. All three responses were attenuated upon stimulation of the affected side.
In the healthy control group, the left-ear response was not significantly different to the right-ear response (Table 2; force: t(21) = 0.72, p = 0.478; velocity: t(21) = 0.45, p = 0.656; displacement: t(21) = 0.36, p = 0.719. In the schwannoma group, stimulation of the unaffected ear evoked significantly larger mean responses than the affected ear (Table 2; force: t(7) = 5.08, p = 0.001; velocity: t(9) = 3.23, p = 0.010; displacement: t(9) = 3.28, p = 0.010.
Table 2.
The magnitude, direction and asymmetry ratios of force, velocity and displacement responses to GVS. All values represent the average of “On” and “Off” responses. Response magnitudes are group arithmetic mean (standard deviation) and response directions are group angular mean (angular deviation). Directions are in degrees according to sign convention in methods, and r is a measure of concentration of direction within the group. For healthy controls, the upper limit of normal reflex asymmetry is calculated as mean + 2SD. N = 8 for force measurements in schwannoma group.
| Group | Side of stimulus | Measure | Force (N) | Velocity (mm/s) | Displacement (mm) |
|---|---|---|---|---|---|
| Control (n = 22) | Left | Magnitude | 1.45 (0.82) | 20.37 (10.19) | 19.00 (9.19) |
| Direction | 1.03 (7.73) | −0.78 (8.54) | 1.84 (8.83) | ||
| r | 0.991 | 0.989 | 0.988 | ||
| Right | Magnitude | 1.40 (0.77) | 19.71 (9.01) | 19.39 (7.95) | |
| Direction | −6.50 (7.39) | −7.39 (6.76) | −6.11 (7.18) | ||
| r | 0.992 | 0.993 | 0.992 | ||
| % AR | 10.21 (7.43) | 11.89 (8.53) | 10.63 (7.05) | ||
| Range | 0.74–22.97 | 0.04–29.85 | 0.4–27.90 | ||
| Upper limit | 25.08 | 28.95 | 24.73 | ||
| Schwannoma (n = 10) | Affected | Magnitude | 0.38 (0.14) | 6.60 (4.69) | 6.19 (2.18) |
| Direction | −12.79 (16.59) | −5.28 (23.04) | 7.30 (21.72) | ||
| r | 0.916 | 0.838 | 0.856 | ||
| Unaffected | Magnitude | 0.93 (0.41) | 17.73 (14.79) | 10.32 (5.53) | |
| Direction | −10.05 (19.80) | −12.57 (20.38) | −3.04 (16.56) | ||
| r | 0.881 | 0.874 | 0.916 | ||
| % AR | 41.17 (10.30) | 40.33 (15.10) | 21.86 (14.57) | ||
| Range | 30.95–58.62 | 19.44–62.3 | 3.65–40.76 | ||
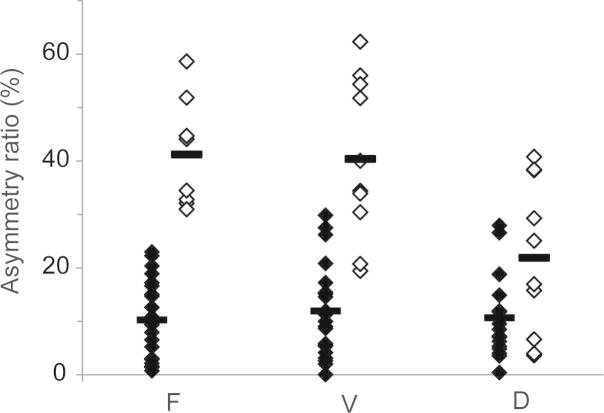
The patients’ asymmetry ratios were significantly larger than control for all three measures (Table 2; force: t(27) = 8.87, p < 0.001; velocity: t(30) = 6.83, p < 0.001; displacement: t(30) = 2.97, p = 0.006). Fig. 3 shows that the asymmetry ratio of the force measure discriminated the patients from controls better than the AR of the other two measures, the displacement measure being the worst. This was reflected in the number of patients who had AR values within the normal range defined as the control mean + 2SD (Table 2). Thus, no patients had a normal force AR, two had a normal velocity AR, and 5 had a normal displacement AR (Table 1).
Figure 3.
Reflex asymmetry ratios for healthy controls (black) and subjects with vestibular schwannoma (white) for force (F), velocity (V) and displacement (D) responses. Group mean values shown by thick horizontal bars.
The directions of the force, velocity and displacement responses are summarised in Table 2. All left-ear responses were mirrored about the mid-sagittal plane to compare with right-ear responses. The r-values, which indicated the degree of response direction concentration within a group, were >0.83 for all measures, indicating highly significant between-subjects clustering of the response directions (p < 0.001 in all cases). In healthy subjects there were small differences (<8°) in response direction for left-ear versus right-ear stimulation, which reached significance only for the displacement measure (Hotelling F = 4.53, p = 0.025). In schwannoma patients, for all measures there were no significant differences in the mean response direction to stimulation of the affected compared to unaffected ear.
4. Discussion
In the present study we have focused on the magnitude and direction of 3 measures of balance response to monaural galvanic vestibular stimulation (GVS). The measures were taken from the ground-reaction shear force, which is proportional to horizontal acceleration of the body’s centre of mass, and the horizontal velocity and displacement of the body at the level of the C7 spinous process. In control subjects, the responses from independent stimulation of the right and left ears fell within the range of asymmetry ratios described for the bithermal caloric test (0–25%) and the sound-evoked VEMP (0–35%) (Tringali et al., 2010; Murofushi et al., 1998). In subjects with schwannoma, average asymmetries were significantly larger than control for all 3 measures. Individual asymmetry ratios fell outside the normal range for the force-response measure in all recorded subjects (8/8), and for the velocity response in all but two subjects (8/10). Individual asymmetry ratios of the displacement measure fell outside the normal range for only 50% subjects (5/10). Overall, this indicates that velocity and force parameters of GVS-evoked sway provide a useful lateralizing measure of vestibulospinal pathways. Displacement measures, which were measured over a longer time interval, were a poor discriminator between controls and schwannomas.
Unlike the caloric test or VEMP, the GVS-evoked sway constitutes a behavioural response to vestibular stimulation. Its magnitude is modifiable by vision, availability of proprioceptive information and musculo-skeletal stability (Day et al., 1997; Britton et al., 1993; Fitzpatrick et al., 1994). The absolute magnitude of the sway thus cannot be considered exclusively representative of vestibular function and needs to be interpreted in the context of other available sensory modalities. However, these complications are unlikely to affect the asymmetry ratio since responses from both ears would be modulated identically. Also, any lateralised neuromuscular asymmetry is unlikely to affect our GVS asymmetry ratio since it has been shown that each labyrinth projects equally to both sides of the body (Day et al., 2010). However, the characteristics of the response in acute and compensated vestibulopathies are not known. Since vestibular schwannomas are slow growing tumors, the responses recorded from our patients are likely to represent partially compensated vestibulopathies. We hypothesize that the relative sparing of the whole body displacement response may perhaps represent the effects of vestibular compensation on these patients. Further studies on acute, well compensated and poorly compensated vestibulopathies will need to explore the dissociation between force and displacement measures.
Following resection of the tumour, a proportion of subjects develops disabling vertigo and ongoing disequilibrium, and fails to rehabilitate satisfactorily (Lynn et al., 1999; Wiegand et al., 1996). It is conceivable that subjects with intact vestibular function prior to surgery are more likely to develop post surgical disequilibrium than those who have pre-existing vestibular loss. The 10 subjects tested in the present study had no complaints of disequilibrium and only two had physical signs of a unilateral vestibulopathy. It may be relevant in this respect that the responses to stimulation of the affected side, although smaller than to stimulation of the unaffected side, were not absent. In contrast, the VEMP yielded “absent responses” in a majority of subjects. This difference may be because GVS is likely to activate both canal and otolith afferents; further, the sway response is most likely produced by stimulation of all semicircular canal afferents (Fitzpatrick and Day, 2004). In contrast, the VEMPs evoked by sound and vibration are likely to represent only otolith afferents and therefore are probably easier to abolish (Curthoys and Vulovic, 2011). The assessment of pre-operative vestibulospinal function with GVS in subjects with vestibular schwannoma is likely to provide a useful pre-surgical baseline and may also enable clinicians to predict the likelihood of postoperative disequilibrium. These measures may also be applicable in the assessment of subjects who are slow to rehabilitate after surgery or radiotherapy.
In conclusion, monaural GVS-evoked body sway measures provide a quantitative and lateralizing test of the vestibulospinal pathways, and may offer a more clinically relevant measure of standing balance than existing vestibular function tests which assess only vestibulo-ocular and vestibulo-collic pathways. Unlike posturography, GVS enables assessment of lateralised vestibular function and is thus applicable to both unilateral and bilateral vestibulopathies.
Acknowledgements
The work was supported by the Medical Research Council. M.W. was supported by the Garnett Passe and Rodney Williams Memorial Foundation and The National Health and Medical Research Council Australia. The authors thank Mr. Ed Bye for technical support.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Asthagiri A.R., Helm G.A., Sheehan J.P. Current concepts in management of meningiomas and schwannomas. Neurol Clin. 2007;25:1209–1230. doi: 10.1016/j.ncl.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Baloh R.W., Honrubia V. Oxford University Press; 2001. Clinical neurophysiology of the vestibular system. [PubMed] [Google Scholar]
- Britton T.C., Day B.L., Brown P., Rothwell J.C., Thompson P.D., Marsden C.D. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res. 1993;94:143–151. doi: 10.1007/BF00230477. [DOI] [PubMed] [Google Scholar]
- Coates A.C., Stoltz M.S. The recorded body sway response to galvanic stimulation of the labyrinth: a preliminary study. Laryngoscope. 1969;79:85–103. doi: 10.1288/00005537-196901000-00004. [DOI] [PubMed] [Google Scholar]
- Curthoys I.S., Vulovic V. Vestibular primary afferent responses to sound and vibration in the guinea pig. Exp Brain Res. 2011;210:347–352. doi: 10.1007/s00221-010-2499-5. [DOI] [PubMed] [Google Scholar]
- Day B.L., Fitzpatrick R.C. Virtual head rotation reveals a process of route reconstruction from human vestibular signals. J Physiol. 2005;567:591–597. doi: 10.1113/jphysiol.2005.092544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B.L., Marsden J.F., Ramsay E., Mian O.S., Fitzpatrick R.C. Non-linear vector summation of left and right vestibular signals for human balance. J Physiol. 2010;588:671–682. doi: 10.1113/jphysiol.2009.181768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B.L., Severac Cauquil A., Bartolomei L., Pastor M.A., Lyon I.N. Human body-segment tilts induced by galvanic stimulation: a vestibularly driven balance protection mechanism. J Physiol. 1997;500:661–672. doi: 10.1113/jphysiol.1997.sp022051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R.C., Burke D., Gandevia S.C. Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol. 1994;478:363–372. doi: 10.1113/jphysiol.1994.sp020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R.C., Day B.L. Probing the human vestibular system with galvanic stimulation. J Appl Physiol. 2004;96:2301–2316. doi: 10.1152/japplphysiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- Goldberg J.M., Smith C.E., Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol. 1984;51:1236–1256. doi: 10.1152/jn.1984.51.6.1236. [DOI] [PubMed] [Google Scholar]
- Kanzaki J., Tos M., Sanna M., Moffat Da. New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol Neurotol. 2003;24:642–649. doi: 10.1097/00129492-200307000-00019. [DOI] [PubMed] [Google Scholar]
- Lynn S.G., Driscoll C.L.W., Harner S.G., Beatty C.W., Atkinson E.J. Assessment of dysequilibrium after acoustic neuroma removal. Am J Otol. 1999;20:484–494. [PubMed] [Google Scholar]
- Murofushi T., Matsuzaki M., Mizuno M. Vestibular evoked myogenic potentials in patients with acoustic neuromas. Arch Otolaryngol Head Neck Surg. 1998;124:509–512. doi: 10.1001/archotol.124.5.509. [DOI] [PubMed] [Google Scholar]
- Tringali S., Charpiot A., Ould M.B., Dubreuil C., Ferber-Viart C. Characteristics of 629 vestibular schwannomas according to preoperative caloric responses. Otol Neurotol. 2010;31:467–472. doi: 10.1097/MAO.0b013e3181cdd8b7. [DOI] [PubMed] [Google Scholar]
- Wiegand D.A., Ojemann R.G., Fickel V. Surgical treatment of acoustic neuroma (vestibular schwannoma) in the United States: report from the acoustic neuroma registry. Laryngoscope. 1996;106:58–66. doi: 10.1097/00005537-199601000-00012. [DOI] [PubMed] [Google Scholar]
- Zar J.H. 5th ed. Pearson Prentice Hall; Upper Saddle River, NJ: 2010. Biostatistical analysis. [Google Scholar]