Abstract
Plasminogen is present in the oviduct, on the zona pellucida (ZP) and on oolemma, and reduces the number of sperm penetrating the oocyte during in vitro fertilization in pig and cow. It is unknown how this reduction occurs. We tested whether plasminogen (1) changed the ZP resistance to enzymatic digestion thus making the passage of the spermatozoa across it difficult; (2) reduced the sperm functionality, assessed by sperm viability, motility, spontaneous acrosome reaction and membrane lipid disorder; or (3) affected the sperm–ZP binding before or after sperm–ZP interaction. The mechanism by which plasminogen/plasmin system contributes to regulate sperm entry into the oocyte is not inducing a ZP hardening or a decrease in sperm functionality but detaching more than 50% of sperm bound to the ZP. It is suggested that the fertilizing spermatozoon activates plasminogen into plasmin at the oocyte surface and that plasmin removes additional spermatozoa attached to the ZP.
Keywords: plasminogen, IVF, sperm-zona pellucida binding, polyspermy, plasmin
Introduction
The plasminogen/plasmin system functions as the most important extracellular protease system in vivo. It is activated when the zymogen plasminogen is converted into the serine protease plasmin by the tissue-type plasminogen activator (tPA) or the uroquinase-type plasminogen activator (uPA). It participates in different processes related to the degradation of protein matrices such as resolution of blood clots or cell migration and dissemination, that is, during embryogenesis, tumor invasion, wound healing, or angiogenesis.1 The fertilization process may include some steps requiring protein degradation such as sperm penetration through the zona pellucida (ZP),2 the remodeling of the ZP, the regulation of the viscosity of the surrounding perivitelline fluid around the oocyte membrane,3 or others. Then, the plasminogen/plasmin system is a candidate to play a role in fertilization, especially in the light of recent studies demonstrating that plasminogen is activated into plasmin during gamete interaction4 and the generated plasmin reduces the number of sperm penetrating the oocyte during in vitro fertilization (IVF).5 However, it is still unknown how this reduction occurs.
Activity of plasminogen activators in oocytes has been demonstrated in different experimental models, most of them indicating that tPA and uPA are secreted by granulose cells and play a role in the ovulation contributing to the degradation of the follicular wall in rat, mouse, cow, pig, and Rhesus monkey.6–11 However, their role in the fertilization process was not partially clarified until recently when it has been showed that plasminogen is present in the oviductal fluid and that plasminogen, tPA, and uPA are immunolocalized on the ZP and oolemma of mature bovine and porcine oocytes.4 These authors showed that after fertilization, plasminogen, tPA, and uPA immunolabeling decreased in oocytes, suggesting the activation of plasminogen into plasmin. Moreover, it has been shown that addition of both plasminogen and plasmin in the IVF medium decreased the sperm penetration suggesting that plasmin is responsible for the observed effect.5 What still remains unclear is the mechanism by which plasminogen/plasmin system reduces penetration of spermatozoa into the oocytes. From the previous data in different species, 3 hypotheses could be considered.
First, it was suggested that tPA is released from cortical granules and could be responsible for the increased resistance of the ZP to protease digestion observed after fertilization in rats, although the mechanism was not explained.12 If this hypothesis was confirmed, the observed reduction in sperm penetration during IVF,5 by addition of plasminogen to the medium, could be due to the binding of plasminogen to oocyte membrane and ZP, and its conversion into plasmin by plasminogen activators. Plasmin, in turn, would induce ZP changes leading to the increased resistance to enzymatic digestion and to sperm entrance, what is known as “ZP hardening.”13 To confirm this hypothesis, in the present study we added plasminogen and plasmin to the IVF medium during gamete interaction and the resistance of the ZP to pronase digestion was evaluated.
Second, tPA and uPA activity in spermatozoa or seminal plasma has also been demonstrated in different mammalian species.14–16 Bearing this in mind, it is plausible that plasminogen added into the IVF medium could be converted into plasmin by tPA or uPA from sperm origin and that such plasmin could be negatively affecting the sperm functionality under the experimental conditions used in the study by Mondéjar et al.4 This hypothesis is contrary to the data from Kim et al17showing that plasminogen increases the number of spermatozoa bound to oocytes and the percentage of oocytes with male pronucleus after fertilization. However, it is necessary to test it in the species (bovine and porcine) and conditions where the reduction in sperm penetration was observed5 before discarding it.
Finally, the hypothesis that plasmin could reduce sperm–ZP binding should also be tested. From the data in Mondéjar et al,4 it was clear that the number of spermatozoa bound to ZP at the end of the in vitro culture (18-20 hours postinsemination) was lower in the presence of plasminogen or plasmin than in their absence. However, this could be due to (1) the avoiding of the initial sperm binding to the ZP or (2) the detaching of the spermatozoa already attached to the ZP by the plasminogen/plasmin system after its activation. To test this last hypothesis, in the present study, we evaluated both the fertilization parameters in bovine and porcine species after addition of plasminogen at different times. Besides, we studied the detachment of spermatozoa from the bovine ZP along the time.
Materials and Methods
This study was developed following institutional approval from the Bioethical Committee in the University of Murcia, and it was performed in accordance with the Animal Welfare regulations of that institution.
Unless otherwise indicated, all chemicals and reagents were purchased from Sigma Aldrich Química SA (Madrid, Spain).
Collection of Oocytes and In Vitro Maturation
Cow
The medium used for in vitro maturation (IVM) of cow oocytes was TCM-199, supplemented in our laboratory as described previously.18 Briefly, cow cumulus–oocyte complexes (COCs) were collected by aspiration from follicles (3-6 mm in diameter) of ovaries of 12- to 18-month-old animals obtained from the slaughterhouse. The COCs were then washed twice in TCM-199 with Hank salts, 10.0 mmol/L (2-Hydroxyethyl)-1-piperazineethanesulfonic acid, 2% fetal bovine serum, 2.0 mmol/L glutamine, 50 U/mL penicillin, and 50 µg/mL streptomycin, and once in maturation medium previously equilibrated for a minimum of 3 hours at 38.5°C under 5% carbon dioxide (CO2) in air. Groups of 35 to 45 COCs were cultured in 500 µL maturation medium for 24 hours at 38.5°C under 5% CO2 in air.
Pig
The medium used for IVM of pig oocytes was North Carolina State University solution-37, prepared in our laboratory as described previously.18 Within 30 minutes of slaughter, ovaries from 6- to 7-month-old animals weighing 80 to 100 kg were transported to the laboratory in saline-containing 100 µg/mL kanamycin sulfate at 38°C, washed once in 0.04% cetrimide solution and twice in saline. The COCs were collected from follicles (3-6 mm diameter), washed twice with Dulbecco phosphate-buffered saline (PBS) supplemented with 1 mg/mL polyvinyl alcohol and 0.005 mg/mL red phenol, and twice more in maturation medium previously equilibrated for a minimum of 3 hours at 38.5°C under 5% CO2 in air. Only COCs with complete and dense cumulus oophorus were used for the experiments. Groups of 35 to 45 COCs were cultured in 500 µL maturation medium for 22 hours at 38.5°C under 5% CO2 in air. After culture, the COCs were washed twice in fresh maturation medium without dibutyryl cyclic adenosisne monophosphate, eCG, and human chorionic gonadotropin and cultured for an additional 20 to 22 hours.19
Assessing of ZP Resistance to Pronase Digestion
For digestion of the ZP, cumulus cells from the in vitro-matured COCs were removed by gently pipetting up and down in a yellow tip adapted to an automatic micropipette with the volume set up at 200 μL. Cumulus-free oocytes were incubated in 50 μL of a 0.5% solution of pronase from Streptomyces griseus (P-8811, Sigma) in PBS.20 Digestion of the ZP was observed continuously at a magnification of ×200 using a stereomicroscope equipped with a hot plate at 38.5°C. The ZP digestion time was defined as the time from placing the oocytes in the pronase solution to the complete disappearance of the ZP. Bovine (n = 215) and porcine (n = 207) oocytes were incubated in IVF medium with plasminogen (150 μg/mL), plasmin (150 μg/mL), or without either for 30 minutes and ZP digestion time was assessed.
In Vitro Fertilization
Cow
The IVF medium for the bovine species consisted of IVF-tyrode albumin lactate pyruvate (TALP) as previously.21 At the end of the maturation period, 35 to 45 COCs were transferred to wells containing 500 µL of IVF-TALP medium. A volume of 25 µL penicillamine–epinephrine–hypotaurine (PHE) were added to each well 30 minutes before insemination.21 Spermatozoa were obtained by centrifugation of two 0.5 mL straws of frozen-thawed semen on a 45/90 discontinuous Percoll (Amersham Pharmacia Biotech Ltd, Uppsala, Sweden) gradient for 10 minutes at 900g. The pellet was resuspended in 10 mL Sperm-TALP medium21 and washed again for 8 minutes at 300g. The final pellet was resuspended in 500 µL IVF-TALP, sperm concentration was adjusted, and cells added at a final concentration of 106 spermatozoa/mL to the IVF-TALP wells containing the COCs.
Pig
Medium used for pig IVF was essentially the same as that used by Rath et al.22 The COCs cultured for a total of 44 hours in maturation medium were washed 3 times with TALP medium and groups of 45 to 55 oocytes were transferred into each well of a 4-well multidish containing 250 µL IVF medium previously equilibrated at 38.5°C under 5% CO2. A sperm-rich fraction of semen from a mature, fertility-tested boar was collected by the gloved hand method and immediately transported to the laboratory diluted at 1:8 in Beltsville thawing solution.23Aliquots of the semen samples (0.5 mL) were centrifuged (700g, 30 minutes) through a discontinuous Percoll (Pharmacia) gradient (45% and 90% v/v) and the resultant sperm pellets were diluted in TALP medium and centrifuged again for 10 minutes at 100g. Finally, the pellet was diluted in TALP and 250 µL of this suspension were added to the wells containing the oocytes, giving a final concentration of 105 cells/mL.
Bovine (n = 387) and porcine (n = 541) oocytes were in vitro fertilized in the absence of plasminogen (control group), in the presence of plasminogen (150 μg/mL) added 30 minutes before adding the spermatozoa (30’ pre-IVF group) or in the presence of plasminogen (150 μg/mL) added at the same time than the spermatozoa (0’ pre-IVF group). Experiments were 4 times replicated including all groups for each species.
Hoechst Staining
At 18 to 20 hours postinsemination, putative zygotes were washed in PBS to remove excess attached sperm, fixed for 30 minutes (0.5% glutaraldehyde in PBS), stained for 15 minutes (1% Hoechst 33342 in PBS), washed in PBS containing 1 mg/mL polyvinylpyrrolidone, and mounted on glass slides. Oocytes were examined under an epifluorescence microscope at ×200 and ×400 magnification. Penetration, number of spermatozoa per oocyte, and number of sperm bound to the ZP (in penetrated oocytes) were assessed in each oocyte.
Sperm Functionality Assessment Processing and Analysis
Different parameters to assess sperm functionality in bull and boar spermatozoa after incubation with or without plasminogen and plasmin were evaluated. The objective was to find out whether the reported decrease in sperm penetration during IVF4 is due to a detrimental effect of plasminogen or plasmin on sperm functionality. The parameters studied were sperm motility, membrane lipid disorder, spontaneous acrosome reaction, and viability based on protocols previously described for bull24 and boar25 spermatozoa.
Assessment of Sperm Motility
Motion parameters were determined using a computer-assisted sperm analysis (CASA) system (ISAS, Valencia, Spain). The CASA-derived motility characteristics studied were the percentage of total motile spermatozoa (Motil %), percentage of motile progressive spermatozoa (Motil prog %), curvilinear velocity (VCL, µm/s), straightline velocity (VSL, µm/s), average path velocity (VAP, µm/s), linearity of the curvilinear trajectory (LIN, ratio of VSL/VCL, %), straightness (STR, ratio of VSL/VAP, %), amplitude of lateral head displacement (ALH, µm), wobble of the curvilinear trajectory (WOB, ratio of VAP/VCL, %), and beat cross-frequency (BCF, Hz).
After sperm processing as described for IVF and incubation for 30 minutes in medium containing either plasminogen (150 μg/mL) or plasmin (150 μg/mL), a 7 µL drop of the sample was placed on a warmed (37°C) slide and covered with a 24 × 24 mm cover slip. The setting parameters were 25 frames in which spermatozoa had to be present in at least 15 in order to be counted. Images were obtained at ×200 magnification in a phase-contrast microscope and spermatozoa with a VAP <20 µm/s were considered immobile. A minimum of 5 fields per sample were evaluated, counting a minimum of 200 spermatozoa per subsample.
Assessment of Seminal Parameters by Flow Cytometry
Flow cytometric analyses were performed on a Coulter Epics XL cytometer (Beckman Coulter Inc, Miami, Florida). A 15 mW argon ion laser operating at 488 nm excited the fluorophores. Data from 10 000 events per sample were collected in list mode, and 4 measures per sample were recorded. Flow cytometric data were analyzed using the program Expo32ADC (Beckman Coulter Inc) using a gate in forward and side scatter to exclude eventual remaining debris and aggregates from the analysis.
Assessment of Sperm Plasma Membrane Lipid Disorder
To detect any increase in plasma membrane lipid packing disorder, sperm samples were stained with merocyanine 540 (M540) and Yo-Pro 1. Stock solutions of M540 (1 mmol/L) and Yo-Pro 1 (25 µmol/L, Molecular Probes, Eugene, Oregon) in dimethyl sulfoxide were prepared. For each of 1 mL diluted semen sample (containing 5-10 × 106 cells), 2.7 µL M540 stock solution (final concentration of 2.7 µmol/L) and 1 µL of Yo-Pro (25 nmol/L final concentration) were added. The M540 fluorescence was collected with a FL2 sensor using a 575 nm bandpass filter and Yo-Pro 1 with a FL1 sensor using a 525 nm bandpass filter. The cells were classified into 3 categories low merocyanine fluorescence (viable, uncapacitated), high merocyanine fluorescence (viable, capacitated), or Yo-Pro-1 positive (dead).
Assessment of Spontaneous Acrosome Reaction
Seminal samples were incubated with 2 µL of fluorescein-labeled lectins (FITC-PNA, 100 mg/mL for boar sperm and FITC-PSA for bull sperm) and 5 µL of propidium iodide (PI) stock solution (500 mg/mL), at room temperature for 10 minutes. Fluorescence was measured using a FL-1 sensor, a 525-nm bandpass filter to detect FITC-PNA, and a FL-2 sensor and a 575-nm bandpass filter to detect PI. Four sperm subsets were detected: live acrosome intact, live acrosome damaged, and dead spermatozoa with and without acrosome intact.
Sperm-ZP Binding Assay
A total of 200 oocytes (bovine species) were used for this assay. Oocytes were washed twice in PBS after IVM and passed repeatedly through a heat-narrowed Pasteur pipette with a pore diameter less than the size of the oocytes. Thus, the oocytes were broken and the ooplasm and empty ZPs were released into the dishes. The ZPs were washed 3 times in PBS and transferred to fertilization medium where they were incubated with 106 spermatozoa/mL in 500 µL IVF-TALP with PHE mix. After removing the sperm not firmly attached by repeated pipetting, the ZPs with firmly bound sperm were stained with Hoechst. The number of sperm bound to each ZP was registered at different times. In the first group, sperms were allowed to be in contact with the ZP for 15 minutes in order to check the initial binding under control conditions. In a second group, sperm–ZP contact lasted for 3 hours but half of the group were under control conditions and half of them were incubated with plasmin (75 μg/mL) added 30 minutes after the beginning of coincubation. In the third group, the sperm–ZP contact lasted 24 hours and, again, half of the ZPs were incubated with sperm under control conditions and half of them with plasmin added 30 minutes after the beginning of coincubation. This experimental design was used to find out whether plasmin-impaired sperm–ZP binding or the effect of plasmin was to detach the spermatozoa already attached.
Statistical Analysis
Data are presented as the mean ± standard error of the mean and all percentages were modeled according to the binomial model of variables. The variables in experiments were analyzed by 1-way analysis of variance (ANOVA; ZP digestion time, motility parameters, membrane lipid disorder, acrosome reaction, viability, and number of sperm bound to the ZP). When ANOVA revealed a significant effect, values were compared by the Tukey test. A P value <.05 was taken to denote statistical significance.
Results
Effect of Plasminogen and Plasmin on ZP Resistance to Pronase Digestion
No effect of incubation for 30 minutes in plasmin or plasminogen at a concentration of 150 μg/mL was observed on ZP digestion times. The mean time for bovine ZP digestion was 143.2 ± 5.9, 155.7 ± 9.9, and 154.0 ± 6.3 seconds in control, plasmin, and plasminogen groups, respectively, and 74.4 ± 4.9, 70.7 ± 3.5, and 76.4 ± 5.0 seconds for porcine ZP.
Effect of Plasminogen and Plasmin on Sperm Functionality
Motility parameters were not affected by the addition of plasminogen or plasmin to the IVF medium (150 μg/mL, 30 minutes incubation), as reflected in Table 1 for the bovine species and in Table 2 for the porcine species.
Table 1.
Effect of Plasminogen and Plasmin on Motility Parameters of Bull Spermatozoa.a
| Group | Motility (%) | Prog. Motility (%) | VCL (µm/s) | VSL (µm/s) | VAP (µm/s) | LIN (%) | STR (%) | WOB (%) | ALH (µm/s) | BCF (Hz) |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 41.4 ± 3.3 | 36.9 ± 3.5 | 129.8 ± 7.9 | 61.7 ± 4.8 | 79.1 ± 4.6 | 47.3 ± 2.5 | 73.8 ± 1.0 | 63.6 ± 2.5 | 2.7 ± 0.1 | 21.1 ± 1.3 |
| Plasminogen | 37.7 ± 2.2 | 32.8 ± 1.9 | 122.3 ± 6.0 | 52.1 ± 3.7 | 75.3 ± 3.8 | 46.4 ± 0.4 | 69.1 ± 5.2 | 65.6 ± 2.2 | 2.7 ± 0.1 | 19.0 ± 1.0 |
| Plasmin | 36.6 ± 1.8 | 32.4 ± 1.6 | 121.8 ± 5.6 | 60.1 ± 3.0 | 75.7 ± 3.3 | 50.1 ± 3.3 | 76.2 ± 2.3 | 64.4 ± 2.7 | 2.6 ± 0.1 | 18.0 ± 0.9 |
| P value | .36 | .34 | .64 | .18 | .77 | .27 | .08 | .73 | .67 | .71 |
Abbreviations: Prog. Motility: progressive motility; VCL: curvilinear velocity; VSL: straight-line velocity; VAP: average path velocity; LIN: linearity of the curvilinear trajectory; STR: straightness; WOB: wobble of the curvilinear trajectory (VAP/VCL); ALH: amplitude of lateral head displacement; BCF: beat cross-frequency.
a Motility parameters measured by computer-assisted sperm analysis (CASA). Bull frozen-thawed spermatozoa incubated in tyrode albumin lactate pyruvate (TALP) medium supplemented with plasminogen or plasmin (150 μg/mL). Data are mean ± standard error of the mean, and were analyzed by analysis of variance.
Table 2.
Effect of Plasminogen and Plasmin on Motility Parameters of Boar Spermatozoa. Motility Parameters Measured by CASA.a
| Group | Motility (%) | Prog. Motility (%) | VCL (µm/s) | VSL (µm/s) | VAP (µm/s) | LIN (%) | STR (%) | WOB (%) | ALH (µm/s) | BCF (Hz) |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 51.2 ± 3.9 | 39.1 ± 4.0 | 44.8 ± 3.0 | 24.6 ± 1.0 | 31.2 ± 1.5 | 59.0 ± 2.1 | 78.4 ± 1.5 | 72.9 ± 1.7 | 1.5 ± 0.1 | 8.8 ± 0.6 |
| Plasminogen | 49.9 ± 4.5 | 37.1 ± 4.4 | 38.5 ± 2.5 | 21.7 ± 1.2 | 26.7 ± 1.4 | 58.9 ± 2.0 | 79.5 ± 1.0 | 72.8 ± 1.9 | 1.3 ± 0.1 | 8.2 ± 0.7 |
| Plasmin | 52.4 ± 3.5 | 38.2 ± 3.0 | 42.8 ± 2.5 | 25.2 ± 1.3 | 30.1 ± 1.5 | 60.6 ± 1.4 | 81.1 ± 0.6 | 72.7 ± 1.5 | 1.4 ± 0.1 | 9.1 ± 0.7 |
| P value | .90 | .66 | .27 | .11 | .09 | .78 | .25 | .99 | .10 | .62 |
Abbreviations: Prog. Motility, progressive motility; VCL, curvilinear velocity; VSL, straight-line velocity; VAP, average path velocity; LIN, linearity of the curvilinear trajectory; STR, straightness; WOB, wobble of the curvilinear trajectory (VAP/VCL); ALH, amplitude of lateral head displacement; BCF, beat cross-frequency.
a Spermatozoa incubated in tyrode albumin lactate pyruvate (TALP) medium supplemented with plasminogen or plasmin (150 μg/mL). Data are mean ± standard error of the mean, and were analyzed by analysis of variance.
Incubation of sperm in IVF medium supplemented with plasminogen had no effect on sperm membrane lipid disorder or on sperm viability either in the bovine or in the porcine species. Plasmin also had no effect in the porcine species although it increased the percentage of viable sperm, assessed by Yo Pro 1, in the bovine species (Table 3). The acrosome reaction was not affected by incubation of sperm in plasminogen or plasmin but again the percentage of viability assessed by PI was higher in the bovine species when plasmin was present in the IVF medium (Table 4).
Table 3.
Effect of Plasmin and Plasminogen (150 µg/mL) Added to the IVF Medium on Bovine Sperm Acrosome Reaction and Viability.a
| Group | Viable Cells | Dead Cells | |
|---|---|---|---|
| Low Lipid Disorder | High Lipid Disorder | ||
| Control | 22.0 ±2.6 | 3.8 ± 09 ab | 74.2 ± 2.7 a |
| Plasminogen | 27.1 ± 3.2 | 2.4 ±0.7 a | 70.5 ±2.7 a |
| Plasmin | 32.2 ± 4.7 | 7.3 ± 1.7 b | 60.5 ± 3.0 b |
| P value | .15 | .02 | <.01 |
Abbreviation: IVF, in vitro fertilization.
a Data are mean ± standard error of the mean, and were analyzed by analysis of variance. Different letters in the same column denote significant differences (P < .05).
Table 4.
Effect of Plasmin and Plasminogen (150 μg/mL) Added to the IVF Medium on Bovine Sperm Acrosome Reaction and Viability.a
| Group | Viable Cells | Dead Cells | |
|---|---|---|---|
| Intact Acrosome | Acrosome Reacted | ||
| Control | 26.5 ± 3.5 a | 2.3 ± 0.4 | 71.2 ± 3.4 a |
| Plasminogen | 30.5 ± 3.5 ab | 2.4 ± 0.6 | 66.9 ± 3.5 ab |
| Plasmin | 41.9 ± 3.1 b | 2.6 ± 0.2 | 55.4 ± 2.9 b |
| P value | <.01 | .79 | <.01 |
Abbreviation: IVF, in vitro fertilization.
a Data are mean ± standard error of the mean, and were analyzed by analysis of variance. Different letters in the same column denote significant differences (P < .05).
Effect of Plasminogen, Added at Different Times, on IVF Parameters
Plasminogen decreased the penetration rates, increased the monospermy rates, and decreased the mean number of spermatozoa per oocyte both in the bovine and in the porcine species (Table 5). The mean number of spermatozoa attached to the ZP also decreased in the porcine species in the presence of plasminogen and did not change in the bovine species. The effect was the same whether the plasminogen was added 30 minutes before adding the spermatozoa or at the same time when sperm was introduced in the dishes containing the oocytes.
Table 5.
Effect of Plasminogen (150 μg/mL), Added at Different Times, on IVF Parameters in Bovine and Porcine Species.a
| Group | N | Penetration (%) | Monospermy (%) | Sperm/oocyte | Sperm/ZP |
|---|---|---|---|---|---|
| Bovine oocytes | |||||
| Control | 134 | 95.5 ± 2.5 a | 67.1 ± 5.9 a | 1.4 ± 0.1 a | 2.5 ± 0.4 |
| 30′ pre-IVF | 123 | 42.2 ± 3.3 b | 90.2 ± 3.1 b | 1.1 ± 0.1 b | 2.6 ± 0.3 |
| 0′-IVF | 130 | 33.7 ± 3.1 b | 92.0 ± 3.1 b | 1.0 ± 0.1 b | 2.7 ± 0.3 |
| Porcine oocytes | |||||
| Control | 160 | 95.6 ± 1.6 a | 18.9 ± 3.1 a | 3.8±0.2 a | 22.5 ± 1.8 a |
| 30′ pre-IVF | 197 | 61.4 ± 3.4 b | 33.8 ± 4.3 b | 2.3±0.1 b | 16.4 ± 1.1 b |
| 0′-IVF | 184 | 59.7 ± 3.6 b | 41.8 ± 4.7 b | 2.2±0.1 b | 12.0 ± 1.1 b |
Abbreviations: IVF, in vitro fertilization; ZP, zona pellucida.
a Data are mean ± standard error of the mean, and were analyzed by analysis of variance. Different letters in the same column for the same species denote significant differences (P < .05).
Effect of Plasmin on Sperm-ZP Binding
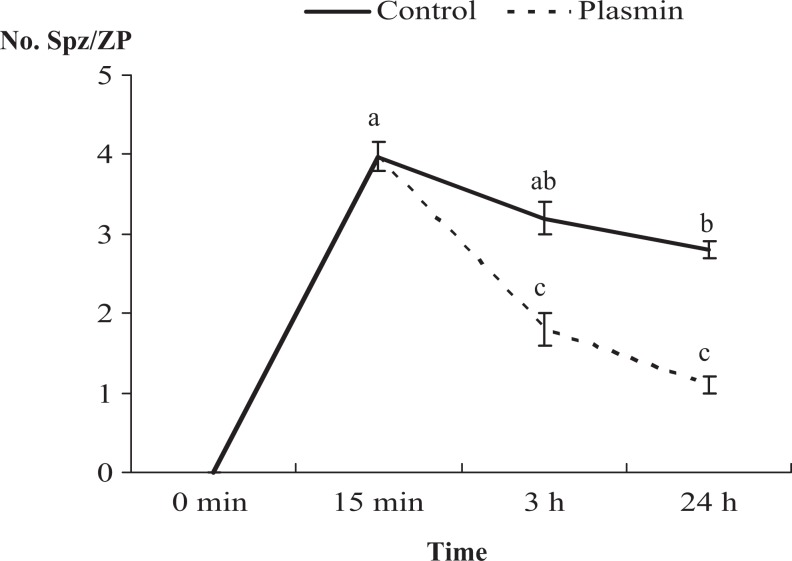
The effect of plasmin on sperm–ZP binding is shown in Figure 1. After 15 minutes of sperm–ZP coincubation around 4 spermatozoa firmly bound to ZP were registered. Addition of plasmin (75 μg/mL) to the IVF medium 30 minutes after initial sperm–ZP coincubation decreased the number of spermatozoa bound to the ZP assessed after 3 hours of contact but it remained steady in absence of plasmin (control). Similarly, 24 hours after initial sperm–ZP contact, the mean number of spermatozoa remaining attached to the ZP decreased in the plasmin group compared to the control group.
Figure 1.
Effect of addition of plasmin (75 μg/mL) on sperm-zona pellucida (ZP) binding in bovine species. Number of spermatozoa (No. Spz/ZP) attached to the ZP after 15 minutes of coincubation and after 3 and 24 hours in the absence (control) or presence of plasmin. A decrease in the number of sperm attached to the ZP was observed in the presence of plasmin. Data are mean ± standard error of the mean, and were analyzed by analysis of variance (ANOVA). Different letters denote significant differences (P < .001). n = 40 ZPs per group.
Discussion
Once the presence of the components of the plasminogen/plasmin system in the oocyte and the reduction in penetration produced by the addition of plasminogen and plasmin to the IVF medium have been demonstrated,4 it would be important to know the mechanism by which this effect occurs.
The first possibility, as mentioned in the introduction section, was the participation of the plasminogen activators, theoretically released from cortical granules during fertilization,26,27 on the increased resistance of the ZP to protease digestion and to sperm penetration (ZP “hardening”).13 This hardening, mediated by proteases from cortical granules, has been observed in the hamster28 and in the mouse,29 but it is different in ungulates because it is mediated by the oviductal fluid and it occurs before fertilization.30 Due to this reason, what we expected exactly happened neither the zymogen plasminogen nor the protease plasmin produced an increase in the ZP resistance to protease digestion in the bovine or porcine species, discarding the possibility that plasminogen activators from cortical granules are the bovine or porcine counterpart for the mouse or hamster protease previously identified.28,29 As we tested both the inactive form of the system, the zymogen plasminogen, and the active serine protease, the enzyme plasmin, the lack of effect cannot be associated to a lack of activity in the additives. Then, we should conclude that the plasminogen–plasmin system is not involved in the chemical ZP hardening.
The second option was that the plasminogen or plasmin had a detrimental effect on sperm functionality, thus reducing their ability to penetrate the oocytes. However, this was not either the case. At the concentrations used, neither plasminogen nor plasmin reduced sperm functionality. In fact, plasmin seemed to protect the sperm in some way, keeping them viable for a longer time. So, a failure in capacitation, a decrease in the viability, or a decrease in the percentage of spermatozoa undergoing the acrosome reaction by the effect of plasminogen or plasmin are not possibilities to explain our IVF results. A similar situation occurs with the sperm motility. Previous studies had demonstrated that plasmin enhances sperm motility 15 when it was added at lower concentrations than that used in the present study, but this effect disappeared when plasmin concentration was increased. Under our experimental conditions, trying to use concentrations similar to those found in the oviduct (higher than those used by Smokovitis et al.15), this was not observed, since sperm motility was no different to the control groups. Then, since we are trying to dissect the reason for the reduced sperm penetration observed under “physiological” concentrations of plasmin, a change in sperm motility cannot be argued as an explanation.
Our last experiment suggests that plasmin generated surrounding the oocyte after sperm contact could induce the detaching of a proportion of spermatozoa previously attached to the ZP, thus explaining why the penetration levels are lower than in control groups. This suggestion comes from the joined interpretation of the IVF assays (in cow and pig) and sperm–ZP binding (in cow) presented here, and the sperm–ZP binding results (in pig) from Coy et al.5 First, data obtained from porcine IVF showed a significant decrease in the number of sperm bound to ZP after 18 to 20 hours of coincubation. It might be suggested that plasminogen or the generated plasmin inhibited the initial sperm–ZP binding, but this was not the case because sperm–ZP binding was similar independently whether plasminogen was added 30 minutes before insemination or at the same time when spermatozoa was added. If the effect of plasminogen was due to stearic hindrance, it would have expected a higher effect in the group where plasminogen was added 30 minutes before adding spermatozoa, but this was not case. This would corroborate the proposed hypothesis by which plasminogen would bind to ZP and oolemma and would become activated into plasmin by the sperm contact with the oocyte.4 So, any observed effect of the plasmin in the system would be triggered after insemination. Although the present data are obtained with in vitro matured oocytes, it is now known that plasminogen is present in the oviductal fluid.4 Consquently, the likely participation of plasminogen–plasmin system in the sperm–oocyte interaction under in vivo conditions is highly probable.
Second, in the bovine species, no differences were found in the number of sperm bound to ZP after 18 to 20 hours of coincubation during the IVF experiment. This can be considered an expected result, bearing in mind that IVF in the bovine species is made with COCs instead of oocytes, as it is explained in the Materials and Methods section, and the number of spermatozoa usually bound to ZP is lower than in the porcine species. However, the question about the possible detaching of spermatozoa from the ZP by plasmin generated during gamete interaction still remained valid and the last experiment helped us to clarify this point. From the sperm–ZP binding assay we could observe that plasmin did not displace the spermatozoa far from the ZP and, after plasmin addition we could not observe differences among the different groups when they were directly evaluated under the stereomicroscope. However, after repeated pipetting to remove spermatozoa loosely bound (or not firmly attached), significant differences were observed among the groups. This confirmed that, once attached, plasmin detached the spermatozoa initially bound to ZP, even though they remained visible on the ZP. This mechanism means that the role of the plasminogen/plasmin system during fertilization would consists of contributing to avoidance of excess of penetration since, in the present experiments, spermatozoa and oocytes remained in contact for 18 to 20 hours and the number of sperm entering the oocyte could have increased through the time. However, this number was higher when no plasminogen or plasmin was present in the IVF medium and lower under their presence in both the bovine (current study) and the porcine5 species.
In conclusion, the results from the present study show a role for the plasminogen/plasmin system in fertilization that could have further implications for IVF in different species, including humans. Since plasminogen is a natural component of human blood serum, its presence in the human tubal fluid is more than probable. Beneficial effects of addition of plasminogen to the IVF media and the corresponding removal of excess of sperm attached to ZP could include not only regulation of polyspermy, which in humans varies from 3% to 30% (revised by van der Ven et al.31), but also better signaling between ZP and tubal epithelium or some advantages in implantation that merit further investigation.
Acknowledgments
We thank Mr Dave Ginsberg, Vice President of Operations from Molecular Innovations, for his technical support with the use of plasminogen/plasmin reagents. We also thank Ruth Tunn for her help with the English. The authors would like to thank Juan A. Carvajal, Darío Abril, and Soledad Rodríguez for their generous technical assistance; Dalland SA farm and Breeders Association of “Asturiana de los Valles” for providing the boar and bull semen samples, respectively; and the staff of the slaughterhouse El Pozo and Orihuela SA for supplying the ovaries.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Spanish Ministry of Science and Innovation and FEDER, Grant AGL2009-12512-C02-01.
References
- 1. Castellino FJ, Ploplis VA. Structure and function of the plasminogen/plasmin system. Thromb Haemost. 2005;93(4):647–654. [DOI] [PubMed] [Google Scholar]
- 2. Zimmerman SW, Manandhar G, Yi YJ, et al. Sperm proteasomes degrade sperm receptor on the egg zona pellucida during mammalian fertilization. PLoS One. 2011;6(2):e17256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hunter RH, Coy P, Gadea J, Rath D. Considerations of viscosity in the preliminaries to mammalian fertilisation. J Assist Reprod Genet. 2011;28(3):191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mondéjar I, Grullón LA, García-Vázquez FA, Romar R, Coy P. Fertilization outcome could be regulated by binding of oviductal plasminogen to oocytes and by releasing of plasminogen activators during interplay between gametes. Fertil Steril. 2012;97(2):453–461. [DOI] [PubMed] [Google Scholar]
- 5. Coy P, Jiménez-Movilla M, García-Vázquez FA, Mondéjar I, Grullón L, Romar R. Oocytes use the plasminogen-plasmin system to remove supernumerary spermatozoa. Hum Reprod. 2012;27(7):1985–1993. [DOI] [PubMed] [Google Scholar]
- 6. Liu YX, Ny T, Sarkar D, Loskutoff D, Hsueh AJ. Identification and regulation of tissue plasminogen activator activity in rat cumulus-oocyte complexes. Endocrinology. 1986;119(4):1578–1587. [DOI] [PubMed] [Google Scholar]
- 7. Liu YX, Cajander SB, Ny T, Kristensen P, Hsueh AJ. Gonadotropin regulation of tissue-type and urokinase-type plasminogen activators in rat granulosa and theca-interstitial cells during the periovulatory period. Mol Cellular Endocrinol. 1987;54(2-3):221–229. [DOI] [PubMed] [Google Scholar]
- 8. Kim NH, Menino AR. Effects of stimulators of protein kinases A and C and modulators of phosphorylation on plasminogen activator activity in porcine oocyte-cumulus cell complexes during in vitro maturation. Mol Reprod Dev. 1995;40(3):364–370. [DOI] [PubMed] [Google Scholar]
- 9. Park KW, Choi SH, Song XX, Funahashi H, Niwa K. Production of plasminogen activators (PAs) in bovine cumulus-oocyte complexes during maturation in vitro: effects of epidermal growth factor on production of PAs in oocytes and cumulus cells. Biol Reprod. 1999;61(1):298–304. [DOI] [PubMed] [Google Scholar]
- 10. Liu YX, Liu K, Feng Q, et al. Tissue-type plasminogen activator and its inhibitor plasminogen activator inhibitor type 1 are coordinately expressed during ovulation in the rhesus monkey. Endocrinology. 2004;145(4):1767–1775. [DOI] [PubMed] [Google Scholar]
- 11. D'Alessandris C, Canipari R, Di Giacomo M, et al. Control of mouse cumulus cell-oocyte complex integrity before and after ovulation: plasminogen activator synthesis and matrix degradation. Endocrinology. 2001;142(7):3033–3040. [DOI] [PubMed] [Google Scholar]
- 12. Zhang X, Rutledge J, Khamsi F, Armstrong D. Release of tissue-type plasminogen activator by activated rat eggs and its possible role in the zona reaction. Mol Reprod Dev. 1992;32(1):28–32. [DOI] [PubMed] [Google Scholar]
- 13. Komar A, Kujawa M. Cortical and zona reactions of heat-activated mouse eggs. J Reprod Fert. 1985;73(2):479–482. [DOI] [PubMed] [Google Scholar]
- 14. Huarte J, Belin D, Bosco D, Sappino AP, Vassalli JD. Plasminogen activator and mouse spermatozoa: urokinase synthesis in the male genital tract and binding of the enzyme to the sperm cell surface. J Cell Biol. 1987;104(5):1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smokovitis A, Kokolis N, Alexopoulos C, Alexaki E, Eleftheriou E. Plasminogen activator activity, plasminogen activator inhibition and plasmin inhibition in spermatozoa and seminal plasma of man and various animal species. Effect of plasmin on sperm motility. Fibrinolysis. 1987;1(1):253–257. [Google Scholar]
- 16. Liu K, Liu Y, Du Q, et al. Preliminary studies on the role of plasminogen activator in seminal plasma of human and rhesus monkey. Mol Hum Reprod. 1996;2(2):99–104. [DOI] [PubMed] [Google Scholar]
- 17. Kim TS, Sa SJ, Shin MY, et al. Stimulation of plasminogen activator activity by free radicals in boar spermatozoa. Anim Reprod Sci. 2009;114(1-3):228–237. [DOI] [PubMed] [Google Scholar]
- 18. Coy P, Grulló L, Cánovas S, Romar R, Matás C, Avilés M. Hardening of the zona pellucida of unfertilized eggs can reduce polyspermic fertilization in the pig and cow. Reproduction. 2008;135(1):19–27. [DOI] [PubMed] [Google Scholar]
- 19. Funahashi H, Cantley TC, Day BN. Synchronization of meiosis in porcine oocytes by exposure to dibutyryl cyclic adenosine monophosphate improves developmental competence following in vitro fertilization. Biol Reprod. 1997;57(1):49–53. [DOI] [PubMed] [Google Scholar]
- 20. Coy P, Gadea J, Romar R, Matás C, García E. Effect of in vitro fertilization medium on the acrosome reaction, cortical reaction, zona pellucida hardening and in vitro development in pigs. Reproduction. 2002;124(2):279–288. [PubMed] [Google Scholar]
- 21. Parrish JJ, Susko-Parrish JL, Leibfried-Rutledge ML, Critser ES, Eyestone WH, First NL. Bovine in vitro fertilization with frozen-thawed semen. Theriogenology. 1986;25(4):591–600. [DOI] [PubMed] [Google Scholar]
- 22. Rath D, Long CR, Dobrinsky JR, Welch GR, Schreier LL, Johnson LA. In vitro production of sexed embryos for gender preselection: high-speed sorting of X-chromosome-bearing sperm to produce pigs after embryo transfer. J Anim Sci. 1999;77(12):3346–3352. [DOI] [PubMed] [Google Scholar]
- 23. Pursel VG, Johnson LA. Freezing of boar spermatozoa: fertilizing capacity with concentrated semen and a new thawing procedure. J Anim Sci. 1975;40(1):99–102. [DOI] [PubMed] [Google Scholar]
- 24. Gadea J, Gumbao D, Cánovas S, García-Vázquez FA, Grullón LA, Gardón JC. Supplementation of the dilution medium after thawing with reduced glutathione improves function and the in vitro fertilizing ability of frozen-thawed bull spermatozoa. Int J Androl. 2008;31(1):40–49. [DOI] [PubMed] [Google Scholar]
- 25. Gadea J, García-Vázquez F, Matás C, Gardón JC, Cánovas S, Gumbao D. Cooling and freezing of boar spermatozoa: supplementation of the freezing media with reduced glutathione preserves sperm function. J Androl. 2005;26(3):396–404. [DOI] [PubMed] [Google Scholar]
- 26. Zhang X, Rutledge J, Khamsi F, Armstrong DT. Release of tissue-type plasminogen activator by activated rat eggs and its possible role in the zona reaction. Mol Reprod Dev. 1992;32(1):28–32. [DOI] [PubMed] [Google Scholar]
- 27. Rekkas CA, Besenfelder U, Havlicek V, Vainas E, Brem G. Plasminogen activator activity in cortical granules of bovine oocytes during in vitro maturation. Theriogenology. 2002;57(7):1897–1905. [DOI] [PubMed] [Google Scholar]
- 28. Cherr GN, Drobnis EZ, Katz DF. Localization of cortical granule constituents before and after exocytosis in the hamster egg. J Exp Zool. 1988;246(1):81–93. [DOI] [PubMed] [Google Scholar]
- 29. Hoodbhoy T, Talbot P. Mammalian cortical granules: contents, fate, and function. Mol Reprod Dev. 1994;39(4):439–448. [DOI] [PubMed] [Google Scholar]
- 30. Coy P, Cánovas S, Mondéjar I, et al. Oviduct-specific glycoprotein and heparin modulate sperm-zona pellucida interaction during fertilization and contribute to the control of polyspermy. PNAS. 2008;105(41):15809–15814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van der Ven HH, Al-Hasani S, Diedrich K, Hamerich U, Lehmann F, Krebs D. Polyspermy in in vitro fertilization of human oocytes: frequency and possible causes. Ann N Y Acad Sci. 1985;442(1):88–95. [DOI] [PubMed] [Google Scholar]