Abstract
We have previously reported that maternal creatine supplementation protects the neonate from hypoxic injury. Here, we investigated whether maternal creatine supplementation altered expression of the creatine synthesis enzymes (arginine:glycine amidinotransferase [AGAT], guanidinoaceteate methyltransferase [GAMT]) and the creatine transporter (solute carrier family 6 [neurotransmitter transporter, creatine] member 8: SLC6A8) in the term offspring. Pregnant spiny mice were fed a 5% creatine monohydrate diet from midgestation (day 20) to term (39 days). Placentas and neonatal kidney, liver, heart, and brain collected at 24 hours of age underwent quantitative polymerase chain reaction and Western blot analysis. Maternal creatine had no effect on the expression of AGAT and GAMT in neonatal kidney and liver, but mRNA expression of AGAT in brain tissues was significantly decreased in both male and female neonates born to mothers who were fed the creatine diet. SLC6A8 expression was not affected by maternal dietary creatine loading in any tissues. Maternal dietary creatine supplementation from midgestation in the spiny mouse did not alter the capacity for creatine synthesis or transport.
Keywords: SLC6A8, arginine:glycine amidinotransferase (AGAT), guanidinoaceteate methyltransferase (GAMT), neonate, placenta
Introduction
The creatine-phosphocreatine system is essential for the maintenance of cellular adenosine triphosphate, serving as a spatial and temporal energy buffer in cells with high and fluctuating energy demands.1 Postnatally, the body’s creatine pool is derived from a diet containing meat, fish, and dairy products, and also by endogenous synthesis. The latter involves the conversion of arginine and glycine to guanidinoacetate by the enzyme arginine:glycine amidinotransferase (AGAT), and then the methylation of guandinoacetate to creatine by the enzyme guanidinoaceteate methyltransferase (GAMT). The principal sites of AGAT and GAMT activity are the kidney and liver, respectively. The brain, pancreas, and testes may synthesize creatine independent of the reno-hepatic axis, although this is thought not to contribute substantially to systemic creatine or to the large pool of creatine contained in muscle.2
Until recently, very little was known about the fetal and neonatal requirement for creatine, and how this might change with advancing pregnancy and early postnatal life, particularly for tissues known to have a high creatine requirement in the adult such as muscle, heart, and brain.3,4 In the spiny mouse, a precocial rodent, we have shown that the creatine content of the placenta and fetal brain increases from midgestation to term, with this increase continuing in the second postnatal week in the brain.5 This study also found that the spiny mouse fetus probably does not synthesize creatine until 0.9 day of gestation, due to the low expression of AGAT and GAMT in the fetal kidney and liver, and the absence of expression of these enzymes in the placenta.5 Such data do not exist for the human fetus and raises important questions, particularly for preterm infants where immaturity of the reno-hepatic axis and intestinal absorption may leave them with insufficient capacity to either synthesize creatine or take it up from breast milk or formula. Whether the preterm or term kidney is able to fully resorb the creatine in glomerular filtrate is currently unknown.
We have recently completed a series of experiments exploring the potential of maternal creatine supplementation to protect the newborn from hypoxia-induced injury.6,7,9 Specifically, we hypothesized that increasing cellular reserves of total creatine (creatine + phosphocreatine) would protect the fetus from the consequences of oxygen deprivation at birth. Indeed, we showed that a maternal diet supplemented with 5% creatine monohydrate from midgestation to term is able to improve offspring survival, normalize postnatal growth which is otherwise delayed,9 and prevent the brain, kidney and diaphragm muscle injury seen in hypoxia-exposed offspring at 24 hours of age.6–8
Before recommending creatine supplementation to pregnant women, it is important to consider whether a high creatine load during pregnancy affects creatine synthesis and transport in the neonate. Creatine supplementation in adult humans, rats, and chick embryos has shown a marked decrease in renal AGAT expression, leading to a negative feedback, downregulation, of endogenous synthesis of creatine.10–13 The consequences of maternal dietary creatine supplementation on creatine synthesis and transport in the newborn of a placental mammal is however unknown. In the current study, we investigated whether maternal dietary creatine supplementation from midgestation to term in the spiny mouse, a period of 19 days, would alter the expression of genes involved in creatine synthesis or transport in selected key tissues in the offspring. We hypothesized that maternal dietary creatine supplementation from midgestation to term would cause a downregulation in the expression of the creatine transporter, solute carrier family 6 (neurotransmitter transporter, creatine) member 8 (SLC6A8) and decrease the expression of AGAT, the first rate-limiting step in the creatine synthesis pathway in the newborn. We also investigated the effect of this creatine supplementation on expression of placental SLC6A8, and specifically examined the labyrinth (exchange region) and spongy zone (germinative zone) of the placenta independently.
Methods
Animals
All experiments were approved in advance by Monash University School of Biomedical Sciences Animal Ethics Committee and conducted according to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. The spiny mice used in this study were obtained from our own laboratory colony and housed, bred, and time-mated as previously described.14
Diet
Pregnant dams were fed either a control diet of standard rat and mouse pellets throughout pregnancy (n = 7), or were fed isocaloric pellets supplemented with 5% creatine monohydrate (Specialty Feeds, Perth, Australia) from day 20 of gestation (n = 7). Water was available ad libitum.
Cesarean Section and Cross Fostering
The pregnant dam was killed by cervical dislocation on day 38 of gestation (term is 39 days) and the fetuses were expelled from the uterus immediately and their mouths cleared of membranes and mucus. The pups were stimulated to breathe by gentle palpation of the chest and abdomen using moist cotton tips. The pups were allowed to recover for 1 to 1.5 hours on a heated pad, and then cross-fostered to a dam that had given birth within the preceding 6 hours.8 The cross-fostered dam was fed a standard rodent diet during pregnancy to avoid postnatal delivery of creatine to the newborn via lactation and thus focus our study on the in utero effects of maternal dietary creatine supplementation.
Tissue Collection and Processing
Placenta samples were collected immediately after pup delivery, weighed, and snap frozen. Tissues for analysis of AGAT, GAMT, and SLC6A8 expression were collected at 24 hours after birth to coincide with a period soon after birth when the newborn is required to synthesize its own creatine. The pups were weighed and killed by decapitation. The heart, brain, liver, and kidney were removed, weighed, and snap frozen for RNA and/or protein extraction. All tissues were stored at −80°C. For all tissue analyses described below, no more than 2 pups, 1 male and 1 female, were taken from any 1 litter.
Messenger RNA Extraction
Samples for quantitative polymerase chain reaction (qPCR; heart, brain, liver, kidney, placenta; n = 4-6/sex/organ/treatment) analysis were prepared by total RNA extraction (RNeasy Midi Kit #75144, Qiagen) and reverse transcribed to form complementary DNA according to manufacturer’s instructions (Reverse Transcription System #A3500, Promega) as previously described,5 with the minor modification that placental samples were separated into labyrinth and spongy zone before RNA extraction.
Protein Extraction
Protein from samples for Western blot was extracted as previously described.5 Briefly, liver and kidney samples (n = 4 male and 3 female/treatment) were homogenized in cell lysis buffer, incubated on ice for 60 minutes followed by centrifugation at 20 800 g for 20 minutes at 4°C. The supernatant was then incubated at 95°C for 5 minutes and stored at −80°C until analysis. A protein assay was performed on all samples (BCA Protein Assay Kit # 23225, Thermo Scientific, Australia) according to manufacturer’s instructions to enable equal loading of protein on the gels. A sample of each organ was collected from untreated adult spiny mice and prepared in the same way to be used as positive controls. The extracted protein samples were then separated based on molecular weight using sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis as reported previously.5
Quantitative PCR
SYBR green PCR chemistry was used to determine relative levels of messenger RNA (mRNA) for AGAT in kidney and brain samples, GAMT in liver and brain samples, and SLC6A8 in placenta, heart, kidney, liver, and brain samples as per the manufacturer’s instructions (Applied BioSystems, Mulgrave, Australia). The primer and probe sequences are as previously described.5 Primer products were generated, sequenced, and confirmed to be the gene of interest using a blastn algorithm within the Basic Local Alignment Search Tool (BLAST, http://blast.ncbi.nlm.nih.gov/; data not shown). The qPCR cycling conditions for all genes consisted of an initial denaturation step of 95°C for 10 minutes, followed by 40 cycles consisting of 95°C for 15 seconds and 60°C for 1 minutes followed by a dissociation curve. Samples were run in triplicate for each gene of interest, and where differences were observed between the groups, the qPCR was repeated.
Data obtained from qPCR were analyzed as follows: the relative expression within the sample (ΔC T) was calculated by subtracting the mean cycle threshold (C T) for the housekeeping gene (RN18S1, 18s ribosomal 1) from the mean C T value for the gene of interest. This number was then inserted into the formula 2−ΔCT to give a final arbitrary expression value.
Western Blotting of AGAT and GAMT Protein
Western blotting was performed as previously described5 with the minor modification that all blocking and primary antibody dilutions were performed using 5% skim milk powder instead of 5% bovine serum albumin. Primary antibody dilutions used were as follows: rabbit anti-GAMT monoclonal 1:4000 and rabbit anti-AGAT polyclonal 1:1000. Secondary antibody (goat anti-rabbit-horseradish peroxidase [HRP]) was diluted 1:10 000 in Tris-buffered saline and Tween 20. Antibodies were removed from the blots using a 1× stripping buffer (70 mmol/L SDS and 63 mmol/L Tris) and reprobed with β-actin as a loading control using the same protocol as above (mouse anti-β-actin, 1:5000, goat anti-mouse HRP, 1:10 000). Densitometric analysis was carried out using ImageJ as per the published protocol.5 To determine relative expression of the protein of interest, each band of interest was normalized to β-actin and creatine-exposed animals were expressed relative to the controls. (Note. due to the lack of a specific SLC6A8 antibody15 we were unable to perform Western blots for this protein.)
Statistical Analysis
All data are presented as mean ± standard error of the mean and were analyzed with a 2-way analysis of variance for diet and sex using the computer-based program SPSS. Significance was set at P < .05.
Results
Effects of Creatine Supplementation on Creatine Synthesizing Enzymes
The effect of the creatine diet on the creatine content of maternal and fetal tissues and the placenta has been presented elsewhere.9 Briefly, the maternal creatine treatment resulted in a 3% to 105% increase in creatine content in fetal tissues (kidney 22%, heart 14%, liver 37%, brain 3.6%) and placenta (105%).9
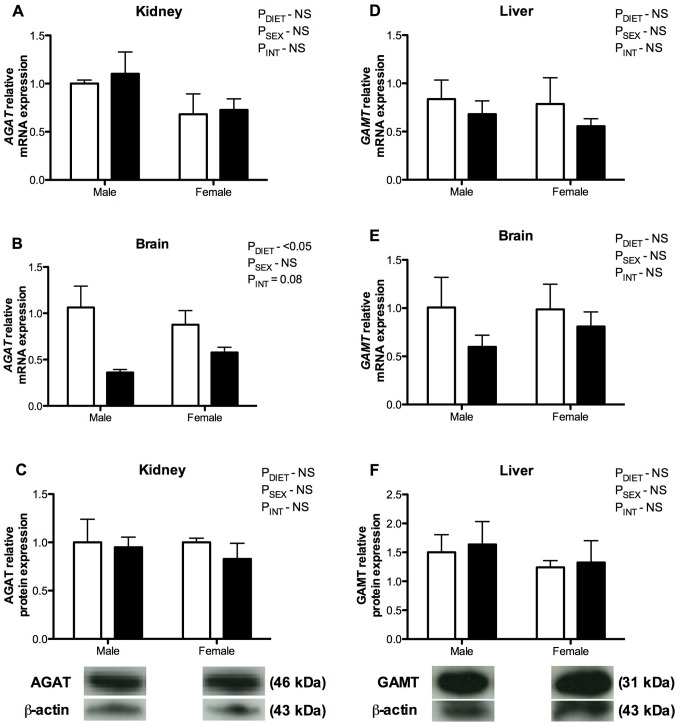
Creatine supplementation from midgestation had no significant effect on the mRNA expression of AGAT in the newborn kidney, although there was a significant decrease in the mRNA expression of AGAT in the brain compared to controls (P DIET < .05; Figure 1A and B). The AGAT mRNA expression was comparable in male and female brains in the control group, but greater in the female brain compared to the male brain in the creatine treatment group (P INT = .08; Figure 1B). The GAMT expression in the neonatal liver and brain at 24 hours of age was not different between creatine-exposed offspring and controls (Figure 1D and E). Analysis of the protein products of these genes showed that creatine supplementation did not significantly alter the protein levels of either AGAT or GAMT in neonatal tissue compared to controls (Figure 1C and F).
Figure 1.
Messenger RNA (mRNA; A, B, D, and E) and protein (C, F) expression of the creatine synthesizing enzymes arginine:glycine amidinotransferase (AGAT; A-C) and guanidinoaceteate methyltransferase (GAMT; D-F) in newborn spiny mouse kidney (A and C), liver (D and F), and brain samples (B and E) from mothers fed a control (open bars) or 5% creatine supplemented diet (closed bars). Group mean ± standard error of the mean (SEM) are shown. The mRNA expression determined by quantitative polymerase chain reaction (qPCR) and protein expression determined by Western blot (representative blots shown). All values expressed relative to an internal control.
Effects of Creatine Supplementation on the Creatine Transporter (SLC6A8)
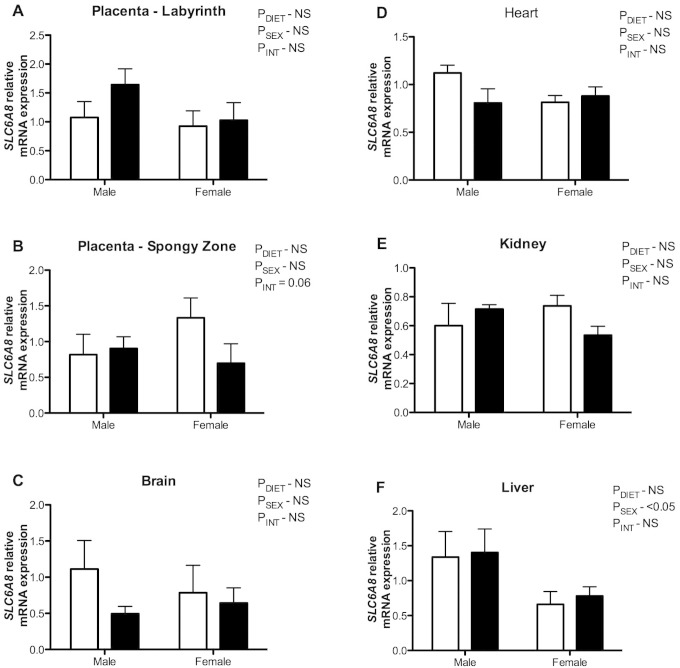
Immediately after birth, the mRNA expression of SLC6A8 within the labyrinthine region of the placenta did not differ between the maternal creatine supplemented group and controls (Figure 2A). In contrast, the expression of SLC6A8 tended to be lower in the spongy zone region of creatine-exposed placentas from female fetuses only, compared to controls (P INT = .06; Figure 2B). The SLC6A8 expression did not differ between diet groups in brain, heart, kidney, or liver samples taken from 24-hour-old neonates (Figure 2C-F).
Figure 2.
Messenger RNA (mRNA) expression of solute carrier family 6 (neurotransmitter transporter, creatine) member 8 (SLC6A8) in spiny mouse placentas at term (labyrinth, A and spongy zone, B) and newborn spiny mouse brain (C), heart (D), kidney (E), and liver (F) samples from mothers fed a control (open bars) or 5% creatine supplemented diet (closed bars). Group mean ± standard error of the mean (SEM) are shown. The mRNA expression determined by quantitative polymerase chain reaction (qPCR) and all values are expressed relative to an internal control.
Interestingly, there was significantly higher expression of SLC6A8 in the liver of male offspring, compared to females at 24 hours of postnatal age independent of maternal diet (P SEX < .05; Figure 2F).
Discussion
Previous studies in adult rat have shown that creatine negatively regulates the expression of the AGAT gene in the kidney.13,15 This study aimed to determine whether our model of maternal dietary creatine supplementation over half of pregnancy, which increases total creatine concentrations in fetal tissues,9 might therefore cause downregulation in expression of AGAT in the neonate—specifically, in the kidney and the brain. The results showed that this did not occur at either the gene or the protein level in the kidney, but that expression of the AGAT gene was decreased in the brain. We also investigated the expression of GAMT, the second enzyme required for endogenous creatine synthesis in the liver and brain, and these gene and protein products were not affected by the maternal creatine load. This suggests that supplementation of the maternal diet with creatine does not impair expression of the enzymes necessary for endogenous creatine synthesis in the key creatine synthesizing organs (kidney and liver), but that local sites of creatine synthesis (eg, brain) may be affected in the neonate.
To our knowledge this is the first evidence that SLC6A8 is expressed in the 2 major regions of the murid placenta. The placenta plays a critical role in the transport of creatine from the maternal circulation to fetal blood via a placental “pool” of creatine. The concept of a placental pool of creatine from which the creatine in fetal blood is derived was first described by Miller in 197416; indeed, we have shown significant loading of creatine within the placenta of the spiny mouse following maternal dietary creatine supplementation.9 This transport of creatine to the fetal blood from the maternal circulation is critical for fetal creatine supplies, as our data suggest that the fetus may not be capable of synthesizing its own creatine until after 0.9 day of gestation.5 The trend for downregulation of SLC6A8 expression in the spongy zone region of the female placenta is an unexpected finding. This could suggest reduced storage of creatine in the placental pool for a female, although we have not detected any sex differences in the amount of creatine loaded in the placentas.
We also observed sex-dependent expression of the SLC6A8 gene in the 24-hour-old neonatal liver. However, this was independent of maternal diet, with the female liver having significantly lower expression than the male in both the diet groups. We observed no change in the expression of SLC6A8 mRNA in the liver of 24-hour-old neonates in response to the maternal creatine diet. The mere presence of the SLC6A8 gene in the newborn spiny mouse liver is, however, a novel and interesting observation, with studies in the adult and near-term rat embryo showing no SLC6A8 expression in the liver.4,17 We have measured the amount of creatine in maternal tissues following our 5% creatine diet from midgestation to term and we observed a 30-fold increase in the total creatine content within the maternal liver at term,9 suggesting that the presence of SLC6A8 in the spiny mouse liver is not restricted to developing or young hepatic tissues. Why creatine supplementation leads to the preferential storage of creatine in the liver, over other nonmuscle sites in the spiny mouse is not known. We suggest that in the transition from fetal to newborn life there may be a shift of creatine storage from the placenta to the newborn liver. During pregnancy the placenta may function to extract excess creatine from the fetal circulation, either storing it itself or returning it to the maternal circulation. After birth, however, with the removal of the placenta, the newborn liver may take over this role, as it appears to do in the adult female.
Determination of SLC6A8 protein is presently limited by the lack of a specific antibody, in particular for the spiny mouse.15 It is unknown whether the observed changes in mRNA expression also correspond to an increase in protein expression. Further work using in situ hybridization to localize placental SLC6A8 mRNA should be done.
Placenta samples were collected at delivery and therefore, at that point in time, were still subject to the creatine concentration prevailing in maternal blood. On the other hand, the neonatal tissues were collected at 24 hours of age, and as all offspring were cross-fostered to a dam who was fed the normal diet, it is possible that some postpartum change of AGAT, GAMT, and SLC6A8 expression had occurred within this time. The half-life of AGAT and GAMT activity has been shown to be 16 to 20 hours,2 and it is therefore possible that in the 24 hours period in which the newborn was not exposed to the creatine load that AGAT and GAMT protein levels had returned to normal. However, this is unlikely, because human studies show that it takes 4 weeks for total creatine levels to return to basal levels after a creatine load,12 and creatine supplementation has been previously shown to inhibit endogenous creatine synthesis.10 We do not know the total creatine content of our tissues at 24 hours; values reported above are those at term, at the time the offspring are removed from the dam and insufficient tissue now prevents us from making further measurements. To our knowledge there is no data on the rate of creatine clearance after loading the fetus or newborn.
Another important consideration is the effect of pregnancy hormones on creatine-synthesizing enzyme expression. Creatine biosynthesis occurs at the highest rates in young vertebrates supported by the good nutrient supply and optimal blood concentrations of insulin (stimulates uptake of blood creatine by muscle), somatotropin, thyroid hormone, and testosterone provided by pregnancy and the early neonatal period.2 The hormone profile during pregnancy may therefore override the inhibition of endogenous creatine synthesis expected to occur with maternal creatine loading.
The lack of change in mRNA and protein expression of AGAT and GAMT in newborn spiny mouse tissues is a significant finding, particularly with respect to the potential benefits of maternal dietary creatine supplementation for the protection of newborn tissues against hypoxia-induced injury,6–8,19 although a limitation of this study is that we do not yet have measures of the activity of these enzymes and have based our conclusions only on gene expression and protein data. Also, the observed decrease in AGAT mRNA expression in the brain is difficult to interpret given that there is no accepted physiological role for guanidinoacetate in the brain. However, recent preliminary findings by Braissant and colleagues20 suggest that AGAT may be involved in the differentiation of neuroblasts and therefore have a role in determining the fate of neural stem cells during early brain development. Also, there appears to be a net exit of guanidinoacetate from cerebrospinal fluid to blood, at the blood–brain barrier, predominantly through the taurine transporter,21 further suggesting a role for this product of AGAT activity other than the cerebral synthesis of creatine. The implications of these findings for fetal brain development and the impact of maternal creatine ingestion on them require further study.
Acknowledgments
The authors would like to thank Mrs Maria Papaioannou for technical assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: funding from the NH&MRC to D.W.W. and R.S. and the Victorian Government’s Operational Infrastructure Support Program supported this work. H.D. is an Australian Research Council Postdoctoral Research Fellow, Z.J.I. was supported by an Australian Postgraduate Award (APA) Scholarship when these experiments were completed and D.A.L., S.E., and B.A.O’C. are currently supported by APA Scholarships.
References
- 1. Wallimann T, Tokarska-Schlattner M, Schlattner U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids. 2011;40(5):1271–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walker JB. Creatine: biosynthesis, regulation, and function. Adv Enzymol Relat Areas Mol Biol. 1979;50:177–242. [DOI] [PubMed] [Google Scholar]
- 3. Brosnan JT, Brosnan ME. Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr. 2007;27:241–261. [DOI] [PubMed] [Google Scholar]
- 4. Braissant O, Henry H, Villard AM, Speer O, Wallimann T, Bachmann C. Creatine synthesis and transport during rat embryogenesis: spatiotemporal expression of AGAT, GAMT and CT1. BMC Dev Biol. 2005;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ireland Z, Russell AP, Wallimann T, Walker DW, Snow R. Developmental changes in the expression of creatine synthesizing enzymes and creatine transporter in a precocial rodent, the spiny mouse. BMC Dev Biol. 2009;9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cannata DJ, Ireland Z, Dickinson H, et al. Maternal creatine supplementation from mid-pregnancy protects the diaphragm of the newborn spiny mouse from intrapartum hypoxia-induced damage. Pediatr Res. 2010;68(5):393–398. [DOI] [PubMed] [Google Scholar]
- 7. Ireland Z, Castillo-Melendez M, Dickinson H, Snow R, Walker DW. A maternal diet supplemented with creatine from mid-pregnancy protects the newborn spiny mouse brain from birth hypoxia. Neuroscience. 2011;194:372–379. [DOI] [PubMed] [Google Scholar]
- 8. Ellery SJ, Ireland ZJ, Kett MM, Snow R, Walker DW, Dickinson H. Creatine pretreatment prevents birth asphyxia-induced injury of the newborn spiny mouse kidney [published online ahead of print November 22, 2012]. Pediatr Res. doi:10.1038/pr.2012.174. [DOI] [PubMed] [Google Scholar]
- 9. Ireland Z, Dickinson H, Snow R, Walker DW. Maternal creatine: does it reach the fetus and improve survival after an acute hypoxic episode in the spiny mouse (Acomys cahirinus)? Am J Obstet Gynecol. 2008;198(4):431 e1–e6. [DOI] [PubMed] [Google Scholar]
- 10. Derave W, Marescau B, Vanden Eede E, Eijnde BO, De Deyn PP, Hespel P. Plasma guanidino compounds are altered by oral creatine supplementation in healthy humans. J Appl Physiol. 2004;97(3):852–857. [DOI] [PubMed] [Google Scholar]
- 11. Fitch CD, Hsu C, Dinning JS. Some factors affecting kidney transamidinase activity in rats. J Biol Chem. 1960;235:2362–2364. [PubMed] [Google Scholar]
- 12. McKenna MJ, Morton J, Selig SE, Snow RJ. Creatine supplementation increases muscle total creatine but not maximal intermittent exercise performance. J Appl Physiol. 1999;87(6):2244–2252. [DOI] [PubMed] [Google Scholar]
- 13. Guthmiller P, Van Pilsum JF, Boen JR, McGuire DM. Cloning and sequencing of rat kidney L-arginine: glycine amidinotransferase. Studies on the mechanism of regulation by growth hormone and creatine. J Biol Chem. 1994;269(26):17556–17560. [PubMed] [Google Scholar]
- 14. Dickinson H, Walker DW. Managing a colony of spiny mice (Acomys cahirinus) for perinatal research. Australian and New Zealand Council for the Care of Animals in Research and Training (ANZCCART) News 2007;20:4-11. [Google Scholar]
- 15. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107–1213. [DOI] [PubMed] [Google Scholar]
- 16. Miller RK, Davis BM, Brent RL, Koszalka TR. Transport of creatine in the human placenta. The Pharmacologist. 1974;16(2):305. [Google Scholar]
- 17. Snow RJ, Murphy RM. Creatine and the creatine transporter: a review. Mol Cell Biochem. 2001;224(1-2):169–181. [DOI] [PubMed] [Google Scholar]
- 18. Speer O, Neukomm LJ, Murphy RM, et al. Creatine transporters: a reappraisal. Mol Cell Biochem. 2004;256-257(1-2):407–424. [DOI] [PubMed] [Google Scholar]
- 19. Wallimann T, Tokarska-Schlattner M, Schlattner U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids. 2011;40(5):1271–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Braissant O, Beard E, Uldry J. Models of creatine deficiency syndromes by RNAi in 3D reaggregated brain cell organotypic cultures. In: XXVth International Symposium on Cerebral Blood Flow, Metabolism, and Function. Barcelona, Spain; 2011:934. [Google Scholar]
- 21. Braissant O. Creatine and guanidinoacetate transport at blood-brain and blood-cerebrospinal fluid barriers. J Inherit Metab Dis. 2012;35(4):655–664. [DOI] [PubMed] [Google Scholar]