Abstract
Objective:
Neutrophil gelatinase–associated lipocalin (NGAL) has emerged as a reliable marker of acute renal injury and is produced at the maternal–fetal interface but its role in preeclampsia has not been systematically examined. This study investigated whether plasma NGAL concentrations changed in patients with preeclampsia at diagnosis compared to normotensive controls.
Study Design:
A case–control study was performed. Plasma was collected from women with preeclampsia and normotensive controls matched for age, gestational age, and body mass index. Plasma NGAL concentrations were measured by specific enzyme-linked immunosorbent assay.
Results:
Patients with preeclampsia had significantly higher NGAL concentrations than controls (median [range]: 203.8 ng/mL [66.1-575.4] vs 122.8 ng/mL [7.0-669.7]; P = .047). In subgroup analysis, patients with severe preeclampsia had significantly higher NGAL concentrations than those with mild preeclampsia. Plasma NGAL concentrations were positively correlated with the amount of proteinuria in women with preeclampsia (P = .003).
Conclusions:
Plasma NGAL concentrations were significantly elevated in women with preeclampsia versus normotensive controls, and concentrations appear to be associated with the severity of the disease.
Keywords: NGAL, preeclampsia, plasma, biomarker, pregnancy
Introduction
Preeclampsia (gestational proteinuric hypertension) complicates 6% to 8% of all pregnancies, and is a major cause of maternal/perinatal morbidity and mortality.1 It cannot be accurately predicted or prevented, and the only effective treatment is delivery. As such, early and accurate diagnosis is critical to optimize pregnancy outcome. Recent studies have shown that a number of biomarkers may be associated with preeclampsia, including several proangiogenic (vascular endothelial growth factor, placental growth factor) and antiangiogenic factors (soluble fms-like tyrosine kinase 1, soluble endoglin).2–13 We propose that neutrophil gelatinase-associated lipocalin (NGAL) may be another such biomarker.
The NGAL is a 25-kDa lipocalin that was originally purified from activated human neutrophils, but has since been shown to be produced by other immune cells, hepatocytes, adipocytes, and renal tubular cells. The NGAL appears to serve primarily as a transport shuttle transferring small molecules (including retinoids, arachidonic acid, prostaglandins, fatty acids, steroids, iron, and matrix metalloproteinases [MMPs]) in a safe and controlled fashion between adjacent cells.14–17 It has also been shown to be a critical component of innate immunity to bacterial infection, a function that appears to be related to its ability to transport and sequester iron into immune cells,18 and has been associated also with cellular growth and differentiation.19,20 The NGAL concentrations are low in healthy human tissues (such as kidney, lung, stomach, liver, and colon), but appear to be upregulated in pathologic conditions following epithelial cell injury.21,22 Several reports suggest a relationship between NGAL and pelvic inflammatory disease,23 chronic obstructive pulmonary disease,24 and atherosclerosis.25 The NGAL expression has also been found in malignancies of the breast, lung, colon, and pancreas,26–28 where it appears to be associated with tumor progression since overexpression of NGAL enhances the enzymatic activity of MMP-9 which, in turn, leads to lysis of basal membranes and extracellular matrix.29–31 The NGAL is not expressed in normal ovaries but is upregulated in ovarian cancer cells.21,32
More recently, NGAL has emerged as a potentially useful diagnostic biomarker for acute kidney injury,33–38 and several studies have reported that elevated NGAL concentration in plasma and/or urine is superior to conventional biomarkers in the prediction of acute kidney injury.38–40 Despite the central role of the kidney in the pathogenesis of preeclampsia and the observation that NGAL is produced at the maternal–fetal interface,41 there have been few studies about the systemic role of NGAL in preeclampsia. In the previous studies, serum NGAL concentrations significantly increased in women who subsequently developed preeclampsia.42,43 The studies showed that NGAL could be used in the prediction of preeclampsia before the clinical diagnosis of the disease. However, comparison of NGAL levels according to the disease severity was not performed in those studies.
The pathogenesis of preeclampsia is not completely understood, but likely involves a series of complex mechanisms including incomplete invasion and remodeling of the maternal spiral arteries,44 high circulating levels of antiangiogenic factors and proinflammatory cytokines,2,45 and generalized endothelial cell dysfunction.46–49 It is promising therefore to posit that the generalized endothelial injury associated with preeclampsia could lead to an upregulation of circulating NGAL levels. This study examined whether plasma NGAL concentrations changed in patients with preeclampsia at diagnosis compared to normotensive controls.
Materials and Methods
Study Population
A case–control study was performed. The study population consisted of women with a singleton pregnancy admitted to Seoul National University Hospital between January 2009 and March 2011. Patients with preeclampsia were selected as cases. Preeclampsia was defined as new-onset hypertension (systolic blood pressure ≥140 mm Hg and/or diastolic pressure ≥90 mm Hg on 2 occasions at least 4 hours but no more than 7 days apart) and significant proteinuria (≥300 mg/24 h or ≥1+ on dipstick) after 20 weeks of gestation in a previously normotensive woman.1 A diagnosis of severe preeclampsia was based on the presence of preeclampsia and one or more of the following criteria: persistent systolic blood pressure ≥160 mm Hg or diastolic blood pressure ≥110 mm Hg, proteinuria ≥5 g in a 24-hour urine collection or ≥3+ dipsick in random urine samples, oliguria (<500 mL/d), cerebral or visual disturbances, pulmonary edema, epigastric or right upper-quadrant pain, impaired liver function, thrombocytopenia (<100 000 platelets/μL), or fetal growth restriction.1 An equal number of normotensive women with a singleton pregnancy admitted in the same period were selected as controls. Age, gestational age at sampling and body mass index (BMI) were considered for the selection of controls. Women with other medical or obstetric complications (such as chronic hypertension, diabetes, systemic lupus erythematosus, acute systemic inflammation, fever, or preterm labor) or with major congenital fetal anomaly were excluded from both groups.
Sample Collection
Maternal blood samples were collected after obtaining written informed consent. The local institutional review board at Seoul National University Hospital approved the study. Maternal venous blood was obtained at the time of hospital admission and centrifuged, and the plasma was stored at −70°C.
NGAL Immunoassay
Plasma NGAL concentrations were measured using a commercially available enzyme-linked immunosorbent assay (Bioporto, Gentofte, Denmark).50 Both intra- and interassay coefficients of variation were <10%. All measurements were performed twice, and the result reported as the average of the 2 values.
Statistical Analysis
Nonparametric techniques were used for statistical analysis. Comparison of the continuous variables was performed using the Mann-Whitney U test. Proportions were compared using Pearson chi-square or Fisher exact test. The nonparametric statistical dependence between 2 variables was measured by Spearman's rho.
Results
Study Population
Demographic and clinical characteristics of the case and control groups (n = 37 for each) are summarized in Table 1. Of the 37 women with preeclampsia, 25 had severe preeclampsia and 12 had mild preeclampsia. There were no statistical differences between the women with and without preeclampsia in terms of maternal age, prepregnancy BMI, and gestational age at sampling. However, compared to normotensive controls, women with preeclampsia were significantly more likely to be nulliparous, has significantly lower median gestational age at delivery, and a significantly lower median birth weight (Table 1).
Table 1.
Clinical Characteristics of the Study Population.
| Control (n = 37) | Preeclampsia (n = 37) | P | |
|---|---|---|---|
| Maternal age,a years | 33.0 (18-43) | 33.0 (15-41) | .999 |
| Nulliparity, n | 16 (43.2%) | 27 (73.0%) | .018 |
| Prepregnancy body mass index,a kg/m2 | 21.1 (15.2-26.4) | 22.0 (17.7-31.7) | .412 |
| Gestational age at sampling,a weeks | 37.0 (24.1-40.4) | 34.0 (26.0-39.9) | .052 |
| Gestational age at delivery,a weeks | 37.7 (34.0-41.3) | 34.9 (28.0-40.0) | .001 |
| Birth weight,a g | 2815 (2110-3820) | 2150 (800-4320) | <.001 |
a Data are given as median (range).
Plasma NGAL Concentrations in Women With/Without Preeclampsia
Patients with preeclampsia had significantly higher circulating NGAL concentrations than normotensive controls (median [range]: 203.8 ng/mL [66.1-575.4] vs 122.8 ng/mL [7.0-669.7], P = .047, Figure 1).
Figure 1.
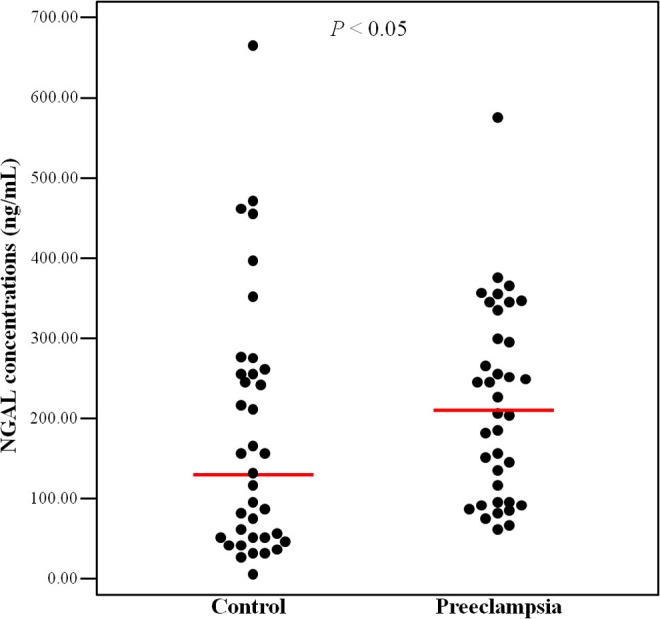
Neutrophil gelatinase–associated lipocalin (NGAL) concentrations in women with/without preeclampsia. The median plasma NGAL concentrations in women with preeclampsia was significantly higher than that of controls (median [range]: 203.77 ng/mL [66.1-575.4] vs 122.77 ng/mL [7.0-669.7], P = .047 by Mann-Whitney U test).
Plasma NGAL Concentrations in Women With Preeclampsia According to the Severity
In subgroup analysis, patients with severe preeclampsia had a significantly lower gestational age at sampling (median [range]: 31.9 weeks [26.0-38.1] vs 38.4 weeks [33.7-39.9], P < .001) and lower gestational age at delivery (median [range]: 33.4 weeks [28.0-38.6] vs 38.8 weeks [34.9-40.0], P < .001) than those with mild preeclampsia, but there were no statistical differences according to the severity of preeclampsia in terms of maternal age, parity and prepregnancy BMI (Table 2). Patients with severe preeclampsia had significantly higher median NGAL concentrations than those with mild preeclampsia (median [range]: 237.5 ng/mL [67.4-575.4] vs 125.9 ng/mL [66.1-295.7], P = .049, Figure 2). The plasma level of NGAL was significantly higher in patients with hypertension of severe criteria (systolic blood pressure ≥160 mm Hg or diastolic blood pressure ≥110 mm Hg) than those without severe hypertension (P = .024). This association could not be accounted for by the difference in gestational age, since plasma NGAL concentrations were not affected by gestational age at sampling in the study population as a whole (P = .630) or in the subgroup analysis (P = .476). There was also no significant difference in the median plasma NGAL concentrations between nulliparous and multiparous women (P = .097). And there were no statistical correlations between the NGAL concentrations and maternal white blood cell count, platelet count, or gestational age at delivery.
Table 2.
Clinical Characteristics of Women With Preeclampsia According to Severity.
| Severe Preeclampsia (n = 25) | Mild Preeclampsia (n = 12) | P | |
|---|---|---|---|
| Maternal age,a years | 34.0 (15-39) | 33.0 (28-41) | .643 |
| Nulliparity, n | 17 (68.0%) | 10 (83.3%) | .445 |
| Prepregnancy body mass index,a kg/m2 | 20.0 (17.7-25.5) | 22.7 (19.0-31.7) | .110 |
| Gestational age at sampling,a weeks | 31.9 (26.0-38.1) | 38.4 (33.7-39.9) | <.001 |
| Gestational age at delivery,a weeks | 33.4 (28.0-38.6) | 38.8 (34.9-40.0) | <.001 |
| Birth weight,a g | 1560 (800-2690) | 2815 (2060-4320) | <.001 |
a Data are given as median (range).
Figure 2.
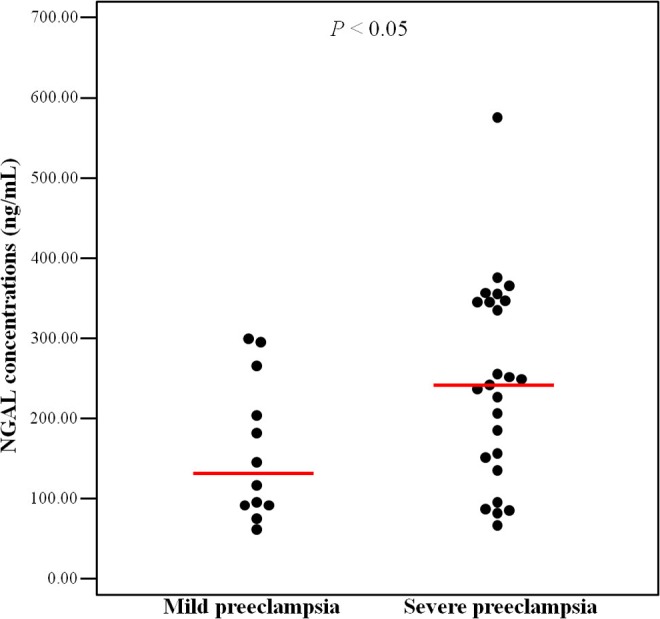
Neutrophil gelatinase–associated lipocalin (NGAL) concentrations in women with mild and severe preeclampsia. The median plasma NGAL concentrations in women with severe preeclampsia was significantly higher than that in women with mild preeclampsia (median [range]: 237.5 ng/mL [67.4-575.4] vs 125.9 ng/mL [66.1-295.7], P = .049 by Mann-Whitney U test).
Correlation Between Plasma NGAL Concentrations and Proteinuria
Plasma NGAL concentrations were positively correlated with the amount of proteinuria in women with preeclampsia (r = .485, P = .003). One patient failed to complete a 24-hour urine collection because she required an emergency cesarean delivery for worsening preeclampsia shortly after admission; she was excluded from this analysis. Of note, her urinary albumin was 4+ on dipstick and the level of plasma NGAL concentration was high (367.6 ng/mL). There were 4 patients with severe preeclampsia who had relatively low levels of proteinuria (300-500 mg/24 h). Of these 4 patients, 3 women had plasma NGAL concentrations <100 ng/mL. The fourth woman had a high plasma NGAL concentration (327 ng/mL) and she went on to develop significant proteinuria (5796 mg/24 h) 1 month later. Serum creatinine concentrations showed a tendency toward a positive correlation with plasma NGAL concentrations in women with preeclampsia but this association failed to reach statistical significance (r = .328, P = .055).
Plasma NGAL Concentrations and Fetal Growth
Thirty-six percent (9 of 25) of patients with severe preeclampsia delivered neonates with a birth weight of <10th percentile for gestational age. These 9 women had a wide range of plasma NGAL concentrations (from 80.7 to 575.4 ng/mL). There was no significant difference in the median plasma NGAL concentrations between women with severe preeclampsia who delivered small-for-gestational age fetuses and those who did not (median [range]: 212.8 ng/mL [80.7-575.4] vs 240.4 ng/mL [67.4-378.3], P = .718). There were no statistical correlations between the NGAL concentrations and birth weight.
Discussion
The Principal Findings of the Study
The principal findings of the study include (1) that patients with preeclampsia had significantly higher circulating NGAL concentrations than gestational age-matched normotensive controls; (2) that patients with severe preeclampsia had significantly higher NGAL concentrations than those with mild preeclampsia; and (3) that plasma NGAL concentrations were positively correlated with the amount of proteinuria in women with preeclampsia. We conclude, therefore, that there is an association between plasma NGAL concentrations and preeclampsia and that circulating NGAL levels may reflect the severity of the disease. The inability to clearly separate mild from severe preeclampsia can be explained by the underlying heterogeneity of preeclampsia with its complex pathophysiology.
A positive correlation between plasma NGAL concentration and degree of proteinuria is consistent with prior observations suggesting that NGAL is a reliable marker of acute renal injury.33–38 In addition, although it failed to reach statistical significance, there did appear to be a trend toward a positive correlation between serum creatinine and plasma NGAL concentrations in women with preeclampsia.
Previous studies showed the relationship between serum NGAL concentration and preeclampsia before the onset of the disease development for prediction of the preeclampsia. 42,43,51,52 On the other hand, we analyzed the association between plasma NGAL concentration and preeclampsia at the time of diagnosis, moreover, we identified NGAL levels based on the severity of preeclampsia.
NGAL in Pathogenesis of Preeclampsia
The elevated concentrations of NGAL in maternal blood with preeclampsia likely represent a consequence of the generalized endothelial dysfunction that characterizes this disease as has been previously proposed.42,43 However, analysis of NGAL concentration according to the severity of preeclampsia or relationship between the concentrations of NGAL and serum creatinine had not been performed in the previous studies. Preeclampsia results from incomplete invasion of the spiral arteries by extravillous cytotrophoblasts. The resultant defective implantation results in a failure of the trophoblast to remodel the maternal vasculature and the persistence of the maternal spiral arteries as small-caliber high-resistance vessels.53–57 Various antiangiogenic factors and proinflammatory mediators then act to aggravate the underlying endothelial cell injury in women who subsequently develop preeclampsia. This generalized endothelial cell injury leads, in turn, to increased levels of NGAL in the maternal circulation. The NGAL was originally identified as a glycoprotein in human neutrophils that complexes with MMP-9,19,20,23 and its expression is upregulated in a number of inflammatory conditions with underlying endothelial cell injury.23–25,35–39 In preeclampsia, defective implantation results in oxidative stress and the upregulation of antiangiogenic factors and proinflammatory mediators in the maternal circulation leading to a variety of clinical symptoms. Therefore, it is reasonable to assume that NGAL may be associated with pathogenesis of preeclampsia.
Weaknesses of the Study and Proposals for Future Research
This study was limited by a relatively small number of participants and the difficulty of identifying adequate normotensive controls, especially at early gestational ages. There were women who delivered at preterm in the control group, however, their causes of preterm delivery were not concerned with the conditions such as acute systemic inflammation, fever, or preterm labor. They delivered at preterm by cesarean section or vaginal delivery after induction of labor because of maternal underlying kyphosis, isolated decreased amniotic fluid volume without fetal growth restriction, vaginal bleeding, or preterm premature rupture of membrane without uterine contractions. There was no growth restricted fetus in the control group. Even though it is not certain that the causes of preterm delivery are associated with elevated NGAL levels and most of the cases had low NGAL concentrations, the poor diagnostic performance of plasma NGAL for preeclampsia in our study might come from the limitation of controls. A prospective study with larger number of samples would be required to confirm and further validate the results of this study. On the other hand, several women of the normotensive group in this study had unexplained elevated plasma NGAL levels without any medical or obstetrical problem, which did not appear to adversely affect pregnancy outcome.
In summary, NGAL concentrations were significantly increased in the circulation of women with preeclampsia as compared with gestational age-matched normotensive controls, and the degree of NGAL elevation appear to be associated with the severity of preeclampsia. Additional studies are needed to further investigate the role of this biomarker in the diagnosis and prediction of preeclampsia.
Footnotes
Authors' Note: This study was presented at the 32nd Annual Meeting of the Society for Maternal–Fetal Medicine, Dallas, Texas (February 11, 2012).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grant on 0320120160 from the SNUH Research Fund.
References
- 1. American College of Obstetricians and Gynecologists. Diagnosis and management of preeclampsia and eclampsia. ACOG Practice Bulletin No. 33. Obstet Gynecol. 2002;99(1):159–167. [DOI] [PubMed] [Google Scholar]
- 2. Chaiworapongsa T, Romero R, Espinoza J, et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Am J Obstet Gynecol. 2004;190(6):1541–1550. [DOI] [PubMed] [Google Scholar]
- 3. Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–683. [DOI] [PubMed] [Google Scholar]
- 4. Thadhani R, Mutter WP, Wolf M, et al. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89(2):770–775. [DOI] [PubMed] [Google Scholar]
- 5. Chaiworapongsa T, Romero R, Kim YM, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17(1):3–18. [DOI] [PubMed] [Google Scholar]
- 6. Levine RJ, Thadhani R, Qian C, et al. Urinary placental growth factor and risk of preeclampsia. JAMA. 2005;293(1):77–85. [DOI] [PubMed] [Google Scholar]
- 7. Buhimschi CS, Norwitz ER, Funai E, et al. Urinary angiogenic factors cluster hypertensive disorders and identify women with severe preeclampsia. Am J Obstet Gynecol. 2005;192(3):734–741. [DOI] [PubMed] [Google Scholar]
- 8. Aggarwal PK, Jain V, Sakhuja V, Karumanchi SA, Jha V. Low urinary placental growth factor is a marker of pre-eclampsia. Kidney Int. 2006;69(3):621–624. [DOI] [PubMed] [Google Scholar]
- 9. Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic gactors in preeclampsia. N Engl J Med. 2006;355(10):992–1005. [DOI] [PubMed] [Google Scholar]
- 10. Erez O, Romero R, Espinoza J, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21(5):279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kusanovic JP, Romero R, Chaiworapongsa T, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22(11):1021–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buhimschi CS, Baumbusch MA, Dulay AT, et al. The role of urinary soluble endoglin in the diagnosis of pre-eclampsia: comparison with soluble fms-like tyrosine kinase 1 to placental growth factor ratio. BJOG. 2010;117(3):321–330. [DOI] [PubMed] [Google Scholar]
- 13. Akolekar R, Syngelaki A, Sarquis R, Zvanca M, Nicolaides KH. Prediction of early, intermediated and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11-13 weeks. Prenat Diagn. 2011;31(1):66–74. [DOI] [PubMed] [Google Scholar]
- 14. Flower DR. The lipocalin family: a role in cell regulation. FEBS Lett. 1994;354(1):7–11. [DOI] [PubMed] [Google Scholar]
- 15. Schmidt-Ott KM, Mori K, Kalandadze A, et al. Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia. Curr Opin Nephrol Hypertens. 2006;15(4):442–449. [DOI] [PubMed] [Google Scholar]
- 16. Bolignano D, Donato V, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008;52(3):595–605. [DOI] [PubMed] [Google Scholar]
- 17. Yang J, Goetz D, Li JY, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10(5):1045–1056. [DOI] [PubMed] [Google Scholar]
- 18. Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432(7019):917–921. [DOI] [PubMed] [Google Scholar]
- 19. Kjeldsen L, Johnsen AH, Sengelv H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268(14):10425–10432. [PubMed] [Google Scholar]
- 20. Kjeldsen L, Bainton DF, Sengelv H, Borregaard N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood. 1994;83(3):799–807. [PubMed] [Google Scholar]
- 21. Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997;45(1):17–23. [DOI] [PubMed] [Google Scholar]
- 22. Xu S, Venge P. Lipocalins as biochemical markers of disease. Biochem Biophys Acta. 2000;1482(1-2):298–307. [DOI] [PubMed] [Google Scholar]
- 23. Tsai HT, Su PH, Lee TH, et al. Significant elevation and correlation of plasma neutrophil gelatinase associated lipocalin and its complex with matrix metalloproteinase-9 in patients with pelvic inflammatory disease. Clin Chim Acta. 2011;412(13-14):1252–1256. [DOI] [PubMed] [Google Scholar]
- 24. Eagan TM, Damas JK, Ueland T, et al. Neutrophil gelatinase-associated lipocalin: a biomarker in COPD. Chest. 2010;138(4):888–895. [DOI] [PubMed] [Google Scholar]
- 25. Elneihoum AM, Falke P, Hedblad B, Lindgarde F, Ohlsson K. Leukocyte activation in atherosclerosis: correlation with risk factors. Atherosclerosis. 1997;131(1):79–84. [DOI] [PubMed] [Google Scholar]
- 26. Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38(3):414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stoesz SP, Friedl A, Haag JD, Lindstrom MJ, Clark GM, Gould MN. Heterogeneous expression of the lipocalin NGAL in primary breast cancers. Int J Cancer. 1998;79(6):565–572. [DOI] [PubMed] [Google Scholar]
- 28. Furutani M, Arii S, Mizumoto M, Kato M, Imamura M. Identification of a neutrophil gelatinase-associated lipocalin mRNA in human pancreatic cancers using a modified signal sequence trap method. Cancer Lett. 1998;122(1-2):209–214. [DOI] [PubMed] [Google Scholar]
- 29. Kubben FJGM, Sier CFM, Hawinkels LJAC, et al. Clinical evidence for a protective role of lipocalin-2 against MMP-9 autodegradation and the impact for gastric cancer. Euro J Cancer. 2007;43(12):1869–1876. [DOI] [PubMed] [Google Scholar]
- 30. Lim R, Ahmed N, Borregaard N, et al. Neutrophil gelatinase-associated lipocalin (NGAL) an early-screening biomarker for ovarian cancer: NGAL is associated with epidermal growth factor-induced epithelio-mesenchymal transition. Int J Cancer. 2007;120(11):2426–2434. [DOI] [PubMed] [Google Scholar]
- 31. Provatopoulou X, Gounaris A, Kalogera E, et al. Circulating levels of matrix metalloproteinase-9 (MMP-9), neutrophil gelatinase-associated lipocalin (NGAL) and their complex MMP-9/NGAL in breast cancer disease. BMC Cancer. 2009;9:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bartsch S, Tschesche H. Cloning and expression of human neutrophil lipocalin cDNA derived from bone marrow and ovarian cancer cells. FEBS Lett. 1995;357(3):255–259. [DOI] [PubMed] [Google Scholar]
- 33. Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14(10):2534–2543. [DOI] [PubMed] [Google Scholar]
- 34. Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–1238. [DOI] [PubMed] [Google Scholar]
- 35. Devarajan P. Emerging biomarkers of acute kidney injury. Contrib Nephrol. 2007;156:203–212. [DOI] [PubMed] [Google Scholar]
- 36. Parikh CR, Devarajan P. New biomarkers of acute kidney injury. Crit Care Med. 2008;36(suppl 4):S159–S165. [DOI] [PubMed] [Google Scholar]
- 37. Nickolas TL, O'Rourke MJ, Yang J, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148(11):810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A; NGAL Meta-analysis Investigator Group. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54(6):1012–1024. [DOI] [PubMed] [Google Scholar]
- 39. Haase-Fielitz A, Bellomo R, Devarajan P, et al. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery--a prospective cohort study. Critical Care Med. 2009;37(2):553–560. [DOI] [PubMed] [Google Scholar]
- 40. Makris K, Markou N, Evodia E, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) as an early marker of acute kidney injury in critically ill multiple trauma patients. Clin Chem Lab Med. 2009;47(1):79–82. [DOI] [PubMed] [Google Scholar]
- 41. Tadesse S, Luo G, Park JS, et al. Intraamniotic infection upregulates neutrophil gelatinase-associated lipocalin (NGAL) expression at the maternal-fetal interface at term: Implications for infection-related preterm birth. Reprod Sci. 2011;18(8):713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. D’Anna R, Baviera G, Giordano D, Todarello G, Corrado F, Buemi M. Second trimester neutrophil gelatinase-associated lipocalin as a potential prediagnostic marker of preeclampsia. Acta Obstet Gynecol Scand. 2008;87(12):1370–1373. [DOI] [PubMed] [Google Scholar]
- 43. D’Anna R, Baviera G, Giordano D, et al. First trimester serum PAPP-A and NGAL in the prediction of late-onset pre-eclampsia. Prenat Diagn. 2009;29(11):1066–1068. [DOI] [PubMed] [Google Scholar]
- 44. Gerretsen G, Huisjes HJ, Elema JD. Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. Br J Obstet Gynaecol. 1981;88(9):876–881. [DOI] [PubMed] [Google Scholar]
- 45. Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006;11(5):309–316. [DOI] [PubMed] [Google Scholar]
- 46. Roberts JM, Taylor RN, Goldfien A. Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia. Am J Hypertension. 1991;4(8):700–708. [DOI] [PubMed] [Google Scholar]
- 47. Schiff E, Ben-Baruch G, Peleg E, et al. Immunoreactive circulating endothelin-1 in normal and hypertensive pregnancies. Am J Obstet Gynecol. 1992;166(2):624–628. [DOI] [PubMed] [Google Scholar]
- 48. Higgins JR, Papayianni A, Brady HR, Darling MR, Walshe JJ. Circulating vascular cell adhesion molecule-1 in pre-eclampsia, gestational hypertension, and normal pregnancy: evidence of selective dysregulation of vascular cell adhesion molecule-1 homeostasis in pre-eclampsia. Am J Obstet Gynecol. 1998;179(2):464–469. [DOI] [PubMed] [Google Scholar]
- 49. Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006;195(1):40–49. [DOI] [PubMed] [Google Scholar]
- 50. Pedersen KR, Ravn HB, Hjortdal VE, Norregaard R, Povlsen JV. Neutrophil gelatinase-associated lipocalin (NGAL): validation of commercially available ELISA. Scand J Clin Lab Invest. 2010;70(5):374–382. [DOI] [PubMed] [Google Scholar]
- 51. Youssef A, Righetti F, Morano D, Rizzo N, Farina A. Uterine artery Doppler and biochemical markers (PAPP-A, PIGF, sFlt-1, P-selectin, NGAL) at 11+0 to 13+6 weeks in the prediction of late (> 34 weeks) pre-eclampsia. Prenat Diagn. 2011;31(12):1141–1146. [DOI] [PubMed] [Google Scholar]
- 52. D'Anna R, Baviera G, Giordano D, et al. Neutrophil gelatinase-associated lipocalin serum evaluation through normal pregnancy and in pregnancies complicated by preeclampsia. Acta Obstet Gynecol Scand. 2010;89(2):275–278. [DOI] [PubMed] [Google Scholar]
- 53. Brosens I. A study of the spiral arteries of the decidua basalis in normotensive and hypertensive pregnancies. J Obstet Gynaecol Br Commonw. 1964;71:222–230. [DOI] [PubMed] [Google Scholar]
- 54. Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–191. [PubMed] [Google Scholar]
- 55. Robertson WB, Brosens I, Dixon G. Maternal uterine vascular lesions in the hypertensive complications of pregnancy. Perspect Nephrol Hypertens. 1976;5:115–127. [PubMed] [Google Scholar]
- 56. Brosens IA. Morphological changes in the utero-placental bed in pregnancy hypertension. Clin Obstet Gynaecol. 1977;4(3):573–593. [PubMed] [Google Scholar]
- 57. Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204(3):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]


