Abstract
Preeclampsia (PE) remains a major cause of maternal/fetal morbidity–mortality worldwide. The first stage of PE is characterized by placental hypoxia due to a relative reduction in uteroplacental blood flow, resulting from restricted trophoblast invasion. However, hypoxia is also an essential element for the success of invasion. Under hypoxic conditions, 2-methoxyestradiol (2-ME) could induce the differentiation of cytotrophoblast cells into an invasive phenotype in culture. 2-Methoxyestradiol is generated by catechol-O-methyltransferase, an enzyme involved in the metabolic pathway of estrogens. During pregnancy, circulating 2-ME levels increase significantly when compared to the menstrual cycle. Interestingly, plasma levels of 2-ME are lower in women with PE than in controls, and these differences are apparent weeks or even months before the clinical manifestations of the disease. This article reviews the metabolic pathways involved in 2-ME synthesis and discusses the roles of these pathways in normal and abnormal pregnancies, with particular emphasis on PE.
Keywords: 2-methoxyestradiol (2-ME), aromatase, preeclampsia, hypoxia, methionine–homocysteine metabolism
Introduction
Preeclampsia (PE) remains a major cause of maternal and fetal morbidity and mortality throughout the world, causing nearly 40% of premature births delivered before 35 weeks of gestation.1 Preeclampsia complicates around 2% to 8% of all pregnancies worldwide and, despite the amount of resources invested in research and treatment of this disorder, it remains difficult to accurately predict and almost impossible to prevent. Preeclampsia is also a major risk factor for the development of cardiovascular and cerebrovascular disease in later life.2,3
Preeclampsia is defined as the new onset of hypertension during the second half of pregnancy accompanied by significant proteinuria.4 However, it begins with an asymptomatic phase during the first half of gestation characterized by deficient trophoblast invasion and incomplete spiral artery remodeling. Both processes are related to the invasive potential of the extravillous cytotrophoblast (EVCT) cells.5 A detailed understanding of the underlying factors that explain this process in normal pregnancies and in patients that subsequently develop PE will help in the development of preventive and/or early therapeutic interventions aimed to reduce the associated morbidity and mortality during pregnancy and potentially also the long-term sequelae associated with PE.
It has been suggested that PE constitutes a clinical spectrum that includes 2 distinct conditions: “maternal PE” and “placental PE.”6 In this model, placental PE refers to otherwise healthy women who develop PE primarily because of abnormal placentation, whereas maternal PE refers to women who develop PE because of a preexisting condition such as cardiovascular disease, chronic arterial hypertension, or diabetes, without abnormal placentation. Placental PE, which results from poor trophoblast invasion, generates oxidative stress at the maternal–fetal interface, leading to the release of various factors from the hypoxic trophoblast cells into maternal circulation, which induces an inflammatory response and generalized epithelial dysfunction.7 In clinical practice, however, it is likely that the majority of patients with PE have elements of both pathologic entities. This difference explains at least in part the great variability in the gestational age at presentation and severity of this syndrome.
Preeclampsia is a multifactorial pregnancy-specific disease that involves the failure of various body systems and whose etiology has been associated with multiple causes. It is important to consider all possible interactions between metabolic pathways involved in the development of this disease in order to have a better understanding of the complexity of the pathophysiology of PE.
Etiopathogenesis of PE: A Placental Disease
Despite recent research advances, the pathophysiology that triggers the disease is still not clearly elucidated. Nevertheless, it seems clear that the development of PE requires the presence of a placenta, since the clinical syndrome will not develop in the absence of a placenta, and it disappears soon after placental delivery.8 It is also widely accepted that the pathophysiological process of PE begins with inadequate trophoblast invasion early in pregnancy, which produces increased oxidative stress contributing to the development of systemic endothelial dysfunction in the later phases of the disease. This leads in turn to the characteristic clinical manifestations of PE, which include hypertension, proteinuria, and nondependent edema.
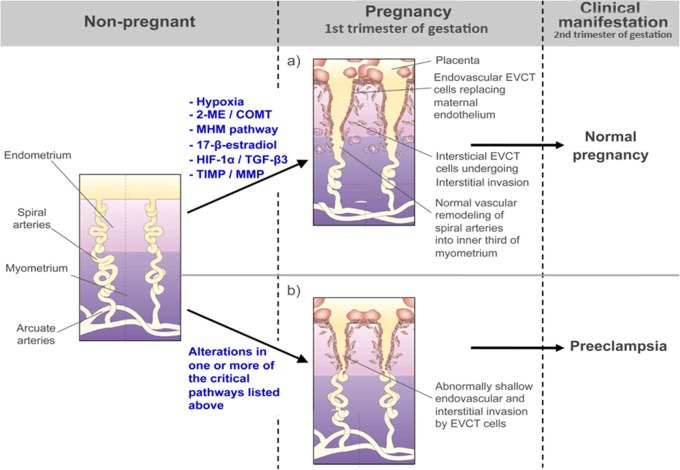
In normal placentation, during the first weeks of gestation after the blastocyst makes contact with the maternal decidua, the cytotrophoblast cells proliferate to form cell columns that intrude into the maternal tissues.9 From the tips of these anchoring villous structures, EVCT cells derived from this proliferating cytotrophoblast invade the maternal decidua and differentiate further into interstitial and endovascular trophoblast cells. The invasion process begins at the center of the placental bed and expands progressively outward toward the lateral areas in the shape of a ring. During interstitial invasion, the compact decidual tissue is “swamped” by interstitial EVCT cells that, from 8 weeks onward, can be seen both clustered around blood vessels and in the inner myometrium zone of the placenta, at which point they stop invading.10 At the same time, endovascular trophoblast cells migrate into the maternal spiral arteries in order to plug these vessels. During this period, the embryo is nourished primarily by the endometrial glands of the maternal decidua and exists in an environment that is relatively hypoxic and hypoglycemic. At around 10 to 12 weeks of gestation, the trophoblast plugs begin to dissolve and endovascular trophoblast cells replace the maternal endothelial lining as far as the inner third of the myometrium, degrading the muscular and elastic component of the vessel walls. This results in the formation of low-resistance vessels required for the establishment of the definitive uteroplacental circulation and for adequate fetal growth9,11 (Figure 1). At this time, maternal blood begins to flow into the intervillous space, where it takes over the responsibility of nourishing the developing embryo. A relative reduction in uteroplacental flow due to deficient trophoblast invasion and failure of the maternal spiral arteries to undergo vascular remodeling may be one of factors that triggers the development of PE5,12 (Figure 1). This was first highlighted by histopathological studies of the placental implantation site, which showed that 80% to 100% of placentas from patients with PE have a deficit of the physiological invasion of the maternal spiral arteries by the EVCT, whereas this finding was rarely observed in placentas from normotensive patients.13,14
Figure 1.
Invasion process scheme during early normal and abnormal pregnancy. A, In normal placentation, EVCT cells derived from proliferating cytotrophoblast invade the maternal decidua and differentiate into interstitial and endovascular trophoblast cells. This process involves critical metabolic pathways necessary for adequate EVCT invasion. During interstitial invasion, the compact decidual tissue is “swamped” by interstitial EVCT cells that cluster around blood vessels and in the inner third myometrium zone of the placenta. At the same time, endovascular trophoblast cells migrate into the maternal spiral arteries in order to replace the maternal endothelial lining as far as the inner third of the myometrium, degrading the muscular and elastic component of the vessel walls. This results in the formation of low-resistance vessels that are required for the establishment of the definitive uteroplacental circulation and for adequate fetal growth, as can be seen in normal pregnancy. B, Alterations in one or more of the critical metabolic pathways involved in the normal invasive process (eg, prolonged hypoxia during invasion process; low 2-ME levels at implantation time due to alteration in COMT enzyme activity, SAM/SAH levels, and/or MHM pathway alteration; 17β-estradiol level alteration due to an aromatase pathway alteration; angiogenic or matrix remodeling imbalance) may result in a reduction of uteroplacental flow due to deficient trophoblast invasion and failure of the maternal spiral arteries to undergo vascular remodeling. This could trigger other local alterations during the second trimester of pregnancy that ultimately contribute to the development of PE. EVCT indicates extravillous cytotrophoblast; PE, preeclampsia; 2-ME, 2-methoxyestradiol; COMT, catechol-O-methyltransferase; HIF-1α, hypoxia-inducible factor-1α; TGF-β3, transforming growing factor-β3; MHM, methionine–homocysteine metabolism; TIMP, tissue inhibitor of metalloproteinases; MMP, matrix metalloproteinases.
It has been postulated that the physiological change that favors the invasive phenotype of EVCT cells is due to the exposure of cytotrophoblast cells to a hypoxic environment. The normal concentration of oxygen in the first trimester placenta is around 3% O2 (±18 mm Hg), and this low oxygen milieu is believed to facilitate trophoblast invasion.15 In the normal placentation process, cytotrophoblast invasion is regulated by the gradient of oxygen concentration between the placenta and maternal arteries. Moreover, the hypoxic environment in which the cytotrophoblast exists at the beginning of placentation changes gradually to a normoxic environment as invasion proceeds.16
The hypoxic placental environment during the first trimester induces an adaptive response that is accompanied by an increase in the expression of a variety of genes within the trophoblast. These genetic responses are designed to optimize cell survival in a hypoxic environment and are regulated by a common oxygen-sensing factor, hypoxia-inducible factor 1 (HIF-1).17,18 This inducible factor is a basic helix-loop-helix PAS (from per, ARNT, and sim, the first genes that contain this motif) transcription factor that binds to DNA as a heteromeric complex composed of 2 subunits the constitutively expressed HIF-1β, and HIF-1α, which are expressed only under hypoxic conditions and are rapidly degraded by the proteosome when oxygen conditions return to normal.19 The expression of HIF-1α parallels the expression of transforming growth factor β3 (TGF-β3), which inhibits the differentiation of the EVCT to an invasive phenotype.20 It has also been found that antisense inhibition of HIF-1 downregulates TGF-β3 messenger RNA (mRNA) expression in villous explants.20 Such suppression of TGF-β3 is associated with inhibition of tissue inhibitor of metalloproteinases (TIMPs) that could produce an imbalance in the synchronized expression patterns of matrix metalloproteinases, leading to a more restricted invasion of the trophoblast, such as that found in PE. The TIMPs have also been reported to directly inhibit cytotrophoblast invasion.21 Moreover, increased expression of HIF-1α, TGF-β3, and TIMP-2 has been observed in placentas from patients with PE, in comparison with the much lower levels of expression in placentas of women with normotensive pregnancies.22
Hypoxia is an essential element for the success of trophoblast invasion, but it does not seem to be the sole regulator of this process. Lee et al recently established that in cell cultures under hypoxic conditions (2.5% oxygen), 2-methoxyestradiol (2-ME), a natural metabolite of 17β-estradiol synthesized by the catechol-O-methyltransferase (COMT) enzyme, induces the differentiation of the cytotrophoblast to an invasive phenotype, thereby allowing for more effective invasion of an extracellular matrix.22 Moreover, the differentiation of these cells was not observed under hypoxic conditions in the absence of 2-ME or by incubation with 2-ME in normoxia.23 These results suggest that appropriate invasion of the trophoblast depends not only on levels of hypoxia but also on the concentration of 2-ME.
In support of this hypothesis, mice genetically deficient for COMT (COMT−/−) show an abnormal accumulation of HIF-1α protein predominantly in the placenta, an effect that was reversed by the administration of 2-ME.24 It has also been suggested that 2-ME counteracts the deleterious effects of HIF-1α-mediated gene expression in the placenta by inhibiting TGF-β3 gene expression and enabling cytotrophoblasts to transdifferentiate into an invasive phenotype. Lee et al have described that, under hypoxia, the expression of HIF-1α and TGF-β3 was decreased.22 These observations are in line with other studies that assigned a deleterious role to HIF-1α accumulation in the placenta20 and suggest that diminished COMT and 2-ME levels could contribute to the elevation of placental HIF-1α. These data also highlight the potential beneficial effects of the use of 2-ME in patients at risk of abnormal trophoblast invasion.
2-Methoxyestradiol and Its Relationship With PE
2-Methoxyestradiol is a naturally occurring metabolite of estradiol. It is generated by the hydroxylation of 17β-estradiol at the 2-position by the enzyme, cytochrome P450, and subsequently by the O-methylation of the catechol ring by the enzyme, COMT. 2-Methoxyestradiol has low estrogenic activity, because it has a 500- and 3200-fold lower affinity than 17β-estradiol for the estrogen receptors α and β, respectively.25 In the last decade, a number of additional action mechanisms have been described for 2-ME, including the inhibition of proliferating tumor cells,25,26 antiangiogenic activity,27 selective disruption of intracellular microtubules,28,29 and the selective inhibition of HIF-1α activity.30 Taken together, these features suggest that 2-ME may be a potent anticancer drug,31 and it is currently being tested in a number of phase I and phase II clinical trials in patients with metastatic breast cancer, prostate cancer, and various other solid tumors.32,33 To date, these trials have shown that 2-ME is well tolerated in humans and has no major toxic effects, even at high doses.
Related to pregnancy, 2-ME has been postulated as a molecule that helps maintain placental homeostasis through the regulation of the cytotrophoblast invasion process during the first stage of pregnancy (first trimester), due to its capacity to induce cytotrophoblast differentiation to an invasive endovascular phenotype, under hypoxic conditions.22 It has also been hypothesized that in the presence of low 2-ME concentrations and placental hypoxia (as observed in PE), cytotrophoblast cells remain with the noninvasive phenotype, associated with a minimal invasive capacity, resulting in a deficient trophoblast invasion as observed in preeclamptic placentas.22
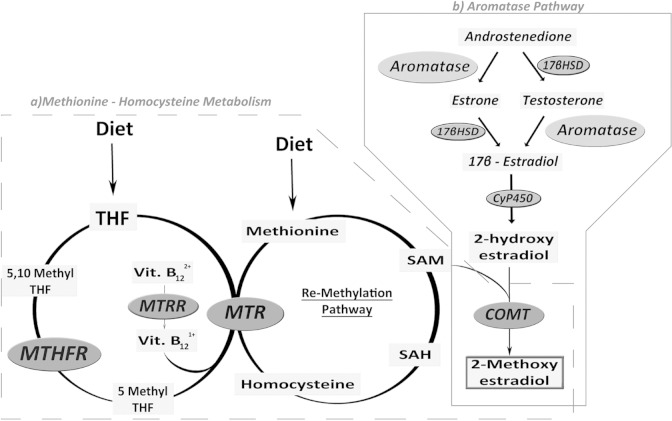
However, the physiological mechanism underlying 2-ME actions are not clearly understood. Throughout the menstrual cycle, picomolar concentrations of 2-ME can be detected in plasma during both the follicular and luteal phase. During normal pregnancy, 2-ME levels increase more than 1000-fold (11th-16th weeks: 0.67 [range between 0.21 and 1.67] ng/mL; 37th-40th weeks: 3.76 [range between 2.03 and 10.69] ng/mL).34 More recently, Barnes et al have described that there is an increase in fetal cord blood 2-ME levels at term of pregnancy (24th–32nd weeks: 62 [range between 38 and 149] pg/mL; 33rd–36th weeks: 72 [range between 43 and 148] pg/mL; 37th–41st weeks: 203 [range between 97 and 509] pg/mL).35 Kanasaki et al have reported that circulating levels of 2-ME during the third trimester of pregnancy are significantly decreased in women with a diagnosis of PE when compared to women with normotensive pregnancies.24 Recent data from our laboratory have shown that 2-ME levels in early pregnancy may also be able to predict the subsequent development of PE, since plasma 2-ME levels at 11 to 14 weeks of gestation were decreased in women who later developed PE (PE: 1.87 ± 2 pg/mL vs normotensive controls: 61.73 ± 27 pg/mL, P < .05).36 Despite these differences, the mechanisms responsible for this reduction are not known. This last step is essential in order to determine whether 2-ME can be used as a potential therapeutic intervention to prevent or treat PE. Several possible explanations exist: (1) decreased expression and/or activity of the COMT enzyme; (2) decreased bioavailability of S-adenosylmethionine (SAM), a methyl donor for COMT, which could be due to the presence of single genetic variants in key enzymes in the methionine–homocysteine pathway that reduce the bioavailability of methionine and SAM or increase levels of homocysteine and/or S-adenosylhomocysteine (SAH); or (3) decreased bioavailability of 17β-estradiol likely due to an alteration in the aromatase pathway (Figure 2). These are discussed in further details below.
Figure 2.
Methionine–homocysteine and aromatase pathways and their involvement in 2-ME synthesis. A, The THF obtained through diet, produces methionine through the remethylation pathway (where MTHFR, MTRR, and MTR are involved) which when added to the diet-supplemented methionine, generates SAM, the main methyl group donor in the methylation reaction. The COMT is a SAM-dependent enzyme that catalyzes the conversion of 2-hydroxyestradiol into 2-ME. B, Androstenedione and testosterone are converted into estrone and 17β-estradiol, respectively, through the action of aromatase. 17β-Estradiol is converted by CyP450 into 2-hydroxyestradiol, the substrate used by COMT for the synthesis of 2-ME. Low levels of 2-ME observed in patients with PE could be explained, among others, by (1) the decreased expression/activity of the COMT enzyme; (2) a decrease in the bioavailability of SAM, likely due to alterations in the methionine–homocysteine pathway that reduce the availability of methionine and SAM, or increase levels of homocysteine and SAH; (3) a decrease in the bioavailability of 17β-estradiol likely due to an alteration in the aromatase pathway. THF indicates tetrahydrofolate; MTHFR, methylenetetrahydrofolate reductase; MTR, methionine synthase; MTRR, methionine synthase reductase; Vit B12, vitamin B12; SAM, S-adenosylmethionine; SAH, S-adenosythomocysteine; 2-ME, 2-methoxyestradiol; PE, preeclampsia; CyP450, cytochromeP450.
Alteration in COMT Expression and/or Activity
As described, 2-ME is synthesized by COMT (Figure 2). This enzyme catalyzes the O-methylation of catechol estrogens. The COMT transfers a methyl group from SAM to one of the hydroxyl groups of the catechol substrate in the presence of magnesium.37 S-Adenosylmethionine is the principal methyl donor inside cells and the methyl groups are key factors for DNA control of cellular proliferation, cellular migration, differentiation, and cell–cell recognition. The production of 2-ME depends on the bioavailability of SAM and 2-hydroxyestradiol as substrates. In this reaction, COMT also produces SAH, a natural occurring noncompetitive inhibitor of COMT. Through this, COMT is involved in the metabolic pathway of estrogens and has a critical role in the methionine–homocysteine metabolic (MHM) cycle.
In humans, there is a single gene for COMT, which has been localized to chromosome 22, band q11.2. This single gene codes for 2 enzymes soluble COMT (S-COMT) and membrane-bound COMT (MB-COMT). Both S-COMT and MB-COMT are considered different enzymes and not simply 2 isoforms of the same enzyme, because MB-COMT is not a precursor of S-COMT.37 These 2 proteins have a ubiquitous and constitutive expression that is distinct for each tissue, including liver, kidney, brain, uterus, placenta, mammary gland, lymphocytes, and erythrocytes.37 Both S-COMT and MB-COMT can be differentiated by their distinct molecular weights (24 and 30 kDa, respectively), but available antibodies are not able to distinguish between these 2 proteins. Palmer et al have reported that COMT enzyme was mainly expressed in the syncytiotrophoblast layer and that placental COMT expression was not altered in severe PE compared to term or preterm normotensive pregnancies.38 Preliminary results of our group, in accordance with Palmer results, show that there are no significant differences in placental COMT protein expression between control and PE patients (unpublished data). With regard to enzyme activity, both enzymes have identical kinetics and a common functional single-nucleotide polymorphism (SNP; rs4680) in codon 108 of S-COMT and codon 158 of MB-COMT.39 Since the COMT gene is characterized by autosomal codominant alleles, this SNP leads to a 3- to 4-fold variation in COMT activity in erythrocytes and liver. The transposition of adenine to guanine at this position gives 1 of 3 different functional variants: that associated with low activity (COMT Met/Met), intermediate activity (COMT Val/Met), or high activity (COMT Val/Val).40 Furthermore, converging lines of evidence suggest that alterations in the activity of COMT may play an important role in the etiology, development, and expression of different diseases, as has been demonstrated for a number of mental disorders and pregnant-related diseases such as fetal growth restriction and PE.39,41 As described above, Kanasaki et al were able to generate a PE-like phenotype (characterized by hypertension, proteinuria, and increased expression of HIF-1 and soluble fms-like tyrosine kinase 1) in a murine COMT (COMT−/−) knockout model, recently confirmed by Stanley et al, which also has low plasma 2-ME concentrations.24,42 Low plasma 2-ME concentrations were associated with absent placental expression of both variants of COMT, S-COMT and MB-COMT. These findings are in keeping with others that have shown a decrease in COMT activity in patients with gestational hypertension.43
Recently, Roten et al showed that the low activity haplotype of the COMT enzyme confers a genetic risk of the development of severe PE in a Norwegian cohort.44 Similarly, Lim et al studying the Val158Met COMT SNP showed that the Met/Met allele of the maternal COMT gene may increase susceptibility to PE.45 More recently, Liang et al have found similar results in the South West Chinese population.46 However, conflicting studies have also appeared and when discussing polymorphisms, it is of high importance to consider not only the sample number in the study but also the origin of the population being studied. Hill et al carried out an allelic variation study of 4 SNPs associated with altered COMT enzyme activity in a Chilean population.47 They found that the maternal low-enzyme activity-related haplotype was associated with a reduced risk of developing PE, and that the risk increased linearly from low-activity to high-activity–associated haplotypes. Our group has recently reported, in a Chilean population, that the placental Val158Met polymorphism was more frequent in controls than in patients with PE, and the presence of this COMT polymorphism in the placenta was associated with a decreased risk of developing PE (PE: 23.1% vs control: 66.6%; χ2 = 10.97, P = .0041).36
The MHM Pathways and Their Relation to 2-ME Synthesis in PE
Methionine–homocysteine metabolism plays a critical role in determining the availability of folate and methionine, which are essential for placental and fetal development. Defects in this pathway reduce the availability of methionine, which is needed for cellular growth. Methionine is transformed into homocysteine through successive reactions that involve the production of SAM and SAH (Figure 2A).
Homocysteine is metabolized by 2 pathways: trans-sulfuration (TS) and remethylation (RM); the latter is the most important and most common metabolic pathway. In TS, homocysteine is metabolized into cysteine and α-ketoglutarate by the action of cystathionine β-synthase and cystathionase, and using pyridoxine as a cofactor, as shown in Figure 2A. In RM, homocysteine is converted into its precursor, methionine. Methionine synthase (MTR) uses vitamin B12 and methyltetrahydrofolate as a cofactor and carbon donor, respectively,48 to convert homocysteine into methionine. The activation of MTR needs the MTR reductase enzyme that catalyzes the generation of reduced vitamin B12, a molecule required for the maintenance of functional MTR.48,49 The critical enzyme in RM of homocysteine is methylenetetrahydrofolate reductase (MTHFR), because it makes folate available to be used as a methyl group donor.48 On the other hand, methionine (obtained from diet or through the remethylation pathway) is the precursor of SAM. When SAM, in turn, donates a methyl group for the methylation reaction it converts into SAH, a precursor of homocysteine (Figure 2A).
The literature indicates that, in normal pregnant women, basal and nonfasting plasma concentrations of homocysteine are low in the first trimester of pregnancy and decrease even further in the second half of gestation.50–52 The reasons for this decrease are not absolutely understood and several hypotheses have been proposed, including a physiological response to hormonal influences, changes in maternal intake of dietary proteins, changes in concentrations of amino acids or albumin, hemodilution due to the plasma volume expansion in pregnancy, supplementation with folic acid, and/or the increase in fetal and maternal demands for methionine and homocysteine.50–53 In patients with preeclampsia, there is strong evidence that plasma homocysteine concentration is increased,54–57 and pregnant women with hyperhomocysteinemia have a 7.7-fold risk of developing PE (confidence interval [CI] 95%: 1.7-34.8) as compared to normotensive controls,58 suggesting that homocysteine may be a useful predictor of the disease. This hypothesis was tested by Cotter et al who observed higher plasma homocysteine levels in early pregnancy in women who subsequently developed severe PE, and postulated that hyperhomocystinemia is a risk factor and good predictor for severe PE.59 Robertson et al conducted a systematic review of 25 studies (n = 11 183) to investigate the association between inherited thrombophilias and the risk of adverse pregnancy outcomes, including PE. They reported that the risk of PE was significantly higher in the presence of MTHFR homozygosity (MTHFR T/T) and hyperhomocysteinemia; the MTHFR C677T SNP had an odds ratio (OR) of 1.37 (95% CI, 1.07-1.76) and hyperhomocysteinemia an OR of 3.49 (95% CI, 1.21-10.11) for developing PE.60 Our laboratory results show that the G allele of the MTR A2756G polymorphism was more frequent in controls than in patients with PE. (control: 70.4% vs PE: 37.5%, P = .036; OR of 0.252, 95% CI 0.079-0.813; unpublished data.) These results suggest that the presence of this polymorphism could be a protective factor against the development of PE.
Some alterations in the MHM pathway could be caused by polymorphisms in other genes that code for enzymes participating in the various pathways or by deficits in one or more of the diverse cofactors involved.49 A review of these alternative pathways has been published elsewhere.61
In 2010, Shenoy et al hypothesized that there is a connection between angiogenic and metabolic pathways in the development of PE. They proposed that high levels of homocysteine could mediate inhibition of COMT, due to an increase in SAH production, a potent COMT inhibitor. An accumulation of SAH, and a consequent inhibition of COMT, could decrease deactivation of endogenous catecholamines, which in turn could cause an overactivation of the sympathetic nervous system leading to an increased risk of cardiovascular morbidity and mortality. In accordance with this hypothesis, if hyperhomocysteinemia inhibits COMT activity through SAH overproduction, it might also suppress COMT production of 2-ME. This could lead to an increase in antiangiogenic factors and vascular defects in the placental bed.62 However, there is no causative evidence that directly connects all the components of this hypothesis. Thus, more research is still needed to elucidate how all the components presented here might explain the development of PE.
The Aromatase Pathways and Their Relation to 2-ME Synthesis in PE
Although the altered synthesis of 2-ME has become an important consideration over the past 3 years, it is also important to take into account the complex metabolic pathways upstream of 2-ME.
As shown in Figure 2B, 2-ME levels depend directly on circulating levels of 17β-estradiol, which in turn are determined by levels of testosterone and estrone. During the last decade, multiple studies have measured the levels of androgens in PE. Most of them have established that the circulating levels of testosterone and androstenedione are increased in PE compared to normotensive pregnancies.63–65 These data have led to suggestions that hyperandrogenism is a risk factor for the development of PE.64
Despite the limited number of studies evaluating estrogen levels in PE, it is possible to infer that not only are androgen levels altered in patients with PE, but also the testosterone/estradiol ratio (T/E2) is increased.66–68 The T/E2 is considered a measure of the functionality of aromatase, the enzyme that catalyzes, among other reactions, the conversion of testosterone into estradiol. Recently, more accurate methods have been used to measure steroids, such as gas chromatography/mass spectrometry (GC/MS), which allows the identification and more specific quantification of androgens, estrogens, and their metabolites and is considered one of the most sensitive steroid-measuring techniques with a limit of detection of 10 pg/mL. This is an important advance since the molecular structure of each component of these steroid groups is highly homologous, thus making their identification difficult. Using GC/MS, Hertig et al measured the concentration of androgens, estrogens, and 2-ME in the plasma of women with PE and controls.69 They demonstrated that both 17β-estradiol and estrone are decreased in PE, whereas the E2/T ratio is decreased (therefore, the T/E2 is increased), and concluded that these are related to the severity of the disease. They also reported that the precursors of these metabolites, testosterone and androstenedione, respectively, are not altered in PE, which is different from what has been previously reported. Taken together, these results suggest that the functionality of aromatase is altered in PE.69 Although the evidence that plasma levels of these metabolites are altered in PE is compelling, the functional state of the aromatase enzyme has not been directly examined in women with PE.
Aromatase is not only responsible for the conversion of androstenedione into estrone and testosterone into 17β-estradiol70 but is also the rate-limiting enzyme in the synthesis of 2-ME69 since the production of 2-ME depends directly on 17β-estradiol concentrations (Figure 2B). This implies that alterations in the functionality or bioavailability of aromatase may affect the levels of 2-ME.
Aromatase is an enzyme complex located in the endoplasmic reticulum and composed of 2 polypeptides: (1) a flavoprotein, nicotinamide adenine dinucleotide phosphate-P450-reductase, expressed in all tissues and cell types of the body; and (2) aromatase P450, expressed only in those tissues that synthesize estrogens, including the gonads, adipose tissue, certain brain nuclei, and the placenta.70–73 Aromatase is coded by a unique gene, CYP19, whose transcription is highly regulated through a series of upstream tissue-specific gene promoters.71 Some of the factors regulating the transcription of this enzyme in the placenta are TGF-β3,74 tumor necrosis factor-α,75 retinoic acid,76 and oxygen reactive species.77 All these molecules are differentially expressed in PE. Supporting this idea, our group’s preliminary results show that patients with preeclampsia have lower placental aromatase RNA and protein than control patients (unpublished data). Further experiments are needed to establish the role of aromatase expression in the development of PE.
Aromatase activity is also regulated posttranscriptionally. This enzyme complex has a heme group in its structure that makes it susceptible to regulation by nitric oxide, just like other enzymes that contain an iron atom in the prosthetic groups (eg, hemoglobin, myoglobin, cytochrome c, and guanylyl cyclase).78 The activity of aromatase is also regulated by hypoxia. Jiang et al observed that both mRNA levels and aromatase activity decrease when trophoblast cells are cultured under hypoxia (2% O2) and return to normal when normoxia is reestablished.79 Considering that placental hypoxia is one of the pathologic hallmarks of PE,80 these data provide an explanation as to why aromatase expression and activity may be altered in PE.
Another way in which the expression and activity of this enzyme can be altered is the presence of polymorphisms in the gene coding for it.23,73,81 Single-nucleotide polymorphisms in the region of exon 1.1 (placenta-specific aromatase regulator) are associated with the changes in aromatase activity as well as alterations in circulating levels of 17β-estradiol.23,82 Additionally, SNPs in exon 9 and in the 3′ region of the gene are associated with the changes in affinity of the aromatase enzyme for its substrate (androstenedione) and to alterations in circulating levels of 17β-estradiol.82 These data suggest that the presence of SNPs in the CYP19 gene might regulate aromatase expression and its activity.
Other Considerations
Inactivation of 2-ME
Considering that diminished circulating levels of 2-ME are found in patients with preeclampsia, even during the asymptomatic phase of the disease, it is relevant to study the possible alterations in the degradation pathway of this estradiol metabolite.
Estrogen, and its metabolites, inactivation occurs, in part, as a result of sulfate conjugation catalyzed by sulfotransferase (SULT) enzymes. More specifically, 2-ME is mainly inactivated by SULT1A183,84 and SULT1E1.85 Sulfotransferase 1A1 is the most highly expressed hepatic SULT,84 whereas SULT1E1 is expressed in many human tissues such as liver,86 testes,87 and endometrium.88 Both enzymes, SULT1A1 and SULT1E1, inactivate 2-ME at physiological concentrations84 and have functional SNPs that can alter their enzymatic activity.84,85
Currently, there is no evidence of alterations in SULT enzymes that might account for altered 2-ME levels related with PE development. This is an unexplored field in the study of possible factors involved in PE development that would be addressed with particular interest.
Conclusions
During pregnancy, 2-ME levels increase significantly when compared to levels during the menstrual cycle; however, plasma levels of 2-ME are lower in patients with clinical PE when compared to normotensive pregnancies. The 2-ME levels are also significantly lower during the first trimester of pregnancy (asymptomatic phase of PE) in patients that will go on to develop this disease compared to normotensive pregnancies. The possible factors involved in this imbalance are still being researched. Alterations in the MHM or 17β-estradiol synthetic pathway could explain, in part, the alteration in 2-ME synthesis during pregnancy. Nevertheless, there are questions around 2-ME synthesis, signaling, and degradations, which are still unresolved. Further studies are necessary to address these questions that would help understand the role of this molecule during normal pregnancy and its relationship with the development of pregnancy-associated diseases such as PE, in order to propose possible treatment strategies.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Fondecyt 1110883.
References
- 1. ACOG technical bulletin. Hypertension in pregnancy. Number 219—January 1996 (replaces no. 91, February 1986). Committee on technical bulletins of the American college of obstetricians and gynecologists. Int J Gynaecol Obstet. 1996;53(2):175–183. [PubMed] [Google Scholar]
- 2. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918–930. [DOI] [PubMed] [Google Scholar]
- 4. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183(1):S1–S22. [PubMed] [Google Scholar]
- 5. Knofler M, Pollheimer J. IFPA award in placentology lecture: molecular regulation of human trophoblast invasion. Placenta. 2012;33(suppl):S55–S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ness RB, Roberts JM. Heterogeneous causes constituting the single syndrome of preeclampsia: a hypothesis and its implications. Am J Obstet Gynecol. 1996;175(5):1365–1370. [DOI] [PubMed] [Google Scholar]
- 7. Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006;11(5):309–316. [DOI] [PubMed] [Google Scholar]
- 8. Redman CW. Current topic: pre-eclampsia and the placenta. Placenta. 1991;12(4):301–308. [DOI] [PubMed] [Google Scholar]
- 9. Pijnenborg R, Vercruysse L, Brosens I. Deep placentation. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):273–285. [DOI] [PubMed] [Google Scholar]
- 10. Hunkapiller NM, Fisher SJ. Chapter 12. Placental remodeling of the uterine vasculature. Methods Enzymol. 2008;445:281–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whitley GS, Cartwright JE. Trophoblast-mediated spiral artery remodelling: a role for apoptosis. J Anat. 2009;215(1):21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lyall F. Mechanisms regulating cytotrophoblast invasion in normal pregnancy and pre-eclampsia. Aust N Z J Obstet Gynaecol. 2006;46(4):266–273. [DOI] [PubMed] [Google Scholar]
- 13. Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–191. [PubMed] [Google Scholar]
- 14. Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93(10):1049–1059. [DOI] [PubMed] [Google Scholar]
- 15. Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80(2):283–285. [PubMed] [Google Scholar]
- 16. Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277(5332):1669–1672. [DOI] [PubMed] [Google Scholar]
- 17. Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8(5):588–594. [DOI] [PubMed] [Google Scholar]
- 18. Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270(3):1230–1237. [DOI] [PubMed] [Google Scholar]
- 19. Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–275. [DOI] [PubMed] [Google Scholar]
- 20. Caniggia I, Mostachfi H, Winter J, et al. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3). J Clin Invest. 2000;105(5):577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Librach CL, Werb Z, Fitzgerald ML, et al. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991;113(2):437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee SB, Wong AP, Kanasaki K, et al. Preeclampsia: 2-methoxyestradiol induces cytotrophoblast invasion and vascular development specifically under hypoxic conditions. Am J Pathol. 2010;176(2):710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180(2 pt 1):499–506. [DOI] [PubMed] [Google Scholar]
- 24. Kanasaki K, Palmsten K, Sugimoto H, et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453(7198):1117–1121. [DOI] [PubMed] [Google Scholar]
- 25. LaVallee TM, Zhan XH, Herbstritt CJ, Kough EC, Green SJ, Pribluda VS. 2-Methoxyestradiol inhibits proliferation and induces apoptosis independently of estrogen receptors alpha and beta. Cancer Res. 2002;62(13):3691–3697. [PubMed] [Google Scholar]
- 26. Schumacher G, Kataoka M, Roth JA, Mukhopadhyay T. Potent antitumor activity of 2-methoxyestradiol in human pancreatic cancer cell lines. Clin Cancer Res. 1999;5(3):493–499. [PubMed] [Google Scholar]
- 27. Fotsis T, Zhang Y, Pepper MS, et al. The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature. 1994;368(6468):237–239. [DOI] [PubMed] [Google Scholar]
- 28. Zacharia LC, Jackson EK, Gillespie DG, Dubey RK. Increased 2-methoxyestradiol production in human coronary versus aortic vascular cells. Hypertension. 2001;37(2 part 2):658–662. [DOI] [PubMed] [Google Scholar]
- 29. Shang W, Konidari I, Schomberg DW. 2-Methoxyestradiol, an endogenous estradiol metabolite, differentially inhibits granulosa and endothelial cell mitosis: a potential follicular antiangiogenic regulator. Biol Reprod. 2001;65(2):622–627. [DOI] [PubMed] [Google Scholar]
- 30. Becker CM, Rohwer N, Funakoshi T, et al. 2-methoxyestradiol inhibits hypoxia-inducible factor-1{alpha} and suppresses growth of lesions in a mouse model of endometriosis. Am J Pathol. 2008;172(2):534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lakhani NJ, Sarkar MA, Venitz J, Figg WD. 2-Methoxyestradiol, a promising anticancer agent. Pharmacotherapy. 2003;23(2):165–172. [DOI] [PubMed] [Google Scholar]
- 32. Pasquier E, Sinnappan S, Munoz MA, Kavallaris M. ENMD-1198, a new analogue of 2-methoxyestradiol, displays both antiangiogenic and vascular-disrupting properties. Mol Cancer Ther. 2010;9(5):1408–1418. [DOI] [PubMed] [Google Scholar]
- 33. Hou Y, Meyers CY, Akomeah M. A short, economical synthesis of 2-methoxyestradiol, an anticancer agent in clinical trials. J Org Chem. 2009;74(16):6362–6364. [DOI] [PubMed] [Google Scholar]
- 34. Berg D, Sonsalla R, Kuss E. Concentrations of 2-methoxyoestrogens in human serum measured by a heterologous immunoassay with an 125I-labelled ligand. Acta Endocrinol (Copenh). 1983;103(2):282–288. [DOI] [PubMed] [Google Scholar]
- 35. Barnes CM, McElrath TF, Folkman J, Hansen AR. Correlation of 2-methoxyestradiol levels in cord blood and complications of prematurity. Pediatr Res. 2010;67(5):545–550. [DOI] [PubMed] [Google Scholar]
- 36. Pérez-Sepúlveda A, Torres MJ, Valenzuela FJ, et al. Low 2-methoxyestradiol levels at the first trimester of pregnancy are associated with the development of pre-eclampsia. Prenat Diagn. 2012;32(11):1–6. [DOI] [PubMed] [Google Scholar]
- 37. Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51(4):593–628. [PubMed] [Google Scholar]
- 38. Palmer K, Saglam B, Whitehead C, Stock O, Lappas M, Tong S. Severe early-onset preeclampsia is not associated with a change in placental catechol O-methyltransferase (COMT) expression. Am J Pathol. 2011;178(6):2484–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–250. [DOI] [PubMed] [Google Scholar]
- 40. Syvanen AC, Tilgmann C, Rinne J, Ulmanen I. Genetic polymorphism of catechol-O-methyltransferase (COMT): correlation of genotype with individual variation of S-COMT activity and comparison of the allele frequencies in the normal population and parkinsonian patients in Finland. Pharmacogenetics. 1997;7(1):65–71. [DOI] [PubMed] [Google Scholar]
- 41. Sata F, Yamada H, Suzuki K, et al. Functional maternal catechol-O-methyltransferase polymorphism and fetal growth restriction. Pharmacogenet Genomics. 2006;16(11):775–781. [DOI] [PubMed] [Google Scholar]
- 42. Stanley JL, Andersson IJ, Poudel R, et al. Sildenafil citrate rescues fetal growth in the catechol-O-methyl transferase knockout mouse model. Hypertension. 2012;59(5):1021–1028. [DOI] [PubMed] [Google Scholar]
- 43. Barnea ER, MacLusky NJ, DeCherney AH, Naftolin F. Catechol-o-methyl transferase activity in the human term placenta. Am J Perinatol. 1988;5(2):121–127. [DOI] [PubMed] [Google Scholar]
- 44. Roten LT, Fenstad MH, Forsmo S, et al. A low COMT activity haplotype is associated with recurrent preeclampsia in a Norwegian population cohort (HUNT2). Mol Hum Reprod. 2011;17(7):439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lim JH, Kim SY, Kim do J, et al. Genetic polymorphism of catechol-O-methyltransferase and cytochrome P450c17alpha in preeclampsia. Pharmacogenet Genomics. 2010;20(10):605–610. [DOI] [PubMed] [Google Scholar]
- 46. Liang S, Liu X, Fan P, et al. Association between Val158Met functional polymorphism in the COMT gene and risk of preeclampsia in a Chinese population. Arch Med Res. 2012;43(2):154–158. [DOI] [PubMed] [Google Scholar]
- 47. Hill LD, York TP, Kusanovic JP, et al. Epistasis between COMT and MTHFR in maternal-fetal dyads increases risk for preeclampsia. PLoS One. 2011;6(1):e16681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ananth CV, Elsasser DA, Kinzler WL, et al. Polymorphisms in methionine synthase reductase and betaine-homocysteine S-methyltransferase genes: risk of placental abruption. Mol Genet Metab. 2007;91(1):104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sharma P, Senthilkumar RD, Brahmachari V, et al. Mining literature for a comprehensive pathway analysis: a case study for retrieval of homocysteine related genes for genetic and epigenetic studies. Lipids Health Dis. 2006;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Walker MC, Smith GN, Perkins SL, Keely EJ, Garner PR. Changes in homocysteine levels during normal pregnancy. Am J Obstet Gynecol. 1999;180(3 pt 1):660–664. [DOI] [PubMed] [Google Scholar]
- 51. Cikot RJ, Steegers-Theunissen RP, Thomas CM, de Boo TM, Merkus HM, Steegers EA. Longitudinal vitamin and homocysteine levels in normal pregnancy. Br J Nutr. 2001;85(1):49–58. [DOI] [PubMed] [Google Scholar]
- 52. Murphy MM, Scott JM, McPartlin JM, Fernandez-Ballart JD. The pregnancy-related decrease in fasting plasma homocysteine is not explained by folic acid supplementation, hemodilution, or a decrease in albumin in a longitudinal study. Am J Clin Nutr. 2002;76(3):614–619. [DOI] [PubMed] [Google Scholar]
- 53. Steegers-Theunissen RP, Wathen NC, Eskes TK, van Raaij-Selten B, Chard T. Maternal and fetal levels of methionine and homocysteine in early human pregnancy. Br J Obstet Gynaecol. 1997;104(1):20–24. [DOI] [PubMed] [Google Scholar]
- 54. Powers RW, Evans RW, Majors AK, et al. Plasma homocysteine concentration is increased in preeclampsia and is associated with evidence of endothelial activation. Am J Obstet Gynecol. 1998;179(6 pt 1):1605–1611. [DOI] [PubMed] [Google Scholar]
- 55. Wang J, Trudinger BJ, Duarte N, Wilcken DE, Wang XL. Elevated circulating homocyst(e)ine levels in placental vascular disease and associated pre-eclampsia. BJOG. 2000;107(7):935–938. [DOI] [PubMed] [Google Scholar]
- 56. D'Anna R, Baviera G, Corrado F, Ientile R, Granese D, Stella NC. Plasma homocysteine in early and late pregnancies complicated with preeclampsia and isolated intrauterine growth restriction. Acta Obstet Gynecol Scand. 2004;83(2):155–158. [DOI] [PubMed] [Google Scholar]
- 57. Makedos G, Papanicolaou A, Hitoglou A, et al. Homocysteine, folic acid and B12 serum levels in pregnancy complicated with preeclampsia. Arch Gynecol Obstet. 2007;275(2):121–124. [DOI] [PubMed] [Google Scholar]
- 58. Lopez-Quesada E, Vilaseca MA, Lailla JM. Plasma total homocysteine in uncomplicated pregnancy and in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2003;108(1):45–49. [DOI] [PubMed] [Google Scholar]
- 59. Cotter AM, Molloy AM, Scott JM, Daly SF. Elevated plasma homocysteine in early pregnancy: a risk factor for the development of nonsevere preeclampsia. Am J Obstet Gynecol. 2003;189(2):391–394. [DOI] [PubMed] [Google Scholar]
- 60. Robertson L, Wu O, Langhorne P, et al. Thrombophilia in pregnancy: a systematic review. Br J Haematol. 2006;132(2):171–196. [DOI] [PubMed] [Google Scholar]
- 61. Valenzuela FJ, Perez-Sepulveda A, Torres MJ, Correa P, Repetto GM, Illanes SE. Pathogenesis of preeclampsia: the genetic component. J Pregnancy. 2012;2012:632732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shenoy V, Kanasaki K, Kalluri R. Pre-eclampsia: connecting angiogenic and metabolic pathways. Trends Endocrinol Metab. 2010;21(9):529–536. [DOI] [PubMed] [Google Scholar]
- 63. Troisi R, Potischman N, Roberts JM, et al. Correlation of serum hormone concentrations in maternal and umbilical cord samples. Cancer Epidemiol Biomarkers Prev. 2003;12(5):452–456. [PubMed] [Google Scholar]
- 64. Ghorashi V, Sheikhvatan M. The relationship between serum concentration of free testosterone and pre-eclampsia. Endokrynol Pol. 2008;59(5):390–392. [PubMed] [Google Scholar]
- 65. Carlsen SM, Romundstad P, Jacobsen G. Early second-trimester maternal hyperandrogenemia and subsequent preeclampsia: a prospective study. Acta Obstet Gynecol Scand. 2005;84(2):117–121. [DOI] [PubMed] [Google Scholar]
- 66. Sowers MR, Wilson AL, Kardia SR, Chu J, Ferrell R. Aromatase gene (CYP 19) polymorphisms and endogenous androgen concentrations in a multiracial/multiethnic, multisite study of women at midlife. Am J Med. 2006;119(9 suppl 1):S23–S30. [DOI] [PubMed] [Google Scholar]
- 67. Somner J, McLellan S, Cheung J, et al. Polymorphisms in the P450 c17 (17-hydroxylase/17,20-Lyase) and P450 c19 (aromatase) genes: association with serum sex steroid concentrations and bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2004;89(1):344–351. [DOI] [PubMed] [Google Scholar]
- 68. Kidokoro K, Ino K, Hirose K, et al. Association between CYP19A1 polymorphisms and sex hormones in postmenopausal Japanese women. J Hum Genet. 2009;54(2):78–85. [DOI] [PubMed] [Google Scholar]
- 69. Hertig A, Liere P, Chabbert-Buffet N, et al. Steroid profiling in preeclamptic women: evidence for aromatase deficiency. Am J Obstet Gynecol. 2010;203(5):477 e471–477 e479. [DOI] [PubMed] [Google Scholar]
- 70. Czajka-Oraniec I, Simpson ER. Aromatase research and its clinical significance. Endokrynol Pol. 2010;61(1):126–134. [PubMed] [Google Scholar]
- 71. Simpson ER, Clyne C, Rubin G, et al. Aromatase--a brief overview. Annu Rev Physiol. 2002;64:93–127. [DOI] [PubMed] [Google Scholar]
- 72. Conley A, Hinshelwood M. Mammalian aromatases. Reproduction. 2001;121(5):685–695. [DOI] [PubMed] [Google Scholar]
- 73. Hong Y, Li H, Yuan YC, Chen S. Molecular characterization of aromatase. Ann N Y Acad Sci. 2009;1155:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhou H, Fu G, Yu H, Peng C. Transforming growth factor-beta inhibits aromatase gene transcription in human trophoblast cells via the Smad2 signaling pathway. Reprod Biol Endocrinol. 2009;7:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Williams MA, Woelk GB, King IB, Jenkins L, Mahomed K. Plasma carotenoids, retinol, tocopherols, and lipoproteins in preeclamptic and normotensive pregnant Zimbabwean women. Am J Hypertens. 2003;16(8):665–672. [DOI] [PubMed] [Google Scholar]
- 76. Zhu SJ, Li Y, Li H, et al. Retinoic acids promote the action of aromatase and 17beta-hydroxysteroid dehydrogenase type 1 on the biosynthesis of 17beta-estradiol in placental cells. J Endocrinol. 2002;172(1):31–43. [DOI] [PubMed] [Google Scholar]
- 77. Milczarek R, Sokolowska E, Hallmann A, Klimek J. The NADPH- and iron-dependent lipid peroxidation in human placental microsomes. Mol Cell Biochem. 2007;295(1-2):105–111. [DOI] [PubMed] [Google Scholar]
- 78. Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev. 2005;57(2):217–252. [DOI] [PubMed] [Google Scholar]
- 79. Jiang B, Kamat A, Mendelson CR. Hypoxia prevents induction of aromatase expression in human trophoblast cells in culture: potential inhibitory role of the hypoxia-inducible transcription factor Mash-2 (mammalian achaete-scute homologous protein-2). Mol Endocrinol. 2000;14(10):1661–1673. [DOI] [PubMed] [Google Scholar]
- 80. Caniggia I, Winter JL. Adriana and Luisa Castellucci Award lecture 2001. Hypoxia inducible factor-1: oxygen regulation of trophoblast differentiation in normal and pre-eclamptic pregnancies—a review. Placenta. 2002;23(suppl A):S47–S57. [DOI] [PubMed] [Google Scholar]
- 81. Tao MH, Cai Q, Zhang ZF, et al. Polymorphisms in the CYP19A1 (aromatase) gene and endometrial cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev. 2007;16(5):943–949. [DOI] [PubMed] [Google Scholar]
- 82. Ma CX, Adjei AA, Salavaggione OE, et al. Human aromatase: gene resequencing and functional genomics. Cancer Res. 2005;65(23):11071–11082. [DOI] [PubMed] [Google Scholar]
- 83. Nagar S, Walther S, Blanchard RL. Sulfotransferase (SULT) 1A1 polymorphic variants *1, *2, and *3 are associated with altered enzymatic activity, cellular phenotype, and protein degradation. Mol Pharmacol. 2006;69(6):2084–2092. [DOI] [PubMed] [Google Scholar]
- 84. Nowell S, Falany CN. Pharmacogenetics of human cytosolic sulfotransferases. Oncogene. 2006;25(11):1673–1678. [DOI] [PubMed] [Google Scholar]
- 85. Adjei AA, Thomae BA, Prondzinski JL, Eckloff BW, Wieben ED, Weinshilboum RM. Human estrogen sulfotransferase (SULT1E1) pharmacogenomics: gene resequencing and functional genomics. Br J Pharmacol. 2003;139(8):1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Aksoy IA, Wood TC, Weinshilboum R. Human liver estrogen sulfotransferase: identification by cDNA cloning and expression. Biochem Biophys Res Commun. 1994;200(3):1621–1629. [DOI] [PubMed] [Google Scholar]
- 87. Song WC, Moore R, McLachlan JA, Negishi M. Molecular characterization of a testis-specific estrogen sulfotransferase and aberrant liver expression in obese and diabetogenic C57BL/KsJ-db/db mice. Endocrinology. 1995;136(6):2477–2484. [DOI] [PubMed] [Google Scholar]
- 88. Falany JL, Azziz R, Falany CN. Identification and characterization of cytosolic sulfotransferases in normal human endometrium. Chem Biol Interact. 1998;109(1-3):329–339. [DOI] [PubMed] [Google Scholar]