Abstract
The control of complement activation in the embryo–maternal environment has been demonstrated to be critical for embryo survival. Complement proteins are expressed in the human endometrium; however, the modulation of this expression by embryo signals has not been explored. To assess the expression of complement proteins in response to human chorionic gonadotropin (hCG), we designed an experimental study using in vivo and in vitro models. Twelve fertile women were treated with hCG or left untreated during the mid-luteal phase, and an endometrial biopsy was performed 24 hours later. The localizations of C3, membrane cofactor protein (MCP; CD46), decay-accelerating factor (DAF; CD55), and protectin (CD59) were assessed by immunohistochemistry, and the messenger RNA (mRNA) levels of these proteins were quantified by real-time reverse transcriptase–polymerase chain reaction (RT-PCR) in cells harvested from endometrial compartments using laser capture microdissection. Endometrial explants were cultured with or without hCG for 24 hours, and the C3 and DAF protein levels were measured by Western blotting. Elevated C3 mRNA levels in stromal cells and elevated DAF levels in epithelial luminal cells were detected after hCG treatment. In the endometrial explant model, the progesterone receptor antagonist RU486 inhibited the increases in the levels of C3 and DAF in response to hCG. The findings of this study indicate that hCG plays a role in embryo–endometrium communication and affects the expression of complement proteins in endometrial compartments during the implantation window.
Keywords: hCG, endometrial receptivity, complement proteins, implantation
Introduction
The implantation process requires synchronized crosstalk between the embryo and the endometrial surface during a specific period of the menstrual cycle.1,2
During implantation, the maternal endometrial mucosa simultaneously facilitates the adhesion and invasion of a semiforeign embryo and prevents attack by opportunistic pathogens. The innate immune response, including the activation of the complement system, is activated under such conditions.3,4 Because the cell damage inflicted by the activated complement system is nonspecific, host cells are protected by complement regulatory proteins including decay-accelerating factor (DAF), membrane cofactor protein (CD46), and protectin (CD59).5
The control of complement activation in the embryo–maternal environment has been demonstrated to be critical for embryo survival. In the mouse model, the deletion of the crry gene, a complement regulatory protein with functional homology to human DAF, results in the deposition of a large amount of activated complement C3, compromising the embryo’s survival and leading to embryo loss.6
The expression of complement system proteins in the human endometrium during the mid-luteal phase has been demonstrated by different methods.7–9 Recent studies using nonhuman primate models have demonstrated that human chorionic gonadotropin (hCG) infusion during the mid-luteal phase elevates the expression levels of several genes, including C3. More recently, using the same model, the modulation of C3 expression by hCG has been shown to be blunted in the endometria of animals with endometriosis.10,11 However, the modulation of complement protein expression by an embryo signal has not been explored in humans. Functional hCG receptors have been found in the endometrium in studies focusing on hCG as an early embryo signal that participates in embryo–maternal communication.12–14 We hypothesized that an embryo signal communicates with the endometrium through hCG, thereby modulating the expression levels of complement proteins. In this study, we designed in vivo and in vitro models to assess the endometrial response to hCG.
Using laser microdissection, we were able to demonstrate that the gene expression levels of complement C3 and DAF are modulated in specific endometrial compartments by the in vivo administration of hCG. Additionally, using the endometrial explant model, we demonstrated that there is cross talk between the hCG signal and the ovarian steroid hormones that regulate the expression of endometrial complement proteins.
Our findings suggest that hCG may function as an embryo signal in embryo–maternal communication and could modulate the maternal immune response by changing the expression levels of complement proteins during implantation.
Materials and Methods
Human Participants
Fertile cycling women who had previously undergone tubal ligation were invited to participate in this study (mean age, 33; range, 27-40).
The Institutional Review Board approved the protocol of this study (Comité de Ética de Investigación en Seres Humanos, Facultad de Medicina, Universidad de Chile and Comité Etico-Científico del Servicio de Salud Metropolitano Central, Santiago, Chile). All participants signed a written consent form before enrolling in this study. At the time of their first visit, volunteers chose which group they wanted to be in (hCG administration or no treatment). Ovulation was detected based on a urinary luteinizing hormone (LH) surge (LH: 0) using the Clearblue ovulation kit (Unipath, UK) and was confirmed by the collapse of the leading follicle using vaginal ultrasound (Sonoace X6, Medison, Korea). Eight days after ovulation, 10 000 UI/mL of urinary hCG (Pregnyl Organon MSD, Withehouse Station, New Jersey) were administered via the IM route to 6 volunteers. The other 6 women did not receive hCG treatment and served as controls. The dose was chosen based on the results of a previous study demonstrating hCG’s effect on physiological processes in the endometrium.15 Blood samples from the peripheral vein were obtained at the time of the endometrial biopsy for the determination of the P and hCG serum concentrations, as previously reported.16
Endometrial Samples
Endometrial biopsies were obtained on day LH + 9 or 24 hours after hCG administration. Four additional biopsies were obtained from fertile controls during the proliferative phase for the localization of complement proteins. A small portion of each endometrial sample was immersed in buffered 10% formalin for histological dating according to Noyes et al’s criteria17 and for immunohistochemical analysis. Another portion was quickly and carefully immersed in optimal cutting temperature (Tissue-Tek, Sakura Finetek Inc, Torrance, California) and stored immediately at −80°C for laser capture microdissection (LCM). For the experiments using endometrial explants, small portions of the endometrial samples obtained from the controls were employed.
Immunohistochemistry
Protein localization was assessed by immunohistochemical staining of paraffin-embedded sections using the LAB-SA detection system (Invitrogen, Camarillo, California), as described in our previous publication.18 The following specific antibodies were employed: mouse antihuman C3 (GeneTex, California), rabbit anti-DAF (Neomakers, North Carolina), mouse anti-CD46, and mouse anti-CD59 (Santa Cruz, California). For the negative control, sections were incubated without any primary antibody. Sections were deparaffinized in toluene and rehydrated with serially graded concentrations of ethanol. Antigen retrieval was achieved by heating sections in a 750-Watt microwave at 80% power in 0.01 mol/L citrate pretreatment buffer. After a phosphate-buffered saline (PBS) rinse, the endogenous peroxidase activity was quenched with a 30-minute incubation with 0.3% H2O2 in absolute ethanol. The samples were then incubated with blocking serum for 10 minutes at room temperature. After the incubation with the primary antibody, the sections were washed with PBS and then treated with blocking serum for an additional 10 minutes. Subsequently, the sections were washed with PBS and incubated with a biotinylated secondary antibody for 60 minutes at room temperature. After the samples were rinsed with PBS, the immunoreactive antigen was visualized using HRP-labeled streptavidin and aminoethylcarbazole (AEC) as the chromogen. Slides were counterstained with hematoxylin f and then dehydrated in a graded series of ethanol, cleared in xylene, and mounted with Permount (Fisher Scientific, Fair Lawn, New Jersey). The staining was evaluated using an Olympus Bx51 microscope (Olympus optical company, Japan). The staining intensity value was assigned using a semiquantitative histological score (HSCORE). The HSCORE was calculated using the following equation: HSCORE = ∑ Pi (I + 1), where I is the intensity of the staining and takes a value of 1, 2, or 3 (weak, moderate, or strong, respectively) and Pi is the percentage of stained epithelial cells for each intensity, varying from 0% to 100%.
Laser Capture Microdissection
As soon as the endometrial sample was obtained, a small portion was immediately placed in OCT and promptly frozen at −80°C. The OCT-embedded tissue was then cut into 8 mm sections using a Leica CM 1510S cryostat (Leica Biosystems, Germany) at −30°C using a sterile blade. Sections were placed on frosted microscope slides (Sigma-Aldrich, St. Louis, Missouri) and stored at −80°C until further use. Sections mounted on slides were then processed. The sections were fixed in 70% ethanol for 30 seconds, washed with RNase-free water, stained with Arcturus HistoGene staining solution (Applied Biosystems, Foster City, California) for 20 seconds, and then washed with RNase-free water. Next, the sections were dehydrated in 75%, 95%, and 100% ethanol for 30 seconds, and then incubated for 5 minutes in fresh xylene. Finally, the slides were air-dried for at least 5 minutes and immediately placed in a vacuum dessicator (Bel-Art, New Jersey). The slides were then placed in the Arcturus LCM system (Molecular Devices Corporation, California) to isolate luminal, glandular epithelial, and stromal cells from the endometrial sections. A single CapSure Macro LCM cap (Arcturus, California) was used for each tissue section. Each cap containing captured cells was tightly fitted to a microcentrifuge tube containing 50 µL of extraction buffer and was placed in a vacuum oven (Sheldom Inc, Cornelius, Oregon) for 30 minutes at 40°C. Cells dissected from various tissue sections from a single biopsy were pooled in a single Eppendorf tube. After cell extraction from each endometrial compartment (lumen, glands, or stroma), the messenger RNA (mRNA) levels of all genes (C3, DAF, CD46, and CD59) were analyzed by real-time reverse transcriptase–polymerase chain reaction (RT-PCR).
Real-Time PCR
Total RNA from each tube containing cells isolated by LCM was extracted using the Arcturus PicoPure Frozen RNA Extraction kit (Applied Biosystems). The RNA integrity was analyzed on a Bioanalyzer (Agilent Technologies), and the RNA concentration was quantified using a Nanodrop 2000c spectrophotometer (Thermo Scientific, Wilmington, Delaware).
To confirm the purity of the dissected cells, the mRNA levels of specific protein markers (cytokeratin 18 for epithelial cells and decorin for stromal cells) were quantified by RT-PCR.
Complementary DNA (cDNA) was generated from RNA (1 µL [100 ng]) using the Superscript III first-strand synthesis system (Invitrogen) in a Mastercycler gradient thermal cycler (Eppendorf, Hauppauge, New York). Quantitative real-time RT-PCR was performed in a 20-µL total reaction volume containing cDNA, sense and antisense primers, 2X QuantiTeck SYBR Green master Mix, and RNAse-free water from the QuantiTeck SYBR Green PCR kit (Qiagen, Valencia, California), and the reactions were run in a PTC-200 thermal cycler (MJ Research, Bio-Rad, Philadelphia, Pennsylvania). The primer sources/sequences and the expected lengths of the resulting PCR products are shown in Table 1.
Table 1.
List of Primers Used for Real-Time RT-PCR.
| Gene name | Primer sequence | Product size (bp) |
|---|---|---|
| C3 | Fw: 5’-CCACCGTATCCACTGGGAAT-3’ | 76 |
| Rv: 3’-TTCCTTTTACTCCCAAAGTGTCAG-5’ | ||
| DAF | Fw: 5’-ACGGCACGGCTCTTCCATCTGTT -3’ | 183 |
| Rv: 3’-AAGTTGGCAGGGACCTGGCACTC -5’ | ||
| CD46 | Fw: 5’-TTTCCTTCCTGGCGCTTTC-3’ | 93 |
| Rv: 5’-AAATGTTGGTGGCTCCTCACA-3’ | ||
| CD59 | Fw: 5’-TCACATGGAACGCTTTCATAAACT-3’ | 161 |
| Rv: 5’-ACCCACATATGGAACATTTGGc-3’ | ||
| Cytokeratin | 18 Fw 5’ CAT CCG GGC CCA ATA TGA C 3’ | 153 |
| Rv: 5’ AAC TCC TCT CGT GGT GTC ACC 3’ | ||
| Decorin | Fw: 5’-AGTTTCCGAGTTGAATGGCAG-3’ | 198 |
| Rv: 5’AGTACATCCAGGTTGTCTACCTT-3’ | ||
| GAPDH | Fw: 5’-GAAGGTGAAGGTCGGAGTC-3’ | 225 |
| Rv: 5’-GAAGATGGTGGGATTTC-3’ |
Abbreviations: RT-PCR, reverse transcriptase–polymerase chain reaction; bp, base pair; DAF, decay-accelerating factor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
The amount of each target DNA was normalized using the constitutively expressed gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and the 2−▵▵CT method was employed to report this ratio as the relative mRNA expression level. Experiments for each analysis were run in triplicate.
In Vitro Endometrial Explant Model
Endometrial explants from biopsies obtained from women not treated with hCG (controls, n = 6) were employed. After the endometrial samples were placed in sterile 60 mm culture plates and washed with sterile PBS to remove the red blood cells, the samples were cut carefully into ∼15 mm3 fragments using a sterile scalpel and were cultured as reported elsewhere.19 Small explants were then incubated in phenol red-free d-MEMF/12 medium (a 1:1 mix of DMEM and Ham F-12) containing 15 mmol/L HEPES buffer, l-glutamine, and pyridoxine hydrochloride plus a 1% antibiotic/antimycotic solution consisting of penicillin, streptomycin, and amphotericin (Invitrogen). The explants were then placed in 35 mm inserts (Orange Scientific, Belgium) containing culture medium supplemented with 10−8 mol/L E2 and 10−7 mol/L medroxyprogesterone acetate (MPA) with or without 5 UI/mL of hCG. These explants were then cultured in the presence or absence of the progesterone receptor antagonist Mifepristone RU486 10−6 mol/L. All reagents were obtained from Sigma. The explants were cultured for 24 hours at 37°C in a humidified 5% CO2 in air environment. The culture experiments were run in triplicate. The concentration of hCG used was chosen based on a time/concentration experiment carried out using 0.5, 5, or 50 UI/mL of hCG at 0, 8, 12, 24, and 48 hours. At the end of the experiment, tissues were collected for protein extraction, and cell viability was determined by measuring the release of lactate dehydrogenase (DHL) using a cell cytotoxicity assay (Promega, Madison, Wisconsin).
Protein Extraction and Western Blot Assay
Endometrial explants were homogenized on ice in 1 mL of tissue extraction reagent I (Invitrogen). The tissue homogenates were incubated on ice for 30 minutes and cleared by centrifugation at 14 000 rpm for 15 minutes at 4°C. The supernatant was collected, and the protein concentration was measured using a bicinchoninic acid (BCA) protein assay kit (Pierce Biotechnology). Then, 25 µg of protein in a 12.5-µL final volume of loading buffer was added per lane. The proteins were resolved by 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Sigma). After blocking nonspecific binding with 5% bovine serum albumin (BSA) in Tris Buffer Saline Tween 20 (TBS-T) for 60 minutes, the blots were incubated at 4°C overnight with the appropriate primary antibody: mouse monoclonal anti-human complement C3 at a 1:1000 dilution or rabbit polyclonal anti-human DAF at a 1:500 dilution (Santa Cruz).
The membranes were washed several times with TBS-T, and specific binding was detected by incubation with rabbit antimouse (C3) and mouse antirabbit (DAF) horseradish peroxidase–conjugated secondary antibodies (Invitrogen) for 1 hour at room temperature. The binding signals were visualized using the Western Lightning Plus-ECL Enhanced Chemiluminescence substrate (Perking Elmer, Waltham, Massachusetts) and an UltraLum Discovery Imaging System (UltraLum, Claremont, California). The area and signal intensity of the detected bands were analyzed using Kodak 1D image analysis software.
Statistical Analysis
Nonparametric Mann-Whitney tests were used for single comparisons of variables. For multiple comparisons, repeated-measures analysis of variance (ANOVA) with the Bonferroni posttest correction or the Kruskal-Wallis test with Dunn posttest correction were employed as appropriate using GraphPad PRISM version 4.0 software (GraphPad, San Diego, California). P < .05 was considered statistically significant. The data are presented as the mean ± standard deviation (SD) or mean ± standard error of the mean (SEM) as appropriate.
Results
The age of the subjects participating in this study was not different between groups (33.3 ± 3.8 and 33.2 ± 3.4, mean ± SD for controls and hCG-treated women, respectively). The serum concentration of β-hCG at the time of the endometrial biopsy was 290 ± 43.5 mUI/mL. The serum concentrations of P before and after hCG administration were 8.4 ± 2.3 and 11.6 ± 3.6 ng/mL, respectively.
Immunolocalization of Complement Proteins in the Endometrium
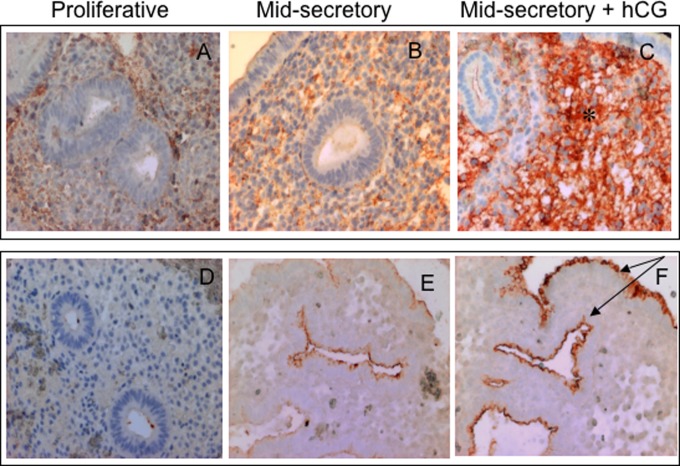
C3 was localized largely in stromal cells but was also identified in epithelial luminal and glandular cells. DAF, CD46, and CD59 were localized mainly in epithelial luminal and glandular cells, with little or no immunostaining in stromal cells. According to the HSCORE, higher C3 levels were detected in the stromal cells from women treated with hCG than in the stromal cells from controls (3.2 ± 0.2 and 1.65 ± 0.4 mean ± SD [n = 6 in each group] p = .0002). In epithelial cells, higher DAF expression was observed in treated women that in controls (2.6 ± 0.5 and 1.8 ± 0.4 mean ± SD [n = 6 in each group] p = .043). Representative microphotographs for C3 and DAF immunostaining are presented in Figure 1.
Figure 1.
Immunolocalization of complement proteins C3 and DAF in representative endometrium specimens from women treated with hCG in vivo or left untreated. The immunostaining for C3 and DAF was more intense during the mid-secretory phase (B and E) than during the proliferative phase (A and D) in normal cycling endometrium. Strong immunostaining for C3 in the stromal compartment and strong immunostaining for DAF in the luminal and glandular compartments are marked by asterisks and arrows, respectively, in the image of an endometrium specimen from a woman treated with hCG relative to the control (C and F, respectively). DAF, decay-accelerating factor; hCG, human chorionic gonadotropin.
Gene Expression of Complement Proteins in Cells Dissected by LCM
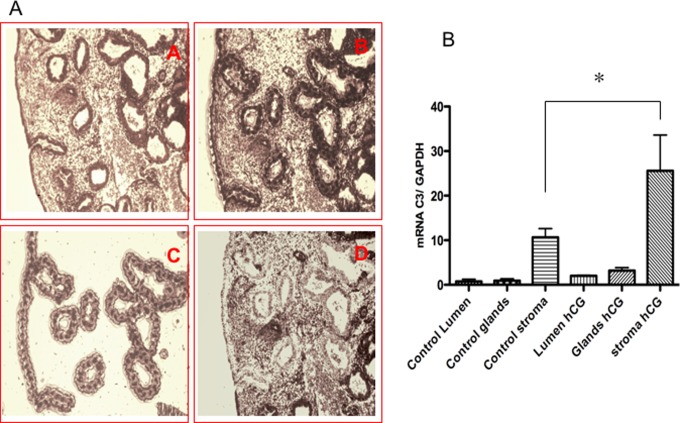
The purity of the epithelial and stromal cells harvested by LCM was analyzed. The mRNA level of decorin (stromal cell protein marker) was 6-fold higher in stromal cells than in epithelial cells (0.81 ± 0.07 and 0.12 ± 0.05, mean ± SD). Conversely, the mRNA levels of cytokeratin 18 (epithelial cell protein marker) were 5-fold higher in epithelial cells than in stromal cells (0.59 ± 0.07 and 0.12 ± 0.05, mean ± SD, respectively), as shown in Supplemental Figure 1.
The C3 mRNA levels in cells dissected from the stromal compartment of women treated with hCG were 4-fold higher than the levels in controls (26.44 ± 9.7 and 7.73 ± 3.27, respectively, mean ± SEM [n = 6 in each group] p = .012), as shown in Figure 2. No differences in the C3 mRNA levels in the epithelial cells dissected from the glandular or luminal compartments cells were detected between groups. The DAF mRNA levels in luminal epithelial cells dissected from women treated with hCG were 2-fold higher than the levels in cells from controls (1.073 ± 0.2 and 0.53 ± 0.1, respectively, mean ± SEM [n = 6 in each group] p < .04). No significant difference was found in the DAF mRNA levels in cells from the glandular dissected compartment. The mRNA levels of CD46 and CD59 in the epithelial luminal and glandular cells dissected from biopsies were not different between women treated with hCG and those who were not treated.
Figure 2.
Representative photomicrographs of laser cell microdissection from functional endometrial compartments (A). Cells dissected from the luminal and glandular compartments are depicted in C. The endometrial specimens before and after laser firing are depicted in A, B, and D. C3 gene expression in cells extracted by the laser from the different endometrial compartments (B). The plot shows higher C3 mRNA levels in stromal cells from women treated with hCG relative to controls. (n = 6) *P = .012. mRNA, messenger RNA; hCG, human chorionic gonadotropin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Regulation of C3 and DAF Expression by hCG in an Endometrial Explant Model
An in vitro endometrial explant model was employed to investigate whether the hCG signal interacts with ovarian steroids in the regulation of C3 and DAF expression.
According to a cell cytotoxicity assay, 87% of cells in the endometrial explant culture were viable after 24 hours. Treatment with hCG (5 UI/mL) for 24 hours was chosen for the treatment of the endometrial explants based on the results of a dose/curve experiment evaluating the endometrial response to hCG, as shown in Supplemental Figure 2.
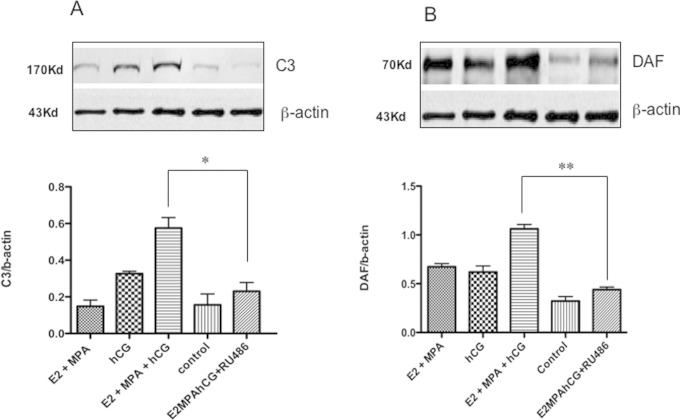
The abundances of the C3 and DAF proteins were increased in response to treatment with estradiol (E2) + MPA plus hCG. Interestingly, the increases in C3 and DAF expression caused by hCG were inhibited when the progesterone receptor antagonist RU486 was added to the culture medium (0.57 ± 0.05 vs 0.23 ± 0.04, mean ± SEM, p = .001 for C3 and 1.06 ± 0.04 vs 0.43 ± 0.02, mean ± SEM, p = .0001 for DAF [n = 6]), as shown in Figure 3.
Figure 3.
Immunoblots to assess the regulation of C3 and DAF expression by hCG and ovarian hormones using an in vitro endometrial explant model (A and B). Densitometric analysis demonstrated increased C3 and DAF protein levels when the endometrial explants were incubated with E2 and MPA plus hCG for 24 hours. Incubation with the progesterone receptor antagonist RU486 abrogated the effect of hCG on C3 and DAF. (n =6) *P = .0016, **P = .0001. DAF, decay-accelerating factor; hCG, human chorionic gonadotropin; E2, estradiol; MPA, medroxyprogesterone acetate.
Discussion
The modulation of the maternal immune response has been proposed to explain how the semiforeign embryo survives and successfully invades the maternal endometrial mucosa while evading immune surveillance. The control of complement activation in the embryo–maternal environment has been demonstrated to be critical for preventing damage to the maternal mucosa and resorption of the embryo.6 Uncontrolled complement activation has also been recently implicated in pathologies associated with recurrent early pregnancy loss, including antiphospholipid syndrome.20
C3 is the central protein of the complement system. C3 activation is followed by the release of C3a (a potent inflammatory agent) and C3b (an opsonin). The activation cascade ends with the formation of a membrane attack complex that causes cellular lysis. DAF acts as a complement regulatory protein and accelerates the dissociation of C3 convertases, limiting C3 activation.21
The expression and regulation of C3 and DAF during endometrial receptivity have been analyzed recently.22,23 In this study, we demonstrated that both proteins are modulated by an embryo signal via hCG during the implantation window.
Functional endometrium comprises a heterogeneous cell population that is organized into different compartments that interact in a paracrine fashion. The current methods for measuring endometrial gene expression include homogenizing methods that disrupt the tissue’s integrity, thus limiting the proper evaluation of hormonal effects on specific endometrial target cells. To overcome this limitation, we employed LCM to procure specific endometrial cells from the different endometrial compartments.24,25 LCM allowed us to measure the gene expression of C3 and DAF in response to hCG in specific cells from the different functional endometrial compartments. The C3 mRNA levels were increased noticeably in stromal cells; whereas in cells from the luminal compartment, a moderate elevation of DAF expression was detected. In agreement with a recent study evaluating the endometrial response to hCG in a nonhuman primate model, we found an increase in the C3 mRNA and protein levels in response to hCG, but these increases were restricted to the stromal compartment.10 This finding suggests that C3 may participate in stromal cell differentiation during implantation. The increase in DAF expression in the luminal compartment in response to hCG may help prevent complement activation, creating a safe environment for embryo adhesion.
To preserve the paracrine communication of endometrial cells, we used the endometrial explant model to investigate the regulation of complement protein expression by hormones. Interestingly, in agreement with previous report, the endometrial responses of the expression level of both C3 and DAF to hCG were abrogated by a progesterone receptor antagonist, suggesting that there is cross talk between the hCG signal and ovarian steroids in the regulation of complement proteins through progesterone receptors.26
Collectively, our findings using in vivo and in vitro models suggest that hCG participates in the embryo–endometrium dialog and modulates the immune response through changes in the expression of complement proteins.
Supplementary Material
Acknowledgment
The author is grateful to Professor Bruce Lessey, MD, PhD, for his invaluable scientific advice.
Footnotes
Authors’ Note: The work was performed at Instituto de Investigaciones Materno Infantil, Facultad de Medicina, Universidad de Chile. Supplemental Figures 1 and 2 are available at http://www.rs.sagepub.com/supplemental.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article:This study was supported by Fondo Nacional de Ciencia y Tecnología (FONDECYT), Grant # 1100217, Santiago, Chile, and in part by FONDAP-CEMC, Grant # 15010006, Santiago, Chile (WAP).
References
- 1. Lessey BA. Endometrial receptivity and the window of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14(5): 775–788. [DOI] [PubMed] [Google Scholar]
- 2. Diedrich K, Fauser BC, Devroey P, Griesinger G. Evian annual reproduction (EVAR) workshop group.The role of the endometrium and embryo in human implantation. Hum Reprod Update. 2007;13(4):365–377. [DOI] [PubMed] [Google Scholar]
- 3. Bulla R, Fischetti F, Bossi F, Tedesco F. Feto-maternal immune interaction at the placental level. Lupus. 2004;13(9):625–629. [DOI] [PubMed] [Google Scholar]
- 4. Lobo SC, Huang ST, Germeyer A, et al. The immune environment in human endometrium during the window of implantation. Am J Reprod Immunol. 2004;52(4):244–251. [DOI] [PubMed] [Google Scholar]
- 5. Murray KP, Mathure S, Kaul R, et al. Expression of complement regulatory proteins CD35, CD46, CD55 and CD 59 in benign and malignant endometrial tissue. Gynecol Oncol. 2000;76(2):176–182. [DOI] [PubMed] [Google Scholar]
- 6. Xu C, Mao D, Holers VM, Palanca B, Cheng AM, Molina H. A critical role for murine complement regulator Crry in feto-maternal tolerance. Science. 2000;287(5452):498–501. [DOI] [PubMed] [Google Scholar]
- 7. Sayegh RA, Tao XJ, Awward JT, Isaacson KB. Localization of the expression of complement C3 in endometrium by in situ hybridization. J Clin Endocrinol Metab. 1996;81:1641–1649. [DOI] [PubMed] [Google Scholar]
- 8. Hasty LA, Lambris JD, Lessey BA, Pruksananonda K, Lyttle CR. Hormonal regulation of complement components and receptors through the menstrual cycle. Am J Obstet Gynecol. 1994;170(1 pt 1):168–175. [DOI] [PubMed] [Google Scholar]
- 9. Nogawa Fonzar-Marana RR, Ferriani RA, Soares SG, Cavalcante-Neto FF, Teixeira JE, Barbosa JE. Expression of complement system regulatory molecules in the endometrium of normal ovulatory and hyperstimulated women correlate with menstrual cycle phase. Fertil Steril. 2006;86(3):758–761. [DOI] [PubMed] [Google Scholar]
- 10. Sherwin JR, Sharkey AM, Cameo P, et al. Identification of novel genes regulated by chorionic gonadotropin in baboon endometrium during the window of implantation. Endocrinology. 2007;148(2):618–626. [DOI] [PubMed] [Google Scholar]
- 11. Sherwin JR, Hastings JM, Jackson KS, Mavrogianis PA, Sharkey AM, Fazleabas AT. The endometrial response to chorionic gonadotropin is blunted in a baboon model of endometriosis. Endocrinology. 2010;151(10):4982–4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Licht P, Von Wolff M, Berkholz A, Wildt L. Evidence for cycle-dependent expression of full-length human chorionic gonadotropin/luteinizing hormone receptor mRNA in human endometrium and deciduas. Fertil Steril. 2003;79 (1):718–723. [DOI] [PubMed] [Google Scholar]
- 13. Fazleabas AT, Kim JJ, Strakova Z. Implantation: embryonic signals and the modulation of the uterine environment-A review. Placenta. 2004;(25 suppl A):S26–S31. [DOI] [PubMed] [Google Scholar]
- 14. Licht P, Russu V, Wildt L. On the role of human chorionic gonadotropin (hCG) in the embryo-endometrial microenvironment: implications for differentiation and implantation. Semin Reprod Med. 2001;19(1):37–47. [DOI] [PubMed] [Google Scholar]
- 15. Lovely LP, Fazleabas AT, Fritz MA, McAdams DG, Lessey BA. Prevention of endometrial apoptosis: randomized prospective comparison of human chorionic gonadotropin versus progesterone treatment in the luteal phase. J Clin Endocrinol Metab. 2005;90(4):2351–2356. [DOI] [PubMed] [Google Scholar]
- 16. Kohen P, Castro O, Palomino A, et al. The steroidogenic response and corpus luteum expression of the steroidogenic acute regulatory protein after human chorionic gonadotropin administration at different times in the human luteal phase. J Clin Endocrinol Metab. 2003;88(7):3421–3430. [DOI] [PubMed] [Google Scholar]
- 17. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1975;122(2):262–263. [DOI] [PubMed] [Google Scholar]
- 18. Palomino WA, Kohen P, Devoto L. A single midcycle dose of Levonorgestrel similar to emergency contraceptive does not alter the expression of the L-selectin ligand or molecular markers of endometrial receptivity. Fertil Steril. 2010;94(5):1589–1594. [DOI] [PubMed] [Google Scholar]
- 19. Fogle RH, Aimin L, Paulson RJ. Modulation of Hoxa 10 and other markers of endometrial receptivity by age and human chorionic gonadotropin in an endometrial explant model. Fertil Steril. 2010;93(4):1255–1259. [DOI] [PubMed] [Google Scholar]
- 20. Francis J, Rai R, Sebire NJ, et al. Impaired expression of endometrial differentiation markers and complement regulatory proteins in patients with recurrent pregnancy loss associated with antiphospholipid syndrome. Mol Hum Reprod. 2006;12(7):435–442. [DOI] [PubMed] [Google Scholar]
- 21. Goldsby RA, Kindt TJ, Osborne BA. Kuby Immunology. 4th ed New York, NY: W.H. Freeman and Company; 2000. [Google Scholar]
- 22. Franchi A, Zaret J, Zhang X, Bocca S, Oehninger S. Expression of immunomodulatory genes, their protein products and specific ligands/receptors during the window of implantation in the human endometrium. Mol Hum Reprod. 2008;14(7):413–421. [DOI] [PubMed] [Google Scholar]
- 23. Young SL, Lessey BA, Fritz MA, et al. In vivo and in vitro evidence suggest that HB-EGF regulates endometrial expression of human decay accelerating factor. J Clin Endocrinol Metab. 2002;87(3):1368–1375. [DOI] [PubMed] [Google Scholar]
- 24. Torres MST, Ace CI, Okulicks WC. Assessment and application of laser microdissection for analysis of gene expression in the rhesus monkey endometrium. Biol Reprod. 2002;67(4):1067–1072. [DOI] [PubMed] [Google Scholar]
- 25. Yanaihara A, Otsuka Y, Iwasaki S, et al. Comparison in gene expression of secretory human endometrium using microdissection. Reprod Biol Endocrinol. 2004;2(17):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Banerjee P, Fazleabas AT. Endometrial responses to embryonic signals in the primate. Int J Dev Biol. 2010;54(2-3):295–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.