Abstract
Retinol (ROL) and its biologically active metabolite, all-trans retinoic acid (ATRA), are essential for a number of reproductive processes. However, there is a paucity of information regarding their roles in ovarian folliculogenesis, oocyte maturation, and early embryogenesis. The objectives of this study were to quantify and compare peripheral plasma (PP) and follicular fluid (FF) retinoid levels, including ATRA in women undergoing in vitro fertilization (IVF) and to investigate the relationship between retinoid levels and embryo quality. Retinoid levels were evaluated in PP and FF from 79 women undergoing IVF at the time of oocyte retrieval and corresponding embryo quality assessed on a daily basis after retrieval for 3 days until uterine transfer. Analysis compared the retinoid levels with day 3 embryo grades and between endometriosis versus control patients. Results demonstrated distinctive levels of retinoid metabolites and isomers in FF versus PP. There was a significantly larger percentage of high-quality grade I embryos derived from the largest versus smallest follicles. An increase in follicle size also correlated with a >50% increase in FF ROL and ATRA concentrations. Independent of follicle size, FF yielding grade I versus nongrade I embryos showed higher mean levels of ATRA but not ROL. In a nested case–control analysis, control participants had 50% higher mean levels of ATRA in their FF and PP than women with endometriosis. These findings strongly support the proposition that ATRA plays a fundamental role in oocyte development and quality, and that reduced ATRA synthesis may contribute to decreased fecundity of participants with endometriosis.
Keywords: retinoic acid, follicular fluid, embryo quality, in vitro fertilization
Introduction
The introduction of in vitro fertilization (IVF) in 1978 and the ability to produce multiple oocytes with controlled ovarian hyperstimulation have revolutionized the treatment of infertility.1,2 However, human reproduction remains an inefficient process with the majority of oocytes failing to produce a live birth.3 Although many factors influence reproductive efficiency, oocyte development is affected by the surrounding micronutrient environment of the ovarian follicle.4
Retinoids are a class of compounds that are chemically related to the micronutrient vitamin A (retinol [ROL]). Retinoids regulate epithelial cell growth and differentiation and have important roles such as maintaining vision, skin health, bone growth, immune function, and hematopoiesis. Although high levels of retinoids can be teratogenic, they are essential for reproduction, including effects on ovarian folliculogenesis, steroid production, oocyte maturation, and early embryogenesis.5
Animal studies have suggested that the extent of reproductive compromise depends on certain circumstances on the degree and timing of ROL deficiency.6 Maternal administration of all-trans retinoic acid (ATRA), the active metabolite of ROL, is able to ameliorate the reproductive and developmental blocks in vitamin A-deficient rats and retinaldehyde dehydrogenase type 2 mutant mice.7–9 Bovine studies have shown that vitamin A is important in ovarian follicular growth, quality, and steroidogenesis and that ATRA stimulates blastocyst development.10–13 In vitro maturation of bovine and porcine oocytes and bovine morulae with retinoids improved embryo quality.14–16 Treatment of mouse embryos in culture or pregnant mice with retinoic acid receptor (RAR) antagonists in vivo demonstrated that RAR-dependent signaling pathways are critical for normal embryonic development.17 To this end, certain RAR isoform null mice produce embryos with developmental abnormalities similar to those observed in vitamin A-deficient animals, including organogenesis defects, limb deformities, male/female germ cell defects, and craniofacial abnormalities.18,19
Given the importance of retinoids in reproduction in animal studies and the ability of retinoids to modulate gene transcription, we designed a prospective observational study to determine the concentrations of retinoids in the peripheral plasma (PP) and follicular fluid (FF) of women undergoing IVF. Because ATRA is essential to oogenesis, we hypothesized that ATRA affects early embryo development and therefore sought to assess whether FF levels of ATRA might correlate with embryo quality. Furthermore, a nested case–control analysis was performed to determine whether there are differences in retinoid metabolism among women with infertility due to endometriosis compared to laparoscopically confirmed controls including those with unexplained, tubal, or male factor infertility. As such, our objectives for this study were to (1) quantify and compare PP and FF retinoid levels in women undergoing IVF; (2) investigate the relationship between retinoid levels and embryo quality; and (3) compare retinoid levels in patients with endometriosis with control patients. These findings will provide significant new evidence that retinoids contribute to oocyte development and quality, and that conditions associated with abnormal retinoid metabolism, such as endometriosis, may be manifested as infertility.
Materials and Methods
Experimental Participants
Following approval by the Emory University Institutional Review Board, all patients undergoing fresh autologous IVF cycles were invited to participate in the study. Informed written consent was provided by 79 women undergoing IVF at the Emory Reproductive Center between August of 2008 and July 2009. Ages ranged from 27 to 46 years. Each participant’s cycle parameters were collated from her clinical records, including infertility diagnosis, age, body mass index (BMI), early follicular phase hormonal assessment of ovarian reserve (follicle-stimulating hormone [FSH] and estradiol [E2]), antral follicle count (AFC), IVF cycle stimulation protocol, duration of controlled ovarian stimulation, total dose of gonadotropins used, day of human chorionic gonadotropin (hCG) administration, total number of ovarian follicles observed, number of ovarian follicles >14 and >18 mm on day of hCG, peak serum E2 and progesterone levels, endometrial thickness on day of hCG, total oocytes retrieved and number of mature oocytes, fertilization rate, number, and cleavage status of each resultant embryo.
Gonadotropin Stimulation
All patients underwent gonadotropin stimulation with FSH administered by subcutaneous injection. Serial E2 levels and 2-dimensional transvaginal ultrasound follicle measurements were performed until at least 2 follicles reached 18 mm or more in diameter. The HCG (10 000 IU intramuscular) was administered followed by transvaginal oocyte retrieval 35 hours later.
Collection of PP and FF
Peripheral plasma was collected from the antecubital vein prior to oocyte retrieval. The first accessible follicle from each ovary was identified and its length, width, and height were measured for volume calculations. These sonographic measurements were only performed on the first follicle aspirated from each ovary. To minimize variances, a single ultrasound machine was used for all studies and a faculty member participated in follicle measurement. There were 4 faculty members participating in the study. The maximum dimensions of the follicle were measured in each plane. The FF from the first follicle aspirated from each ovary was collected and the collection tube was changed to avoid contamination with blood or fluid from other follicles. Both PP and FF aspirates were centrifuged at 3000g for 15 minutes, and the supernatants were stored at −80°C until assayed. All fluids were processed and stored in light-protected tubes.
Retinoid Quantification
All samples were thawed on ice and prepared for retinoid analysis under yellow lights, where 100 to 300 mL of fluid or plasma were extracted as described elsewhere.20–23 Retinoic acid was quantified by liquid chromatography–tandem mass spectrometry with atmospheric pressure chemical ionization in positive-ion mode on an API-4000 or 5500 Qtrap (AB Sciex, Foster City, California).20,22,23 Retinol and total retinyl ester (RE) were quantified by high-performance liquid chromatography with ultraviolet detection on an Alliance 2690 or Aquity H-Class (Waters, Milford, Massachusetts) apparatus.21,23 Fluid retinoid concentrations are expressed in International System of Units as mol/mL.
Embryo Quality
To correlate embryo quality with FF retinoid levels, all embryos studied were derived from the first follicle aspirated from each ovary. Fertilization was assessed 17 to 18 hours after insemination. Embryo quality was evaluated by the same faculty embryologist on a daily basis and scored by morphological assessment on day 3.24 The day 3 embryo grading is scored I (best) to III (worst) based on the number and evenness of blastomeres and the degree of fragmentation.25 Single embryo culture was done to track embryos according to their corresponding FF sample. Ultrasound-guided fresh embryo transfer was performed 3 days after fertilization.
Statistical Analysis
Data are presented as mean ± standard error of the mean. Spearman correlation coefficients, Fisher 2-tailed exact test, and Mann-Whitney tests were used for statistical analysis. The potential association of factors (age, BMI, day 3 FSH, peak E2, total gonadotropins, and AFC) that can influence embryo quality were evaluated by comparing these factors between women with at least 1 grade I embryo and women without grade 1 embryos using a 1-way analysis of variance (SAS Proc GLM, version 9.3). Repeated-measure analyses of the ATRA concentration from each ovary were done with a means model with SAS Proc Mixed (version 9.3) providing separate estimates of the means by embryo quality (grade I and nongrade I embryos). A compound symmetric variance–covariance form in repeated measurements was assumed for ATRA, and robust estimates of the standard errors of parameters were used to do statistical tests and construct 95% confidence intervals. The model-based means were unbiased with unbalanced and missing data, so long as the missing data were noninformative (missing at random). Statistical tests were 2-sided. A P value<.05 was considered statistically significant. All these analyses were also performed for the subset of grade I versus grade II or III embryos.
The generalized estimating equations (GEEs) approach of Zeger and Liang26 was used to perform logistic regression to help identify the factors associated with embryo quality. The statistical model accounts for the correlation between multiple embryos from the same participant. The GEE analysis used an exchangeable correlation binomial-logit model implemented using SAS Proc Genmod, version 9.3. Odds ratios were calculated to measure the degree of association between the ATRA concentration (using tertiles) and embryo quality. Patient factors significant to at least a value of P ≤ .10 in univariable analyses were used in the GEE multiple logistic regression. The odds ratio and its 95% confidence interval were calculated for each factor in the presence of others in the final model.
Results
Retinoid Levels in Plasma and FF
Collection of PP and FF from the first follicle aspirated from each ovary was successful in 79 participants participating in the study, yielding 147 FF samples. In all, 132 FF volumes were adequate to be analyzed. Eleven ovaries could not be sampled due to prior oopherectomy, inaccessibility of the ovary, or technical difficulties encountered during the retrieval. There was insufficient quantity of FF to allow for analysis of 15 samples.
Similar retinoid isoforms and metabolites were identified in both PP and FF (Table 1). On average, the PP retinoid concentrations were approximately twice those measured in FF. 9-cis RA was not detected (ND) in either the plasma or the FF (limit of detection for 9-cis and ATRA was 0.05 pmol/mL). Retinol and REs were more abundant than the various retinoic acid isomers, falling in the nmol/mL range. The ratio of plasma to FF concentrations of ROL and retinoic acid isomers ranged from 1.6 to 2.3 (Table 1). Retinyl esters, the storage form of retinoids, were more abundant in the plasma, with a PP to FF ratio of 4.2.
Table 1.
Mean Retinoid Levels in Plasma and Follicular Fluid From Women Undergoing In Vitro Fertilization.
| Plasmaa mean (SEM) pmol/mL | Follicular fluidb mean (SEM) pmol/mL | Ratio of plasma to follicular fluid | |
|---|---|---|---|
| Retinol | 2112 (62) | 1306 (56) | 1.6 |
| All-trans retinoic acid | 8.6 (0.5) | 5.2 (0.3) | 1.7 |
| 9-cis retinoic acid | Not detected | Not detected | N/A |
| 9,13-cis retinoic acid | 0.7 (0.1) | 0.4 (0.03) | 1.6 |
| 13-cis retinoic acid | 2.2 (0.1) | 0.9 (0.1) | 2.3 |
| Total retinoic acid | 11.3 (0.6) | 6.5 (0.4) | 1.7 |
| Retinyl esters | 550 (22) | 130 (8) | 4.2 |
Abbreviations: N/A, not available; SEM, standard error of the mean.
a N = 79.
b N = 132.
Plasma ATRA levels ranged from 3.5 to 29.9 pmol/mL. Follicular fluid ATRA ranged from ND-20.3 pmol/mL for left FF aspirates and ND-17.5 pmol/mL for right FF aspirates. Left and right FF ATRA concentrations from the same women correlated moderately with plasma ATRA levels (P = .59). Similarly, FF ROL and RE correlated with plasma ROL and RE (P = .53 and P = .47, respectively). Although FF ATRA weakly correlated with FF ROL and RE (P = .39 and P = .34), plasma ATRA did not correlate with plasma ROL and RE (P = .04 and P = −.06).
Embryo Quality
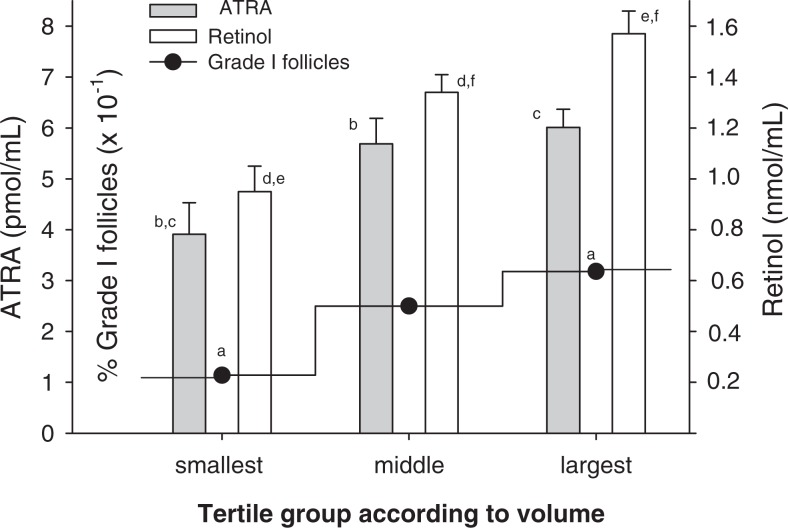
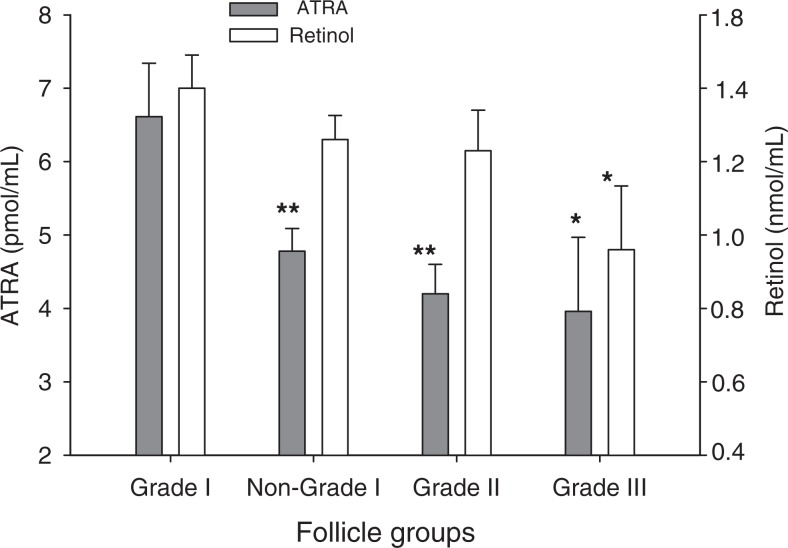
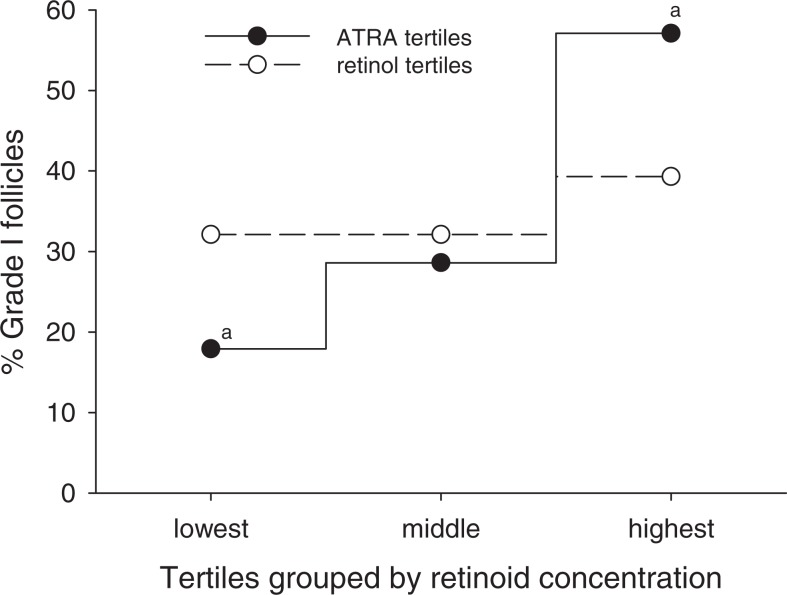
Previous studies have suggested an association between follicle size and oocyte quality in women undergoing IVF27; however, a relationship between resultant embryo quality and follicle size has, to our knowledge, not been reported. When all follicles were stratified by tertiles according to volume, 31% of the largest follicles yielded grade I embryos, whereas the smallest follicles yielded 11.4% of grade I embryos (Figure 1). In these calculations, volumetric determination of follicles was assigned independent of oocyte quality or recovery. Hence, follicles could have yielded immature oocytes (eg, M1, GV), oocytes yielding abnormal embryos (eg, 3 pronuclei or fractured zonae) and even those in which recovery of an oocyte failed. Increased follicle size also correlated significantly with higher FF ATRA and ROL concentrations, with each found to be >50% higher in the largest versus the smallest tertile. To further explore a potential relationship between retinoid levels in the FF and the quality of embryos derived from the follicles, additional analyses were performed. Independent of follicle size, the mean FF concentration of ATRA was significantly higher in follicles yielding grade I embryos (grade I follicles) than the other (nongrade I) follicles (Figure 2), 6.6 ± 0.7 and 4.8 ± 0.3 pmol/mL, respectively. The mean ATRA concentrations of the FF in follicles which yielded grade II and grade III embryos were 4.2 ± 0.4 and 4.0 ± 1.0 pmol/mL, respectively, significantly less than that found in the grade I follicles. In contrast, FF ROL concentrations in grade I follicles were not significantly different from that in nongrade I follicles although, interestingly, a comparison of ROL levels in grade I versus grade III follicles did reach significance. For follicles from which a mature egg was retrieved (n = 84), an increase in the percentage of grade I embryos was observed across the tertiles of ATRA distribution (Figure 3); 57% of the embryos generated from oocytes in the highest ATRA tertile were classified as grade I versus only 18% of those derived from oocytes in the lowest tertile. There was no significant difference in the distribution of grade I embryos according to tertiles of ROL concentrations. None of the other patient characteristics or menstrual cycle parameters differed across the tertiles of ATRA and ROL (not shown).
Figure 1.
Follicular fluid retinol and all-trans retinoic acid (ATRA) concentrations and percentage of follicles yielding grade I embryos (percentage of grade I follicles) stratified by tertiles according to follicle volume. Values represent mean ± standard error of the mean (SEM) of retinoid concentrations or percentage of grade I embryos obtained from all follicles in the indicated volume tertile. Volumetric determination was assigned independent of oocyte quality or recovery. Values that are labeled with identical letters showed statistically significant differences according to the following P values: a<.05, b<.005, c<.005, d<.005, e<.0001, and f<.05.
Figure 2.
Increased all-trans retinoic acid (ATRA) levels in follicles yielding grade I embryos (grade I follicles). Values represent the mean ± standard error of the mean (SEM) of retinoid concentrations in follicular fluid (FF) from indicated follicle groups yielding grade I, II, or III embryos or all nongrade I follicles (regardless of oocyte quality or recovery). Retinoid concentrations are compared to corresponding retinoid in grade I follicles. *P < .05; **P < .01.
Figure 3.
Percentage of grade I embryos derived from “mature egg follicles” (n = 84) stratified by tertiles according to all-trans retinoic acid (ATRA) or retinol (ROL) concentrations. Percentage of grade I follicles = number of grade I follicles/28 (number of mature egg follicles in each tertile). aSignificant difference between labeled values at P < .005.
Univariate and Multivariate Analyses of Factors Associated With Embryo Quality
Univariable analyses of patient factors and embryo grade (grade I vs grade II, III) were performed in 50 women and 66 follicles, 23 women with at least 1 grade I follicle (29 grade I follicles) versus 27 women with grades II and/or III follicles (37 grade II/III follicles). Results showed no difference between age, day 3 FSH, peak E2, total gonadotropins or AFC, and development of grade I versus grade II/III embryos (Table 2). Using an independent logistic regression model, higher levels of ATRA concentration was a significant predictor of grade I embryos (Table 3). The odds of a grade I embryo relative to a grade II/III embryo was over 7 times higher (7.29) for the women with ATRA concentration >5.81 pmol/mL (ie, the third/highest ATRA tertile) compared to the first/lowest ATRA tertile (≤3.62 pmol/mL). The GEE approach of Zeger and Liang26 was used to analyze the clustered/repeated binary responses within participants (1 or 2 embryos from each study participant; 29 grade I embryos and 37 grade II/III embryos from 50 women). Increased ATRA concentration was a significant predictor of grade I embryos (P = .027). The odds of a grade I embryo relative to a grade II/III embryo was over 3 times higher (3.08) for the women with ATRA concentration in the highest ATRA tertile compared to the lowest ATRA tertile. The preferred analysis is the logistic regression for clustered data since the model properly accounts for the correlation between multiple observations/embryos from the same participant. After adjusting for BMI (multivariate analysis), the odds of a grade I embryo relative to a grade II/III embryo was 2.77 times higher for the women with ATRA concentration in the highest ATRA tertile compared to the lowest ATRA tertile. The adjusted ATRA odds ratio and 95% confidence interval were 2.77 and 1.26 to 6.09, respectively.
Table 2.
Univariable Analyses of Patient Factors Associated With Embryo Grade.a
| Risk factors | Grade I embryos | Grade II/III embryos | P valueb |
|---|---|---|---|
| ATRA, pmol/mL | 7.0 (4.4-9.7) 29c | 4.2 (2.6-5.8) 37 | .06 |
| Age, years | 35.9 (33.9-37.8) 23d | 33.9 (32.1-35.7) 27e | .14 |
| BMI, kg/m2 | 28.5 (25.9-31.1) 23 | 24.8 (22.4-27.3) 27 | .05 |
| Day 3, FSH, IU/L | 8.6 (6.9-10.2) 22 | 8.7 (7.2-10.2) 25 | .90 |
| Peak E2, pg/mL | 1958 (1520-2396) 23 | 1934 (1530-2338) 27 | .94 |
| Total gonadotropins, pmol/mL | 3538 (2745-4331) 23 | 3327 (2595-4059) 27 | .70 |
| Antral follicle count (AFC) | 19.0 (12.1-25.8) 23 | 24.7 (18.4-31.0) 27 | .22 |
Abbreviations: ATRA, all-trans retinoic acid; FSH, follicle-stimulating hormone.
a A total of 50 women and 66 follicles were evaluated, 23 women (29 follicles) with grade I and 27 women (37 follicles) with grade II and/or III.
b Grade I versus grade II/III.
c Mean (95% confidence interval) number of follicles. Means are model-based means (not raw means) obtained from a repeated measures analysis of ATRA concentration from each ovary. The model-based means are the estimated means taking into consideration the fact that not all women have ATRA measurements from both ovaries.
d Mean (95% confidence interval) number of women with at least 1 grade I embryo from the 2 follicles studied.
e Mean (95% confidence interval) number of women with at least 1 grade II/III embryo (but no grade I embryo) from the 2 follicles studied.
Table 3.
Univariable Analyses of Logistic Regression Models of Embryo Gradea by ATRA Concentrations.
| Tertile groups | Grade I | Grade II/III | Odds ratio (OR)b | OR (95% CI)c | P valued |
|---|---|---|---|---|---|
| ATRA, pmol/mL | |||||
| ≤3.62e | 17.2% (5/29) | 46.0% (17/37) | 1.0 | 1.0 | .027 |
| >3.62-5.81 | 31.0% (9/29) | 35.1% (13/37) | 2.35 | 1.78 (0.80-3.93) | |
| >5.81 | 51.7% (15/29) | 18.9% (7/37) | 7.29 | 3.08 (1.36-6.98) |
Abbreviations: ATRA, all-trans retinoic acid; CI, confidence interval; SEM, standard error of the mean.
a Grade I versus grade II/III.
b Independence model.
c Generalized estimating equations (GEEs) for clustered data model.
d Calculated from the GEE clustered data model.
e The first/lowest ATRA tertile (≤3.62 pmol/mL) is the reference level.
Similar analyses were performed for grade I (23 women, 29 grade I follicles) versus all nongrade I follicles regardless of oocyte quality or recovery (53 women, 102 nongrade I follicles). As above, logistic regression for clustered data indicated that follicles with concentrations of ATRA in the highest tertile was a significant predictor of grade I embryos compared to those in the lowest ATRA tertile (Supplemental Table 1). After adjusting for BMI, the odds of a grade I embryo relative to a nongrade I embryo was 2.64 times higher for follicles with ATRA concentration in the highest compared to the lowest ATRA tertile. The adjusted odds ratio and 95% confidence interval were 2.64 and 1.23 to 5.67, respectively.
Retinoids and Endometriosis
A nested case–control study was performed to analyze whether there were differences in retinoid levels between women with or without endometriosis. All women underwent laparoscopic evaluation by subspeciality trained infertility experts, and those without visible evidence of endometriosis were assigned to the control group. Their infertility diagnoses included unexplained, tubal, or male factor infertility. All cases of endometriosis were confirmed by pathologic examination of biopsied tissue. The baseline characteristics between the endometriosis and control groups were comparable (Table 4). Both the groups had similar stimulation parameters and response to gonadotropins. Despite equal size follicles being aspirated in each group, women with endometriosis had significantly lower levels of ATRA in FF (3.9 ± 0.3 vs 5.6 ± 0.6 pmol/mL, P = .01) and even in PP (6.8 ± 0.6 vs 9.9± 0.7 pmol/mL, P = .005) than the controls. No significant differences were observed in the FF or PP levels of ROL or REs between the 2 groups. Subanalyses were performed in an attempt to correlate FF and PP ATRA levels after stratifying for stage of endometriosis (based on the Revised American Society for Reproductive Medicine Classification of Endometriosis28) but these were underpowered and revealed no significant differences of ATRA between endometriosis stages for either FF or PP (P > .30).
Table 4.
Comparison of Baseline Characteristics of Endometriosis and Control Groups Undergoing in Vitro Fertilization.a
| Controls N = 15, mean (SEM) | Endometriosis N = 16, mean (SEM) | P value | |
|---|---|---|---|
| Age, years | 33.2 (1.1) | 33.8 (1.1) | .64 |
| BMI, kg/m2 | 27.4 (1.7) | 24.6 (1.2) | .29 |
| Day 3 FSH, IU/L | 7.3 (0.41) | 7.5 (1.0) | .86 |
| Antral follicle count | 21.8 (2.4) | 24.8 (3.7) | .40 |
| Days of stimulation | 10.1 (0.4) | 9.8 (0.2) | .33 |
| Total number of follicles | 16.4 (0.9) | 16.9 (1.0) | .68 |
| Number of follicles >14 mm | 8.4 (1.1) | 9.9 (1.0) | .26 |
| Number of follicles >18 mm | 1.6 (0.4) | 2.5 (0.7) | .28 |
| Average follicle size, mm3 | 2376 (360) | 2412 (356) | .93 |
| Units of FSH | 2831 (347) | 2472 (412) | .59 |
| EM thickness, mm | 12.4 (0.8) | 12.1 (0.5) | .69 |
| Peak E2, pg/mL | 1831 (220) | 2320 (257) | .10 |
| Peak prog, ng/mL | 1.5 (0.2) | 1.3 (0.1) | .78 |
| Plasma ROL, pmol/mL | 2139 (128) | 2082 (116) | .74 |
| Plasma ATRA, pmol/mL | 9.9 (0.7) | 6.8 (0.6) | .002* |
| Plasma RE, pmol/mL | 571 (57) | 504 (37) | .49 |
| FFb ROL, pmol/mL | 1256 (140) | 1158 (88) | .55 |
| FF ATRA, pmol/mL | 5.6 (0.6) | 3.9 (0.3) | .01* |
| FF RE, pmol/mL | 137 (26) | 128 (17) | .77 |
Abbreviations: EM, endometrial; ROL, retinol; ATRA, all-trans retinoic acid; RE, retinyl esters; FF, follicular fluid; SEM, standard error of the mean.
a Control group consisted of women with either unexplained or tubal factor infertility with laparoscopic confirmation of no endometriosis or male factor infertility.
b Number of FF samples: controls, n = 26; endometriosis, n = 29.
Discussion
The essential actions of retinoids in reproductive biology have long been recognized.29,30 During the human menstrual cycle, expression of retinoid receptors and synthesis of ATRA in reproductive tissue are influenced by the changing pattern of steroid exposure, and localized ATRA production is known to regulate numerous aspects of endometrial behavior.31–33 In addition, emerging evidence indicates important effects of retinoids on oocyte development and likely on oocyte fertilization, transport, uterine implantation, and development into a health fetus.10,34–40 To this end, in cattle, sheep, and pigs, retinoids have been shown to enhance competence of oocyte development.36 Although concentrations of ROL in the FF of women and animals have previously been quantified,13,16,41 direct determination of the active metabolic products including ATRA has not, to our knowledge, been reported. Our results indicate that the ratio of plasma to FF concentrations of ROL and the ATRA isomers ranged from 1.6 to 2.3 while that of the REs showed a ratio of 4.2. This reduced concentration of ROL in FF compared to plasma is consistent with that reported by other investigators.41 Our finding that RE shows an even greater disparity between FF and plasma levels may reflect differences in blood–follicle transport of RE compared to the other retinoids.42,43 It is also possible that the RE concentration in FF may be affected by vitamin A consumption within the follicles themselves.44 Thus, the greater reduction of RE in FF in comparison to the other retinoids may reflect relatively high vitamin A utilization in localized follicular processes.
In bovine ovary, concentrations of ROL were found to be greater in the fluid of large follicles in some studies10,41 but were negatively related to follicle size in others.16 Our study in humans was consistent with results of the former; when grouping the follicles by tertiles according to volume, the data indicated significantly lower concentrations of both ROL and ATRA in the smallest (third) tertile compared to the other larger groups. Correspondingly, third tertile follicles yielded oocytes that gave rise to fewer grade I embryos than those from the larger follicles. Interestingly, Scott et al27 showed that follicles with diameters <15 mm yielded a significantly lower proportion of mature oocytes when compared to larger follicles. This cutoff diameter roughly corresponds to the upper limit of our third tertile value. Taken together, these findings indicate a direct association between follicle size, retinoid levels, and embryo quality. This association does not allow for drawing conclusions regarding the “cause-and-effect” relationship between these parameters. Thus, reduced retinoid levels in the smallest follicles may be an associated manifestation of aberrant follicle development that results in both poor oocyte quality and decreased ATRA concentrations. However, in support of an independent causal relationship between ATRA levels and oocyte properties that affect embryo quality, our results showed significantly higher concentrations of ATRA in FF from follicles yielding grade I embryos compared to fluid from other follicles. Moreover, when follicles yielding mature oocytes were stratified according to FF ATRA concentrations, there were significantly more grade I embryos derived from those in the highest tertile. It should be noted that there were no similar relationships between ROL FF levels and embryo quality suggesting that local metabolic conversion of ROL to ATRA, as opposed to the ROL precursor itself, may be important for influencing oocyte fertilization and embryo quality. However, in studies of bovine follicles, concentrations of ROL were shown to be higher in nonatretic versus atretic follicles leading to the suggestion that ROL may have the potential for local control of follicular development.13 Whether this control might be mediated through conversion of ROL to functionally active ATRA was not addressed.
Our results demonstrated that FF from women with endometriosis undergoing IVF has a significantly lower mean concentration of ATRA than control participants. Surprisingly, although they were well within the normal concentration range,45,46 the mean concentration of ATRA in the plasma of the patients with endometriosis was also significantly lower than in controls. In contrast, ROL concentrations in the 2 groups did not differ in either compartment. These data suggest that on a systemic level, there is a relative reduction in ATRA synthesis from ROL in these patients. Future studies will address this possibility in endometriosis and control populations that are not undergoing IVF. Nevertheless, our results in these IVF patients posit the interesting hypothesis that women whose overall retinoid metabolism is in the low normal range of activity may be more susceptible to development of endometriosis. Such a possibility would address the quintessential question of why only some women develop endometriosis in spite of the fact that retrograde menstruation seeds the peritoneal cavity with endometrial cells in most women.47 There are a number of observations that are consistent with an association of low ATRA production and endometriosis. These include the expression of several ATRA-regulated genes and cytokines that are aberrantly expressed in eutopic endometrium of women with endometriosis, including matrix metalloproteinases, tumor necrosis factor-β, interleukin (IL)-6, IL-11, various integrins, bax, and fas ligand.48–50 As such, a number of seemingly discordant features of endometriosis including decreased cell death, increased growth and migration, and enhanced invasive properties of intraperitoneally seeded endometrial cells could be accounted for by dysregulation of ATRA synthesis in endometrial tissue. In further support of this concept, it has recently been shown that endometriotic tissue is characterized by a gene expression pattern that is consistent with a decrease in ATRA synthesis and/or action in situ.51 Such processes could also contribute to the decrease in fecundity of patients with endometriosis through the reduced or defective functional capacity of their endometrium. Finally, lower ATRA levels on a systemic level including that in immune cells could help explain the myriad observations of abnormal immunological parameters reported in the patients with endometriosis.52,53
In summary, these studies have quantified the concentration of ROL and its metabolic products including ATRA in the FF of women undergoing IVF. Here, we provide the first direct quantitative measurements of endogenous ATRA levels in FF. Results showed a direct association between small follicle size, low retinoid levels, and poor embryo quality. Independent of size, FF from follicles yielding grade I embryos had significantly higher mean levels of ATRA compared to other follicles and, correspondingly, higher ATRA levels in FF containing mature oocytes conferred an increased probability of generating grade I embryos. Finally, mean ATRA concentrations in the FF and plasma from patients with endometriosis were significantly lower than those from controls. Together, these results strongly support the contention that tight regulation of in vivo ATRA plays a fundamental role in oocyte development and that reduced ATRA synthesis may contribute to the inflammatory phenotype and decreased fecundity widely reported among patients with endometriosis.
Supplementary Material
Acknowledgment
The authors are indebted to Zhaoju Shen for her outstanding technical support.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NIH RO1HD55379, U54HD55787, UO166439, RO1AA017927, RO1DK090522.
References
- 1. Jones HW., Jr The use of controlled ovarian hyperstimulation (COH) in clinical in vitro fertilization: the role of Georgeanna Seegar Jones. Fertil Steril. 2008;90(5):e1–e3. [DOI] [PubMed] [Google Scholar]
- 2. Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2(8085):366. [DOI] [PubMed] [Google Scholar]
- 3. Patrizio P, Sakkas D. From oocyte to baby: a clinical evaluation of the biological efficiency of in vitro fertilization. Fertil Steril. 2009;91(4):1061–1066. [DOI] [PubMed] [Google Scholar]
- 4. Ashworth CJ, Toma LM, Hunter MG. Nutritional effects on oocyte and embryo development in mammals: implications for reproductive efficiency and environmental sustainability. Philos Trans R Soc Lond B Biol Sci. 2009;364(1534):3351–3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blomhoff R. Transport and metabolism of vitamin A. Nutr Rev. 1994;52(2 pt 2):S13–S23. [DOI] [PubMed] [Google Scholar]
- 6. Clagett-Dame M, DeLuca HF. The role of vitamin A in mammalian reproduction and embryonic development. Annu Rev Nutr. 2002;22:347–381. [DOI] [PubMed] [Google Scholar]
- 7. Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21(4):444–448. [DOI] [PubMed] [Google Scholar]
- 8. Thompson SY. Factors affecting the absorption of carotene and its conversion into vitamin A. Exp Eye Res. 1964;3:392–404. [DOI] [PubMed] [Google Scholar]
- 9. Wang X, Penzes P, Napoli JL. Cloning of a cDNA encoding an aldehyde dehydrogenase and its expression in Escherichia coli. Recognition of retinal as substrate. J Biol Chem. 1996;271(27):16288–16293. [DOI] [PubMed] [Google Scholar]
- 10. Brown JA, Eberhardt DM, Schrick FN, Roberts MP, Godkin JD. Expression of retinol-binding protein and cellular retinol-binding protein in the bovine ovary. Mol Reprod Dev. 2003;64(3):261–269. [DOI] [PubMed] [Google Scholar]
- 11. Graves-Hoagland RL, Hoagland TA, Woody CO. Effect of beta-carotene and vitamin A on progesterone production by bovine luteal cells. J Dairy Sci. 1988;71(4):1058–1062. [DOI] [PubMed] [Google Scholar]
- 12. Schweigert FJ, Lutterbach A, Rambeck WA, Zucker H. Vitamin A- and beta-carotene concentrations in bovine follicular fluid in relationship to follicle size. Zentralbl Veterinarmed A. 1986;33(5):360–364. [DOI] [PubMed] [Google Scholar]
- 13. Schweigert FJ, Zucker H. Concentrations of vitamin A, beta-carotene and vitamin E in individual bovine follicles of different quality. J Reprod Fertil. 1988;82(2):575–579. [DOI] [PubMed] [Google Scholar]
- 14. Alminana C, Gil MA, Cuello C, et al. In vitro maturation of porcine oocytes with retinoids improves embryonic development. Reprod Fertil Dev. 2008;20(4):483–489. [DOI] [PubMed] [Google Scholar]
- 15. Gomez E, Caamano JN, Rodriguez A, De Frutos C, Facal N, Diez C. Bovine early embryonic development and vitamin A. Reprod Domest Anim. 2006;(41 suppl 2):63–71. [DOI] [PubMed] [Google Scholar]
- 16. Haliloglu S, Baspinar N, Serpek B, Erdem H, Bulut Z. Vitamin A and beta-carotene levels in plasma, corpus luteum and follicular fluid of cyclic and pregnant cattle. Reprod Domest Anim. 2002;37(2):96–99. [DOI] [PubMed] [Google Scholar]
- 17. Kochhar DM, Jiang H, Penner JD, Johnson AT, Chandraratna RA. The use of a retinoid receptor antagonist in a new model to study vitamin A-dependent developmental events. Int J Dev Biol. 1998;42(4):601–608. [PubMed] [Google Scholar]
- 18. Lohnes D, Kastner P, Dierich A, Mark M, LeMeur M, Chambon P. Function of retinoic acid receptor gamma in the mouse. Cell. 1993;73(4):643–658. [DOI] [PubMed] [Google Scholar]
- 19. Lufkin T, Lohnes D, Mark M, et al. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc Natl Acad Sci U S A. 1993;90(15):7225–7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kane MA, Chen N, Sparks S, Napoli JL. Quantification of endogenous retinoic acid in limited biological samples by LC/MS/MS. Biochem J. 2005;388(pt 1):363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kane MA, Folias AE, Napoli JL. HPLC/UV quantitation of retinal, retinol, and retinyl esters in serum and tissues. Anal Biochem. 2008;378(1):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kane MA, Folias AE, Wang C, Napoli JL. Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal Chem. 2008;80(5):1702–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kane MA, Napoli JL. Quantification of endogenous retinoids. Methods Mol Biol. 2010;652:1–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dayal MB, Frankfurter D, Athanasiadis I, Peak D, Dubey A, Gindoff PR. Day 2 embryo transfer (ET) and day 3 ET afford similar reproductive outcomes in the poor responder. Fertil Steril. 2011;95(3):1130–1132. [DOI] [PubMed] [Google Scholar]
- 25. Desai NN, Goldstein J, Rowland DY, Goldfarb JM. Morphological evaluation of human embryos and derivation of an embryo quality scoring system specific for day 3 embryos: a preliminary study. Hum Reprod. 2000;15(10):2190–2196. [DOI] [PubMed] [Google Scholar]
- 26. Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 27. Scott RT, Hofmann GE, Muasher SJ, Acosta AA, Kreiner DK, Rosenwaks Z. Correlation of follicular diameter with oocyte recovery and maturity at the time of transvaginal follicular aspiration. J In Vitro Fert Embryo Transf. 1989;6(2):73–75. [DOI] [PubMed] [Google Scholar]
- 28. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–821. [DOI] [PubMed] [Google Scholar]
- 29. Bo WJ, Smith MS. The effect of retinol and retinoic acid on the morphology of the rat uterus. Anat Rec. 1966;156(1):5–9. [DOI] [PubMed] [Google Scholar]
- 30. Thompson JN, Howell JM, Pitt GA. Vitamin A and reproduction in rats. Proc R Soc Lond B Biol Sci. 1964;159:510–535. [DOI] [PubMed] [Google Scholar]
- 31. Deng L, Shipley GL, Loose-Mitchell DS, et al. Coordinate regulation of the production and signaling of retinoic acid by estrogen in the human endometrium. J Clin Endocrinol Metab. 2003;88(5):2157–2163. [DOI] [PubMed] [Google Scholar]
- 32. Ulven SM, Gundersen TE, Weedon MS, et al. Identification of endogenous retinoids, enzymes, binding proteins, and receptors during early postimplantation development in mouse: important role of retinal dehydrogenase type 2 in synthesis of all-trans-retinoic acid. Dev Biol. 2000;220(2):379–391. [DOI] [PubMed] [Google Scholar]
- 33. Zheng WL, Sierra-Rivera E, Luan J, Osteen KG, Ong DE. Retinoic acid synthesis and expression of cellular retinol-binding protein and cellular retinoic acid-binding protein type II are concurrent with decidualization of rat uterine stromal cells. Endocrinology. 2000;141(2):802–808. [DOI] [PubMed] [Google Scholar]
- 34. Bowles J, Knight D, Smith C, et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312(5773):596–600. [DOI] [PubMed] [Google Scholar]
- 35. Bowles J, Koopman P. Retinoic acid, meiosis and germ cell fate in mammals. Development. 2007;134(19):3401–3411. [DOI] [PubMed] [Google Scholar]
- 36. Hidalgo C, Diez C, Duque P, et al. Oocytes recovered from cows treated with retinol become unviable as blastocysts produced in vitro. Reproduction. 2005;129(4):411–421. [DOI] [PubMed] [Google Scholar]
- 37. Huang JC. The role of peroxisome proliferator-activated receptors in the development and physiology of gametes and preimplantation embryos. PPAR Res. 2008;2008:732303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ikeda S, Kitagawa M, Imai H, Yamada M. The roles of vitamin A for cytoplasmic maturation of bovine oocytes. J Reprod Dev. 2005;51(1):23–35. [DOI] [PubMed] [Google Scholar]
- 39. Livera G, Rouiller-Fabre V, Valla J, Habert R. Effects of retinoids on the meiosis in the fetal rat ovary in culture. Mol Cell Endocrinol. 2000;165(1-2):225–231. [DOI] [PubMed] [Google Scholar]
- 40. Zheng WL, Bucco RA, Sierra-Rievera E, Osteen KG, Melner MH, Ong DE. Synthesis of retinoic acid by rat ovarian cells that express cellular retinoic acid-binding protein-II. Biol Reprod. 1999;60(1):110–114. [DOI] [PubMed] [Google Scholar]
- 41. Schweigert FJ, Steinhagen B, Raila J, Siemann A, Peet D, Buscher U. Concentrations of carotenoids, retinol and alpha-tocopherol in plasma and follicular fluid of women undergoing IVF. Hum Reprod. 2003;18(6):1259–1264. [DOI] [PubMed] [Google Scholar]
- 42. Hess KA, Chen L, Larsen WJ. The ovarian blood follicle barrier is both charge- and size-selective in mice. Biol Reprod. 1998;58(3):705–711. [DOI] [PubMed] [Google Scholar]
- 43. Schweigert FJ, Gericke B, Wolfram W, Kaisers U, Dudenhausen JW. Peptide and protein profiles in serum and follicular fluid of women undergoing IVF. Hum Reprod. 2006;21(11):2960–2968. [DOI] [PubMed] [Google Scholar]
- 44. Smith FR, Goodman DS. Vitamin A transport in human vitamin A toxicity. N Engl J Med. 1976;294(15):805–808. [DOI] [PubMed] [Google Scholar]
- 45. Moulas AN, Gerogianni IC, Papadopoulos D, Gourgoulianis KI. Serum retinoic acid, retinol and retinyl palmitate levels in patients with lung cancer. Respirology. 2006;11(2):169–174. [DOI] [PubMed] [Google Scholar]
- 46. Sedjo RL, Ranger-Moore J, Foote J, et al. Circulating endogenous retinoic acid concentrations among participants enrolled in a randomized placebo-controlled clinical trial of retinyl palmitate. Cancer Epidemiol Biomarkers Prev. 2004;13(11 pt 1):1687–1692. [PubMed] [Google Scholar]
- 47. Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64(2):151–154. [PubMed] [Google Scholar]
- 48. Elias JA, Zheng T, Einarsson O, et al. Epithelial interleukin-11. Regulation by cytokines, respiratory syncytial virus, and retinoic acid. J Biol Chem. 1994;269(35):22261–22268. [PubMed] [Google Scholar]
- 49. Sago K, Teitelbaum SL, Venstrom K, Reichardt LF, Ross FP. The integrin alphavbeta5 is expressed on avian osteoclast precursors and regulated by retinoic acid. J Bone Miner Res. 1999;14(1):32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sharpe-Timms KL. Endometrial anomalies in women with endometriosis. Ann N Y Acad Sci. 2001;943:131–147. [DOI] [PubMed] [Google Scholar]
- 51. Pavone ME, Dyson M, Reirstad S, et al. Endometriosis expresses a molecular pattern consistent with decreased retinoid uptake, metabolism and action. Hum Reprod. 2011;26(8):2157–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75(1):1–10. [DOI] [PubMed] [Google Scholar]
- 53. Sidell N, Han SW, Parthasarathy S. Regulation and modulation of abnormal immune responses in endometriosis. Ann N Y Acad Sci. 2002;955:159–173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.