Abstract
We assessed FAS and FAS-L gene polymorphisms and messenger RNA (mRNA) levels in patients with recurrent pregnancy loss (RPL). This case–control study compared 129 women with RPL with 235 healthy multiparous women (control group). Genomic DNA and total mRNA were extracted from whole blood, and polymorphisms genotyping was performed by polymerase chain reaction (PCR). Messenger RNA expression levels were analyzed by real-time PCR. Data were analyzed by chi-square and Fisher exact tests; P < .05 was considered significant. There were no significant differences in the FAS (670 A/G) genotype or allelic frequencies between the RPL and control groups. We found significant differences in the FAS-L (844 C/T) genotype and allelic frequencies between women with RPL and controls. Patients with RPL had significantly higher FAS-L expression. Our data suggest that FAS-L gene polymorphism is associated with increased susceptibility to RPL. Moreover, women with RPL seem to abnormally express FAS-FAS-L molecules.
Keywords: recurrent pregnancy loss, polymorphism, FAS, FAS-L, apoptosis
Introduction
Recurrent pregnancy loss (RPL) occurs in approximately 1% of pregnant women and is a distressing condition for those affected. Despite extensive investigations, etiology remains unknown in approximately half of all cases.1,2
Limited trophoblast invasion is one of the events implicated in the pathogenesis of RPL. Apoptosis, or programmed cell death, is a physiological process necessary for adequate remodeling of maternal deciduas, to control trophoblast invasion and to maintain maternal–fetal immune tolerance in balance.3 Accordingly, several studies have reported an association between abnormal trophoblast apoptosis and RPL.4–7
The FAS-FAS-L system is one of the most important inducers of apoptosis.7–10 Activation of this complex is essential for trophoblast development and invasion and also to protect trophoblasts from being attacked by the maternal immune system.11 Abnormal expression of pro- and antiapoptotic molecules have been identified in tissues from patients with RPL, and higher expression of FAS and FAS-L genes have been found in chorionic villi from patients with RPL.8,12
Single-nucleotide polymorphisms (SNPs) have been identified in the promoter regions of FAS-FAS-L genes. Experimental results suggest that a polymorphism at the −670 position (A to G) of the FAS gene leads to decreased FAS production, while a polymorphism at the −844 position (C to T) of the FAS-L gene leads to increased expression of this molecule.13 FAS and FAS-L gene polymorphisms have been linked with several autoimmune diseases14,15 and with cancer.16,17 However, to the best of our knowledge, these genetic variants have not been evaluated in women with RPL. This motivated us to conduct this investigation.
The aim of this study was to evaluate FAS and FAS-L gene polymorphisms and messenger RNA (mRNA) expression in women with RPL.
Materials and Methods
This case–control study enrolled patients from the Obstetrics Department of São Paulo Federal University Medical School (UNIFESP-EPM), between 2008 and 2010. The study group consisted of 129 women with unexplained RPL, and without any previous live births, being managed at the university’s infertility clinic. All these participants had a history of at least 3 spontaneous miscarriages (range: 3-9, 70% of participants had up to 4 miscarriages) and normal results on the following examinations that are routinely performed to exclude known causes of spontaneous abortion: hysteroscopy; hysterosalpingography; serial ultrasound; karyotype (of the couple); investigation of luteal phase insufficiency (serum progesterone concentration measurement); prolactin concentration; glycemic curve; thyroid hormone levels; antibody screen for toxoplasmosis, cytomegalovirus, rubella, HIV, hepatitis B and C, and antiphospholipid antibodies (anticardiolipin antibody and lupus anticoagulant). The control group included 235 ethnically matched women with 2 or more successful pregnancies and no history of miscarriage, preeclampsia, ectopic pregnancy or preterm delivery. Ethnicity was self-reported. Control women were recruited at the antenatal care clinics or obstetric wards at UNIFESP-EPM. All pregnancies in both groups were conceived naturally.
Upon enrollment, each participant provided a 20-mL peripheral blood sample collected in tubes containing ethylenediamine tetraacetic acid (EDTA; BD Diagnostics, Franklin Lakes, New Jersey). Half (10 mL) was centrifuged to obtain a buffy coat layer containing polymorphonuclear cells and DNA was extracted by the Dodecyltrimethylammonium bromide/Hexadecyltrimethylammonium bromide (DTAB/CTAB) method.18 The other half was used for RNA extraction.
Polymorphism Genotyping
Primer sequences and polymerase chain reaction (PCR), cycling conditions have been previously described as well as genotyping methods for FAS (rs1800682)19 and FAS-L (rs763110)16 gene polymorphisms. The two allelic variants were genotyped using the restriction fragment length polymorphism (RFLP) method. Briefly, PCR products were digested with BstNI and BsrDI restriction endonucleases (New England Biolabs Inc, Beverly, Massachusetts).
The FAS gene polymorphism genotyping resulted in fragment sizes of 233 bp (A) or 189 bp (G) and the FAS-L gene polymorphism genotyping resulted in fragment sizes of 401 bp (T) or 232 and 168 bp (C).16,19
All PCR products were visualized using electrophoresis on an agarose gel stained with bromide ethidium.
Determination of FAS and FAS-L mRNA Levels by Real-Time PCR
PBMC were isolated by centrifugation on Ficoll-Isopaque (GE Bio-Science, Uppsala, Sweden) at 1800 rpm for 30 minutes at 20°C to –23°C, and the cells were washed in phosphate-buffered saline (PBS; Sigma-Aldrich, St Louis, Missouri). Total RNA was extracted using the TRIzol reagent (Life Technologies, Grand Island, New York) from isolated PBMCs (approximately 80% lymphocytes, 20% monocytes). RNA solution was quantified using a spectrophotometer at 260 nm (NanoDrop 2000; Thermo Fisher Scientific, Wilmington, Delaware) and RNA purity was assessed by the ratio of absorbance at 260 and 280 nm. Only samples with a ratio >1.8 were used in this study. Total RNA (1 μg) was reverse transcribed into complementary DNA (cDNA) using the RevertAidTM H Minus First Strand cDNA Synthesis kit (Fermentas, Thermo Fisher Scientific), in accordance with the manufacturer's instructions.
RNA expression levels of the 2 target genes (FAS and FAS-L) and 2 housekeeping genes (glyceraldehyde 3-phosphate dehydrogenase [GAPDH] and beta-actin [ACTB]) were analyzed by real-time PCR in a Rotor Gene 6000 instrument (Corbett Research, Qiagen, Hombrechtikon, Switzerland). TaqMan Gene Expression Assays (Applied Biosystems, Foster City, California) were used for the target genes FAS (Hs00907759_m1) and FAS-L (Hs00181225_m1) and the endogenous controls GAPDH (Hs99999903_m1) and ACTB (Hs99999905_m1). PCR was performed in separate tubes for each reaction, and each sample was run in triplicate. For each gene, case and control samples were run in the same RT-PCR cycle to minimize the intracycle variation. The reaction efficiencies of each assay were calculated from cDNA serial dilutions.
Sample Size
The sample size estimates were based on the FAS (670 A/G) gene polymorphism (rs1800682) frequency, according to Hap Map Project data (http://www.hapmap.org/cgi-perl/gbrowse/hapmap27_B36/), which defined an allele risk of 42% in the European Caucasian population. Applying allele risk data in the Power and Sample Size Program Version 2.1.30,20 and considering an 80% power and a 2-tailed α of .05, a sample size of 110 patients per group was calculated to detect an association between the alleles and RPL.
Data Analysis
Hardy-Weinberg equilibrium tests were performed by calculating the expected frequencies of each genotype and comparing them with the observed values. The single allelic and single genotype frequencies (obtained by direct count) were analyzed by Fisher exact or chi-square tests, with the level of significance set at P < .05. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Chi-square and Student’s t tests were used to analyze categorical and continuous variables, respectively. Statistical analyses were performed with standard software (SPSS for the Social Science, v13.1 for Windows).
All quantitative PCR data were analyzed using the relative expression software Relative Expression Software Tool 2009 (REST), version 2.0.13.21 This software estimates gene up- and downregulation, accounting for reaction efficiency and reference gene normalization.
All procedures were conducted in accordance with the ethical standards in human experimentation and the Helsinki Declaration of 1975. The study was approved by the university’s ethics committee, and informed consent was obtained from all participants.
Results
The mean age of cases and controls was similar (30.1 ± 5.9 vs 31.4 ± 8.8 years, respectively, P = .089). Racial distribution was similar in both groups, with a predominance of Caucasian (self-reported) participants (71% cases vs 67% controls, P = .081).
All SNPs in the RPL and control groups were in Hardy-Weinberg equilibrium. The genotype frequencies for the FAS-670 polymorphism were 24% AA, 44.8% GA, and 31.2% GG in the RPL group and 25.5% AA, 49.8% GA, and 24.7% GG in the control group. For the FAS-L-844 polymorphism, the genotype frequencies were 11.6% CC, 41.1% CT, and 47.3% TT in the RPL group and 20.4% CC, 45.1% CT, and 34.5% TT in the control group.
We detected no significant association between RPL and FAS-670 gene polymorphisms, but there was a significant difference in FAS-L (-844) genotype and allelic frequencies between the RPL patients and controls (χ2 test; P = .023; Fisher exact test P = .004, respectively), as presented in Table 1.
Table 1.
FAS and FAS-L Gene Polymorphisms Allele Frequencies in Women With Recurrent Pregnancy Loss (RPL) and Controls.
| Alleles | RPL | Controls | OR (95% CI) | P value* | |
|---|---|---|---|---|---|
| FAS | n = 250 | n = 454 | |||
| −670G/A | A | 116 (46.4%) | 229 (50.4%) | 0.85 (0.62 – 1.16) | 0.307 |
| G | 134 (53.6%) | 225 (49.6%) | |||
| FAS-L | n = 258 | n = 470 | |||
| −844C/T | C | 83 (32.2%) | 202 (43%) | 0.63 (0.45 – 0.86) | 0.004* |
| T | 175 (67.8%) | 268 (57%) |
Abbreviations: OR, odds ratio; CI, confidence interval.
*Fisher exact test.
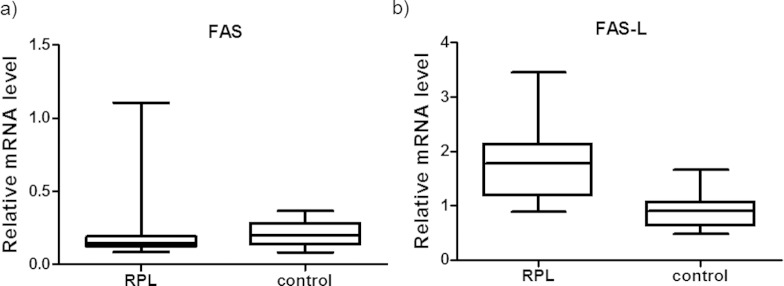
The RT-PCR results were analyzed with REST 2009 software and presented in Tables 2 and 3. As shown in Figure 1A, patients with RPL and controls presented similar FAS gene mRNA expression (P(H1) = 0.694; hypothesis test).
Table 2.
Gene Expression Pattern in Cases of Recurrent Pregnancy Loss.
| Gene | Expression | CI (95%) | P(H1) | Result |
|---|---|---|---|---|
| FAS | 0.904 | 0.305-7.121 | 0.694 | ND |
| FAS-L | 2.000 | 0.729-5.346 | 0.000 | UP |
Abbreviations: CI, confidence interval; P(H1), probability of the alternate hypothesis that the difference between the sample and control groups is due only to chance; ND, sample group is not different than the control group; UP, upregulated.
Table 3.
Gene Expression Pattern in the FAS −670 and FAS-L −844 Genotypes.
| Gene | Expression | CI (95%) | P(H1) | Result |
|---|---|---|---|---|
| FAS-670, GG × GA + AA | 0.601 | 0.124-2.157 | 0.027 | DOWN |
| FAS-L-844 CC × CT + TT | 0.914 | 0.500-1.693 | 0.764 | ND |
Abbreviations: CI, confidence interval; P(H1), probability of the alternate hypothesis that the difference between the sample and control groups is due only to chance; ND, sample group is not different than the control group; DOWN, downregulated.
Figure 1.
Normalized mRNA expression values for the FAS and FAS-L genes in the RPL and control groups. mRNA, messenger RNA; RPL, recurrent pregnant loss.
FAS-L mRNA was upregulated in patients with RPL compared to control women (P(H1) = 0.000; hypothesis test; Figure 1B).
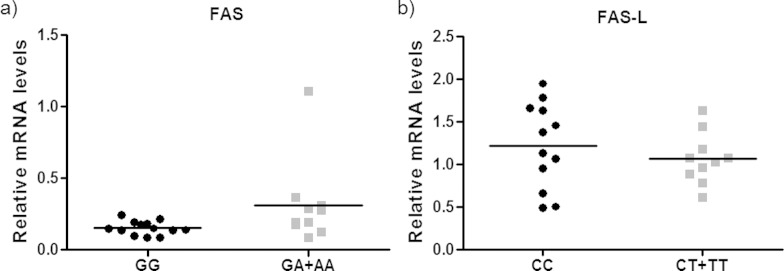
There was a significant association between FAS-670 GG genotype polymorphism and lower FAS mRNA levels (P(H1) = 0.027; hypothesis test; Figure 2A).
Figure 2.
Normalized mRNA expression values for the FAS and FAS-L genes and the FAS-670 and FAS-L-844 genotypes, respectively. mRNA, messenger RNA.
There was no association between FAS-L-844 polymorphism and FAS-L mRNA levels (P(H1) = 0.764; hypothesis test; Figure 2B).
Discussion
In accordance with our hypothesis, our results indicate that FAS-L (-844) gene polymorphism was associated with RPL. We found higher FAS-L mRNA expression in this group when compared to women with successful pregnancy. However, there was no association between FAS (-670) gene polymorphism or abnormal expression of this molecule in women with RPL. We only found an association between the GG genotype and lower FAS mRNA levels.
Abnormal trophoblast apoptosis has been associated with adverse pregnancy outcomes, including spontaneous abortion. Although previous studies have reported altered expression of FAS and FAS-L molecules and genes in tissues of patients with RPL,3,8,12 to the best of our knowledge, this is the first study that assessed FAS and FAS-L gene polymorphisms and PBMC mRNA levels in patients with RPL.
Our findings contribute to the body of conflicting evidence regarding the role of FAS-L in pregnancy. While some studies indicate that reduced FAS-L expression may compromise the immune privileged status of the decidua and, in theory, could be involved in early pregnancy loss, others suggest that increased levels of FAS-L may affect trophoblast invasion, leading to obstetric disorders.4,22–24 Increased FAS-L expression has been detected in chorionic villi from patients with RPL 8 and in decidual leukocytes of extravillous trophoblasts associated with spontaneous abortion.12 At present, the role of FAS-L-844 CC genotype and subsequent FAS-L expression in RPL is not well understood. Part of the existing contradictions may be due to the possible interference of other factors such as the type of tissue, cytokine microenvironment, and gestational age3,4 and also to heterogeneity in study protocol and technical methods.
Although there was an association between RPL with FAS-L-844 gene polymorphism and also abnormal mRNA FAS-L levels in the peripheral blood of these patients, we did not detect a correlation between genotype and phenotype. Up to the moment, few studies have analyzed the effect of FAS-L-844 polymorphism on FAS-L production. Wu et al14 reported a relationship between this SNP and FAS-L expression on peripheral blood fibrocytes evaluated by flow cytometry. A study by Sun et al13 suggested that the FAS-L-844T→C polymorphism affects the expression of FAS-L ex vivo on stimulated T cells and that the FAS-L-844C allele seems to be associated with an enhanced rate of activation-induced cell death (AICD) of T cells. On the other hand, we analyzed FAS-L expression at the basal state, without stimulation in peripheral blood leukocytes. Besides differences in the protocols used for the analysis of gene expression, a combination of SNPs may affect the production of the molecule,25 environmental agents can modify gene expression,26 and different intracellular mechanisms may interfere with maintenance or degradation of mRNAs and proteins.27 Indeed, several factors influence the production of a particular protein, and this influence can hinder the detection of a genotype/phenotype correlation.
We did not detect a relationship between the FAS-670 gene polymorphism or FAS mRNA expression and RPL. This genetic variant has been associated with other pregnancy disorders, such as preeclampsia, fetal growth restriction, and preterm labor,2,14,28 but there is only one study that evaluated its relationship with RPL. Nair et al29 evaluated FAS-670 and -1377 gene polymorphisms but did not analyze FAS expression in patients with RPL. In agreement with our findings, these investigators did not find an association between FAS-670 genetic variants and RPL; however, they reported that FAS-1377 gene polymorphism was associated with an increased risk of this condition.
Others have previously suggested that this polymorphism can affect FAS levels30 and that FAS genetic polymorphisms are associated with the decreased expression of FAS in activated T lymphocytes.13 Accordingly, we observed an association between the GG genotype of the FAS-670 polymorphism and lower FAS mRNA levels; however, we did not detect a relationship between RPL and FAS mRNA levels or this genotype.
Our results indicate that patients with RPL have higher mRNA FAS-L levels in peripheral blood leukocytes than controls. Based on our findings, we can hypothesize that patients with RPL may have altered trophoblast invasion due to increased FAS-L expression. Because both FAS and FAS-L are important for maintaining maternal–fetal immune tolerance, impaired expression of one these molecules may be sufficient to affect trophoblast apoptosis and compromise the implantation process or interfere with pregnancy development.3,11
In conclusion, this study suggests that the FAS-L gene polymorphism is associated with RPL in Brazilian women and that these women also have impaired FAS-FAS-L systems due to abnormal FAS-L expression. This finding is biologically plausible, but further studies are needed to confirm these results. In addition, the correlation between genotype and expression requires further investigation. Future research should not only investigate other SNPs but also test other protocols to evaluate expression.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was suported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP # 09/50236-5) and Conselho Nacional de Desenvolvimento Científico e Tecnoógico (CNPq # 301886/2009-1).
References
- 1. Ford HB, Schust DJ. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol. 2009;2(2):76–83. [PMC free article] [PubMed] [Google Scholar]
- 2. Kiwi R. Recurrent pregnancy loss: evaluation and discussion of the causes and their management. Cleve Clin J Med. 2006;73(10):913–921. [DOI] [PubMed] [Google Scholar]
- 3. Aschkenazi S, Straszewski S, Verwer KM, et al. Differential regulation and function of the Fas/Fas ligand system in human trophoblast cells. Biol Reprod. 2002;66(6):1853–1861. [DOI] [PubMed] [Google Scholar]
- 4. Abrahams VM, Straszewski-Chavez SL, Guller S, Mor G. First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol Hum Reprod. 2004;10(1):55–63. [DOI] [PubMed] [Google Scholar]
- 5. Crocker IP, Cooper S, Ong SC, Baker PN. Differences in apoptotic susceptibility of cytotrophoblasts and syncytiotrophoblasts in normal pregnancy to those complicated with preeclampsia and intrauterine growth restriction. Am J Pathol. 2003;162(2):637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoshimoto K, Hayashi M, Ohkura T. Plasma levels of soluble Fas during normal pregnancy. Gynecol Obstet Invest. 2001;51(2):96–98. [DOI] [PubMed] [Google Scholar]
- 7. Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006;20(1):1–15. [DOI] [PubMed] [Google Scholar]
- 8. Choi HK, Choi BC, Lee SH, Kim JW, Cha KY, Baek KH. Expression of angiogenesis- and apoptosis-related genes in chorionic villi derived from recurrent pregnancy loss patients. Mol Reprod Dev. 2003;66(1):24–31. [DOI] [PubMed] [Google Scholar]
- 9. Levy R, Nelson DM. To be, or not to be, that is the question. Apoptosis in human trophoblast. Placenta. 2000;21(1):1–13. [DOI] [PubMed] [Google Scholar]
- 10. Patel T, Gores GJ. Apoptosis in liver transplantation: a mechanism contributing to immune modulation, preservation injury, neoplasia, and viral disease. Liver Transpl Surg. 1998;4(1):42–50. [DOI] [PubMed] [Google Scholar]
- 11. Neale DM, Mor G. The role of Fas mediated apoptosis in preeclampsia. J Perinat Med. 2005;33(6):471–477. [DOI] [PubMed] [Google Scholar]
- 12. Minas V, Jeschke U, Kalantaridou SN, et al. Abortion is associated with increased expression of FasL in decidual leukocytes and apoptosis of extravillous trophoblasts: a role for CRH and urocortin. Mol Hum Reprod. 2007;13(9):663–673. [DOI] [PubMed] [Google Scholar]
- 13. Sun T, Zhou Y, Li H, et al. FASL -844C polymorphism is associated with increased activation-induced T cell death and risk of cervical cancer. J Exp Med. 2005;202(7):967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu J, Metz C, Xu X, et al. A novel polymorphic CAAT/enhancer-binding protein beta element in the FasL gene promoter alters Fas ligand expression: a candidate background gene in African American systemic lupus erythematosus patients. J Immunol. 2003;170(1):132–138. [DOI] [PubMed] [Google Scholar]
- 15. Liakouli V, Manetti M, Pacini A, et al. The -670G>A polymorphism in the FAS gene promoter region influences the susceptibility to systemic sclerosis. Ann Rheum Dis. 2009;68(4):584–590. [DOI] [PubMed] [Google Scholar]
- 16. Kang S, Dong SM, Seo SS, Kim JW, Park SY. FAS -1377 G/A polymorphism and the risk of lymph node metastasis in cervical cancer. Cancer Genet Cytogenet. 2008;180(1):1–5. [DOI] [PubMed] [Google Scholar]
- 17. Li C, Larson D, Zhang Z, et al. Polymorphisms of the FAS and FAS ligand genes associated with risk of cutaneous malignant melanoma. Pharmacogenet Genomics. 2006;16(4):253–263. [DOI] [PubMed] [Google Scholar]
- 18. Gustincich S, Manfioletti G, Del Sal G, Schneider C, Carninci P. A fast method for high-quality genomic DNA extraction from whole human blood. Biotechniques. 1991;11(3):298–300, 302. [PubMed] [Google Scholar]
- 19. Fuks A, Parton LA, Polavarapu S, et al. Polymorphism of Fas and Fas ligand in preterm premature rupture of membranes in singleton pregnancies. Am J Obstet Gynecol. 2005;193(3 pt 2):1132–1136. [DOI] [PubMed] [Google Scholar]
- 20. Dupont WD, Plummer WD., Jr Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11(2):116–128. [DOI] [PubMed] [Google Scholar]
- 21. Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30(9):e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstet Gynecol 2000;96(2):271–276. [DOI] [PubMed] [Google Scholar]
- 23. Hammer A, Blaschitz A, Daxbock C, Walcher W, Dohr G. Fas and Fas-ligand are expressed in the uteroplacental unit of first-trimester pregnancy. Am J Reprod Immunol. 1999;41(1):41–51. [DOI] [PubMed] [Google Scholar]
- 24. Eide IP, Isaksen CV, Salvesen KA, et al. Fetal growth restriction is associated with reduced FasL expression by decidual cells. J Reprod Immunol 2007;74(1-2):7–14. [DOI] [PubMed] [Google Scholar]
- 25. Turner AK, Begon M, Jackson JA, Bradley JE, Paterson S. Genetic diversity in cytokines associated with immune variation and resistance to multiple pathogens in a natural rodent population. PLoS Genet. 2011;7(10):e1002343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Novakovic B, Saffery R. The ever growing complexity of placental epigenetics - Role in adverse pregnancy outcomes and fetal programming. Placenta. 2012;33(12):959–970. [DOI] [PubMed] [Google Scholar]
- 27. Hebert DN, Molinari M. In and out of the ER: protein folding, quality control, degradation, and related human diseases. Physiol Rev. 2007;87(4):1377–1408. [DOI] [PubMed] [Google Scholar]
- 28. Ciarmela P, Boschi S, Bloise E, et al. Polymorphisms of FAS and FAS ligand genes in preeclamptic women. Eur J Obstet Gynecol Reprod Biol. 2010;148(2):144–146. [DOI] [PubMed] [Google Scholar]
- 29. Nair RR, Khanna A, Singh K. Association of FAS -1377 G>A and FAS -670 A>G functional polymorphisms of FAS gene of cell death pathway with recurrent early pregnancy loss risk. J Reprod Immunol. 2012;93(2):114–118. [DOI] [PubMed] [Google Scholar]
- 30. Huang QR, Morris D, Manolios N. Identification and characterization of polymorphisms in the promoter region of the human Apo-1/Fas (CD95) gene. Mol Immunol. 1997;34(8-9):577–582. [DOI] [PubMed] [Google Scholar]