Abstract
Glycosylation abnormalities have been observed in autoimmune diseases and cancer. Here, we compare mechanisms of aberrant O-glycosylation, i.e., formation of Tn and sialyl-Tn structures, on MUC1 in breast cancer, and on IgA1 in an autoimmune disease, IgA nephropathy. The pathways of aberrant O-glycosylation, although different for MUC1 and IgA1, include dysregulation in glycosyltransferase expression, stability, and/or intracellular localization. Moreover, these aberrant glycoproteins are recognized by antibodies, although with different consequences. In breast cancer, elevated levels of antibodies recognizing aberrant MUC1 are associated with better outcome, whereas in IgA nephropathy, the antibodies recognizing aberrant IgA1 are part of the pathogenetic process.
Keywords: IgA nephropathy, O-glycosylation, IgA, MUC1, Anti-glycan antibodies
Introduction
Alterations in glycan moieties of cell-surface or secreted glycoproteins are now recognized as a factor that can cause, or contribute to, defects in the development and function of cells and organs, leading in some instances to autoimmune diseases as well as cancers (for review see [1–6]).
Autoimmune disease immunoglobulin A (IgA) nephropathy (IgAN) is associated with alterations in mucin-type O-glycans [7–9]. In patients with IgAN, a fraction of IgA1 molecules has some O-glycans deficient in galactose (Gal) leading to exposition of N-acetylgalactosamine (GalNAc) residues attached to serine or threonine (Tn antigen, also called CD175). These Tn-antigen-containing IgA1 molecules are recognized by IgG and/or IgA1 antibodies, resulting in the formation of nephritogenic immune complexes [7, 9, 10].
Some cancer cells, on the other hand, produce similar immature truncated aberrant O-glycans on various cellular glycoproteins. For example, adenocarcinoma cells of breast and other tissues over-express the cell-membrane mucin MUC1 with truncated non-branched O-glycans consisting of Tn and sialyl-Tn (STn, CD175s) antigens as well as of Core 1 glycans such as T (GalNAc–Gal disaccharide, also called CD176) and sialyl-T antigens (ST, CD176s), whereas branched O-glycans of Core 2 and, to a lesser degree Core 3, are typical for the glycoproteins in the normal counterpart cells [1, 11, 12]. The aberrant O-glycosylation appears to play functional roles for the malignant phenotype of cancer cells. Truncation of O-glycans to STn on the surface glycoproteins of cancer cell lines changes growth morphology and adhesiveness important for metastatic behavior [13, 14]. These aberrant glycoproteins are recognized by antibodies recognizing Tn, STn and T O-glycopeptide-associated neoepitopes or the core peptides [15–17]. Such antibodies, primarily those specific for aberrantly glycosylated glycoproteins, seem to be protective in cancer [18], in contrast to those recognizing IgA1 O-glycans in IgAN.
The O-glycosylation pathways leading to expression of the immature truncated Tn, STn, and T O-glycans on glycoproteins are diverse and not fully clarified. In this review, we will discuss molecular mechanisms leading to the glycosylation changes in O-glycans on IgA1 in patients with IgAN and on MUC1 in patients with breast cancer. We will also discuss characteristics of antibodies in both types of diseases that target these neo-glycoproteins through their truncated O-glycans and O-glycopeptide neoepitopes (i.e., neoepitopes involving glycan(s) or glycan(s) in the context of the amino-acid sequence) and their potential for use in diagnostic and prognostic applications.
Molecular structure of human IgA1
Monomeric human IgA1 has two heavy chains and two light chains connected by disulphidic bridges. The heavy chains are composed of three constant (Cα) domains and one variable (V) domain, and light chains of one C domain and one V domain. A hinge region of IgA1, located between Cα1 and Cα2, has a unique amino acid sequence containing two octapeptide repeats (Fig. 1) with nine potential O-glycosylation sites (POGSs) of which three to six are glycosylated (PVPST 225PPT 228PS 230PS 232 T 233PPT 236PSPSC; POGSs are in bold and the six sites utilized on circulatory IgA1 are numbered; Fig. 1) [19, 20]. There are also two N-glycosylation sites per heavy chain. Monomers of IgA1 can be covalently associated during the production in plasma cells with a 17-kDa J-chain and thus be present as dimers and higher oligomers, called collectively polymeric IgA1. In serum, most of IgA1 (80–99 %) is monomeric [21].
Fig. 1.
Molecular structures of human IgA1 and MUC1. IgA1 monomer (top) is composed of two light chains (L) and two heavy chains (H), with O-glycans (orange oval symbols) attached in the hinge region between the first (Cα1) and second (Cα2) constant domains of the heavy chain. Two N-glycans (blue oval symbols) are attached to each heavy chain. The hinge-region sequence is composed of two octapeptide repeats with up to six O-glycans attached (numbered S/T residues in the hinge-region amino-acid sequence) [19, 20]. Constant and variable domains of light chains (CL, VL) and heavy chains (Cα1-3, VH) are in yellow and green color, respectively. Potential O-glycosylation sites (POGS) are in red and the six residues that had been shown to be glycosylated on circulatory IgA1 are numbered [19, 20, 22]. MUC1 (bottom) is a transmembrane glycoprotein composed of two noncovalently associated subunits. The N-terminal extracellular subunit contains variable number of tandem repeats (VNTR) of 20 amino acids that may carry up to five O-glycans (orange oval symbols) [24]. N-glycans are shown by blue oval symbols. The extracellular subunit is in pink color and contains N-terminal flanking sequence (NFS), VNTR, and C-terminal flanking sequence (CFS). The transmembrane subunit is in blue color and contains extracellular segment (EC), transmembrane domain (TM), and cytoplasmic domain (CD). POGS in VNTR amino-acid sequence are in red color. The numbers are used for orientation in reference to a single VNTR
The carbohydrate composition of the O-linked glycans on normal serum IgA1 is variable but the prevailing form is the GalNAc with a β1,3-linked Gal (Core 1), and its mono- and di-sialylated forms [22].
Aberrant O-glycosylation of IgA1 in patients with IgAN
IgAN, the most common primary glomerulonephritis, is characterized by IgA1 immune deposits in glomerular mesangium usually with complement C3, and frequently with co-deposits of IgG and/or IgM (for review see [9]). Serum of patients with IgAN exhibits elevated levels of IgA1 molecules that have some O-glycans aberrantly glycosylated, i.e., deficient in Gal [7]. Furthermore, IgA1 in the mesangial deposits is enriched for this aberrantly glycosylated IgA1 form (for review see [9]). IgA1 with Gal-deficient hinge-region glycans are recognized by IgG and/or IgA1 antibodies [7, 10], resulting in the formation of circulating immune complexes. These complexes are relatively large, are not efficiently cleared from the circulation, and tend to deposit in the renal mesangium where they bind to the resident mesangial cells and activate them. This activation results in cellular proliferation, production of cytokines, and overproduction of extracellular matrix proteins leading to glomerular fibrosis and, ultimately, to renal failure [9].
Molecular structure of human MUC1
MUC1 is a large type I transmembrane mucin glycoprotein that consists of two non-covalently-linked subunits released from a precursor polypeptide by proteolytic cleavage (Fig. 1). The large N-terminal extracellular subunit contains a domain with variable number of tandem repeats (VNTRs) of 20 highly conserved amino acids with dense O-glycosylation (HGVT 4 S 5APDT 9RPAPGS 15 T 16APPA; POGSs are in bold; Fig. 1); MUC1 is polymorphic with variable number of VNTRs from 25 to 125. Notably, carriers of alleles with a low number of VNTRs may exhibit increased susceptibility to cancer. The N-terminal subunit associates with the C-terminal transmembrane subunit consisting of short extracellular domain, membrane-spanning domain, and C-terminal cytoplasmic tail [23]. MUC1 is one of the most extensively studied cancer-associated mucins and serves as a model of aberrant O-glycosylation and cancer-associated O-glycoprotein immunogenicity.
The MUC1 VNTRs contain five POGSs, all of which can be O-glycosylated [24], although MUC1 isolated from normal human milk contains only 2-3 O-glycans per VNTR. MUC1 also contains potential N-glycosylation sites, one of which is located in the membrane-associated subunit and four near the C-terminal end of the extracellular subunit. MUC1 expressed by normal mammary gland epithelial cells is generally glycosylated with larger branched Core 2 O-glycans extended by polylactosamine and, to a lesser extent, capped Core 1 O-glycans, whereas the truncated Tn, STn, and T structures are absent [12].
Aberrant glycosylation of MUC1 in breast cancer
MUC1 is characteristically overexpressed in breast adenocarcinomas as well as adenocarcinomas of other organs, including ovary, lung, pancreas, and prostate. The O-glycosylation of MUC1 VNTRs produced by cancer cells is altered in density and core structures and elongation/capping of the O-glycans. In breast cancer, MUC1 is greatly overexpressed and has lost in polarized expression on the apical surface of the cell. The aberrant O-glycosylation of MUC1 includes an increase in the density of O-glycans (average POGS occupancy 4.2–4.8 per one VNTR in breast cancer T47D cell line compared to 2.6 in normal human milk) and a switch from branched Core 2 to truncated Core 1 O-glycans [23–26]. MUC1 is shed from cancer cells and patients with late-stage breast cancer have elevated levels of MUC1 in the circulation [17, 27]. Circulating MUC1 in these patients is glycosylated mainly with the Core 1 ST structures [28], which suggests that truncated glycoforms with T, Tn, and STn structures shed from cancer cells are cleared from the circulation by lectin receptors and antibodies [15, 29, 30]. In this respect, it has been found that dendritic cells selectively take up Tn-containing MUC1 glycoforms via their macrophage Gal-recognizing C-type lectin (MGL) receptor that exhibits significant affinity for αGalNAc. It is likely that this process is involved in the induction of Tn-MUC1 glycopeptide autoantibodies found in some cancer patients [15, 29].
The biosynthetic pathways for O-glycans on IgA1 and MUC1
Mucin-type O-glycosylation is a highly complex process potentially involving more than 50 glycosyltransferases, enzymes that add one monosaccharide to a growing O-glycan. O-glycosylation is initiated by attachment of GalNAc to Ser/Thr residues by a large family of at least 20 UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases (GalNAc-Ts) with different but partly overlapping functions (for review see [31, 32]). The repertoire of GalNAc-Ts in a particular cell determines sites and patterns of O-glycan attachments to proteins, but the specific roles of individual GalNAc-T isoforms and how these enzymes cooperate is not fully understood. Initiation of O-glycosylation generally occurs in Golgi apparatus [33]. However, a recent report revealed that activation of Src proto-oncogene leads to selective relocation of some GalNAc-Ts to the ER, resulting in the early initiation of O-glycosylation characterized by Tn structures on target proteins and, consequently, leading to presence of more dense O-glycans on glycoproteins such as MUC1 [34]. Although it is tempting to speculate that such a mechanism may play a role in the generation of malignant glycophenotype in cancer, further studies are needed to address this issue and elucidate the mechanisms directing relocation of GalNAc-Ts, but not other glycosyltransferases.
Initiation of IgA1 O-glycosylation
Initiation of O-glycosylation of the hinge region of IgA1 has been originally attributed to the ubiquitously expressed GalNAc-T2 [35]. However, the IgA1 hinge region resembles mucin-type sequence that can be an excellent acceptor for many different GalNAc-Ts. Indeed, a more recent report has shown that other GalNAc-Ts, including GalNAc-T1 and GalNAc-T11 can also initiate O-glycosylation of IgA1 hinge-region peptide (Fig. 2a) [36]. In fact, so far, all GalNAc-Ts analyzed for the peptide specificity can O-glycosylate one or more of the six POGS in hinge-region peptide (Clausen, pers. comm.). Supporting this hypothesis, our recent data indicated that not only GalNAc-T2 [8] but also other GalNAc-Ts are abundantly expressed in IgA1-producing cells derived from peripheral blood of IgAN patients and healthy controls [37]. Specifically, GalNAc-T14 was among the major GalNAc-Ts transcribed in IgA1-producing cells and its expression was fivefold greater in the cells from the patients versus the cells from healthy controls. The expression of GalNAc-T2, and other GalNAc-Ts, did not differ between patients and healthy controls. It remains to be determined whether the over-expression of GalNAc-T14 may contribute to the aberrant glycosylation of IgA1 in IgAN. Synthesis of Tn structures on Gal-deficient IgA1 produced by the cells from patients with IgAN appears to occur in the Golgi apparatus, but not in the ER, suggesting that the above-mentioned ER-based initiation of O-glycosylation may be specific for epithelial cells producing membrane-associated MUC1 and other glycoproteins [8].
Fig. 2.
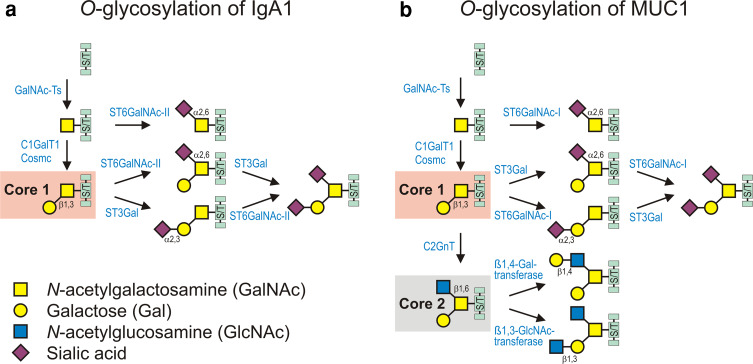
Biosynthetic pathways of O-linked glycans on human circulatory IgA1 (a) and MUC1 on mammary epithelial cells (b). O-glycosylation is initiated by a family of enzymes known as the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases (GalNAc-Ts); these enzymes catalyze the transfer of GalNAc from UDP-GalNAc to the hydroxyl group on Ser or Thr residues (S/T). a Biosynthesis of O-linked glycans of circulatory IgA1 can be initiated by GalNAc-T1, 2, 11, and possibly by other GalNAc-Ts and followed by addition of Gal from UDP-Gal catalyzed by the Core 1 β1,3-galactosyltransferase 1 (C1GalT1). Synthesis of the stable enzyme C1GalT1 depends on Core 1 β1,3-galactosyltransferase-specific chaperone (Cosmc). The Core 1 disaccharide can be elongated with sialic acid residue(s) transferred from CMP-N-acetylneuraminic acid (CMP-NeuAc) by sialyltransferases to either Gal or GalNAc or both. The reactions are catalyzed by a Galβ1,3GalNAc α2,3-sialyltransferase (ST3Gal) and a α2,6-sialyltransferase (ST6GalNAc), respectively. In IgA1-producing cells, sialylation of GalNAc is catalyzed exclusively by ST6GalNAc-II [8, 64]. Patients with IgAN have elevated serum levels of aberrantly glycosylated IgA1, with some of their O-glycans Gal-deficient and, thus, consisting of GalNAc or sialylated GalNAc [8]. This aberrancy is a result of changes in the activities of two glycosyltransferases, decreased C1GalT1 and increased ST6GalNAc-II, in the IgA1-producing cells in the patients with IgAN [8]. Sialylated GalNAc represents a terminal step of the O-glycosylation pathway, as it cannot be further modified (for review see [9]). b Biosynthesis of O-linked glycans of MUC1 on mammary epithelial cells is initiated by GalNAc-T1, 2, 3, 4, 6, 7, 10, and followed by addition of Gal from UDP-Gal catalyzed by C1GalT1. The Core 1 disaccharide can be elongated with sialic acid residue(s) transferred from CMP-NeuAc by sialyltransferases to either Gal or GalNAc or both. The reactions are catalyzed by a Galβ1,3GalNAc α2,3-sialyltransferase (ST3Gal) and a α2,6-sialyltransferase (ST6GalNAc), respectively. In breast epithelial cells, sialylation of GalNAc is catalyzed by ST6GalNAc-I. The Core 1 can be branched by addition of GlcNAc, catalyzed by Core 2 β1,6-GlcNAc transferase (C2GnT), and further elongated by β1,4-Gal-transferase and β1,3-GlcNAc-transferase. In breast cancer epithelial cells, MUC1 is aberrantly expressed, and it contains less Core 2 glycans, more Core 1 glycans, and elevated level of sialylated Core 1 glycans and sialylated GalNAc. The aberrant glycosylation stems from decreased activity of C2GnT and increased activity of sialyltransferases, ST3Gal, and ST6GalNAc-I [12, 61, 68–70]. Furthermore, ST6GalNAc-I, C1GalT1, and C2GnT compete for the same acceptor, GalNAc [1, 59, 68]. Biosynthetic pathway of Core 3 O-glycans is not shown for simplicity, as these glycans are relatively uncommon on MUC1
Initiation of MUC1 O-glycosylation
The initiation of O-glycosylation of MUC1 VNTR with its five POGSs (HGVT 4 S 5APDT 9RPAPGS 15 T 16APPA) has been studied in a great detail. Many GalNAc-Ts, including GalNAc-T1, 2, 3, and 6, can O-glycosylate Thr4 in the VTSA sequence and Ser15 and Thr16 in the GSTA sequence of the MUC1 VNTR (Fig. 2b) [25, 38, 39]. GalNAc-T11 only glycosylates Thr4 and Thr16 [40]. Other GalNAc-Ts appear to have similar activities toward MUC1, but detailed analyses of GalNAc attachment sites in MUC1 need to be performed [41–43]. Only GalNAc-T4 has so far been shown to be able to glycosylate the remaining two residues in the MUC1 VNTR, Ser5 and Thr9. This reaction is rather unique in that GalNAc-T4 requires prior GalNAc attachment at any of the other sites in order to initiate glycosylation [44]. GalNAc-T4 is therefore predicated to play a major role in the higher density of O-glycan attachments to the MUC1 VNTR in cancer cells, likely due to the lectin domain that directs the glycopeptide specificity [44]. Although overexpression of GalNAc-T4 in breast cancer cell lines has been observed [45], further studies of breast tissues are needed.
The expression of the GalNAc-Ts controlling the initiation glycosylation step is differentially regulated during maturation and differentiation of cells [46] and markedly altered in cancer [47–49]. This is especially evident for the GalNAc-T3 and GalNAc-T6 isoforms, which constitute a subfamily of GalNAc-Ts with similar functions. GalNAc-T6 has been reported to be a biomarker of breast cancer [49, 50], and recently, it was discovered that GalNAc-T6 plays a direct role in O-glycosylation of MUC1 in breast cancer and expression of GalNAc-T6 in breast-cancer cell lines reduces cell adhesion and enhances growth properties [51]. Another enzyme, GalNAc-T14, is overexpressed in breast cancer [52]. This GalNAc-T isoform is highly similar to GalNAc-T2 and, could contribute to increase in the O-glycan density of MUC1 observed in breast cancer. Further studies are clearly needed to address the importance of individual GalNAc-Ts in cancer, namely using strategies that would directly link expression of individual enzymes with altered glycosylation and functions of particular glycoproteins in cells and tissues. Genome-wide association studies (GWAS) support direct functions of GalNAc-Ts in carcinogenesis [53, 54] and germline and somatic mutations in GalNAc-T12 have been found in patients with colon cancer [55].
In summary, the first step of O-glycosylation—the attachment of GalNAc to multiple Ser and Thr residues in IgA1 and MUC1 involves the coordinated function of multiple GalNAc-Ts and changes in expression of the repertoire of GalNAc-Ts and, in at least some instances, changes in GalNAc-Ts localization. These changes likely affect the density and pattern of O-glycans attached to these molecules, i.e., how many Ser/Thr residues and which ones will receive GalNAc. One of the major research challenges is to correlate differential expression of various GalNAc-Ts with detailed structural determination of sites of O-glycan attachments in O-glycoproteins. Although modern mass spectrometric methods can identify specific O-glycosylation sites in glycoproteins, there are still considerable hurdles to overcome to make screening the sites of mucin-type dense O-glycans in glycoproteins a routine protocol [20, 56]. Also, further studies are needed to decipher the specific molecular changes occurring in disease and the underlying changes in GalNAc-Ts repertoire.
Extension of Tn structures
After GalNAc had been attached, the biosynthesis of O-glycans continues. Additional monosaccharides are attached to the GalNAc residues in a stepwise manner. The processing/extension step is cell specific; for example, B cells produce glycoproteins with sialylated Core 1 O-glycans (Fig. 2), whereas normal epithelial breast cells produce glycoproteins with branched Core 2 structures.
In both B cells and mammary gland epithelial cells, the processing starts with formation of the Core 1 disaccharide structure. This reaction is catalyzed by a single UDP-Gal:GalNAc-α-Ser/Thr β1,3-galactosyltransferase (C1GalT1) [57], and this step is therefore without genetic back-up; a lack of C1GalT1 results in truncated O-glycans [58]. Interestingly, expression of active C1GalT1 in the Golgi apparatus depends on a specific chaperone (Cosmc) [59]. Cosmc is mutated in several cell lines (LSC and Jurkat) and in several diseases (for review see [58]) and Cosmc mutation is one of the mechanisms that lead to expression of the truncated Tn and STn cancer antigens. Surprisingly, the Jurkat cell line with Cosmc mutation was shown to revert to Core 1 and 2 synthesis by expression of SHIP-1 (Src homology 2-containing inositol 5′-phosphatase-1), suggesting that the requirement of Cosmc for the production of active C1GalT1 can be bypassed by another, yet unknown mechanism [60].
Subsequently, the Core 1 structures are either capped by sialylation (IgA1) or further modified toward Core 2 structures (MUC1).
Extension of Tn structures on IgA1
In normal IgA1, Core 1 structures are modified by attaching the sialic acid from CMP-N-acetylneuraminic acid (CMP-NeuAc) to the Gal residues in a reaction catalyzed by a Galβ1,3GalNAc α2,3-sialyltransferase (ST3Gal) and/or attaching the sialic acid to the GalNAc residues catalyzed by an α2,6-sialyltransferase (ST6GalNAc) (for review see [61, 62]). The biosynthetic basis for deficient O-glycosylation of IgA1 in IgAN patients has been extensively studied, and although substantial progress has been made there are still many open questions [8, 9, 63]. Studies with IgA1-producing cell lines from patients with IgAN [8, 64] showed that the cells secreted polymeric IgA1 with Gal-deficient O-glycans with exposed terminal GalNAc, i.e., Tn. Neuraminidase treatment of the secreted IgA1 markedly enhanced reactivity with GalNAc-specific lectin HAA, suggesting that some Tn O-glycans are likely capped with sialic acids, i.e., STn antigens. Real-time RT-PCR showed that these aberrancies were associated with altered expression of specific glycosyltransferases: elevated expression of ST6GalNAc-II and decreased expression of C1GalT1 and the Cosmc [8]. These results were consistent with the measured enzyme activities in cell extracts. Observed decrease in expression of Cosmc would potentially further reduce the amount of intact C1GalT1 enzyme due to the C1GalT1 degradation in the absence of the protein chaperone [59]. Another factor that may affect O-glycosylation of IgA1 is relative localization of the specific glycosyltransferases within the Golgi apparatus and/or their turnover, although no data are available on this aspect.
Surprisingly, ST6GalNAc-I, the enzyme responsible for sialylation of Tn antigen in man, is not expressed in the IgA1-producing cells from IgAN patients [8, 64], and the candidate enzyme for biosynthesis of STn in IgAN is therefore ST6GalNAc-II. Overexpression of ST6GalNAc-II in epithelial cells does not result in production of STn O-glycans [65], but preliminary siRNA studies in IgA1-producing cells suggest that ST6GalNAc-II may participate in synthesis of STn O-glycans on IgA1 [66]. Analysis of IgA1 O-glycans by mass spectrometry did not provide a clear answer to the presence of STn [19, 20, 56], but these studies did not address IgA1 from patients with IgAN. Premature sialylation of GalNAc blocks further modifications and the observation that Gal-deficient sialylated GalNAc-containing IgA1 is present throughout the entire Golgi [8] would lend support to the notion of possible abnormal localization of the sialyltransferases. However, studies of subcellular localization of individual enzymes will be needed to support such hypotheses. Moreover, future studies of ST6GalNAc-II and C1GalT1 enzymes and their substrate specificities and kinetics for various hinge-region glycoforms may uncover additional mechanisms potentially involved in the generation of Tn and STn antigens on IgA1 in IgAN.
Extension of Tn structures on MUC1
In normal breast glandular epithelial cells, the Core 1 glycans on MUC1 are extended to generate the branched Core 2 structure (Fig. 2b) by addition of GlcNAc catalyzed mainly by Core 2 β1,6-GlcNAc-transferase1 (C2GnT1) which is down-regulated in 50 % of breast cancers [67]. The Core 2 glycans are further elongated by adding Gal or GlcNAc in reactions catalyzed by β1,4-Gal-transferase and β1,3-GlcNAc-transferase, respectively, forming Core 2 polylactosamine protrusions [1].
In breast adenocarcinoma cells, the characteristic shift from Core 2 to Core 1 on MUC1 has been associated with reduction in expression of the Core 2 enzyme, C2GnT1, and increased expression of the sialyltransferase ST3Gal-I [67]. C2GnT1 and ST3Gal-I competes for the same Core 1 substrate and premature sialylation of Core 1 blocks Core 2 formation [12, 68, 69], so increased ST3Gal-I expression and perhaps alteration in its topology blocks the Core 2 glycosylation pathway. Data on expression of T, Tn, and STn in breast cancer varies greatly among different studies, presumably because of differences in specificity and sensitivity of different lectin and monoclonal-antibody reagents used for detection. STn is generally believed to be present in 30 % of breast cancers, and it appears to be due to overexpression of ST6GalNAc-I [70], which utilizes Tn on MUC1 VNTR as substrate and produces the STn structures in vitro [1, 65]. Overexpression of ST6GalNAc-I may override the function of other glycosyltransferases, including C1GalT1, but mutations in Cosmc and, thus, lack of C1GalT1 enzyme could also result in truncation of O-glycans and expression of the Tn and STn structures [71], but further studies are needed to evaluate whether this mechanism is more widely involved in breast cancer. In summary, in breast cancer, aberrant glycosylation of MUC1 with increased proportion of STn and ST antigens is consequence of aberrant expression and localization of GalNAc-Ts, increased expression of both α2,3- and α2,6-specific sialyltransferases, and reduced expression of C2GnT. The action of sialyltransferases is further enhanced by the recycling, up ten cycles, of the overexpressed MUC1 between the cell membrane and trans-Golgi network in breast-cancer cells, thus further increasing the extent of MUC1 sialylation [72].
Antibodies targeting aberrantly glycosylated IgA1 and MUC1
Tumor-associated oligosaccharides are recognized predominantly by naturally occurring IgM antibodies
Immune response to normal and modified autoantigens, including those appearing during cancer development (aberrantly glycosylated MUC1) or those generated in association with autoimmune conditions (aberrantly O-glycosylated glycoproteins in IgA nephropathy or in Tn syndrome), is covered in human and other animals by natural polyreactive antibodies, which bind a broad spectrum of different antigens [10, 73, 74]. These polyreactive antibodies are mostly of IgM isotype, followed by IgG and IgA isotypes (for review see [75]). Natural antibodies contain predominantly germ-line-encoded variable domains of H and L chains (VH and VL) recognizing carbohydrate-containing epitopes on various surface molecules; these antibodies play a role in the elimination of bacteria, viruses, and other pathogenic agents, regulation of B and T cells activities, cytokine production, and inflammation, presentation of antigens to T cells, development of autoimmunity, removal of apoptotic cells, including tumor and senescent cells, as well as many other functions [75–79]. Brandlein et al. analyzed more than 18,000 B cell hybridomas from over 60 cancer patients. Tumor-specific monoclonal antibodies detected in this cohort were exclusively of IgM isotype without signs of affinity-maturation. All of the IgM antibodies were germ-line coded and belonged to distinct VH and VL gene families. In addition, most of them bound to carbohydrates on cell surfaces and induced cellular apoptosis in vitro [78]. In human cancer, the appearance of T epitope on cancer cells is accompanied by an increase in the titer of T-specific IgM antibodies [80]. However, during the progression of cancer, these T-specific IgM antibodies tend to decrease in most patients [81]. These changes are not mirrored by changes in the levels of other natural antibodies, indicating specificity of such downregulation [81]. In conclusion, natural antibodies are responding to cancer carbohydrate antigens preferentially in the incipient stages, but their increase is modest and not sufficient to prevent further cancer progression, probably because of tumor-associated immune suppression. In contrast, the level of natural antibodies against ABO blood group system could rapidly increase in response to presence of xenoantigen (α-Gal epitope expressed on xenotransplantates from pigs). Nevertheless, such B cells are probably selected by very unique mechanisms, although sharing some steps with other antibody-producing B cells, as their levels are affected by the presence of autoantigens [82, 83]. Although B cells producing these natural antibodies respond to tumor antigens and immunization by antibody production, they have only a limited isotype switching and do not produce high-affinity antibodies [84].
Antibodies against aberrantly glycosylated IgA1 are of IgG and IgA1 isotypes
Whereas antibodies against aberrantly glycosylated MUC1 in patients with adenocarcinoma have potentially protective function [18], the antibodies against Gal-deficient IgA1 play a key role in the pathogenesis of IgAN. In IgAN patients, the aberrantly glycosylated IgA1 is recognized by IgG and IgA1 antibodies. This interaction is glycan-specific and can be blocked by GalNAc-binding reagents, such as lectins [10], suggesting a direct participation of Tn structure in the antibody binding. Conversely, IgA1 molecules with GalNAc modified by Gal or sialic acid are not recognized by these antibodies. Sequence analysis of the cloned heavy-chain antigen-binding domains (VH) of IgG antibodies reactive with Gal-deficient IgA1 identified unique features in complementarity-determining region 3 (CDR3). Specifically, the motif YCSR/K with S in the third position plays a prominent role in binding to Gal-deficient IgA1 [10], and, thus, promotes formation of pathogenic immune complexes. The antibody binding to the aberrantly glycosylated IgA1 may be based on a unique genetic background (i.e., variable heavy-chain genes encoding for or driving the somatic mutation to YCSR/K motif) [85]. These antibodies may be induced by an infection of upper respiratory tract and may just happen to be cross-reacting with the aberrantly glycosylated IgA1 in a genetically susceptible organisms. Levels of these antibodies may be further enhanced by recurrent upper-respiratory tract infection or, in some individuals, by mounting immune response to the cross-reactive aberrantly glycosylated IgA1. Irrespective of the mechanism(s), these processes would thus lead to increased formation of nephritogenic immune complexes (see for review [9]). At present, there are no experimental approaches for specific suppression of production of such autoantibodies and therefore interference with the formation of the pathogenic immune complexes seems to be the only possible causative therapeutic approach.
Experimental efforts to induce high-affinity antibodies recognizing antigens containing T, Tn, and STn epitopes in adenocarcinoma patients
Two types of antibodies specific for MUC1 are found in cancer patients. The first type is directed to the MUC1 VNTR peptide sequence [16, 17], whereas the second type recognizes aberrant glycopeptides in the VNTR containing truncated Tn, STn, and T O-glycans [15]. It has been speculated that the shorter glycans (T, Tn, and STn antigens) allow better access of peptide-specific antibodies to the amino-acid backbone (first type). Accordingly, several experiments attempted to elicit MUC1-specific antibody responses by immunizing cancer patients with nonglycosylated MUC1 VNTR variants. Although a few studies showed that this kind of immunization extended significantly the survival of patients with breast cancer (for review see [16, 25]), it is questionable whether this vaccine-driven elicitation represents a broadly acceptable approach as such antibodies likely react also with MUC1 on normal cells. Therefore, parallel efforts were focused on elicitation of antibodies targeting cancer-specific MUC1 glycans or glycoproteins. For example, immunization of colorectal cancer patients with partially desialylated ovine submaxillary mucin (with about equal amounts of Tn and STn) resulted in production of Tn- and STn-specific IgM and IgG antibodies [86]. Immunization of patients with various preparation of STn-KLH with or without adjuvant induced production of STn-specific IgG and IgM or only IgG [87, 88]. In general, STn- or Tn-specific immune responses elicited by natural antigens expressing STn epitopes, such as ovine submaxillary mucin, were much lower than those elicited by synthetic immunogens, suggesting a significant contribution of non-sugar epitope moieties. Synthetic vaccine based on STn-KLH (Theratope) was tested in combination with adjuvant Detox in more than 1,200 patients with breast cancer, with or without concurrent use of cyclophosphamide to inhibit CD4+CD25+-regulatory T cells [88–90]. Although IgM and IgG antibodies, including complement-dependent cytotoxic isotypes, were induced, there were no statistically significant clinical benefits [91]. Similarly, attempts to use vaccines mimicking clustered glycan patterns typical for MUC1 in cancer pattern have failed to provide a significant clinical impact [92]. One of the reasons for these disappointing results may be related to the fact that IgM antibodies were predominantly induced by these vaccines without significant IgG responses. Moreover, the induced antibodies showed minimal reactivity with MUC1 on cancer cells [93, 94]. These observations suggest that the immune responses occurred probably mostly through activation of natural antibody producing B1 cells without substantial affinity maturation and class switching and likely in T cell-independent fashion. B1 cells are naturally resistant to induction of somatic hypermutation and therefore to the development of high-affinity isotype switched glycans-specific IgG or IgA. Genetic models associated with autoimmune diseases revealed that autoantibodies can be stimulated by excessive antigenic drive from antigens associated with fragments of dead cells, as well as by enhanced responses associated with intrinsic abnormalities in B or T lymphocytes [95]. In contrast, classical B2 cells, which easily undergo somatic hypermutation and isotype switch, do not respond to glycan antigen probably because of their “self-nature” leading to tolerance. This mechanism may be related to negative selection of specifically reacting B2 cells, because the naturally weak interactions of B-cell receptor with glycans may not be strong enough to reach the necessary threshold. Therefore, alternative efforts currently tested in experimental animals are focused on the development of peptide glycoconjugates, i.e., glycopeptides, instead of clustered glycans conjugated to a protein carrier, to elicit glycan- or glycoprotein-specific high-affinity IgG antibodies. These efforts are based on the considerations that formerly used carrier proteins are highly immunogenic in themselves and could inevitably elicit strong B cell responses associated with carrier-induced carbohydrate epitope suppression [96], which in particular is a problem when “self-antigens”, such as tumor-associated glycans, are used. Several fully synthetic vaccines were tested. For example: (a) MUC-1 derived glycopeptide carrying a single STn moiety linked to a ovalbumin-derived CD4+ T cell epitope coupled together by polar non-immunogenic linker [97]; (b) nonglycosylated, Tn-, and TF-modified MUC-1 as a B cell epitope linked to universal PADRE peptide helper T-epitope [98]; (c) CD4+ peptide Th epitopes derived from Polio virus or the PADRE linked each to trimeric Tn as B cell epitope coupled on four-arm lysine core [99]; and (d) linear polypeptide consisting of MUC1-derived single Tn glycopeptide as a B cell epitope, T cell epitope derived from Polio virus and Pam2CysSK4 or Pam3CysSK4 as built-in TLR ligand as an adjuvant [100]. All these constructs induced specific IgG recognizing glycosylated MUC1 or Tn. In case of construct (b), antibodies recognized selectively the native tumor epitopes expressed by human mammary adenocarcinoma cells. These experiments thus provide a promising strategy to develop well-defined vaccines capable of eliciting tumor glycan-specific IgG antibodies that could potentially control especially minimal residual disease.
Characterization of antibodies specific for IgA1- or MUC1-associated antigens in diseases such as IgAN or breast cancer revealed their distinct heterogeneity, specificity, and clinical and pathophysiological significance. Suppression of such antibodies may be of therapeutic significance in IgAN, whereas induction of antibodies specific to cancer-associated aberrantly glycosylated MUC1 is a therapeutic approach being tested for the treatment of various MUC1-expressing cancers. These examples illustrate that the same simple glycan structure (i.e., Tn antigen) expressed in different pathophysiological conditions on different glycoproteins through different molecular mechanisms may have diverse biological and immunological responses. Recent progress in our understanding of the glycobiology and immunology of IgAN and breast cancer provides justification for a guarded optimism that effective and specific therapies for these diseases may be indeed developed and introduced in the clinical practice.
Future directions
Explore the relative roles of natural carbohydrate antibodies to Tn, STn, and T and IgG and IgA1 antibodies with glycopeptide specificity that target specific aberrantly glycosylated molecules, such as IgA1 in IgAN and MUC1 in breast cancer;
Explore the mechanism for induction and clinical significance of the glycopeptide-specific autoantibodies;
Further explore the biochemical, genetic, and epigenetic mechanisms leading to aberrant O-glycosylation in IgAN and cancer;
Develop potential diagnostic, prognostic, and therapeutic applications based on the understanding of mechanisms of aberrant O-glycosylation and functions of autoantibodies recognizing the aberrant glycoproteins in different diseases.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health DK082753, DK078244, DK083663, DK075868, DK077279, and GM098539, a grant from the IGA Nephropathy Foundation of America, and by grants and LH11046 Ministry of School, Youth, and Sport, GAP302/10/1055 Czech Science Foundation, NT11081 Grant Agency of the Ministry of the Health, Czech Republic.
Contributor Information
Milan Raška, Phone: +420-58-5632752, Email: raskamil@uab.edu.
Jan Novak, Phone: +1-205-9344480, FAX: +1-205-9343894, Email: jannovak@uab.edu.
References
- 1.Brockhausen I. Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 2006;7:599–604. doi: 10.1038/sj.embor.7400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chui D, et al. Genetic remodeling of protein glycosylation in vivo induces autoimmune disease. Proc Natl Acad Sci USA. 2001;98:1142–1147. doi: 10.1073/pnas.98.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobata A. A retrospective and prospective view of glycopathology. Glycoconj J. 1998;15:323–331. doi: 10.1023/A:1006961532182. [DOI] [PubMed] [Google Scholar]
- 4.Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291:2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 5.Ju T, Cummings RD. Protein glycosylation: chaperone mutation in Tn syndrome. Nature. 2005;437:1252. doi: 10.1038/4371252a. [DOI] [PubMed] [Google Scholar]
- 6.Tabak LA. The role of mucin-type O-glycans in eukaryotic development. Semin Cell Dev Biol. 2010;6:616–621. doi: 10.1016/j.semcdb.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomana M, et al. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73–81. doi: 10.1172/JCI5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki H, et al. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest. 2008;118:629–639. doi: 10.1172/JCI33189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki H, et al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011;22:1795–1803. doi: 10.1681/ASN.2011050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki H, et al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest. 2009;119:1668–1677. doi: 10.1172/JCI38468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh R, Bandyopadhyay D. MUC1: a target molecule for cancer therapy. Cancer Biol Ther. 2007;6:481–486. doi: 10.4161/cbt.6.4.4201. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd KO, Burchell J, Kudryashov V, Yin BW, Taylor-Papadimitriou J. Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. Demonstration of simpler and fewer glycan chains in tumor cells. J Biol Chem. 1996;271:33325–33334. doi: 10.1074/jbc.271.52.33317. [DOI] [PubMed] [Google Scholar]
- 13.Julien S, et al. Stable expression of sialyl-Tn antigen in T47-D cells induces a decrease of cell adhesion and an increase of cell migration. Breast Cancer Res Treat. 2005;90:77–84. doi: 10.1007/s10549-004-3137-3. [DOI] [PubMed] [Google Scholar]
- 14.Pinho S, et al. Biological significance of cancer-associated sialyl-Tn antigen: modulation of malignant phenotype in gastric carcinoma cells. Cancer Lett. 2007;249:157–170. doi: 10.1016/j.canlet.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Wandall HH, et al. Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes. Cancer Res. 2010;70:1306–1313. doi: 10.1158/0008-5472.CAN-09-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 17.von Mensdorff-Pouilly S, et al. Humoral immune response to polymorphic epithelial mucin (MUC-1) in patients with benign and malignant breast tumours. Eur J Cancer. 1996;32A:1325–1331. doi: 10.1016/0959-8049(96)00048-2. [DOI] [PubMed] [Google Scholar]
- 18.Blixt O, et al. Autoantibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis. Breast Cancer Res. 2011;13:R25. doi: 10.1186/bcr2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi K, et al. Naturally occurring structural isomers in serum IgA1 O-glycosylation. J Proteome Res. 2012;11:692–702. doi: 10.1021/pr200608q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi K, et al. Clustered O-glycans of IgA1: defining macro- and microheterogeneity by use of electron capture/transfer dissociation. Mol Cell Proteomics. 2010;9:2545–2557. doi: 10.1074/mcp.M110.001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novak J, Mestecky J (2009) IgA Immune-complex. In: Lai KN (ed) Recent advances in IgA nephropathy, Imperial College Press and the World Scientific Publisher, Hong Kong, p 177–191
- 22.Mattu TS, et al. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fcα receptor interactions. J Biol Chem. 1998;273:2260–2272. doi: 10.1074/jbc.273.4.2260. [DOI] [PubMed] [Google Scholar]
- 23.Sihlbom C, et al. Localization of O-glycans in MUC1 glycoproteins using electron-capture dissociation fragmentation mass spectrometry. Glycobiology. 2009;19:375–381. doi: 10.1093/glycob/cwn144. [DOI] [PubMed] [Google Scholar]
- 24.Muller S, Hanisch FG. Recombinant MUC1 probe authentically reflects cell-specific O-glycosylation profiles of endogenous breast cancer mucin. High density and prevalent core 2-based glycosylation. J Biol Chem. 2002;277:26103–26112. doi: 10.1074/jbc.M202921200. [DOI] [PubMed] [Google Scholar]
- 25.Tarp MA, Clausen H. Mucin-type O-glycosylation and its potential use in drug and vaccine development. Biochim Biophys Acta. 2008;1780:546–563. doi: 10.1016/j.bbagen.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Backstrom M, et al. Recombinant MUC1 mucin with a breast cancer-like O-glycosylation produced in large amounts in Chinese-hamster ovary cells. Biochem J. 2003;376:677–686. doi: 10.1042/BJ20031130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mall AS. Analysis of mucins: role in laboratory diagnosis. J Clin Pathol. 2008;61:1018–1024. doi: 10.1136/jcp.2008.058057. [DOI] [PubMed] [Google Scholar]
- 28.Storr SJ, et al. The O-linked glycosylation of secretory/shed MUC1 from an advanced breast cancer patient’s serum. Glycobiology. 2008;18:456–462. doi: 10.1093/glycob/cwn022. [DOI] [PubMed] [Google Scholar]
- 29.Napoletano C, et al. Tumor-associated Tn-MUC1 glycoform is internalized through the macrophage galactose-type C-type lectin and delivered to the HLA class I and II compartments in dendritic cells. Cancer Res. 2007;67:8358–8367. doi: 10.1158/0008-5472.CAN-07-1035. [DOI] [PubMed] [Google Scholar]
- 30.Wahrenbrock MG, Varki A. Multiple hepatic receptors cooperate to eliminate secretory mucins aberrantly entering the bloodstream: are circulating cancer mucins the “tip of the iceberg”? Cancer Res. 2006;66:2433–2441. doi: 10.1158/0008-5472.CAN-05-3851. [DOI] [PubMed] [Google Scholar]
- 31.Bennett EP, et al. Control of mucin-type O-glycosylation—a classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22:736–756. doi: 10.1093/glycob/cwr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerken TA, et al. Emerging paradigms for the initiation of mucin-type protein O-glycosylation by the polypeptide GalNAc transferase family of glycosyltransferases. J Biol Chem. 2011;286:14493–14507. doi: 10.1074/jbc.M111.218701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rottger S, et al. Localization of three human polypeptide GalNAc-transferases in HeLa cells suggests initiation of O-linked glycosylation throughout the Golgi apparatus. J Cell Sci. 1998;111(Pt 1):45–60. doi: 10.1242/jcs.111.1.45. [DOI] [PubMed] [Google Scholar]
- 34.Gill DJ, Chia J, Senewiratne J, Bard F. Regulation of O-glycosylation through Golgi-to-ER relocation of initiation enzymes. J Cell Biol. 2010;189:843–858. doi: 10.1083/jcb.201003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwasaki H, et al. Initiation of O-glycan synthesis in IgA1 hinge region is determined by a single enzyme, UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase 2. J Biol Chem. 2003;278:5613–5621. doi: 10.1074/jbc.M211097200. [DOI] [PubMed] [Google Scholar]
- 36.Wandall HH, et al. The lectin domains of polypeptide GalNAc-transferases exhibit carbohydrate-binding specificity for GalNAc: lectin binding to GalNAc-glycopeptide substrates is required for high density GalNAc-O-glycosylation. Glycobiology. 2007;17:374–387. doi: 10.1093/glycob/cwl082. [DOI] [PubMed] [Google Scholar]
- 37.Raska M, et al. Role of GalNAc-transferases in the synthesis of aberrant IgA1 O-glycans in IgA nephropathy. J Am Soc Nephrol. 2011;22:625A. [Google Scholar]
- 38.Bennett EP, et al. Cloning and characterization of a close homologue of human UDP-N-acetyl-alpha-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase-T3, designated GalNAc-T6. Evidence for genetic but not functional redundancy. J Biol Chem. 1999;274:25362–25370. doi: 10.1074/jbc.274.36.25362. [DOI] [PubMed] [Google Scholar]
- 39.Wandall HH, et al. Substrate specificities of three members of the human UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase family, GalNAc-T1, -T2, and -T3. J Biol Chem. 1997;272:23503–23514. doi: 10.1074/jbc.272.38.23503. [DOI] [PubMed] [Google Scholar]
- 40.Schwientek T, et al. Functional conservation of subfamilies of putative UDP-N-acetylgalactosamine:polypeptide N-acetylgalactosaminyltransferases in Drosophila, Caenorhabditis elegans, and mammals. One subfamily composed of l(2)35Aa is essential in Drosophila . J Biol Chem. 2002;277:22623–22638. doi: 10.1074/jbc.M202684200. [DOI] [PubMed] [Google Scholar]
- 41.Cheng L, et al. Characterization of a novel human UDP-GalNAc transferase, pp-GalNAc-T15. FEBS Lett. 2004;566:17–24. doi: 10.1016/j.febslet.2004.03.108. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, et al. Cloning and characterization of a new human UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase, designated pp-GalNAc-T13, that is specifically expressed in neurons and synthesizes GalNAc α-serine/threonine antigen. J Biol Chem. 2003;278:573–584. doi: 10.1074/jbc.M203094200. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, et al. Cloning and characterization of a novel UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase, pp-GalNAc-T14. Biochem Biophys Res Commun. 2003;300:738–744. doi: 10.1016/S0006-291X(02)02908-X. [DOI] [PubMed] [Google Scholar]
- 44.Hassan H, et al. The lectin domain of UDP-N-acetyl-d-galactosamine: polypeptide N-acetylgalactosaminyltransferase-T4 directs its glycopeptide specificities. J Biol Chem. 2000;275:38197–38205. doi: 10.1074/jbc.M005783200. [DOI] [PubMed] [Google Scholar]
- 45.Brooks SA, Carter TM, Bennett EP, Clausen H, Mandel U. Immunolocalisation of members of the polypeptide N-acetylgalactosaminyl transferase (ppGalNAc-T) family is consistent with biologically relevant altered cell surface glycosylation in breast cancer. Acta Histochem. 2007;109:273–284. doi: 10.1016/j.acthis.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Mandel U, et al. Expression of polypeptide GalNAc-transferases in stratified epithelia and squamous cell carcinomas: immunohistological evaluation using monoclonal antibodies to three members of the GalNAc-transferase family. Glycobiology. 1999;9:43–52. doi: 10.1093/glycob/9.1.43. [DOI] [PubMed] [Google Scholar]
- 47.Marcos NT, et al. Polypeptide GalNAc-transferases, ST6GalNAc-transferase I, and ST3Gal-transferase I expression in gastric carcinoma cell lines. J Histochem Cytochem. 2003;51:761–771. doi: 10.1177/002215540305100607. [DOI] [PubMed] [Google Scholar]
- 48.Cooper LS, et al. Expression of GalNAc transferases in breast tissues and cell lines. J Pathol. 1999;187:26A–26A. [Google Scholar]
- 49.Berois N, et al. UDP-N-acetyl-d-galactosamine: polypeptide N-acetylgalactosaminyltransferase-6 as a new immunohistochemical breast cancer marker. J Histochem Cytochem. 2006;54:317–328. doi: 10.1369/jhc.5A6783.2005. [DOI] [PubMed] [Google Scholar]
- 50.Freire T, et al. UDP-N-acetyl-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase 6 (ppGalNAc-T6) mRNA as a potential new marker for detection of bone marrow-disseminated breast cancer cells. Int J Cancer. 2006;119:1383–1388. doi: 10.1002/ijc.21959. [DOI] [PubMed] [Google Scholar]
- 51.Park JH, et al. Critical roles of mucin 1 glycosylation by transactivated polypeptide N-acetylgalactosaminyltransferase 6 in mammary carcinogenesis. Cancer Res. 2010;70:2759–2769. doi: 10.1158/0008-5472.CAN-09-3911. [DOI] [PubMed] [Google Scholar]
- 52.Wu C, et al. N-Acetylgalactosaminyltransferase-14 as a potential biomarker for breast cancer by immunohistochemistry. BMC Cancer. 2010;10:123. doi: 10.1186/1471-2407-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner KW, et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- 54.Gray-McGuire C, et al. Confirmation of linkage to and localization of familial colon cancer risk haplotype on chromosome 9q22. Cancer Res. 2010;70:5409–5418. doi: 10.1158/0008-5472.CAN-10-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guda K, et al. Inactivating germ-line and somatic mutations in polypeptide N-acetylgalactosaminyltransferase 12 in human colon cancers. Proc Natl Acad Sci USA. 2009;106:12921–12925. doi: 10.1073/pnas.0901454106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wada Y, et al. Comparison of methods for profiling O-glycosylation: Human Proteome Organisation Human Disease Glycomics/Proteome Initiative multi-institutional study of IgA1. Mol Cell Proteomics. 2010;9:719–727. doi: 10.1074/mcp.M900450-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ju T, Brewer K, D’Souza A, Cummings RD, Canfield WM. Cloning and expression of human core 1 β1,3-galactosyltransferase. J Biol Chem. 2002;277:178–186. doi: 10.1074/jbc.M109060200. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, et al. Cosmc is an essential chaperone for correct protein O-glycosylation. Proc Natl Acad Sci USA. 2010;107:9228–9233. doi: 10.1073/pnas.0914004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 β 3-galactosyltransferase. Proc Natl Acad Sci USA. 2002;99:16613–16618. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Charlier E, et al. SHIP-1 inhibits CD95/APO-1/Fas-induced apoptosis in primary T lymphocytes and T leukemic cells by promoting CD95 glycosylation independently of its phosphatase activity. Leukemia. 2010;24:821–832. doi: 10.1038/leu.2010.9. [DOI] [PubMed] [Google Scholar]
- 61.Dall’Olio F, Chiricolo M. Sialyltransferases in cancer. Glycoconj J. 2001;18:841–850. doi: 10.1023/A:1022288022969. [DOI] [PubMed] [Google Scholar]
- 62.Harduin-Lepers A, et al. The human sialyltransferase family. Biochimie. 2001;83:727–737. doi: 10.1016/S0300-9084(01)01301-3. [DOI] [PubMed] [Google Scholar]
- 63.Gharavi AG, et al. Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol. 2008;19:1008–1014. doi: 10.1681/ASN.2007091052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raska M, et al. Identification and characterization of CMP-NeuAc:GalNAc-IgA1 α2,6-sialyltransferase in IgA1-producing cells. J Mol Biol. 2007;369:69–78. doi: 10.1016/j.jmb.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marcos NT, et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004;64:7050–7057. doi: 10.1158/0008-5472.CAN-04-1921. [DOI] [PubMed] [Google Scholar]
- 66.Suzuki H, et al. Mechanisms of aberrant glycosylation of IgA1 in patients with IgA nephropathy. J Am Soc Nephrol. 2009;20:301A. [Google Scholar]
- 67.Dalziel M, et al. The relative activities of the C2GnT1 and ST3Gal-I glycosyltransferases determine O-glycan structure and expression of a tumor-associated epitope on MUC1. J Biol Chem. 2001;276:11007–11015. doi: 10.1074/jbc.M006523200. [DOI] [PubMed] [Google Scholar]
- 68.Brockhausen I, Yang JM, Burchell J, Whitehouse C, Taylor-Papadimitriou J. Mechanisms underlying aberrant glycosylation of MUC1 mucin in breast cancer cells. Eur J Biochem. 1995;233:607–617. doi: 10.1111/j.1432-1033.1995.607_2.x. [DOI] [PubMed] [Google Scholar]
- 69.Hanisch FG, Stadie TR, Deutzmann F, Peter-Katalinic J. MUC1 glycoforms in breast cancer—cell line T47D as a model for carcinoma-associated alterations of O-glycosylation. Eur J Biochem. 1996;236:318–327. doi: 10.1111/j.1432-1033.1996.00318.x. [DOI] [PubMed] [Google Scholar]
- 70.Sewell R, et al. The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. J Biol Chem. 2006;281:3586–3594. doi: 10.1074/jbc.M511826200. [DOI] [PubMed] [Google Scholar]
- 71.Ju T, et al. Human tumor antigens Tn and sialyl Tn arise from mutations in Cosmc. Cancer Res. 2008;68:1636–1646. doi: 10.1158/0008-5472.CAN-07-2345. [DOI] [PubMed] [Google Scholar]
- 72.Litvinov SV, Hilkens J. The epithelial sialomucin, episialin, is sialylated during recycling. J Biol Chem. 1993;268:21364–21371. [PubMed] [Google Scholar]
- 73.Avrameas S, Ternynck T (1998) Natural antibodies. In: Delves PJ, Roitt IM (eds) Encyclopedia of immunology. Academic Press, San Diego, p 1806–1809
- 74.Brandlein S, et al. Cysteine-rich fibroblast growth factor receptor 1, a new marker for precancerous epithelial lesions defined by the human monoclonal antibody PAM-1. Cancer Res. 2003;63:2052–2061. [PubMed] [Google Scholar]
- 75.Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. 1995;7:812–818. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 76.Avrameas S, Ternynck T, Tsonis IA, Lymberi P. Naturally occurring B-cell autoreactivity: a critical overview. J Autoimmun. 2007;29:213–218. doi: 10.1016/j.jaut.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 77.Vollmers HP, Brandlein S. Natural antibodies and cancer. J Autoimmun. 2007;29:295–302. doi: 10.1016/j.jaut.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 78.Brandlein S, et al. Natural IgM antibodies and immunosurveillance mechanisms against epithelial cancer cells in humans. Cancer Res. 2003;63:7995–8005. [PubMed] [Google Scholar]
- 79.Mouthon L, et al. Analysis of the normal human IgG antibody repertoire. Evidence that IgG autoantibodies of healthy adults recognize a limited and conserved set of protein antigens in homologous tissues. J Immunol. 1995;154:5769–5778. [PubMed] [Google Scholar]
- 80.Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med. 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- 81.Bray J, MacLean GD, Dusel FJ, McPherson TA. Decreased levels of circulating lytic anti-T in the serum of patients with metastatic gastrointestinal cancer: a correlation with disease burden. Clin Exp Immunol. 1982;47:176–182. [PMC free article] [PubMed] [Google Scholar]
- 82.Macher BA, Galili U. The Galα1,3Galβ1,4GlcNAc-R (α-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim Biophys Acta. 2008;1780:75–88. doi: 10.1016/j.bbagen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Galili U, Mandrell RE, Hamadeh RM, Shohet SB, Griffiss JM. Interaction between human natural anti-α-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun. 1988;56:1730–1737. doi: 10.1128/iai.56.7.1730-1737.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26:347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 85.Gharavi AG, et al. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet. 2011;43:321–327. doi: 10.1038/ng.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O’Boyle KP, et al. Immunization of colorectal cancer patients with modified ovine submaxillary gland mucin and adjuvants induces IgM and IgG antibodies to sialylated Tn. Cancer Res. 1992;52:5663–5667. [PubMed] [Google Scholar]
- 87.MacLean GD, et al. Immunization of breast cancer patients using a synthetic sialyl-Tn glycoconjugate plus Detox adjuvant. Cancer Immunol Immunother. 1993;36:215–222. doi: 10.1007/BF01740902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Longenecker BM, Reddish M, Koganty R, MacLean GD. Immune responses of mice and human breast cancer patients following immunization with synthetic sialyl-Tn conjugated to KLH plus detox adjuvant. Ann N Y Acad Sci. 1993;690:276–291. doi: 10.1111/j.1749-6632.1993.tb44016.x. [DOI] [PubMed] [Google Scholar]
- 89.Longenecker BM, Reddish M, Koganty R, MacLean GD. Specificity of the IgG response in mice and human breast cancer patients following immunization against synthetic sialyl-Tn, an epitope with possible functional significance in metastasis. Adv Exp Med Biol. 1994;353:105–124. doi: 10.1007/978-1-4615-2443-4_11. [DOI] [PubMed] [Google Scholar]
- 90.MacLean GD, Miles DW, Rubens RD, Reddish MA, Longenecker BM. Enhancing the effect of THERATOPE STn-KLH cancer vaccine in patients with metastatic breast cancer by pretreatment with low-dose intravenous cyclophosphamide. J Immunother Emphasis Tumor Immunol. 1996;19:309–316. doi: 10.1097/00002371-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 91.Guo Z, Wang Q. Recent development in carbohydrate-based cancer vaccines. Curr Opin Chem Biol. 2009;13:608–617. doi: 10.1016/j.cbpa.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gilewski TA, et al. Immunization of high-risk breast cancer patients with clustered STn-KLH conjugate plus the immunologic adjuvant QS-21. Clin Cancer Res. 2007;13:2977–2985. doi: 10.1158/1078-0432.CCR-06-2189. [DOI] [PubMed] [Google Scholar]
- 93.Slovin SF, et al. Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with α-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J Clin Oncol. 2003;21:4292–4298. doi: 10.1200/JCO.2003.04.112. [DOI] [PubMed] [Google Scholar]
- 94.Slovin SF, et al. A bivalent conjugate vaccine in the treatment of biochemically relapsed prostate cancer: a study of glycosylated MUC-2-KLH and Globo H-KLH conjugate vaccines given with the new semi-synthetic saponin immunological adjuvant GPI-0100 OR QS-21. Vaccine. 2005;23:3114–3122. doi: 10.1016/j.vaccine.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 95.Elkon K, Casali P. Nature and functions of autoantibodies. Nat Clin Pract Rheumatol. 2008;4:491–498. doi: 10.1038/ncprheum0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ni J, Song H, Wang Y, Stamatos NM, Wang LX. Toward a carbohydrate-based HIV-1 vaccine: synthesis and immunological studies of oligomannose-containing glycoconjugates. Bioconjug Chem. 2006;17:493–500. doi: 10.1021/bc0502816. [DOI] [PubMed] [Google Scholar]
- 97.Dziadek S, Hobel A, Schmitt E, Kunz H. A fully synthetic vaccine consisting of a tumor-associated glycopeptide antigen and a T-cell epitope for the induction of a highly specific humoral immune response. Angew Chem Int Ed Engl. 2005;44:7630–7635. doi: 10.1002/anie.200501594. [DOI] [PubMed] [Google Scholar]
- 98.Cremer GA, et al. Synthesis and biological evaluation of a multiantigenic Tn/TF-containing glycopeptide mimic of the tumor-related MUC1 glycoprotein. ChemMedChem. 2006;1:965–968. doi: 10.1002/cmdc.200600104. [DOI] [PubMed] [Google Scholar]
- 99.Lo-Man R, et al. A fully synthetic therapeutic vaccine candidate targeting carcinoma-associated Tn carbohydrate antigen induces tumor-specific antibodies in nonhuman primates. Cancer Res. 2004;64:4987–4994. doi: 10.1158/0008-5472.CAN-04-0252. [DOI] [PubMed] [Google Scholar]
- 100.Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons GJ. Robust immune responses elicited by a fully synthetic three-component vaccine. Nat Chem Biol. 2007;3:663–667. doi: 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]