Abstract
Isochorismate synthase 1 (ICS1) is a crucial enzyme in the salicylic acid (SA) synthesis pathway, and thus it is important for immune defences. The ics1 mutant is used in experiments on plant–pathogen interactions, and ICS1 is required for the appropriate hypersensitive disease defence response. However, ICS1 also takes part in the synthesis of phylloquinone, which is incorporated into photosystem I and is an important component of photosynthetic electron transport in plants. Therefore, photosynthetic and molecular analysis of the ics1 mutant in comparison with wild-type and SA-degrading transgenic NahG Arabidopsis thaliana plants was performed. Photosynthetic parameters in the ics1 mutant, when compared with the wild type, were changed in a manner observed previously for state transition-impaired plants (STN7 kinase recessive mutant, stn7). In contrast to stn7, deregulation of the redox status of the plastoquinone pool (measured as 1–q p) in ics1 showed significant variation depending on the leaf age. SA-degrading transgenic NahG plants targeted to the cytoplasm or chloroplasts displayed normal (wild-type-like) state transition. However, ics1 plants treated with a phylloquinone precursor displayed symptoms of phenotypic reversion towards the wild type. ics1 also showed altered thylakoid structure with an increased number of stacked thylakoids per granum which indicates the role of ICS1 in regulation of state transition. The results presented here suggest the role of ICS1 in integration of the chloroplast ultrastructure, the redox status of the plastoquinone pool, and organization of the photosystems, which all are important for optimal immune defence and light acclimatory responses.
Key words: Chlorophyll fluorescence, photosynthetic electron transport (PET), phylloquinone, plastoquinone pool (PQ pool), salicylic acid (SA), state transitions (ST).
Introduction
One of the key factors affecting photosynthesis in the natural environment is the amount of available light. Plants possess the natural capacity to absorb light energy in excess of what is sufficient for photochemistry; thus, in full sunlight, only a portion of the light energy absorbed by chlorophylls is used for CO2 fixation (Asada, 1999; Ruban et al., 2004). The quantity of absorbed light energy that exceeds the amount needed for photochemistry is referred to as excess excitation energy (EEE). EEE must be dissipated through both fluorescence and heat. The failure to dissipate and quench EEE is highly damaging to plants, and often leads to chlorosis, bleaching, or bronzing of leaves due to imbalanced reactive oxygen species (ROS) and hormonal cellular homeostasis (Karpinski et al., 1999; Niyogi, 1999; Apel and Hirt, 2004; Ruban et al., 2004; Laloi et al., 2007; Van Breusegem et al., 2008; Muhlenbock et al., 2008; Li et al., 2009).
Non-photochemical quenching (NPQ) is a protective mechanism which, when it is in balance with the photochemical processes, ensures the optimal use of absorbed light energy for photochemistry with minimal damage due to, for example, heat and ROS generation, and foliar cell death induction (Mateo et al., 2004; Baker, 2008; Li et al., 2009; Szechynska-Hebda et al., 2010). It consists of state transition (ST), photoinhibition, and EEE dissipation. ST is a mechanism which allows absorbed light energy to be adequately distributed between two photosystems; thus photosynthesis is optimally efficient under variable light conditions. For decades, the molecular basis of this process was unknown, but recent experiments have demonstrated a key protein involved in the regulation of ST (Bellafiore et al., 2005; Pribil et al., 2010; Shapiguzov et al., 2010). STN7 is a kinase that is crucial for phosphorylation of the mobile antenna, light-harvesting complex II (LHCII), during ST. Phosphorylated LHCII (e.g. in low light conditions) migrates to photosystem I (PSI) and enhances its light energy absorption—this is called state 2. In the case of PSI-favouring conditions (e.g. far-red light), LHCII is dephosphorylated by phosphatase PPH1/TAP38 and joins PSII (state 1), which results in a more efficient energy transfer to PSII. This process lasts a few minutes (Bellafiore et al., 2005; Pribil et al., 2010; Shapiguzov et al., 2010). Furthermore, the role of ST in the regulation of Darwinian fitness in field conditions was reported and confirmed (Frenkel et al., 2007).
However, an energy imbalance may also be compensated by plants in a different manner. During long-lasting non-optimal light conditions, plants adjust the number of reaction centres, LHCs, and the ratio of photosystems (Chow et al., 1990; Pfannschmidt et al., 1999). This takes at least a few hours, is more noticeable after a few days, and is called the long-term response (LTR). ST and LTR changes are brought about by modifications of the redox state of the plastoquinone (PQ) pool as well as PSI and PSII protein expression modifications, and lead to an alteration of the ultimate proportion of chlorophyll a and b (Pfannschmidt et al., 2001; Brautigam et al., 2009). It is also believed that the redox status of the PQ pool regulates immune defences, cell death, and light acclimatory responses induced by EEE conditions (Mateo et al., 2004; Muhlenbock et al., 2008; Szechynska-Hebda et al., 2010; Karpinski et al., 2013).
The role of the hormone salicylic acid (SA) in plant defence has been well studied (Loake and Grant, 2007; Vlot et al., 2009; An and Mou, 2011). The ROS burst drives the local accumulation of SA in cells surrounding the infection sites to induce defence and transcription of antioxidant genes. Loss of SA accumulation suppresses immune defences (Aviv et al., 2002), and it is widely accepted that SA is the main player in the regulation of cell death (Coll et al., 2011; Karpinski et al., 2013). However, SA is not only important for regulation of biotic stress responses. It has been shown that SA signalling is induced during light acclimatory responses and EEE dissipation, and that SA has a regulatory effect on photosynthesis, stomatal conductance, and cell death during light acclimation (Karpinski et al., 2003; Mateo et al., 2004; Muhlenbock et al., 2008). Additionally, SA is involved in physiological and molecular processes, such as the regulation of flowering or thermogenesis (Clarke et al., 2004; Vlot et al., 2009). These observations suggest that SA synthesis could regulate the cross-talk between light acclimatory and immune defence responses in plants.
There are at least two pathways of SA production in plants. In the first metabolic pathway, ~5% of SA is synthesized from benzoic acid by benzoic acid-2-hydrolase. In the second pathway, isochorismate plays a key role, and up to 95% of SA originates from this pathway (Vlot et al., 2009). There are two genes encoding isochorismate synthase (ICS), named ICS1 and ICS2, in Arabidopsis. ICS catalyses isochorismate formation from chorismate (Garcion et al., 2008). Previous analyses indicate that ics1 (also called salicylic acid induction deficient 2, sid2) is the main source of isochorismate, while the ics2 null mutant does not have significantly decreased levels of SA (Garcion et al., 2008). Isochorismate is also a precursor of phylloquinone, known as vitamin K1, which is a secondary electron acceptor from PSI and transfers an electron to the iron–sulphur cluster. The double null ics1/ics2 mutant is pale green, smaller than the wild type (WT), and is totally devoid of phylloquinone (Garcion et al., 2008). This suggests that this pathway of phylloquinone biosynthesis is the only one in Arabidopsis.
Based on the information presented above, the following hypotheses have been formulated: (i) phylloquinone (PQ) as a PSI component could be involved in the regulation of ST through SA or the phylloquinone synthesis pathway; and (ii) ICS1 might play an important role in maintaining the balance between photosystems in variable light conditions.
Materials and methods
Plant material and growth condition
All plants used in this study were derived from the Col-0 ecotype. The ics1 mutant, NahG, RbsC–NahG, and RbsC–NahG–green fluorescent protein (GFP) (RbsC is a chloroplast-targeting sequence) transgenic lines were described earlier (Gaffney et al., 1993; Wildermuth et al., 2001; Fragnière et al., 2011). The stn7 mutant with impaired STs was described in Bonardi et al. (2005). Plants were grown for 4–5 weeks in Jiffy pots (Jiffy Products) in short-day conditions, 8h light/16h dark, at 22 °C under fluorescent white light: 80 μmol m–2 s–1. If different growth conditions were applied, this is indicated in the text.
Chlorophyll a fluorescence measurements
Chlorophyll a fluorescence parameters were determined using a pulse amplitude-modulated FluorCam 800 MF PSI device (Brno, Czech Republic). The plants were kept in darkness for 30min to determine F 0 and F m, exposed to 5min of actinic red light (90 μmol m–2 s–1) to determine F t and F mʹ, then the actinic light was switched off and, after incubation with far-red light, F 0ʹ was determined. The maximum quantum efficiency of PSII, F v/F m=(F m–F o)/F m, non-photochemical quenching, NPQ=(F m–F mʹ)/F mʹ, photochemical quenching, q p=(F mʹ–F t)/(F mʹ–F 0ʹ), and the operating quantum efficiency of PSII, ΦPSII=(F mʹ−F s)/F mʹ were determined as described earlier (Baker, 2008). Steady-state fluorescence (F s) was calculated as F s=F t–F 0ʹ, and used to determine the F s/F m parameter, which reflects the structural differences in the photosynthetic apparatus (Pfannschmidt et al., 2001). ST was determined on whole rosettes with a pulse amplitude-modulated FluorCam 800 MF device. F 0 was determined on dark-acclimated whole plants, then a 1 s flash of saturating light was applied to determine F m. Plants were pre-illuminated with both blue (PSII light) and far-red light (PSI light) for 5min, and subsequently only blue light (PSII light) was switched on for 15min. The far-red light was turned on for 15min and the maximum fluorescence in state 1 was determined (F m1). Next, the far-red light was turned off and fluorescence was monitored for 15min, after which the maximum fluorescence in state 2 was determined (F m2). F i and F ii indicate fluorescence in the presence of PSI light in state 1 and state 2, respectively. F iʹ and F iiʹ denote fluorescence in the absence of PSI light in state 1 and state 2, respectively. The q T and q S parameters were calculated as [(F m1–F m2)/F m1] and [(F iʹ–F i)–(F iiʹ–F ii)]/(F iʹ–F i), respectively (Damkjaer et al., 2009).
Phylloquinone determination
Phylloquinone was determined by the method of van Oostende et al. (2008) with the following modifications: the zinc post-column was 1×0.4cm, and the solvent was methanol containing 10mM ZnCl2, 5mM sodium acetate and 5mM acetic acid. The fluorescence detection was performed at 245nm excitation and 430nm emission (Kruk et al., 1994).
Feeding with the precursor of phylloquinone
To check the role of phylloquinone in ST, 3-week-old ics1 plants were sprayed with 100 μM 1,4-dihydroxy-2-naphthoic acid (NA; Sigma-Aldrich) in 0.2% dimethylsulphoxide (DMSO; Sigma-Aldrich) with 0.1% Tween-20 (Sigma-Aldrich) for 2 weeks, three times a week. Control ics1 plants were treated with an analogous solution without NA. Adequate experiments on WT plants were conducted.
Sample preparation for light and transmission electron microscopy
Segments from the mid-lamina regions of mature, low light-acclimated (state 2-favouring conditions) leaves were cut from the Col-0 as the WT and from ics1 plants directly into cold Karnovsky’s fixative (Karnovsky, 1965). The segments were fixed for 2h under a slight vacuum at room temperature. After 0.1M cacodylate buffer rinses were repeated four times, the leaf tissue was post-fixed in 2% OsO4 for 4h. Subsequently, it was dehydrated through a cold ethanol series (10–100%), followed by several changes of propylene oxide, embedded in resin Epon 812 (Fluka), and polymerized for 24h at 60 °C. Sections (3 μm) were cut onto glass slides using microtomes (Jung RM 2065 and Ultracut UCT, Leica), mounted in methylene blue and azure A, and examined using an Olympus AX70 Provis light microscope. Ultrathin sections were collected on copper grids and stained with uranyl acetate, followed by lead citrate for 1min, and examined under a Morgagni 268C (FEI) transmission electron microscope.
Gas exchange and chlorophyll fluorescence analysis
Photosynthetic parameters in variable light conditions were measured using the Gas Exchange Fluorescence System GFS-3000 (Walz GmbH, Effeltrich, Germany) and calculated according to the manufacturer’s instructions and Wituszyńska et al. (2013). Three-week-old plants cultivated at 120 μmol m–2 s–1 were examined.
Chlorophyll a/b ratio measurement
Four-week-old Arabidopsis plants were frozen in liquid nitrogen and 50–100mg of frozen tissue was homogenized in a TissueLyser LT (Qiagen) (5min; 50 s–1, 4 °C) with 1ml of cold acetone (–20 °C). The homogenate was evaporated under a nitrogen stream, dissolved in cold solvent A (acetonitrile:methanol; 90:10 v/v), and re-homogenized for 1min. The extract was filtered through a syringe filter (0.2 μm nylon filter, Whatman) into an autosampler vial, capped, and stored in the dark at –80 °C until HPLC analysis (Shimadzu Liquid Chromatography System). Pigments were separated on a Synergi 4u MAX-RP 80A 250×4.6 column (Phenomenex) at 30 °C. A low-pressure gradient method was used: solvent A for 10min, followed by solvent B (methanol:ethyl acetate; 68:32 v/v) for 10min at a flow rate of 1ml min–1. Absorbance spectra were recorded at 440nm by diode array detector. Pigments were identified by using standards obtained from Sigma. The results were expressed as a chlorophyll a/b ratio.
Thermoluminescence (TL) measurements
TL measurements of detached leaves were performed with the thermoluminescence TL 200/PMT system (Brno, Czech Republic). After 2min of dark adaptation at 20 °C, the leaves were cooled to –6 °C and excited with one or a multiple number of single-turnover flashes. Then the samples were warmed up to 65 °C at a heating rate of 0.5 °C s–1, and TL light emission was measured.
Microarray analysis
In the present analysis, data from Affymetrix ATH1 GeneChips published by Behringer et al. (2011) and deposited in the Gene Expression Omnibus (GEO) with the number GSE25489 were used. The CEL files of non-treated Col-0 and ics1 were downloaded. The data were pre-processed and analysed using R and Bioconductor software (Gentleman et al., 2004). The array intensities were normalized using gcrma from the simpleaffy package (Wilson and Miller, 2005). The limma package (Smyth, 2005) was used to compare the hybridization intensities of Col-0 and ics1. Up- and down-regulated genes in ics1 were defined as those whose hybridization intensities were significantly higher or lower than in Col-0 (P < 0.05, Student’s t-test). Gene ontology (GO) terms were retrieved from the TAIR website (http://arabidopsis.org).
Analyses of quantum efficiencies of PSI
To examine the activity of PSI P700, absorbance changes at 830nm and 875nm were measured using a Dual-PAM-100 (Walz GmbH). The experiments were performed on the fifth to seventh detached leaves which had been dark adapted for a minimum of 30min. Both slow kinetics and the light curve were determined. The parameters of PSI activity were calculated as described both by the manufacturer and by Niewiadomska et al. (2011). Moreover, the cyclic electron flow around PSI, YCEF=Y(I)–Y(II), was calculated as described earlier (Miyake et al., 2005).
Results
Inhibition of state transitions in ics1 is phylloquinone dependent
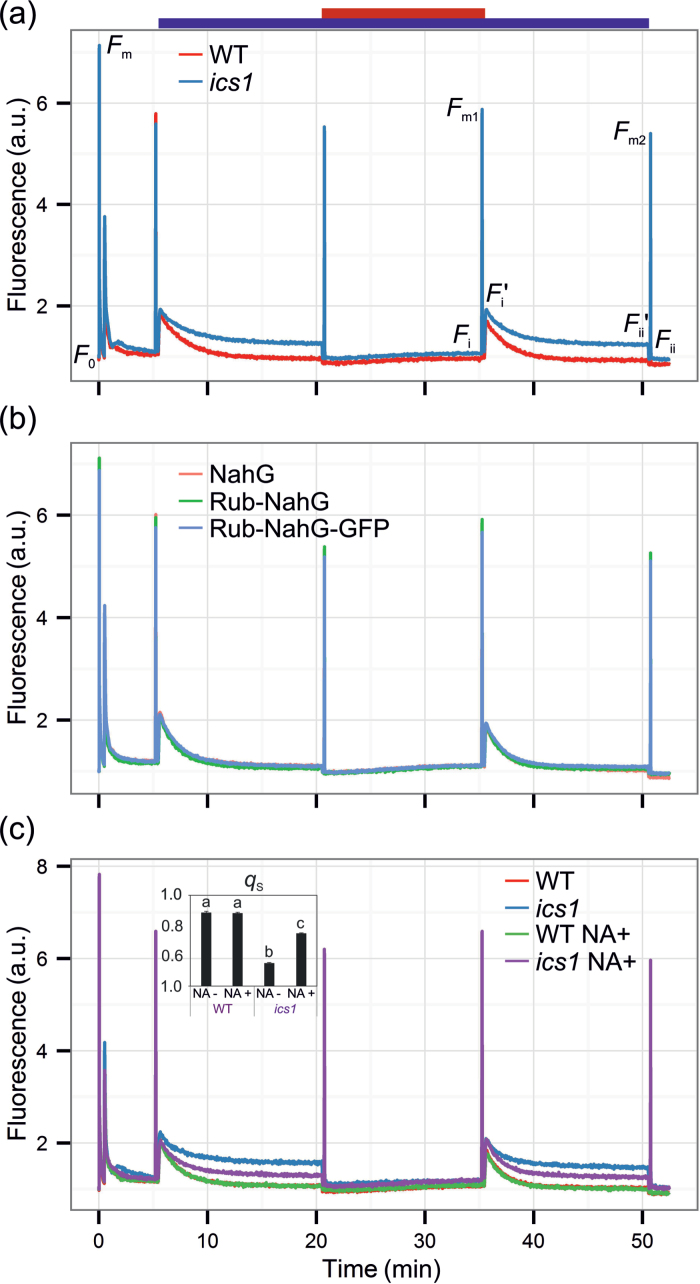
To investigate the role of ICS1 in the regulation of photosynthesis in Arabidopsis, chlorophyll a fluorescence in the ics1 mutant and the WT plants was tested. The maximum quantum yield of photosystem II (F v/F m) was similar in ics1 to that in the WT (Table 1). However, upon illumination with actinic light, the ics1 plants were unable to quench chlorophyll a fluorescence (Fig. 1a), which is reflected in the increased F s/F m parameter (Table 1), indicating that perturbations in photosynthesis may be located beyond PSII. The PQ pool was more reduced in plants lacking ICS1, as indicated by the 1–q p value (Table 1). To check if the ics1 plants had an impaired mechanism of ST, changes of chlorophyll a fluorescence in state 1- and state 2-favouring light conditions were investigated (Fig. 1a). Transitions from state 1 to state 2 can be described by the q T and q S parameters (as described in the Materials and methods). The q T value in the WT plants was 0.114±0.006, while in ics1 it was decreased to 0.037±0.003. Similarly, the q S value in the WT plants was 0.883±0.006, while in ics1 it was decreased to 0.549±0.002 (Table 1). To test if perturbation in ST in ics1 was due to strongly diminished SA levels, the chlorophyll a fluorescence parameters were measured in transgenic NahG plants carrying a bacterial gene encoding salicylate hydroxylase (which converts SA to the catechol) localized in the cytosol and in the chloroplast. However, neither plants with a cytosolic localization of NahG nor those with chloroplast-targeted NahG demonstrated ST disorders (Fig. 1b). Therefore, the role of SA in ST was excluded. The analysis of the phylloquinone content showed that the content of phylloquinone in the ics1 mutant was 55% of that in the WT when calculated on a fresh weight (FW) basis, and 58% of that of the WT in relation to chlorophyll (Table 1). The obtained results are similar to, although higher than, those reported by Garcion et al. (2008) where the phylloquinone content (per g FW) in the mutant was 35% of the value found for the WT. To examine if the observed ST alterations in ics1 were due to a depleted phylloquinone synthesis pathway (another metabolic pathway dependent upon ICS1 activity), the ics1 plants were treated with the precursor of phylloquinone, NA. Two weeks of systematic treatment with NA significantly increased q T in the ics1 plants from 0.037±0.003 to 0.102±0.002, and q S from 0.549±0.002 to 0.743±0.003 (Fig. 1c, Table 1). No significant changes in ST in NA-treated WT plants in comparison with the non-treated WT plants were observed (Fig. 1c, Table 1).
Table 1.
Photosynthetic parameters of WT and ics1 plants
| WT | ics1 | |
|---|---|---|
| F s/F m | 0.048±0.008 (n=6) | 0.110±0.009 (n=6) |
| 1–q P | 0.088±0.004 (n=6) | 0.200±0.015 (n=6) |
| F v/F m | 0.847±0.010 (n=6) | 0.842±0.016 (n=6) |
| NPQ | 0.445±0.113 (n=6) | 0.407±0.105 (n=6) |
| Chl a/b | 2.70±0.09 (n=7) | 2.50±0.07 (n=5) |
| Phylloquinone (μg g FW–1)a | 5.62±0.55 (n=5) | 3.07±0.64 (n=5) |
| Phylloquinone (mol 100mol–1 Chl)a | 1.04±0.07 (n=5) | 0.60±0.10 (n=5) |
| q T | 0.114±0.006 (n=6) | 0.037±0.003 (n=6) |
| q T (+NA)b | 0.116±0.010 (n=6) | 0.102±0.002 (n=6) |
| q S | 0.883±0.006 (n=6) | 0.549±0.002 (n=6) |
| q S (+NA)b | 0.880±0.005 (n=6) | 0.743±0.003 (n=6) |
| YCEF | 0.23±0.03 (n=8) | 0.34±0.04 (n=8) |
Values are means ±SD.
a The experiment was repeated twice with similar results.
b Values for plants sprayed with NA (for details, see the Materials and methods).
Fig. 1.
State transition in wild-type and ics1 plants (a), NahG transgenic plants with both a cytosolic- and chloroplast-targeted SA-degrading enzyme (b), and wild-type and ics1 plants supplemented with the phylloquinone precursor (1,4-dihydroxy-2-naphthoic acid; NA) (c). The top bar depicts lights applied during a particular period of the measurement; blue, actinic light on; red, far-red light on. The graph shows representative measurements of six whole rosettes for each genotype and treatment. The inset in (c) shows the q S parameter (mean ±SD) for the analysed genotypes and treatments. Homogeneous groups were calculated using Tukey HSD analysis. For details, see the Materials and methods.
Transmission electron microscopy
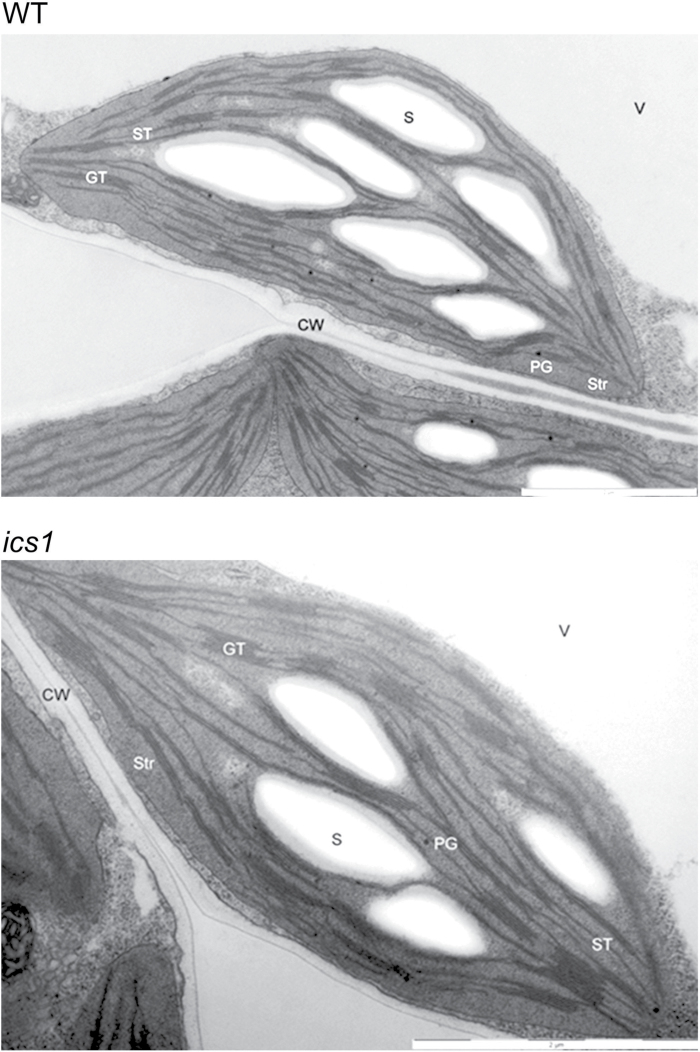
Transmission electron microscopy was used to determine the organization of internal membranes in the chloroplasts of the WT and ics1. Thylakoid membranes were differentiated into two domains that were different morphologically and functionally, namely grana and unstacked (non-appressed) membranes called stroma lamellae. The grana were interconnected by the stroma thylakoids. The thylakoids were flattened and oriented towards the longitudinal axis of the thylakoid system. The large starch grains and a few and relatively small plastoglobuli were observed in the stroma (Fig. 2).
Fig. 2.
Representative pictures of the chloroplast structure from WT and ics1 plants. The WT chloroplasts show a typical structure with a lamellar system of granal and stromal thylakoids, while in ics1 enlargement of the granum showing numerous stacked thylakoid sheets can be observed. CW, cell wall; GT, grana thylakoids; PG, plastoglobuli; S, starch; ST, stroma thylakoids; Str, stroma; V, vacuole. Bar=2 μm.
In general, the organization of the thylakoid membranes in the WT was similar to that observed in ics1 (Fig. 2). However, observations revealed some differences. The ics1 grana stacks had more thylakoids when compared with the chloroplasts from the WT. Moreover, the grana in ics1 were rare in comparison with the WT chloroplasts. Although thylakoids form characteristic granum structures, an increased number of layers in the stacks can be seen in ics1 (Fig. 2).
Photosynthetic parameters in ics1
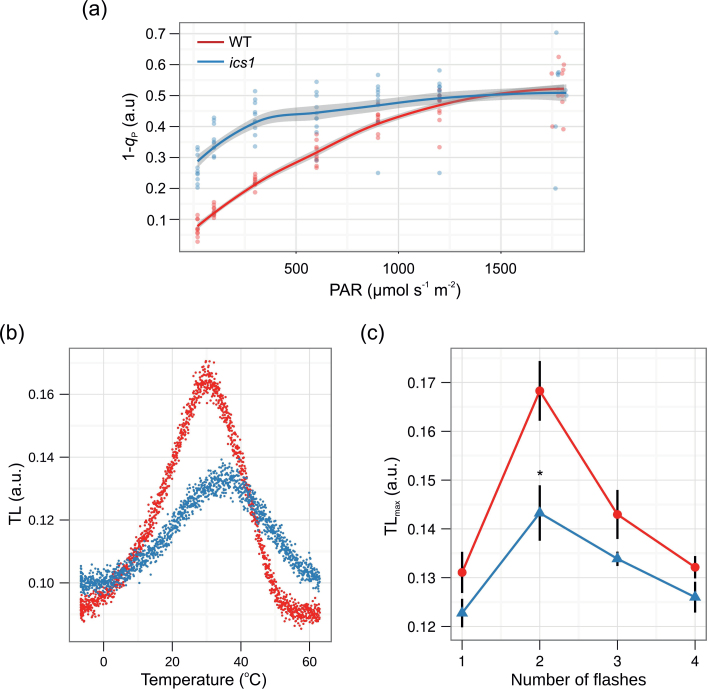
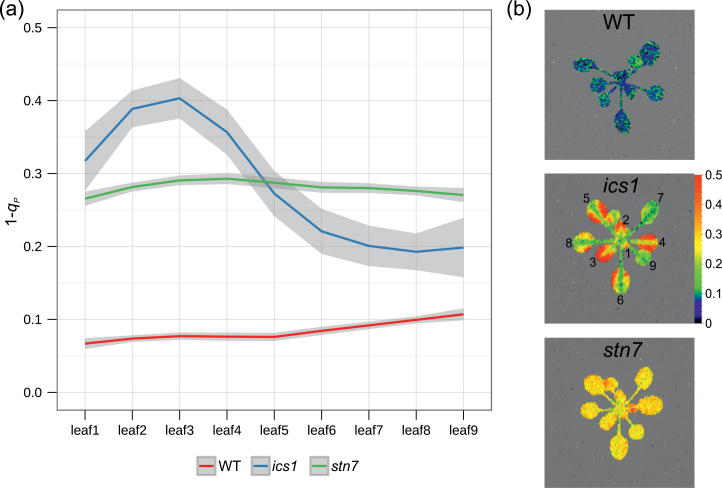
To assay if the observed changes in fluorescence in the ics1 mutant are due to alterations in the mechanisms of NPQ in the WT and ics1, the plants were grown at 120 μmol m–2 s–1 and the rosettes were challenged with increasing light intensities. No significant differences in NPQ between the ics1 and WT plants were observed (Table 1; Supplementary Fig. S1a available at JXB online). In the ics1 rosettes, the PQ pool, expressed as a 1–q P, was more reduced than in the WT plants (Table 1, Fig. 3a), while this difference was more pronounced at lower light intensities. The level of reduction of the PQ pool (expressed as 1–q P) in light-exposed ics1 plants was significantly higher in young than in old, well-developed leaves and oscillates around values obtained for stn7 that were similar in all leaves (Fig. 4). However, 1–q P values in WT control plants were significantly lower than those in ics1 and stn7, and were also homogeneous in the whole rosette (Fig. 4). Higher PQ pool reduction levels correlate with an increased yield of cyclic electron flow (YCEF) around PSI observed in ics1 in comparison with the WT (Table 1). However, CO2 assimilation in ics1 was similar to that in the WT plants (Supplementary Fig. S1c). These data are consistent with the fact that there was no significant difference in growth between ics1 and WT in laboratory conditions (Mateo et al., 2006).
Fig. 3.
Redox status of the plastoquinone pool. (a) Light curve of the redox status of the PQ pool (1–q P). Data represent an average from 10 different rosettes (n=10, grey shading indicates the 95% confidence intervals for the analysed genotypes). (b and c) Thermoluminescence (TL) measurements. (b) TL glow curves in wild-type (WT, red dots) and ics1 (blue dots) plants. The figure shows representative measurement of the leaves excited with two flashes. (c) Flash-induced oscillation of the B-band amplitude (TLmax) in wild-type (WT, red line and dots) and ics1 (blue line and triangles) plants. Values are means ±SEM of seven independent measurements (leaves from seven independent plants). Asterisks indicate a significant difference from the control at P < 0.05 (according to Student’s t-test).
Fig. 4.
Different patterns of the PQ pool reduction in ics1, stn7, and WT Arabidopsis rosettes. (a) The redox status of the plastoquinone pool (expressed as 1–q p) in different leaves of ics1, stn7, and the WT. Data represent an average from six different rosettes (n=6; grey shading indicates the 95% confidence intervals for the analysed genotypes). (b) Photos show false-colour images of 1–q p for ics1, stn7, and the WT.
Thermoluminescence
TL was further used to investigate the redox properties of the acceptor and donor sides of PSII in the WT and ics1 plants. In order to confirm that the redox state of the PQ pool is more reduced in the ics1 mutants, the flash-induced patterns of TL in the WT and mutants were measured. The best characterized TL band is the B-band. Illumination of a single turnover flash with the plant sample after a short dark adaptation induces a major B-band, which appears at ~30–35 ºC (Misra et al., 2001; Ducruet and Vass, 2009; Sane et al., 2012). It is correlated with the activity of the oxygen-evolving complex (OEC). As is shown in Fig. 3b, the temperature peak for the TL glow curves was ~30–35 ºC, thus indicating that the B-band was responsible for TL emission in both the WT and ics1 plants, but in the ics1 plants the B-band amplitude (TLmax) was strongly decreased (Fig. 3b). When the B-band amplitude (TLmax) is plotted against the flash number, a periodicity-of-four oscillation is clearly seen, with maxima occurring on the second and sixth flashes (Fig. 3c; Inoue, 1996). When the dark-adapted PSII centre is exposed to a series of short turnover flashes, formation of the redox pairs: S2QB –, S2/S3QB –, and S3QB – is expected after one, two, and three flashes, respectively (Sane et al., 2012). It was observed that after two turnover flashes, the transformation of the S2QB – charge pairs to S2/S3QB – was inhibited in ics1 in comparison with the WT (Fig. 3c). However, the oscillatory pattern of the B-band TLmax showed similar rhythmicity to that in the WT leaves (Fig. 3c).
Microarray analysis
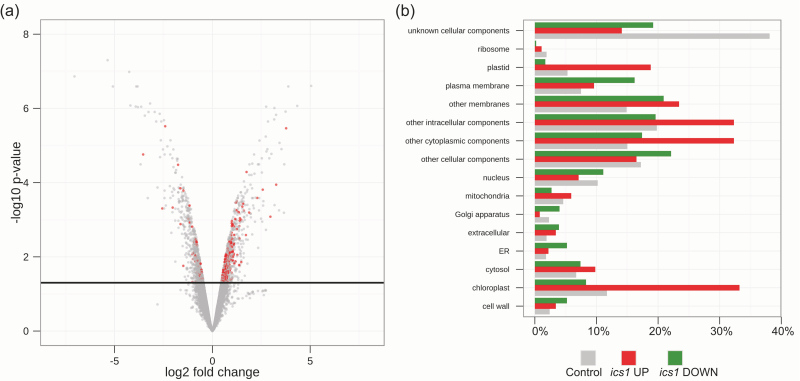
The redox status of the PQ pool may influence the expression of nuclear and chloroplast genes (Escoubas et al., 1995; Karpinski et al., 1997, 1999; Pfannschmidt et al., 1999, 2001; Muhlenbock et al., 2008). To test whether the mRNA profile of the non-treated ics1 mutant is different from that of the WT, publicly available microarray data (Behringer et al., 2011), deposited in GEO (GSE25489), were analysed. The total number of differentially regulated genes at P < 0.05 (in comparison with the WT) in ics1 was 1558. Of these, 773 were induced while 785 were inhibited in ics1. Interestingly, according to GO analysis, the protein products of genes induced in ics1 are predicted to be predominantly targeted to the chloroplasts (33.2%) (Fig. 5a, b). Such an enhancement of the chloroplast-targeted proteins was not observed among the inhibited transcripts in ics1 (8.3%) (Fig. 5a, b).
Fig. 5.
Microarray analysis of the ics1 mutant. (a) Volcano plot of microarray data. The log fold change is plotted on the x-axis (down-regulated genes on the left side, up-regulated on the right) and the negative log10 P-value is plotted on the y-axis. The black, solid line represents the P-value cut-off (0.05, Student’s t-test). Points above the line have P-values <0.05 and points below the line have P-values >0.05. The red points represent genes whose protein products are targeted to the chloroplasts. (b) Gene ontology (Cellular Component) of genes induced (red) and suppressed (green) in ics1. As a control, whole-genome categorization (grey) was used.
Efficiency of PSI in ics1
As the mutation in ICS1 causes a deficiency of phylloquinone, it was expected that the efficiency of PSI in the ics1 mutant would be decreased. However, the results show that there are no significant changes in the efficiency of PSI in ics1 as compared with the WT plants (Supplementary Fig. S2 at JXB online). It is worth noting that there are slight differences in the yield of PSI [Y(I)] and the acceptor-side limitation of PSI (YNA) (Supplementary Fig. S2). The donor-side limitation of PSI, YND, does not seem to be affected in the ics1 mutant (Supplementary Fig. S2).
Discussion
Two isochorismate synthase genes, ICS1 (AT1G74710) and ICS2 (AT1G18870), exist in the Arabidopsis genome. The protein products of these genes, ICS1 and ICS2, possess plastid-targeting signals and are indeed localized in the chloroplasts (Garcion et al., 2008). ICS converts chorismate to isochorismate, which is a precursor of SA as well as phylloquinone (Gross et al., 2006; Garcion et al., 2008). In contrast to the SA synthesis pathway, ICS-dependent phylloquinone synthesis is the only known pathway of phylloquinone synthesis in Arabidopsis. However, ics1 is commonly used as a model mutant with abolished SA synthesis, especially in experiments concerning plant pathogenesis (Wildermuth et al., 2001; Ichimura et al., 2006; Mishina and Zeier, 2007; Genger et al., 2008; Chen et al., 2009); the role of ICS1 in phylloquinone synthesis is often omitted. Therefore, the role of ICS1 in the regulation of photosynthesis, chloroplast membrane structure, and chloroplast to nucleus retrograde signalling was analysed.
To test if ICS1 influences ST, the changes of chlorophyll a fluorescence in light conditions which preferentially excite PSII (blue light) or PSI (blue light and far-red light) were measured (Fig. 1a). It was also shown that ST inhibition in ics1 is phylloquinone dependent (Fig. 1c) and is not caused by a strongly decreased level of SA in ics1 (Fig. 1b, c). The complete reversion of the q T and q S parameters in NA-treated ics1 plants was not observed, possibly due to a limitation of the treatment (spraying 3-week-old plants which had already developed leaves could influence the measured parameters, which were averaged from whole rosettes). The results obtained from NahG-overexpressing plants (localized in the cytoplasm and the chloroplast) indicate that SA is not directly involved in the regulation of ST. Furthermore, it was found that the q T and q S values are significantly decreased in ics1 (Table 1). To check whether the observed changes in fluorescence are due to impaired NPQ, the photosynthetic parameters were also measured in rosettes challenged with different light intensities. NPQ and CO2 assimilation in WT and ics1 plants were unaltered (Supplementary Fig. S1a, b at JXB online).
The results presented here prove that phylloquinone is an important element of the photosynthetic apparatus and not only plays a role in electron transfer through PSI, but is also essential for optimal energy distribution between photosystems. At present it cannot be asserted that the lack of phylloquinone causes conformational modifications of PSI so that it cannot bind the antennae complexes. It is also possible that due to efficient electron transfer through PSI, phylloquinone ensures an energetic equilibrium in both PSI and PSII and maintains the PQ pool in an optimally oxidized state. However, in PSI efficiency measurements for the ics1 mutant, a slightly increased yield of PSI [Y(I)] and a decreased acceptor side limitation (YNA) (Supplementary Fig. S2 at JXB online) were observed, which can be caused by an increased cyclic electron flow (CEF) around PSI (Table 1). These observations remain consistent with the measurements of the redox state of the PQ pool (expressed as 1–q P) in ics1, where PQ was more reduced than in the WT (Fig. 3a, Table 1). Variation in the PQ pool reduction levels between leaves in different developmental stages of ics1 plants was observed, while in WT and stn7 plants, the redox state of the PQ pool is independent of the leaf age (Fig. 4). This result suggests that the function of ICS1 is to stabilize the redox status of the PQ pool independently of the leaf age. This is required for optimization of immune defences and light acclimatory responses in the whole Arabidopsis thaliana rosette (Karpinski et al., 1999, 2013; Muhlenbock et al., 2008; Szechynska-Hebda et al., 2010).
Furthermore, glutathione reductase (GR) activity in ics1 was decreased, and changes in foliar SA and reduced glutathione levels in A. thaliana were positively correlated (Mateo et al., 2006). Therefore, lowered GR activity could be explained by an increased NADPH consumption by the CEF (Munekage and Shikanai, 2005), which in turn may cause increased reduction of the PQ pool (Karpinska et al., 2000).
The redox status of the PQ pool is well known as an important regulator of integrated light acclimatory and immune defence processes, and retrograde signalling from the chloroplasts to the nucleus in plant cells (Escoubas et al., 1995; Karpinski et al., 1997, 1999, 2013; Pfannschmidt et al., 1999; Kruk and Karpinski, 2006; Muhlenbock et al., 2008; Rochaix, 2011). For instance, it regulates ST by activation/deactivation of kinase/phosphatase (Allen, 2003; Depège et al., 2003). To confirm deregulation in the redox state of the PQ pool in the ics1 mutant, TL measurements were performed. TL originates from PSII via thermally stimulated delayed light which is emitted by singlet excited chlorophylls and generated by a recombination of the S2QA –, S2QB –, and S3QB – charge pairs, where S2 and S3 denote the oxidation states of the manganese OEC and the primary (QA) and secondary (QB) quinone electron acceptors in PSII (Ducruet, 2003; Ducruet and Vass, 2009). The charge pairs involved can be identified by their emission temperatures, which strongly depend on the redox potentials of the charge pairs. It is assumed that the dark-adapted samples contain ~25% S0 and 75% S1, and the distribution of QB and QB – has been shown to be 50:50 (Inoue, 1996). According to the previously described model (Inoue, 1996; Sane et al., 2012), in the dark-adapted samples the proportion of charge pairs is predicted as 25% S0/QB/QB – and 75% S1/QB/QB –. After first saturating, the turnover flash QB is converted to QB – and QB – to QB 2–. After two-electron reduction, QB 2– is transformed into a protonated form (QBH2), which is replaced by a new QB from the reoxidized PQ pool. After two turnover flashes, the TL signal originates predominantly from S3QB – (the lower intensity of TL is also emitted by the S2QB – charge pair) (Sane et al., 2012). The decreased amplitude of the B-band in the ics1 mutant after two turnover flashes strongly suggests that the PQ pool which provides QB at the acceptor side of PSII (the quinone reduction cycle) is more reduced than in the WT (Fig. 3b, c). These results strongly support the measurements of chlorophyll a fluorescence, which also indicate a more reduced state of the PQ pool in the ics1 mutant (Fig. 3a, Table 1).
Changes in the redox status of the PQ pool also affect expression of chloroplast- and nuclear-encoded genes (Escoubas et al., 1995; Karpinski et al., 1997, 1999; Pfannschmidt et al., 1999, 2001, 2009; Muhlenbock et al., 2008). These changes may adjust PSI and PSII stoichiometry in response to PSI- or PSII-favouring light (Pfannschmidt et al., 2009; Rochaix, 2011) or may induce expression of genes encoding ROS-scavenging enzymes when the plants are exposed to excess light (Karpinski et al., 1997, 1999) and immune defence-related genes (Mullineaux et al., 2000; Muhlenbock et al., 2008). Here it was shown that nuclear-encoded genes induced in the ics1 mutant encoded proteins predominantly targeted toward the chloroplasts (Fig. 5a, b). This supports the hypothesis that ICS1 is important for optimal photosynthesis (Mateo et al., 2006), and probably affects the whole cell through chloroplast to nucleus retrograde signalling dependent on the redox state of PQ pool (Figs 4, 5).
To test if the observed changes in the ST processes are connected with altered stoichiometry of the photosystems, the chlorophyll a/b ratio was measured (Pfannschmidt et al., 2001). Chlorophyll a/b was decreased in ics1 when compared with Col-0 (Table 1), which suggests that the PSII/PSI ratio is increased in ics1. PSI can only be located in unstacked thylakoids and at the ends of the thylakoid stacks, while PSII is located only in stacked grana (Dekker and Boekema, 2005). The increased level of PSII in relation to PSI in ics1 was further confirmed by the presence of higher numbers of stacked grana in ics1 (Fig. 2). On the other hand, the increased number of grana in ics1 can be explained by inhibited ST. During the state 1 to state 2 transition, thylakoid membrane remodelling occurs and results in the unstacking of layers in the grana. This process involves breakage of the connections between the layers in the grana domains, thus reducing the number of layers in the grana (Chuartzman et al., 2008). In the present experiment, the transmission electron microscopy samples were prepared from state 2-favouring conditions (low light, short photoperiod). The increased number of layers in ics1, in comparison with the Col-0 grana (Fig. 2), can be explained by the inhibited unstacking of grana when the ics1 plants are placed in state 2-favouring conditions.
The role of phylloquinone in the regulation of STs is unequivocal, but the mechanism of this process is still unknown. The results of the present experiments do not allow the exclusion of the role of the structural changes in PSI, leading to a steric obstacle for LHCII, or the higher reduction of the PQ pool, which is commonly known as a regulator of ST. Nevertheless, the results demonstrate that plant light acclimatory and immune defence responses are genetically, molecularly, physiologically, and biochemically linked to the functions of ICS1. These results have important implications for understanding how a plant’s immune defences have evolved and for understanding a plant’s biotic and abiotic integrated environmental stress response strategies. Therefore, the results may help in the development of the smart amelioration of plant performance under multivariable environmental conditions.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Light curves of various photosynthetic parameters in the WT and ics1 mutant.
Figure S2. PSI activity in the WT and ics1 mutant.
Acknowledgements
We thank Jean-Pierre Métraux and Floriane L’Haridon (University of Fribourg) for NahG seeds, and Ewa Niewiadomska and Zbigniew Miszalski (Institute of Plant Physiology, Polish Academy of Sciences) for help with Dual-Pam measurements. This research was supported by the Welcome2008/1 grant operated within the framework of the Foundation for Polish Science, co-financed by the European Regional Development Fund and by the REGPOT project FP7-286093 WULS-PLANT HEALTH.
References
- Allen JF. 2003. State transitions—a question of balance. Science 299, 1530–1532. [DOI] [PubMed] [Google Scholar]
- An C, Mou Z. 2011. Salicylic acid and its function in plant immunity. Journal of Integrative Plant Biology 53, 412–428. [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55, 373–399. [DOI] [PubMed] [Google Scholar]
- Asada K. 1999. The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology 50, 601–639. [DOI] [PubMed] [Google Scholar]
- Aviv DH, Rustérucci C, Holt BF, Dietrich RA, Parker JE, Dangl JL. 2002. Runaway cell death, but not basal disease resistance, in lsd1 is SA- and NIM1/NPR1-dependent. The Plant Journal 29, 381–391. [DOI] [PubMed] [Google Scholar]
- Baker NR. 2008. Chlorophyll fluorescence: a probe of photosynthesis in vivo . Annual Review of Plant Biology 59, 89–113. [DOI] [PubMed] [Google Scholar]
- Behringer C, Bartsch K, Schaller A. 2011. Safeners recruit multiple signalling pathways for the orchestrated induction of the cellular xenobiotic detoxification machinery in Arabidopsis. Plant, Cell and Environment 34, 1970–1985. [DOI] [PubMed] [Google Scholar]
- Bellafiore S, Barneche F, Peltier G, Rochaix JD. 2005. State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433, 892–895. [DOI] [PubMed] [Google Scholar]
- Bonardi V, Pesaresi P, Becker T, Schleiff E, Wagner R, Pfannschmidt T, Jahns P, Leister D. 2005. PSII core phosphorylation and photosynthetic long-term acclimation require two different kinases in Arabidopsis thaliana . Nature 437, 1179–1182. [DOI] [PubMed] [Google Scholar]
- Brautigam K, Dietzel L, Kleine T, et al. 2009. Dynamic plastid redox signals integrate gene expression and metabolism to induce distinct metabolic states in photosynthetic acclimation in Arabidopsis. The Plant Cell 21, 2715–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Xue L, Chintamanani S, et al. 2009. ETHYLENE INSENSITIVE3 and ETHYLENE INSENSITIVE3-LIKE1 repress SALICYLIC ACID INDUCTION DEFICIENT2 expression to negatively regulate plant innate immunity in Arabidopsis. The Plant Cell 21, 2527–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow WS, Melis A, Anderson JM. 1990. Adjustments of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proceedings of the National Academy of Sciences, USA 87, 7502–7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuartzman SG, Nevo R, Shimoni E, Charuvi D, Kiss V, Ohad I, Brumfeld V, Reich Z. 2008. Thylakoid membrane remodeling during state transitions in Arabidopsis. The Plant Cell 20, 1029–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SM, Mur LAJ, Wood JE, Scott IM. 2004. Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. The Plant Journal 38, 432–447. [DOI] [PubMed] [Google Scholar]
- Coll NS, Epple P, Dangl JL. 2011. Programmed cell death in the plant immune system. Cell Death and Differentiation 18, 1247–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damkjaer JT, Kereïche S, Johnson MP, Kovacs L, Kiss AZ, Boekema EJ, Ruban AV, Horton P, Jansson S. 2009. The photosystem II light-harvesting protein Lhcb3 affects the macrostructure of photosystem II and the rate of state transitions in Arabidopsis. The Plant Cell 21, 3245–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker JP, Boekema EJ. 2005. Supramolecular organization of thylakoid membrane proteins in green plants. Biochimica et Biophysica Acta 1706, 12–39. [DOI] [PubMed] [Google Scholar]
- Depège N, Bellafiore S, Rochaix JD. 2003. Role of chloroplast protein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science 299, 1572–1575. [DOI] [PubMed] [Google Scholar]
- Ducruet JM. 2003. Chlorophyll thermoluminescence of leaf discs: simple instruments and progress in signal interpretation open the way to new ecophysiological indicators. Journal of Experimental Botany 54, 2419–2430. [DOI] [PubMed] [Google Scholar]
- Ducruet JM, Vass I. 2009. Thermoluminescence: experimental. Photosynthesis Research 101, 195–204. [DOI] [PubMed] [Google Scholar]
- Escoubas JM, Lomas M, LaRoche J, Falkowski PG. 1995. Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinone pool. Proceedings of the National Academy of Sciences, USA 92, 10237–10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragnière C, Serrano M, Abou-Mansour E, Métraux JP, L’Haridon F. 2011. Salicylic acid and its location in response to biotic and abiotic stress. FEBS Letters 585, 1847–1852. [DOI] [PubMed] [Google Scholar]
- Frenkel M, Bellafiore S, Rochaix JD, Jansson S. 2007. Hierarchy amongst photosynthetic acclimation responses for plant fitness. Physiologia Plantarum 129, 455–459. [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J. 1993. Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261, 754–756. [DOI] [PubMed] [Google Scholar]
- Garcion C, Lohmann A, Lamodière E, Catinot J, Buchala A, Doermann P, Métraux JP. 2008. Characterization and biological function of the ISOCHORISMATE SYNTHASE2 gene of Arabidopsis. Plant Physiology 147, 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genger RK, Jurkowski GI, Mcdowell JM, Lu H, Jung HW, Greenberg JT, Bent AF. 2008. Signaling pathways that regulate the enhanced disease resistance of Arabidopsis ‘defense, no death’ mutants. Molecular Plant-Microbe Interactions 21, 1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman C, Carey VJ, Bates DM, et al. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biology 5, R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Cho WK, Lezhneva L, Falk J, Krupinska K, Shinozaki K, Seki M, Herrmann RG, Meurer J. 2006. A plant locus essential for phylloquinone (vitamin K1) biosynthesis originated from a fusion of four eubacterial genes. Journal of Biological Chemistry 281, 17189–17196. [DOI] [PubMed] [Google Scholar]
- Ichimura K, Casais C, Peck SC, Shinozaki K, Shirasu K. 2006. MEKK1 is required for MPK4 activation and regulates tissue-specific and temperature-dependent cell death in Arabidopsis. Journal of Biological Chemistry 281, 36969–36976. [DOI] [PubMed] [Google Scholar]
- Inoue Y. 1996. Photosynthetic thermoluminescence as a simple probe of photosystem II electron transport. In: Amesz J, Hoff AJ, eds. Biophysical techniques in photosynthesis. Berlin: Springer, 93–107. [Google Scholar]
- Karnovsky MJ. 1965. A formaldehyde–glutaraldehyde fixative of high osmolarity for use in electron microscopy. Journal of Cell Biology 27, 137–138. [Google Scholar]
- Karpinska B, Wingsle G, Karpinski S. 2000. Antagonistic effects of hydrogen peroxide and glutathione on acclimation to excess excitation energy in Arabidopsis. IUBMB Life 50, 21–26. [DOI] [PubMed] [Google Scholar]
- Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux PM. 1997. Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. The Plant Cell 9, 627–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski S, Gabrys H, Mateo A, Karpinska B, Mullineaux PM. 2003. Light perception in plant disease defence signalling. Current Opinion in Plant Biology 6, 390–396. [DOI] [PubMed] [Google Scholar]
- Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P. 1999. Systemic signalling and acclimation in response to excess excitation energy in Arabidopsis. Science 284, 654–657. [DOI] [PubMed] [Google Scholar]
- Karpinski S, Szechynska-Hebda M, Wituszynska W, Burdiak P. 2013. Light acclimation, retrograde signalling, cell death and immune defences in plants. Plant, Cell and Environment 36, 736–44. [DOI] [PubMed] [Google Scholar]
- Kruk J, Karpinski S. 2006. An HPLC-based method of estimation of the total redox state of plastoquinone in chloroplasts, the size of the photochemically active plastoquinone-pool and its redox state in thylakoids of Arabidopsis. Biochimica et Biophysica Acta 1757, 1669–1675. [DOI] [PubMed] [Google Scholar]
- Kruk J, Strzałka K, Schmid GH. 1994. Antioxidant properties of plastoquinol and other biological prenylquinols in liposomes and solution. Free Radical Research 21, 409–416. [DOI] [PubMed] [Google Scholar]
- Laloi C, Stachowiak M, Pers-Kamczyc E, Warzych E, Murgia I, Apel K. 2007. Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 104, 672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wakao S, Fischer BB, Niyogi KK. 2009. Sensing and responding to excess light. Annual Review of Plant Biology 60, 239–260. [DOI] [PubMed] [Google Scholar]
- Loake G, Grant M. 2007. Salicylic acid in plant defence—the players and protagonists. Current Opinion in Plant Biology 10, 466–472. [DOI] [PubMed] [Google Scholar]
- Mateo A, Funck D, Muhlenbock P, Kular B, Mullineaux PM, Karpinski S. 2006. Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. Journal of Experimental Botany 57, 1795–1807. [DOI] [PubMed] [Google Scholar]
- Mateo A, Muhlenbock P, Rusterucci C, Chang CC-C, Miszalski Z, Karpinska B, Parker JE, Mullineaux PM, Karpinski S. 2004. LESION SIMULATING DISEASE 1 is required for acclimation to conditions that promote excess excitation energy. Plant Physiology 136, 2818–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina TE, Zeier J. 2007. Pathogen-associated molecular pattern recognition rather than development of tissue necrosis contributes to bacterial induction of systemic acquired resistance in Arabidopsis. The Plant Journal 50, 500–513. [DOI] [PubMed] [Google Scholar]
- Misra AN, Dilnawaz F, Misra M, Biswal AK. 2001. Thermoluminescence in chloroplasts as an indicator of alterations in photosystem 2 reaction centre by biotic and abiotic stresses. Photosynthetica 39, 1–9. [Google Scholar]
- Miyake C, Miyata M, Shinzaki Y, Tomizawa K. 2005. CO2 response of cyclic electron flow around PSI (CEF-PSI) in tobacco leaves—relative electron fluxes through PSI and PSII determine the magnitude of non-photochemical quenching (NPQ) of Chl fluorescence. Plant and Cell Physiology 46, 629–637. [DOI] [PubMed] [Google Scholar]
- Mullineaux PM, Ball L, Escobar C, Karpinska B, Creissen G, Karpinski S. 2000. Are diverse signalling pathways integrated in the regulation of arabidopsis antioxidant defence gene expression in response to excess excitation energy? Philosophical Transactions of the Royal Society B: Biological Sciences 355, 1531–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlenbock P, Szechynska-Hebda M, Plaszczyca M, Baudo M, Mullineaux PM, Parker JE, Karpinska B, Karpinski S. 2008. Chloroplast signaling and LESION SIMULATING DISEASE1 regulate crosstalk between light acclimation and immunity in Arabidopsis. The Plant Cell 20, 2339–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munekage Y, Shikanai T. 2005. Cyclic electron transport through photosystem I. Plant Biotechnology 22, 361–369. [Google Scholar]
- Niewiadomska E, Bilger W, Gruca M, Mulisch M, Miszalski Z, Krupinska K. 2011. CAM-related changes in chloroplastic metabolism of Mesembryanthemum crystallinum L. Planta 233, 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK. 1999. Photoprotection revisited: genetic and molecular approaches. Annual Review of Plant Physiology and Plant Molecular Biology 50, 333–359. [DOI] [PubMed] [Google Scholar]
- Pfannschmidt T, Bräutigam K, Wagner R, Dietzel L, Schröter Y, Steiner S, Nykytenko A. 2009. Potential regulation of gene expression in photosynthetic cells by redox and energy state: approaches towards better understanding. Annals of Botany 103, 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannschmidt T, Nilsson A, Allen JF. 1999. Photosynthetic control of chloroplast gene expression. Nature 397, 625–628. [Google Scholar]
- Pfannschmidt T, Schütze K, Brost M, Oelmüller R. 2001. A novel mechanism of nuclear photosynthesis gene regulation by redox signals from the chloroplast during photosystem stoichiometry adjustment. Journal of Biological Chemistry 276, 36125–36130. [DOI] [PubMed] [Google Scholar]
- Pribil M, Pesaresi P, Hertle A, Barbato R, Leister D. 2010. Role of plastid protein phosphatase TAP38 in LHCII dephosphorylation and thylakoid electron flow. PLoS Biology 8, e1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochaix JD. 2011. Regulation of photosynthetic electron transport. Biochimica et Biophysica Acta 1807, 375–383. [DOI] [PubMed] [Google Scholar]
- Ruban A, Lavaud J, Rousseau B, Guglielmi G, Horton P, Etienne AL. 2004. The super-excess energy dissipation in diatom algae: comparative analysis with higher plants. Photosynthesis Research 82, 165–175. [DOI] [PubMed] [Google Scholar]
- Sane PV, Ivanov AG, Öquist G, Hüner NPA. 2012. Thermolumin escence. In Eaton-Rye JJ, Tripathy BC, Sharkey TD. (eds), Photosynthesis: plastid biology, energy conversion and carbon assimilation. Advances in Photosynthesis and Respiration volume 34.: Dordrecht: Springer, PP. 445–474. [Google Scholar]
- Shapiguzov A, Ingelsson B, Samol I, Andres C, Kessler F, Rochaix JD, Vener AV, Goldschmidt-Clermont M. 2010. The PPH1 phosphatase is specifically involved in LHCII dephosphorylation and state transitions in Arabidopsis. Proceedings of the National Academy of Sciences, USA 107, 4782–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth GK. 2005. Limma: linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry R, Huber H, eds. Bioinformatics and computational biology solutions using R and Bioconductor. New York: Springer, 397–420. [Google Scholar]
- Szechynska-Hebda M, Kruk J, Gorecka M, Karpinska B, Karpinski S. 2010. Evidence for light wavelength-specific systemic photoelectrophysiological signalling and cellular light memory of excess light episode in Arabidopsis. The Plant Cell 22, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breusegem F, Bailey-Serres J, Mittler R. 2008. Unraveling the tapestry of networks involving reactive oxygen species in plants. Plant Physiology 147, 978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oostende C, Widhalm JR, Basset JC. 2008. Detection and quantification of vitamin K1 quinol in leaf tissues. Phytochemistry 69, 2457–2462. [DOI] [PubMed] [Google Scholar]
- Vlot AC, Dempsey DA, Klessig DF. 2009. Salicylic acid, a multifaceted hormone to combat disease. Annual Review of Phytopathology 47, 177–206. [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM. 2001. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414, 562–565. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Miller CJ. 2005. Simpleaffy: a BioConductor package for Affymetrix quality control and data analysis. Bioinformatics 21, 3683–3685. [DOI] [PubMed] [Google Scholar]
- Wituszynska W, Galazka K, Rusaczonek A, Vanderauwera S, Van Breusegem F, Karpinski S. 2013. Multivariable environmental conditions promote photosynthetic adaptation potential in Arabidopsis thaliana. Journal of Plant Physiology 170, 548–559. [DOI] [PubMed] [Google Scholar]