Abstract
Antisense-mediated down-regulation of the fruit-specific polygalacturonase (PG) gene FaPG1 in strawberries (Fragaria×ananassa Duch.) has been previously demonstrated to reduce fruit softening and to extend post-harvest shelf life, despite the low PG activity detected in this fruit. The improved fruit traits were suggested to be attributable to a reduced cell wall disassembly due to FaPG1 silencing. This research provides empirical evidence that supports this assumption at the biochemical, cellular, and tissue levels. Cell wall modifications of two independent transgenic antisense lines that demonstrated a >90% reduction in FaPG1 transcript levels were analysed. Sequential extraction of cell wall fractions from control and ripe fruits exhibited a 42% decrease in pectin solubilization in transgenic fruits. A detailed chromatographic analysis of the gel filtration pectin profiles of the different cell wall fractions revealed a diminished depolymerization of the more tightly bound pectins in transgenic fruits, which were solubilized with both a chelating agent and sodium carbonate. The cell wall extracts from antisense FaPG1 fruits also displayed less severe in vitro swelling. A histological analysis revealed more extended cell–cell adhesion areas and an enhanced tissue integrity in transgenic ripe fruits. An immunohistological analysis of fruit sections using the JIM5 antibody against low methyl-esterified pectins demonstrated a higher labelling in transgenic fruit sections, whereas minor differences were observed with JIM7, an antibody that recognizes highly methyl-esterified pectins. These results support that the increased firmness of transgenic antisense FaPG1 strawberry fruits is predominantly due to a decrease in pectin solubilization and depolymerization that correlates with more tightly attached cell wall-bound pectins. This limited disassembly in the transgenic lines indicates that these pectin fractions could play a key role in tissue integrity maintenance that results in firmer ripe fruit.
Key words: Cell wall, Fragaria×ananassa, fruit ripening, fruit softening, pectins, polygalacturonase.
Introduction
The cultivated strawberry (Fragaria×ananassa Duch.) is the most economically important soft fruit. This fruit has a short post-harvest life due to its rapid softening, which results in large post-harvest losses due to bruising, over-softening, and fungal infections. In addition to their economic importance, strawberries have been mostly studied as a non-climacteric model species to elucidate the molecular basis of fruit softening. Currently, strawberries are probably the second most important fruit, after the tomato, in which a high number of ripening-related genes have been analysed using transgenesis (Mercado et al., 2011; Posé et al., 2011).
Fruit softening occurs predominantly by the disassembly of the cell wall and the dissolution of the middle lamella, which attenuates cell–cell adhesion (Goulao and Oliveira, 2008; Mercado et al., 2011). These processes involve the coordinate action of numerous enzymes that act on different cell wall polysaccharides. Thus, the genetic manipulation of genes encoding proteins that participate in wall disassembly has been explored as a biotechnological approach to reduce fruit softening. Strawberry cell wall disassembly during ripening is mainly characterized by pectin solubilization, galactose and arabinose loss, and xyloglucan depolymerization (Perkins-Veazie, 1995; Santiago-Doménech et al., 2008; Posé et al., 2011). Among these processes, pectin solubilization is the most consistent wall modification during strawberry softening. Pectin is solubilized at the expense of the pectin fraction that is covalently bound to the cell wall (Posé et al., 2011), which can be initiated by different processes, including a loss of the calcium-stabilized pectin gel structure (Knee et al., 1977; Lara et al., 2004), cleavage of pectin and hemicellulose connections (Nogata et al., 1996), disentanglement of pectin complexes due to the hydrolysis of pectin neutral side chains (Redgwell et al., 1997a ; Trainotti et al., 2001), and, finally, pectin depolymerization (Rosli et al., 2004; Santiago-Doménech et al., 2008; Figueroa et al., 2010). This last process was initially considered to be of minor importance in strawberries due to the low or undetectable polygalacturonase (PG) activity levels (Huber, 1984; Nogata et al., 1996). However, the transgenic manipulation of ripening-related genes encoding pectin-degrading enzymes has challenged this assumption. Thus, the silencing of a pectate lyase gene, an enzyme that catalyses the cleavage of the unesterified galacturonosyl linkage via a β-elimination reaction, significantly increased fruit firmness and reduced post-harvest softening (Jiménez-Bermúdez et al., 2002; Youssef et al., 2009, 2013). This firmer genotype correlated not only with a lower pectin solubilization but also with a decreased depolymerization of covalently bound pectins that were extracted after treating the cell walls with sodium carbonate (Santiago-Doménech et al., 2008). Two ripening-related PG genes, FaPG1 and FaPG2, which are up-regulated during fruit ripening, have been cloned (Salentijn et al., 2003; Villarreal et al., 2008; Quesada et al., 2009a ). FaPG1 silencing by antisense transformation significantly reduced strawberry fruit softening without affecting other ripening-related traits, such as colour, weight, or soluble solids (Quesada et al., 2009a ). Furthermore, at ripening, antisense FaPG1 fruits were slightly firmer than antisense pectate lyase fruits (Quesada et al., 2009b ). A preliminary characterization of the cell wall of transgenic fruits with down-regulated FaPG1 demonstrated that the firmer phenotype correlated with decreased pectin solubilization (Quesada et al., 2009a ), but detailed analyses of wall fraction characteristics were not presented. Accordingly, the main objective of the present work was to ascertain the role of the pectinase gene FaPG1 in fruit softening via a detailed analysis of cell wall changes induced by the down-regulation of this gene. Thus, characteristics of strawberry fruits with low levels of FaPG1 expression were analysed, with a particular focus on the pectic matrix, as this is the putative target of this enzyme. Although the experimental study mainly relied on analyses of cell wall extracts, histological and microscopic analyses were also performed to generate a general overview of the function of the FaPG1 gene in strawberry fruit softening.
Materials and methods
Plant material and phenotypic analysis
Control, non-transformed, strawberry (Fragaria×ananassa Duch. cv. ‘Chandler’) plants and two independent transgenic antisense PG lines (APG29 and APG62; described in Quesada et al., 2009a ) were grown in a greenhouse under the natural temperature and photoperiod. Transgenic ripe fruits exhibited a strong reduction in FaPG1 mRNA levels, ~95% for both transgenic lines. The fruits were harvested at the red ripe stage, frozen in liquid nitrogen, and stored at –25 ºC. A minimum of 25 fresh fruits per line were measured for soluble solid content and firmness. Their firmness was assessed using a hand-held penetrometer (Effegi) with a cylindrical needle with a 9.62mm2 area. The soluble solid percentage was estimated using an Atago N1 refractometer. The ripening stage was also characterized by measuring the anthocyanin content according to Nunes et al. (2006). This study was conducted during three consecutive years, where the plants from each year were derived using runner propagation from mother plants.
Cell wall analysis
The cell walls were extracted from frozen ripe fruits following the method of Redgwell et al. (1992) with some modifications as previously described in Santiago-Doménech et al. (2008). Briefly, 10–15 frozen fruits were ground to a powder in liquid N2, and 20g was homogenized in 40ml of PAW (phenol:acetic acid:water, 2:1:1, w/v/v). The homogenate was centrifuged at 4000 g for 15min and the supernatant filtered through Miracloth (Merck, Bioscience, UK). The pellet obtained after PAW extraction was treated with 90% aqueous dimethylsulphoxide (DMSO) to solubilize the starch. The extract was then centrifuged at 4000 g and the pellet washed twice with distilled water. The water fraction was discarded, and the de-starched pellet, which is considered the cell wall material (CWM), was lyophilized and weighed. The CWM was sequentially extracted as previously described by Santiago-Doménech et al. (2008). Briefly, 150mg of the CWM fraction was sequentially extracted with deionized water; 0.05M trans-1,2-diaminocyclohexane-N,N,N′N′-tetra-acetic acid (CDTA) in 0.05M sodium acetate buffer, pH 6; 0.1M Na2CO3 containing 0.1% NaBH4; 1M KOH containing 0.1% NaBH4; and 4M KOH containing 0.1% NaBH4. All fractions were extensively dialysed against distilled water. Then, the extracts were concentrated with a rotary evaporator and freeze-dried. Before dialysis, the alkaline fractions were neutralized using concentrated HCl. Three independent complete sets of fractionations per CWM sample were performed.
The neutral sugar content was colorimetrically quantified following the orcinol method (Rimington, 1931) with glucose as the standard. The uronic acid (UA) content was determined by the carbazole method (Filisetti-Cozzi and Carpita, 1991) with galacturonic acid (GalA) as the standard. The absorbance data were corrected as described by Montreuil et al. (1997) to eliminate mutual interferences between neutral sugars and the UA in the carbazole and orcinol methods, respectively.
Size exclusion chromatography
Polymers present in the different cell wall fractions were fractionated as described by Santiago-Domenech et al. (2008). A manually poured column (1×40cm) of Sepharose CL-6B (Sigma-Aldrich Química SA, Spain) was used to analyse the polymers in the PAW and water fractions. The gel medium was equilibrated with 0.2M acetate buffer, pH 5, and the cell wall fractions (5–7mg) were dissolved in 1ml of buffer, loaded on the column (250 μl), and eluted at 14ml h–1. Fractions of 1ml were collected and assayed for UA and total sugar contents. Chromatographies of the CDTA, Na2CO3, and both KOH fractions were performed with similarly sized Sepharose CL-2B columns. For the CDTA extracts, the gel medium was equilibrated in the same buffer as above, but the columns were equilibrated with 0.05M TRIS-HCl buffer, pH 8.5, for the alkaline extracts. The results presented here correspond to the average profile of at least two independent chromatographic assays per sample.
PACE analysis of pectin fractions
CDTA pectin fractions were analysed with carbohydrate gel electrophoresis (PACE). The pectin samples, diluted to a concentration of 0.5mg ml–1 of galacturonic acid in 0.1M ammonium acetate buffer, pH 5.5, were treated with endo-PG (EC 3.2.1.15; from Aspergillus aculeatus; Megazyme) at 25 ºC for 0, 30, and 60min. Then, the reaction was stopped by boiling the samples for 10min. The pectins in the CDTA fraction were previously de-esterified by incubating the samples with 1M NaOH at room temperature for 30min as previously described (Barton et al., 2006). The samples were derivatized with 2-aminoacridone (AMAC) as described by Goubet et al. (2003). The derivatized samples were resuspended in 100 μl of 6M urea and stored at –20 °C.
Samples (5 μl) were separated using the Hoefer SE 660 vertical slab gel electrophoresis apparatus (Amersham, UK) with 24cm plates and a 0.75mm spacer with 0.25cm wide wells. Electrophoresis was performed at 4 °C. The resolving gel contained 25% (w/v) polyacrylamide and 0.5% (w/v) N,N-methylenebisacrylamide, and the stacking gel (2cm) was composed of 8% (w/v) polyacrylamide and 0.2% (w/v) N,N-methylenebisacrylamide. Both resolving and stacking gels were prepared in 0.1M TRIS-HCl, pH 8.2. A discontinuous electrophoresis buffer system was used: 0.15M TRIS brought to pH 8.5 with 0.15M glycine as the cathode reservoir buffer and 0.1M TRIS-HCl, pH 8.2, as the anode reservoir buffer. The samples were electrophoresed initially at 200V for 20min and then at 1000V for 3h. The gels were scanned using a BIORAD Gel DocXR+ Imaging System. Galacturonic acid (GalA), GalA2, and GalA3 (Sigma-Aldrich) were used as standards. All of the gels were run at least twice using independent samples.
In vitro cell wall swelling
The cell wall material was freeze-dried, and 7mg was suspended in 0.4ml of water in a 1.5ml glass vial (4mm i.d.). The suspension was vortexed several times and allowed to settle for 48h at room temperature. Then, the height of the cell wall column was measured, and these data were used as an index of wall swelling. The sample was quickly pelleted in a centrifuge, and the water was replaced with 250 μl of 0.05M CDTA in 0.05M sodium acetate buffer, pH 6, and treated as described above. After 48h of incubation, the wall column height was recorded, and the sample was washed twice with water and then treated sequentially with 0.05M Na2CO3 and 1M KOH, both containing 0.1% NaBH4. Each sample was generated in triplicate.
Immunolabelling of fruit sections
Small cylinders (12 × 5mm) were obtained from ripe fruits harvested from control and transgenic plants. All cylinders included cortical and pith tissues and were extracted from the region of the fruit with the largest diameter. They were immediately fixed in a solution containing 0.1M phosphate buffer (pH 7.3), 4% (w/v) formaldehyde, and 2.5% (v/v) glutaraldehyde, and subjected to a mild vacuum. The sections were left in a vacuum for 2h and stored at 4 °C overnight in a fixation solution. The samples were dehydrated in an ethanol series and subsequently infiltrated with resin Unicryl (1:1) at –20 ºC overnight and pure Unicryl for 2 d at –20 ºC. Embedded samples were cured at a low temperature with UV light for 4 d. Thin sections (4 μm) were cut using a hard tissue microtome (Microm HM 355S), mounted on glass slides, and stained with 1% toluidine blue buffered in phosphate-citrate buffer, pH 4, for 5min to visualize the tissue anatomy.
After assessing the histological integrity and quality of the samples, sections were processed for immunohistological analysis using the monoclonal antibodies JIM5 and JIM7, which were kindly provided by Professor Knox (University of Leeds, UK). JIM5 is an anti-pectin antibody that recognizes low methyl esterification levels on homogalacturonan (HGA) pectins, whereas JIM7 recognizes highly methyl-esterified HGA (Clausen et al., 2003). Briefly, the sections were blocked in PBT [0.1M phosphate-buffered saline, pH 7.2, 0.3% Triton X-100, 0.3% bovine serum albumin (BSA)] for 90min. The sections were incubated with a primary antibody in blocking buffer for 90min, rinsed with PBS (0.1M phosphate-buffered saline, pH 7.3; 0.3M NaCl) and then incubated with a secondary antibody (Alexa fluor 488; goat anti-rat IgG; Invitrogen #A11006; 1:1000 dilution) in blocking buffer for 1h. The sections were rinsed with PBS four times. The control sections for the immunoassay were subjected to the same treatment excluding the primary antibody incubation. The sections were mounted with Mowiol, and the green fluorescence was examined using a fluorescein isothiocyanatee (FITC) filter block in an epifluorescence microscope (Nikon Eclipse E800). A minimum of five independent fruits per genotype were processed.
Scanning electron microscopy
Small pieces of ripe control and transgenic fruits were fixed in a mixture of 4% formaldehyde and 2.5% glutaraldehyde in 0.1M phosphate buffer (pH 7.3). The samples were dehydrated in an ethanol series, dried with CO2, mounted, sputter-coated with evaporated gold, and observed in a JEOL JSM-840 (Japan) scanning electron microscope.
Statistical analysis
The SPSS software package (v.19.0, IBM Corp., Route 100, Somers, NY, USA) was used for all the statistical analyses. Analysis of variance (ANOVA) was used to compare genotypes, and post-hoc mean comparisons were performed using Tukey’s HSD test (P=0.05).
Results
Stability of the firmer fruit phenotype and the ripening stage
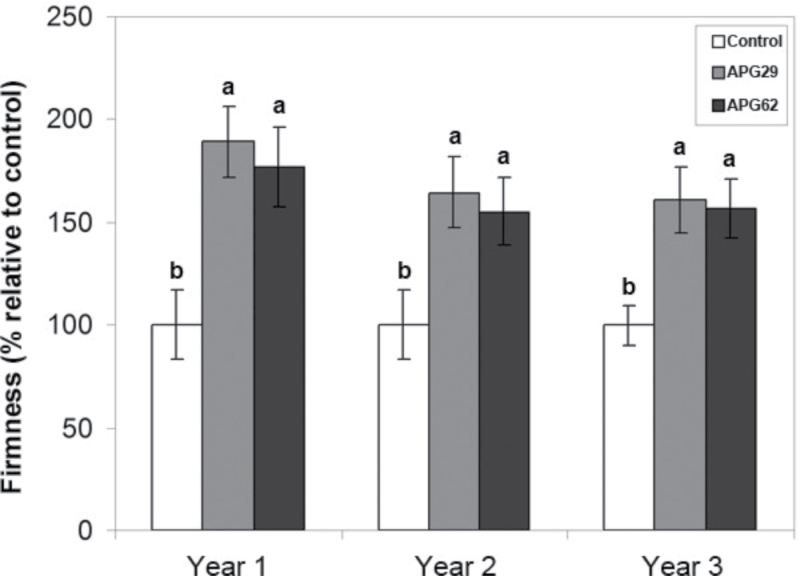
The transgenic fruits were evaluated for three consecutive years, where each year the plants were renewed by vegetative propagation through runners. Figure 1 demonstrates the consistency and stability of the ‘firmer fruit’ phenotype previously reported for FaPG1 antisense strawberry lines (Quesada et al., 2009a ). Transgenic fresh fruits were significantly firmer than control fruits at the red-ripe stage during all three consecutive growing seasons, where both transgenic lines were at least 55% firmer than the controls (Fig. 1). This study focused on the cell walls of the red-ripe fruit because the phenotype is consistently displayed at this stage, and the differences between the control and transgenic lines become more prominent. To ensure that both control and transgenic fruits were harvested at the same ripening stage, soluble solids and the anthocyanin content were measured as stage estimators. The soluble solid content was similar in control and transgenic lines and there were no differences during all three years of analysis (Supplementary Fig. S1 available at JXB online). Likewise, anthocyanin levels were similar in control and transgenic lines, which displayed an average value of 50.9±1.1mg pelargonidin (Pg-3-glu) 100g–1 of fruit (Supplementary Fig. S2). Altogether, these results indicate that control and transgenic fruits sampled for cell wall analysis were harvested at equivalent ripening stages.
Fig. 1.
Firmness of control and transgenic ripe fruits of the APG29 and APG62 lines was evaluated for three consecutive years. The data represent the means ±SD of 30 fruits per line. The bars with different letters within each year are significantly different by the Tukey-HSD test (P=0.05).
Cell wall fraction yields
The CWM from ripe control and transgenic fruit lines, APG29 and APG62, respectively, was extracted according to Santiago-Doménech et al. (2008) with PAW as an enzyme inhibitor. Total CWM yields were statistically higher in the transgenic lines (Table 1). In contrast, the amount in the soluble PAW fraction was significantly lower from transgenic fruits than from controls (Table 1). The CWM was sequentially fractionated with water, CDTA, sodium carbonate, 1M KOH, and 4M KOH to extract fractions enriched in water-soluble pectins, ionically bound pectins (CDTA), covalently bound pectins (CO3), and hemicellulosic polymers (KOH fractions), respectively. Interestingly, the amounts of the ionically bound, and especially covalently bound, fractions were higher in transgenic fruits than in controls, whereas only minor differences were observed for the other fractions (Table 1).
Table 1.
Yield of PAW-soluble polymers, total CWM, and its sequentially extracted fractions from control and transgenic APG ripe fruits
| PAW (g 100g–1 FW) | CWM (g 100g–1 FW) | Cell wall fractions (g 100g–1 CWM) | ||||||
|---|---|---|---|---|---|---|---|---|
| Water | CDTA | Na2CO3 | KOH 1 M | KOH 4 M | Residue | |||
| Control | 0.10 a | 0.87 b | 3.3 a | 7.5 a | 8.4 b | 6.5 a | 6.5 a | 20.2 a |
| APG29 | 0.08 a,b | 1.04 a,b | 3.3 a | 9.1 a | 11.9 a | 7.3 a | 7.2 a | 18.5 a |
| APG62 | 0.07 b | 1.12 a | 2.9 a | 9.7 a | 12.5 a | 7.7 a | 7.8 a | 19.8 a |
Data correspond to the mean ±SD. Within each column, means with different letters are significantly different by the Tukey-HSD test at P=0.05.
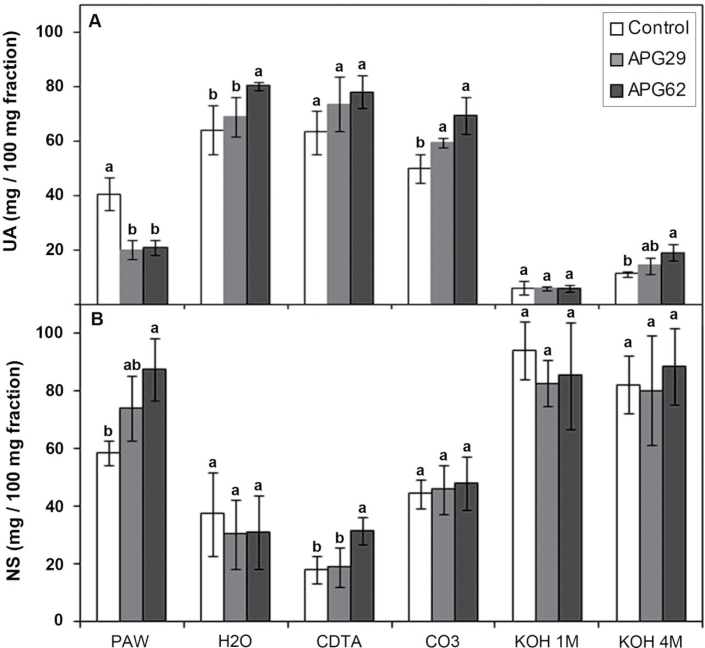
The water, CDTA, and CO3 fractions were predominantly composed of UA, whereas the KOH fractions were enriched for neutral sugars (Fig. 2). The carbohydrate composition of the PAW fractions displayed a contrasting behaviour depending on the genotype. Thus, the control PAW exhibited an almost equal amount of UA and neutral sugars. In contrast, transgenic PAW fractions harboured a significantly lower proportion of UA (Fig. 2). The total pectin amount in the cell wall, estimated by the sum of the UA content in the different fractions, was similar in control and transgenic APG29 lines, but this value was slightly higher in the APG62 line (15.7 versus 20.4mg 100mg–1 CWM in the control and APG62 lines, respectively). According to Redgwell et al. (1992), polymers soluble in PAW and water are freely soluble in the apoplast and were solubilized by the in vivo processes. Interestingly, transgenic lines displayed a 42% reduction in the amount of soluble pectin but higher levels of ionic and CO3 pectins when the UA content was expressed on a cell wall basis (Supplementary Fig. S3 at JXB online). This bound pectin increment was due to both increased amounts of these fractions in the transgenic CWM (Table 1) and a higher content of UA in the CDTA and CO3 fractions (Fig. 2A). Thus, the ratio of the soluble (PAW plus water) to the bound (CDTA plus CO3) pectins was significantly higher in control (0.7) than in transgenic lines (0.3). The KOH fractions harboured a lower amount of UA than the other fractions. However, both transgenics displayed higher residual pectin contents in the 4M KOH fraction than the controls (Fig. 2A). The amount of neutral sugars per fraction was similar in control and transgenic lines, with the exception of the transgenic PAW fractions and the CDTA fraction from line APG62, which exhibited higher neutral sugar levels than the control (Fig. 2B). Altogether, these results suggest a lower pectin solubilization in the transgenic APG fruits. Notably, both APG lines displayed a similar cell wall modification pattern, although APG62 exhibited more conspicuous differences in some fractions.
Fig. 2.
Amount of uronic acids (A) and neutral sugars (B) in the cell wall fractions extracted from ripe strawberry fruits. The data correspond to the mean ±SD from three independent extractions. The different letters within the same fraction indicate significant differences by the Tukey-HSD test (P=0.05).
Analysis of cell wall fractions by gel permeation chromatography
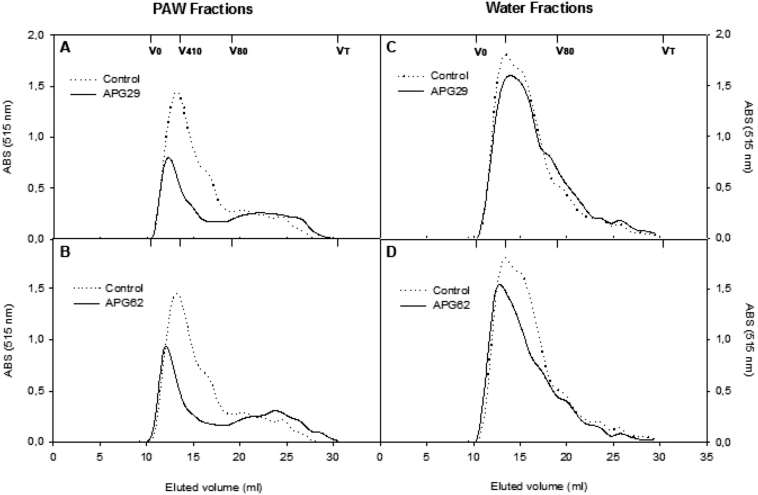
Chromatographic profiles were developed for the different cell wall fractions. The profiles of the pectic polymers present in the soluble fractions, PAW and water, are represented in Fig. 3. The PAW-soluble polyuronides formed a main polymer peak with a molecular mass >410kDa and a tail of lower molecular mass pectins extending throughout the separation range of the column (Fig. 3A, B). The main control sample peak was wider than in the transgenic samples, and the transgenic profiles were enriched in low molecular weight polyuronides with an average molecular mass of <80kDa. As observed for the PAW fractions, water-soluble polyuronides formed a unique main peak with an average molecular mass of >410kDa and a tail of lower molecular mass polymers. In this case, minor differences were observed between the control and transgenic lines (Fig. 3C, D).
Fig. 3.
Molecular mass profiles of soluble polyuronides extractable by PAW (A, B) and water (C, D) from fruit cell walls of control and two independent APG transgenic lines. The profiles were obtained by gel filtration chromatography on Sepharose CL6B. The fractions were assayed for uronic acid, and the pectin contents were estimated by the absorbance values at 515nm. The elution volumes for the different dextran standards and acetone used for column calibration are presented.
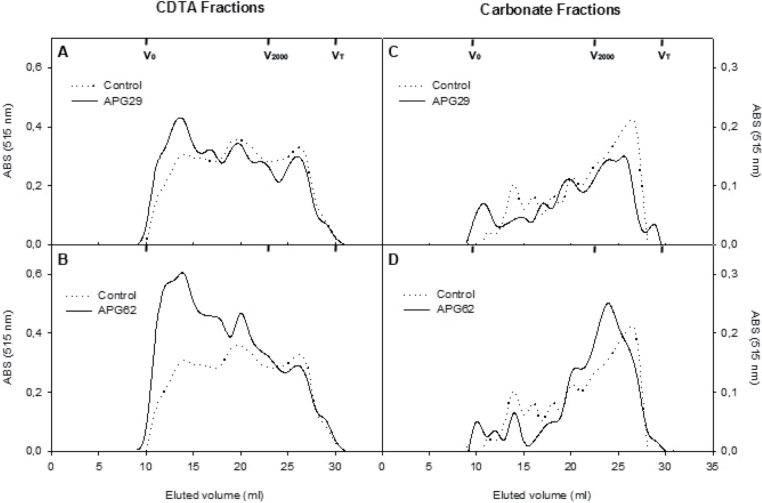
Polymers present in the CDTA fraction were eluted throughout the fractionation range of the column (Fig. 4A, B). Three peaks eluting at 14, 20, and 26.5ml were present in the control profile, similar to what was previously observed by Santiago-Doménech et al. (2008). These three peaks were also observed in the CDTA fractions of the two transgenic lines analysed, but an increased relative abundance of the first peak, with a higher average molecular mass, was observed in the profiles of the two APG lines.
Fig. 4.
Molecular mass profiles of bound polyuronides extractable by CDTA (A, B) and carbonate (C, D) from fruit cell walls of control and two independent APG transgenic lines. The profiles were obtained by gel filtration chromatography on Sepharose CL2B. The fractions were assayed for uronic acid, and the pectin contents were estimated by the absorbance values at 515nm. The elution volumes for the different dextran standards and acetone used for column calibration are presented.
The chromatograms obtained for polyuronides solubilized with Na2CO3 are displayed in Fig. 4C and D. In the control line, a peak of high molecular mass polymers eluting at 14ml was observed and was followed by a broad range of polymers of medium size that eluted between 15ml and 20ml. However, ~50% of the polyuronides present in this fraction exhibited low molecular sizes of <2000kDa, and they eluted at a main peak at 27ml. The profiles displayed by the two transgenic lines significantly differed from the control profile. The main pectin peak, which eluted at 27ml in the control line, was slightly displaced towards a higher molecular mass in the two transgenic lines, and this difference was more pronounced in the APG62 line. In addition, a peak containing high molecular mass polymers that eluted at 11ml was present in both transgenics but not observed in the control profiles. Furthermore, the APG62 line displayed a reduction in the relative abundance of the pool of medium-size polymers, which eluted between 15ml and 20ml. These results suggest that a reduction in the depolymerization of the CO3 polyuronides occurred due to polygalacturonase FaPG1 silencing.
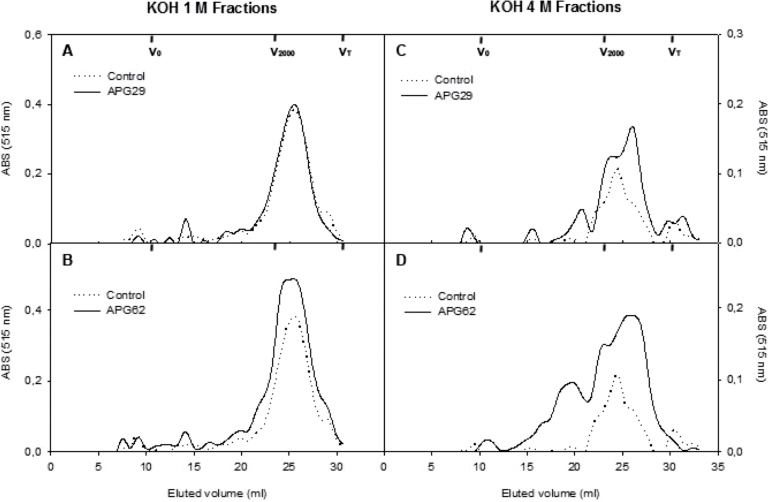
Regarding hemicellulosic polysaccharides, the gel chromatography profiles of xyloglucans from both KOH fractions harboured a unique polymer population, which eluted in one sharp peak at 25ml in all of the genotypes (Supplementary Fig. S4 at JXB online). Furthermore, absolute absorbance profiles displayed a higher xyloglucan content in 4M KOH samples with a broader peak. As expected, the neutral sugar profiles from the control and transgenic lines were also similar (results not shown). The profiles for the UA content in the KOH fractions are presented in Fig. 5. The polyuronides in the controls formed a single peak of ~2000kDa, which eluted at 26ml and 24ml in the 1M and 4M KOH fractions, respectively. No differences were observed between the control and transgenic profiles for the 1M KOH samples. However, the transgenic 4M KOH profiles exhibited a slight displacement of the main peak towards a lower molecular mass. Additionally, the APG62 transgenic profile exhibited the presence of medium-size polyuronides, >2000kDa, that eluted between 14ml and 21ml and were absent in the control profiles.
Fig. 5.
Molecular mass profiles of hemicellulosic polyuronides extractable by 1M KOH (A, B) and 4M KOH (C, D) from fruit cell walls of control and two independent APG transgenic lines. The profiles were obtained by gel filtration chromatography on Sepharose CL2B. The fractions were assayed for uronic acid, and the pectin contents were estimated by the absorbance values at 515nm. The elution volumes for the different dextran standards and acetone used for column calibration are presented.
PACE analysis of CDTA pectins
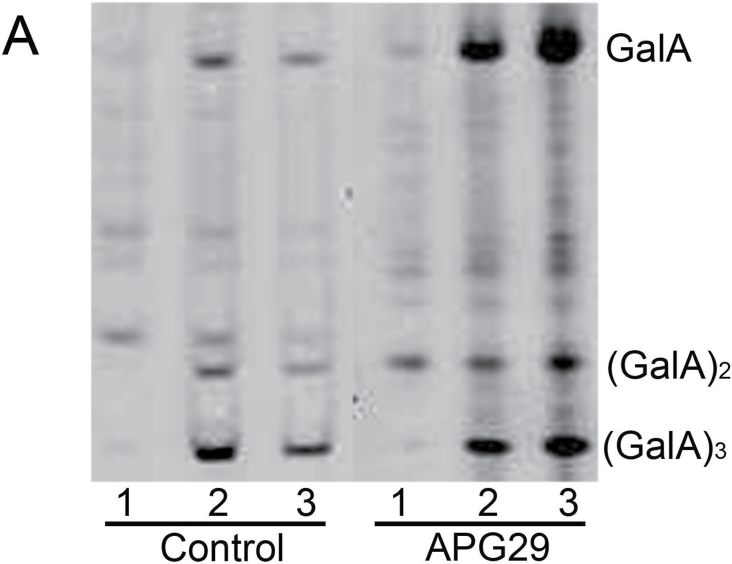
The CDTA-soluble pectins from the control and transgenic APG29 fruits were digested with endo-PG, and the released oligosaccharides were derivatized with AMAC and analysed by gel electrophoresis. The released oligosaccharides were predominantly oligogalacturonans, which displayed two or three degrees of polymerization, and free galacturonic acid (Fig. 6), as previously observed by Barton et al. (2006) in Arabidopsis cell wall material digested with endo-PG. In the CDTA fraction from the control line, the sample was completely digested after 30min, at which point any additional digestion did not increase the amount of oligogalacturonans (Fig. 6). Interestingly, the transgenic CDTA fraction was completely digested after 1h, and significantly higher amounts of GalA, (GalA)2 and (GalA)3, were released in the digested transgenics than in the control (Fig. 6). A similar result was observed with esterified CDTA samples, which were not treated with NaOH. However, the samples were less digested due to the inability of the enzyme to break methyl-esterified HGA (result not shown). In the CO3 fraction, minor changes were observed after digestion with endo-PG; however, a slight increase in oligogalacturonans and GalA was detected after 30min of digestion in the digested CO3 samples (result not shown). Overall, the higher digestibility of CDTA pectins from transgenic fruits suggests that these polyuronides were less degraded during fruit ripening.
Fig. 6.
PACE analysis of CDTA-soluble pectins that were isolated from control and transgenic ripe fruits. The pectin samples were digested with fungal endo-PG, derivatized with AMAC, and separated in polyacrylamide gels. Lane 1, undigested sample; lanes 2 and 3, samples digested with endo-PG for 30min and 60min, respectively. The same amount of pectin was loaded in each lane.
In vitro cell wall swelling
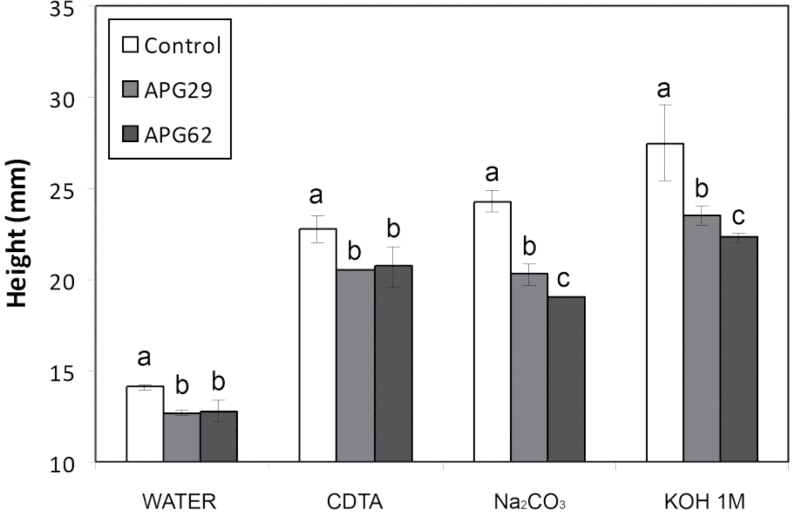
The cell wall material from transgenic fruits incubated in water displayed a lower degree of in vitro swelling than control CWM (Fig. 7). The sequential chemical extraction of the CWM with CDTA, sodium carbonate, and 1M KOH notably increased wall swelling in all genotypes. However, in all of the treatments, the degree of swelling was significantly lower in transgenic walls than in the control. The highest genotypic differences were detected after pectin solubilization with sodium carbonate, where the degree of swelling in transgenic lines was 20% lower than the control level (Fig. 7).
Fig. 7.
In vitro swelling of cell walls from control and transgenic APG lines, estimated as the height reached by the cell wall column after sequential extraction with water, CDTA, Na2CO3, and 1M KOH. The data represent the average of three replicates, and the bars represent the SD. Different letters indicate statistically significant differences by ANOVA and the Tukey-HSD test (P=0.05).
Analysis of histological sections from ripe fruits
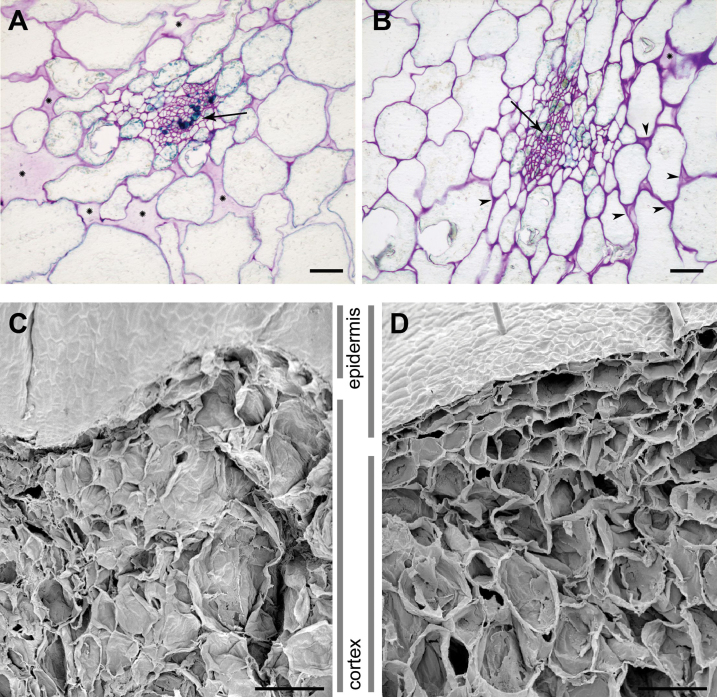
Histological sections of ripe control and transgenic fruits were stained with toluidine blue to visualize the microscopic tissue structure and cell adhesion. Control fruits exhibited little contact between cells, which were often separated by large intercellular spaces (Fig. 8A). In contrast, transgenic sections displayed smaller intercellular spaces and more cellular adhesion. Additionally, transgenic cell walls were densely stained, and some typical tri-cellular junctions could be observed (Fig. 8B). The higher tissue integrity in transgenic fruits was confirmed by scanning electron microscopy (SEM). The SEM images of control fruits revealed that most cells displayed a crushed appearance, most probably due to the low resistance of the tissue to the vacuum treatment applied during sample processing (Fig. 8C). However, transgenic tissues appeared more organized, and most cells maintained their volume and were not collapsed (Fig. 8D).
Fig. 8.
Light microscopy micrographs (A, B) and scanning electron micrographs (C, D) of control (A, C) and APG (B, D) strawberry fruit tissues. Tissue sections for light microscopy were stained with toluidine blue. Arrows indicate vascular bundles, and arrowheads indicate small, well-stained tri-cellular junctions. The large spaces without cells are indicated by asterisks. Scale bars correspond to 10 μm (A, B) or 200 μm (C, D). (This figure is available in colour at JXB online.)
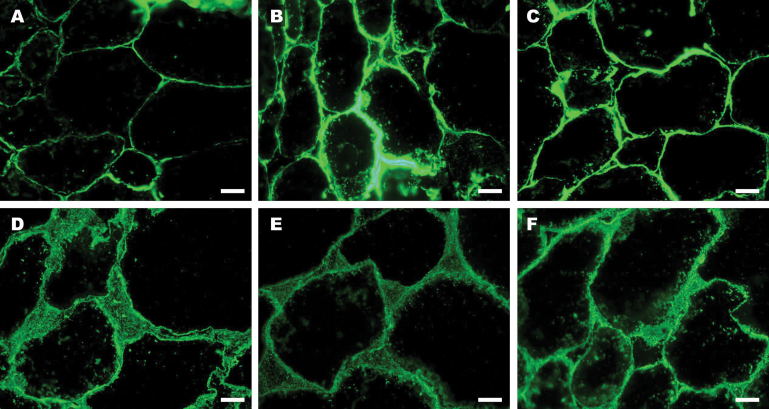
Immunohistochemical assays were also performed using the JIM5 and JIM7 antibodies against low and high methyl-esterified HGA pectic domains, respectively (Fig. 9). Immunolabelling the cortex tissue from ripe fruits with JIM5 revealed a fluorescence that was restricted to the cell wall. With this antibody, more fluorescence was observed in the tissue sections from the transgenic lines than from the control (Fig. 9A–C). In contrast to JIM5, JIM7 labelling was more disperse; that is, the fluorescent signal appeared not only in the cell wall but also in large intercellular spaces (Fig. 9D–F). Minor differences between control and transgenic fruits were observed with this antibody.
Fig. 9.
Immunolabelling of pectic components in cell walls in ripe controls (A, D) and transgenic fruit lines APG29 (B, E) and APG62 (C, F). Tissue sections were incubated with JIM5 (A–C) or JIM7 (D–F). Scale bars correspond to 50 μm. (This figure is available in colour at JXB online.)
Discussion
PGs are known to participate in developmental processes that require cell separation such as fruit softening (Hadfield and Bennett, 1998). PG enzymatic activity is low and difficult to detect from strawberry protein extracts when compared with other fruits. This fact suggested to researchers that this enzyme plays a minor role in strawberry softening (Huber, 1984; Nogata et al., 1996). However, the down-regulation of the PG gene FaPG1 significantly improved fruit firmness and reduced post-harvest softening (Quesada et al., 2009a , b ). Similarly, the silencing of a PG gene in apples, another fruit that harbours low PG activity during ripening, also resulted in firmer fruits with improved textural characteristics (Atkinson et al., 2012). PG silencing increased strawberry fruit firmness, but it did not affect other ripening parameters, such as anthocyanin content or soluble solids. Because this species is propagated by runners instead of seeds, it is important to determine the stability of the transgenic phenotype through several vegetative propagation cycles. Although most researchers had not investigated the stability of transgenic phenotypes in the strawberry, Lunkenbein et al. (2006) reported a frequency of 1:20 for a stable antisense chalcone synthase phenotype after 4 years of propagation. In the present case, the firmer fruit phenotype observed in two transgenic antisense FaPG1 lines was stable after three cycles of vegetative propagation.
Suppression of FaPG1 in fruit reduces pectin solubilization and depolymerization
To elucidate the role of FaPG1 in strawberry softening, changes in the cell wall induced by the suppression of this gene were evaluated. A sequential series of solvents was used to obtain fractions enriched in pectins that differed in their relative affinities for the primary cell wall and middle lamella (Redgwell et al., 1992, 1997b ; Santiago-Doménech et al., 2008). Pectin solubilization is the most generally reported cell wall modification related to the softening process of numerous fleshy fruits, including strawberries (Brummell, 2006; Mercado et al., 2011; Posé et al., 2011). Redgwell et al. (1997b ) observed that unripe strawberries harboured 40% less PAW-soluble pectins than ripe fruits. Interestingly, the suppression of FaPG1 also reduces soluble pectins to a similar degree (42%) and significantly increases both CDTA- and NaCO3-soluble pectic polymers. Additionally, the amount of pectin extracted with 4M KOH was also higher in transgenic fruits, although the total amount of polyuronide in this fraction was lower than that in other cell wall fractions. These results suggest that pectin solubilization occurs at the expense of bound pectins, mainly sodium carbonate-soluble pectins, and that the product of FaPG1 expression may play an important role in this process. Similarly, Carrington et al. (1993) observed a lower amount of water-soluble pectins concurrently with an equivalent increase in carbonate-soluble pectins in transgenic tomato fruits that harboured a silenced PG gene. Furthermore, the overexpression of the PG gene in the non-softening tomato mutant rin, which harbours low levels of PG activity and reduced pectin solubilization, restores polyuronide solubilization to wild-type levels (Giovannoni et al., 1989). A lower amount of water-soluble pectins has also been observed in Cnr (Colourless non-ripening) tomato mutants than in the wild type (Orfila et al., 2002). This mutant displays an aberrant fruit-ripening phenotype that yields fruit that do not soften but exhibit a severe reduction in pericarp cell adhesion. Interestingly, among the pleiotropic effects of the Cnr mutation, an absence of the PG transcript during ripening has been reported (Thompson et al., 1999). In the strawberry, the down-regulation of a pectate lyase gene also reduced pectin solubilization (Santiago-Doménech et al., 2008); however, the reduction of soluble pectins was lower than the down-regulation of FaPG1.
Previous reports have demonstrated that pectin solubilization could be partially due to the depolymerization of covalently bound pectins (Rosli et al., 2004; Santiago-Doménech et al., 2008; Figueroa et al., 2010). The modifications observed in the gel filtration chromatographic profiles of pectins from transgenic fruits support this hypothesis. In the two transgenic lines analysed, CDTA pectic profiles displayed a shift towards a higher abundance of large molecular mass pectic polymers and a reduction of medium and small molecular mass polyuronides. The average molecular mass of Na2CO3-solubilized polyuronides was lower than in the CDTA-extracted pectic polymers, as previously observed by Posé et al. (2012). In contrast to the control profiles, the main CO3 peaks for both transgenic lines were shifted in the chromatograms to the left, indicating a higher molecular mass for this group of polymers. Furthermore, a small peak of large polyuronides was observed in CO3 transgenic samples but was absent in the control. Altogether, these results suggest that both ionically and covalently bound pectins in lines APG29 and APG62 exhibit a lower depolymerization. These CDTA and CO3 profiles in control fruits resemble those reported by Brummel and Labavitch (1997) for ripe tomatoes. However, the silencing of an endo-PG in this species did not modify pectic profiles.
Notably, the suppression of a PG gene in the apple, a fruit that harbours a crisp texture when ripe, causes changes in the cell wall similar to those observed in strawberries. Thus, transgenic apples displayed a lower amount of water-soluble pectins and a lower depolymerization of CDTA pectins than the control, although the CO3 fraction was not analysed in this study (Atkinson et al., 2012). The silencing of a pectate lyase gene in strawberries also reduces pectin depolymerization (Santiago-Doménech et al., 2008). However, the changes observed in the chromatographic profiles were slightly distinct from the modifications detected in antisense FaPG1 plants. Pectate lyase has been suggested to depolymerize subsets of strongly bound pectic polymers, increasing their solubility (Santiago-Doménech et al., 2008). This contrasts with the FaPG1 mode of action, as the product of this gene may display a more ubiquitous activity in muro, where it depolymerizes covalently as well as ionically bound pectins. The lower depolymerization of CDTA polyuronides due to FaPG1 silencing was also supported by the PACE results. The transgenic samples were significantly more digested in vitro when incubated with fungal endo-PG than the control samples. A different pectin composition could explain this result; however, the carbohydrate composition was similar in control and transgenic samples (results not shown), suggesting that transgenic CDTA polyuronides contain more sites for endo-PG action due to FaPG1 silencing.
FaPG1 suppression in fruit increases intercellular adhesion and reduces wall swelling
Histological studies have clearly demonstrated that transgenic APG fruits exhibit higher tissue integrity than controls due to FaPG1 silencing. A denser cell wall staining, smaller and less abundant intercellular spaces, and a greater cell–cell adhesion were observed in transgenic tissue sections. Immunohistological studies using the JIM5 and JIM7 antibodies confirmed the higher HGA pectin content of APG fruits. Interestingly, striking differences between control and transgenic fruits were observed with JIM5. This antibody binds to a wide range of HGA epitopes with varying degrees of methyl-esterification and also weakly to completely de-esterified pectins (Willats et al., 2000). In contrast, JIM7 binds predominantly to highly methyl-esterified pectins, which include polyuronides that cannot be degraded by PG. Similarly, an increase in JIM5 labelling was observed in transgenic ripe apples with a silenced PG gene (Atkinson et al., 2012).
The degradation of calcium-chelated pectins from the middle lamella is widely accepted to reduce intercellular adhesion during fruit ripening (Orfila et al., 2002; Brummell, 2006). However, in the sugarbeet root parenchyma, Marry et al. (2006) observed that cell–cell adhesion was only disrupted by successive extractions with a calcium chelator and sodium carbonate. This finding suggests an important role for this last fraction. More recently, Molina-Hidalgo et al. (2013) observed a lower middle lamella dissolution relative to control fruits when a rhamnogalacturonate lyase gene was down-regulated in strawberries transiently transformed by agroinfiltration. Although rhamnogalacturonan I is located within the primary wall, it could participate in anchoring HGA to the middle lamella (Vincken et al., 2003). Thus, the reduced degradation of the sodium carbonate fraction could also be related to the better preserved integrity of the middle lamella and the maintenance of the cell adhesion observed in transgenic APG fruits. Altogether, these tissue characteristics contribute to the increased firmness of transgenic ripe fruits.
Most fleshy fruits display incremental cell wall swelling during ripening that parallels the dissolution of the middle lamella. This process may result from water penetrating into enlarged intermicrofibrillar spaces due to pectin solubilization. According to Redgwell et al. (1997b ), a moderate to high degree of pectin solubilization during ripening is accompanied by increased wall swelling, both in vivo and in vitro, but this process is not affected by pectin depolymerization. The present results indicate that the degree of wall swelling in strawberries depends mainly on ionically bound pectins, as the largest swelling increase was observed when cell wall extracts were treated with CDTA, and the subsequent pectin extractions with sodium carbonate and 1M KOH increased swelling only slightly. In contrast, kiwifruit cell wall swelling was only induced by sodium carbonate treatment, and the removal of CDTA-soluble pectins exerted little effect on swelling (Redgwell et al., 1997b ). In this fruit, treatment of the CWM with PG caused a similar degree of swelling to Na2CO3. The suppression of FaPG1 in strawberries exerted a great effect on in vitro wall swelling, where the degree of swelling was significantly lower than in the control wall even when pectic polymers were extracted with different solvents. Notably, the extraction of covalently bound pectins with sodium carbonate from both transgenic lines did not induce additional swelling. Accordingly, PG gene expression affects the physical properties of the cell wall. Hence, in addition to the direct role of FaPG1 expression in pectin solubilization and depolymerization, the suppression of this gene could also limit substrate access for other cell wall hydrolases in muro.
Conclusion
The results support the notion that the firmer fruit genotype displayed by ripe strawberries with a silenced FaPG1 gene is predominantly due to a decrease in pectin solubilization and depolymerization. The main changes observed in the cell wall occurred in the CDTA and CO3 fractions, and, notably, the contents of the CO3 fractions were higher in both transgenic lines. These bound pectic fractions represent good candidates for further study as possible PG targets. The down-regulation of this gene also reduces middle lamella dissolution, thereby increasing cell–cell adhesion and decreasing in vitro cell wall swelling.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Soluble solid content of control and transgenic ripe fruits from the transgenic lines APG29 and APG62 were evaluated for three consecutive years.
Figure S2. Anthocyanin content, estimated as mg Pg-3-glu per 100g of fruit, of control and transgenic ripe fruits from the APG29 and APG62 lines were evaluated for two years.
Figure S3. Pectin content, expressed as mg UA per 100mg of CWM, in the cell wall fractions obtained from control and transgenic ripe fruits.
Figure S4. Molecular mass profiles of xyloglucans extractable by 1M KOH (A, B) and 4M KOH (C, D) from fruit cell walls of control and two independent APG transgenic lines
Acknowledgements
This work was supported by the Ministerio de Educación y Ciencia of Spain and Feder EU Funds (grant reference AGL2011-24814). SP and CP were supported by FPI fellowships from the Spanish Government (grant references BES-2006-13626 and BES-2009-027985). The authors thank Dr Fernando Pliego-Alfaro (Departamento de Biología Vegetal, Universidad de Málaga) and Dr José M. López-Aranda (IFAPA, Centro de Churriana, Málaga, Spain) for their support and advice on growing the plants.
References
- Atkinson RG, Sutherland PW, Johnston SL, Gunaseelan K, Hallett IC, Mitra D, Brummell DA, Schröder R, Johnston JW, Schaffer RJ. 2012. Down-regulation of POLYGALACTURONASE1 alters firmness, tensile strength and water loss in apple (Malus×domestica) fruit. BMC Plant Biology 12, 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton CJ, Tailford LE, Welchman H, Zhang Z, Gilbert HJ, Dupree P, Goubet F. 2006. Enzymatic fingerprinting of Arabidopsis pectic polysaccharides using polysaccharide analysis by carbohydrate gel electrophoresis (PACE). Planta 224, 163–174. [DOI] [PubMed] [Google Scholar]
- Brummell DA. 2006. Cell wall disassembly in ripening fruit. Functional Plant Biology 33, 103–119. [DOI] [PubMed] [Google Scholar]
- Brummell DA, Labavitch JM. 1997. Effect of antisense suppression of endopolygalacturonase activity on polyuronide molecular weight in ripening tomato fruit and in fruit homogenates. Plant Physiology 115, 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington CMS, Greve LC, Labavitch JM. 1993. Cell wall metabolism in ripening fruit. VI. Effect of the antisense polygalacturonase gene on cell wall changes accompanying ripening in transgenic tomatoes. Plant Physiology 103, 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen MH, Willats WGT, Knox JP. 2003. Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydrate Research 338, 1797–1800. [DOI] [PubMed] [Google Scholar]
- Figueroa CR, Rosli HG, Civello PM, Martínez GA, Herrera R, Moya-León MA. 2010. Changes in cell wall polysaccharides and cell wall degrading enzymes during ripening of Fragaria chiloensis and Fragaria×ananassa fruits. Scientia Horticulturae 124, 454–462. [Google Scholar]
- Filisetti-Cozzi TMCC, Carpita NC. 1991. Measurement of uronic acids without interference from neutral sugars. Analytical Biochemistry 197, 157–162. [DOI] [PubMed] [Google Scholar]
- Giovannoni JJ, DellaPenna D, Bennett AB, Fischer RL. 1989. Expression of a chimeric polygalacturonase gene in transgenic rin (ripening inhibitor) tomato fruit results in polyuronide degradation but not fruit softening. The Plant Cell 1, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubet F, Morriswood B, Dupree P. 2003. Analysis of methylated and unmethylated polygalacturonic acid structrure by polysaccharide analysis using carbohydrate gel electrophoresis. Analytical Biochemistry 321, 174–182. [DOI] [PubMed] [Google Scholar]
- Goulao LF, Oliveira CM. 2008. Cell wall modification during fruit ripening: when a fruit is not the fruit. Trends in Food Science and Technology 19, 4–25. [Google Scholar]
- Hadfield KA, Bennett AB. 1998. Polygalacturonases: many genes in search of a function. Plant Physiology 117, 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber DJ. 1984. Strawberry (Fragaria×ananassa) fruit softening, the potential roles of polyuronides and hemicelluloses. Journal of Food Science 49, 1310–1315. [Google Scholar]
- Jiménez-Bermúdez S, Redondo-Nevado J, Muñoz-Blanco J, Caballero JL, López-Aranda JM, Valpuesta V, Pliego-Alfaro F, Quesada MA, Mercado JA. 2002. Manipulation of strawberry fruit softening by antisense expression of a pectate lyase gene. Plant Physiology 128, 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knee M, Sargent JA, Osborne DJ. 1977. Cell wall metabolism in developing strawberry fruits. Journal of Experimental Botany 28, 377–396. [Google Scholar]
- Lara I, García P, Vendrell M. 2004. Modifications in cell wall composition after cold storage of calcium-treated strawberry (Fragaria×ananassa Duch.) fruit. Postharvest Biology and Technology 34, 331–339. [Google Scholar]
- Lunkenbein S, Coiner H, De Vos CHR, Schaart JG, Boone MJ, Krens FA, Schwab W, Salentijn EMJ. 2006. Molecular characterization of a stable antisense chalcone synthase phenotype in strawberry (Fragaria×ananassa). Journal of Agricultural and Food Chemistry 54, 2145–2153. [DOI] [PubMed] [Google Scholar]
- Marry M, Roberts K, Jopson SJ, Huxham M, Jarvis MC, Corsar J, Robertson E, McCann MC. 2006. Cell–cell adhesion in fresh sugar-beet root parenchyma requires both pectin esters and calcium cross-links. Physiologia Plantarum 126, 243–256. [Google Scholar]
- Mercado JA, Pliego-Alfaro F, Quesada MA. 2011. Fruit shelf life and potential for its genetic improvement. In: Jenks MA, Bebeli PJ, eds. Breeding for fruit quality. Oxford: John Wiley & Sons, Inc., 81–104. [Google Scholar]
- Molina-Hidalgo FJ, Franco AR, Villatoro C, Medina-Puche L, Mercado JA, Hidalgo MA, Monfort A, Caballero JL, Muñoz-Blanco J, Blanco-Portales R. 2013. The strawberry (Fragaria×ananassa) fruit-specific rhamnogalacturonate lyase 1 (FaRGLyase1) gene encodes an enzyme involved in the degradation of cell-wall middle lamellae. Journal of Experimental Botany 64, 1471–1483. [DOI] [PubMed] [Google Scholar]
- Montreuil GS, Spik G, Fournet B, Tollier MT. 1997. Nonenzymatic determinations of carbohydrates. In: Multon JL, ed. Analysis of food constituents. New York: Wiley-VCH, 109–123. [Google Scholar]
- Nogata Y, Yoza K, Kusumoto K, Ohta H. 1996. Changes in molecular weight and carbohydrate composition of cell wall polyuronide and hemicellulose during ripening in strawberry fruit. In: Visser J, Voragen AGJ, eds. Pectins and pectinases. Amsterdam: Elsevier Science, 591–596. [Google Scholar]
- Nunes MCN, Brecht JK, Morais AMMB, Sargent SA. 2006. Physicochemical changes during strawberry development in the field compared with those that occur in harvested fruit during storage. Journal of the Science of Food and Agriculture 86, 180–190. [Google Scholar]
- Orfila C, Huisman MMH, Willats WGT, van Alebeek GJWM, Schols HA, Seymour GB, Knox JP. 2002. Altered cell wall disassembly during ripening of Cnr tomato fruit: implications for cell adhesion and fruit softening. Planta 215, 440–447. [DOI] [PubMed] [Google Scholar]
- Perkins-Veazie P. 1995. Growth and ripening of strawberry fruit. Horticultural Reviews 17, 267–297. [Google Scholar]
- Posé S, García-Gago JA, Santiago-Doménech N, Pliego-Alfaro F, Quesada MA, Mercado JA. 2011. Strawberry fruit softening: role of cell wall disassembly and its manipulation in transgenic plants. Genes, Genomes and Genomics 5, 40–48. [Google Scholar]
- Posé S, Kirby AR, Mercado JA, Morris VJ, Quesada MA. 2012. Structural characterization of cell wall pectin fractions in ripe strawberry fruits using AFM. Carbohydrate Polymers 88, 882–890. [Google Scholar]
- Quesada MA, Blanco-Portales R, Posé S, García-Gago JA, Jiménez-Bermúdez S, Muñoz-Serrano A, Caballero JL, Pliego-Alfaro F, Mercado JA, Muñoz-Blanco J. 2009a. Antisense down-regulation of the FaPG1 gene reveals an unexpected central role for polygalacturonase in strawberry fruit softening. Plant Physiology 150, 1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada MA, Posé S, Santiago-Doménech N, et al. 2009b. Effect of silencing of cell wall degrading enzymes on strawberry fruit softening. Acta Horticulturae 842, 931–934. [Google Scholar]
- Redgwell RJ, Fischer M, Kendall E, MacRae EA, Perry J, Harker R. 1997a. Galactose loss and fruit ripening: high-molecular-weight arabinogalactans in the pectic polysaccharides of fruit cell walls. Planta 203, 174–181. [Google Scholar]
- Redgwell RJ, MacRae EA, Hallett I, Fischer M, Perry J, Harker R. 1997b. In vivo and in vitro swelling of cell walls during fruit ripening. Planta 203, 162–173. [Google Scholar]
- Redgwell RJ, Melton LD, Brasch DJ. 1992. Cell-wall dissolution in ripening kiwifruit (Actinidia deliciosa). Solubilisation of the pectic polymers. Plant Physiology 98, 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimington C. 1931. The carbohydrate complex of the serum proteins: improved method for isolation and re-determination of structure. Isolation of glucosaminodimannose from proteins of ox blood. Biochemical Journal 25, 1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosli HG, Civello PM, Martinez GA. 2004. Changes in cell wall composition of three Fragaria×ananassa cultivars with different softening rate during ripening. Plant Physiology and Biochemistry 42, 823–831. [DOI] [PubMed] [Google Scholar]
- Salentijn EMJ, Aharoni A, Schaart JG, Boone MJ, Krens FA. 2003. Differential gene expression analysis of strawberry cultivars that differ in fruit-firmness. Physiologia Plantarum 118, 571–578. [Google Scholar]
- Santiago-Doménech N, Jiménez-Bermúdez S, Matas AJ, Rose JKC, Muñoz-Blanco J, Mercado JA, Quesada MA, 2008. Antisense inhibition of a pectate lyase gene supports a role for pectin depolymerization in strawberry fruit softening. Journal of Experimental Botany 59, 2769–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Tor M, Barry CS, Vrebalov J, Orfila C, Jarvis MC, Giovannoni JJ, Grierson D, Seymour GB. 1999. Molecular and genetic characterization of a novel pleiotropic tomato-ripening mutant. Plant Physiology 120, 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainotti L, Spinello R, Piovan A, Spolaore S, Casadoro G. 2001. β-Galactosidases with a lectin-like domain are expressed in strawberry. Journal of Experimental Botany 52, 1635–1645. [PubMed] [Google Scholar]
- Villarreal NM, Rosli HG, Martínez GA, Civello PM. 2008. Polygalacturonase activity and expression of related genes during ripening of strawberry cultivars with contrasting fruit firmness. Postharvest Biology and Technology 47, 141–150. [Google Scholar]
- Vincken JP, Schols HA, Oomen RJFJ, McCann MC, Ulvskov P, Voragen AGJ, Visser RGF. 2003. If homogalacturonan were a side chain of rhamnogalacturonan I. Implications for cell wall architecture. Plant Physiology 132, 1781–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WGT, Limberg G, Buchholt HC, van Alebeek GJ, Benen J, Christensen TMIE, Visser J, Voragen A, Mikkelsen JD, Knox JP. 2000. Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation. Carbohydrate Research 327, 309–320. [DOI] [PubMed] [Google Scholar]
- Youssef SM, Jiménez-Bermúdez S, Bellido ML, et al. 2009. Fruit yield and quality of strawberry plants transformed with a fruit specific strawberry pectate lyase gene. Scientia Horticulturae 119, 120–125. [Google Scholar]
- Yousseff SM, Amaya I, López-Aranda JM, Sesmero R, Valpuesta V, Casadoro G, Blanco-Portales R, Pliego-Alfaro F, Quesada MA, Mercado JA. 2013. Effect of simultaneous down-regulation of pectate lyase and endo-β-1,4-glucanase genes on strawberry fruit softening. Molecular Breeding 31, 313–322. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.