Abstract
The beneficial endophytic fungus Piriformospora indica colonizes the roots of many plant species, including the model plant Arabidopsis thaliana. Its colonization promotes plant growth, development, and seed production as well as resistance to various biotic and abiotic stresses. In the present work, P. indica was tested as potential antagonist of the sedentary plant-parasitic nematode Heterodera schachtii. This biotrophic cyst-forming nematode induces severe host plant damage by changing the morphogenesis and physiology of infected roots. Here it is shown that P. indica colonization, as well as the application of fungal exudates and cell-wall extracts, significantly affects the vitality, infectivity, development, and reproduction of H. schachtii.
Key words: Antagonist, Arabidopsis, cell-wall extract, culture filtrate, Heterodera schachtii, Piriformospora indica.
Introduction
The sedentary endoparasitic beet cyst nematode Heterodera schachtii is an economically important pest that causes yield losses on a number of different Chenopodiaceae and Brassicaceae crop species (Jung and Wyss, 1999). It also infects roots of Arabidopsis thaliana, which can be used as a powerful model to study plant–nematode interactions (Sijmons et al., 1991). In general, root exudates of host plants stimulate the hatching of mobile second-stage juveniles (J2s) that are dormant in nematode cysts. Attracted by a concentration gradient of these exudates of presently unknown composition, the mobile J2 moves towards plant roots, where it breaks through the epidermis and migrates intracellularly towards the vascular cylinder. There it punctures a single cell with its stylet and injects nematode gland secretions (Golinowski et al., 1996; Sobczak et al., 1999). This initial cell fuses with neighbouring cells by local cell-wall dissolutions, thus forming a feeding site of a syncytium type (Golinowski et al., 1996; Grundler et al., 1998). Within few days, the syncytium is characterized by enlarged nuclei, proliferated mitochondria, and plastids, a disintegration of the central vacuoles, and an electron-dense cytoplasm (Golinowski et al., 1996). As soon as the parasite starts to withdraw nutrients, the feeding site becomes a strong sink in the plant solute circulation system (Böckenhoff et al., 1996). During the first 2 weeks the juvenile remains sedentary, feeds from its syncytium, and passes through two further juvenile stages to finally develop into adult. Adult males become vermiform and mobile to find female mating partners. After fertilization, the females start to produce eggs in their bodies that turn into brown cysts and serve as a lasting stage.
To date, pest management against sedentary endoparasitic nematodes is difficult due to their complex life cycle and severe ecological threats of highly toxic synthetic nematicides. However, there are successful biological approaches against these nematodes applying different antagonists such as mycorrhizal and endophytic fungi, as well as endophytic bacteria and rhizobacteria (Dababat and Sikora, 2007a; Mendoza, 2008; Elsen et al., 2008; Le et al., 2009). These natural antagonists interfere with nematodes’ ability to find, penetrate, and complete their life cycle in the hosts, through direct competition and production of antibiotics as well as induction of systemic resistance (Kerry, 2000; Sikora et al., 2007). The application of a nematode-antagonistic endophytic fungus showed the first successful results under greenhouse conditions (Dababat and Sikora, 2007b; Hallmann et al., 2009).
Piriformospora indica, a root endophytic fungus from the Sebacinaceae family, has been described to be beneficial for plant growth (Farkya et al., 2010) and productivity (Varma et al., 1999). Besides this direct beneficial interaction, autoclaved cell-wall extracts (CWE) as well as the application of culture filtrates (CF) containing fungal exudates were shown to promote plant growth (Vadassery et al., 2009). However, there is only a limited knowledge about the chemical compositions of the CWE and CF. P. indica has also been reported to increase plant stress tolerance towards abiotic stresses like drought, acidity, and heavy metals (Kumari et al., 2004) and against biotic stresses such as plant pathogens (Waller et al., 2008; Zuccaro et al., 2009). In barley, P. indica triggers resistance against Fusarium head blight (Fusarium graminearum) (Deshmukh and Kogel, 2007) as well as against the leaf pathogen Blumeria graminis f. sp. hordei (Waller et al., 2005). Thus, it is suggested that P. indica may induce systemic resistance in plants.
The aim of the present work was to test P. indica as a potential antagonist of H. schachtii. Therefore, the nematode’s life cycle was studied in detail on P. indica-colonized and non-colonized A. thaliana roots. Moreover, nematode infection and development was studied on plants treated with fungal CF and CWE in order to analyse their potential as biocontrol agents. The presented results provide novel information about nematode-antagonistic abilities of the fungus and give new insights on nematode interactions with other organisms.
Materials and methods
Plant, nematode, and fungus cultivation
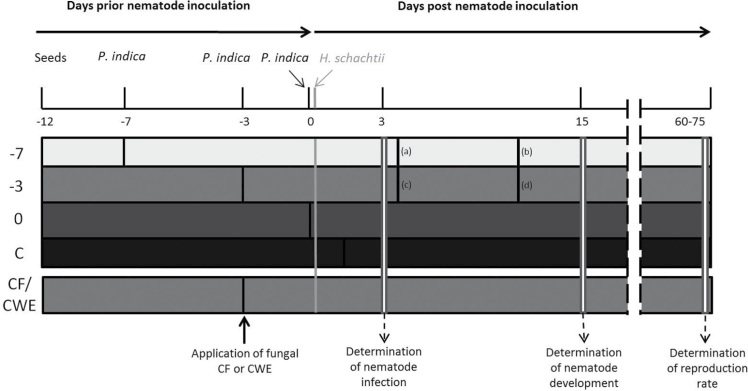
Sterile A. thaliana L. (Col-0) seeds were cultivated on 0.2% Knop medium with a 16/8 light/dark cycle at 25 °C (Sijmons et al., 1991). P. indica was cultured either on potato dextrose agar (Fluka, Germany) or on modified Aspergillus nidulans minimal medium (Varma et al., 1999) at 28 °C in the dark. A. thaliana Col-0 plants aged 5, 9, and 12 days were inoculated with potato dextrose agar plugs (diameter 5mm) containing fungal hyphae 1cm away from the roots. Nematode inoculation was always conducted on 12-day-old plants using 50–60 freshly hatched J2s obtained from the sterile cyst-stock culture (Sijmons et al., 1991). The fungal inoculation was carried out at 7 or 3 days before nematode inoculation (–7 and –3, respectively) or at the same time as the nematode inoculation (0) (Fig. 1). CF and CWE (50 μl) were applied directly onto the roots 3 days before nematode inoculation (Fig. 1).
Fig. 1.
Scheme of the experimental set up. Horizontal bars represent different plant treatments: 5-day-old plants were inoculated with P. indica at 7 days before nematode inoculation –7; 9-day-old plants were inoculated with P. indica at 3 days before nematode inoculation –3; 12-day-old plants were inoculated with P. indica concomitantly with the nematodes (0); control plants were inoculated only with H. schachtii (C); application of fungal CF and CWE occurred 3 days before nematode inoculation (CF/CWE). The number of nematode infection sites was counted at 3 dpi, the number of developed males and females at 15 dpi, and reproduction rate 60–75 days after inoculation with H. schachtii second-stage juveniles. Samples for transmission electron microscopy were collected at 7/4 (c), 11/4 (a), 13/10 (d) and 17/10 (b) days after inoculation with P. indica/H. schachtii, respectively.
Quantification of fungal colonization by quantitative PCR
Genomic DNA was extracted from roots with the Plant DNeasy Kit (Qiagen; www.qiagen.com) according to the manufacturer’s instructions. Quantitative PCR (qPCR) was carried out with the ABI PRISM 7300 Sequence Detector (Applied Biosystems, Foster City, CA, USA), and SYBR Green was used as fluorescent DNA binding dye. Each qPCR sample contained 12.5 μl of Platinum SYBR Green qPCR SuperMix containing UDG and ROX (Invitrogen, Carlsbad, CA, USA), 0.5 μl of forward and reverse primer (10mM), 2 μl of genomic DNA, and water to a total reaction volume of 25 μl. Fungal colonization was determined by the 2–ΔΔCt method (Deshmukh and Kogel, 2007; Schmittgen and Livak, 2008) using AtUBQ5 (forward primer: 5′-CCAAGCCGAAGAAGATCAAG-3′; reverse: 5′-ATGACTCGCCATGAAAGTCC-3′) as reference for plant-derived DNA and PiTFF1 (forward primer: 5′-ACCGTCTTGG GGTTGTATCC-3′; reverse primer: 5′-TCGTCGGTGTCAACA AGATG-3′) to quantify fungal DNA. Samples were analysed in three biological and three technical replicates. At the end of each PCR run, a dissociation curve was added to rule out unspecific reactions or primer dimmers.
Light and transmission electron microscopy
Samples of P. indica-infected root segments containing nematode-feeding sites were dissected and immediately immersed in a fixative composed of 2% (w/v) paraformaldehyde (Sigma, St Louis, MI, USA) and 2% (v/v) glutaraldehyde (Fluka, Buchs, Switzerland) in 0.05M sodium cacodylate buffer (pH 7.2; Sigma) for 2h at room temperature. Samples were collected at 7/4, 11/4, 13/10, and 17/10 days post infection (dpi, Fig. 1) after inoculation with P. indica/H. schachtii, respectively. Probes were post fixed in osmium tetroxide (Merck, Darmstadt, Germany), dehydrated in ethanol and propylene oxide (Sigma), and infiltrated and embedded in EPON epoxy-resin (Fluka) as described by Golinowski et al. (1996) and Sobczak et al. (1999). Semi-thin sections (3 μm thick) were taken on a Leica RM2165 microtome (Leica Microsystems, Wetzlar, Germany). They were collected on glass slides and stained with hot 1% (w/v) aqueous solution of crystal violet (Sigma) for 60 s at 65 °C and examined using a AX70 Provis light microscope equipped with an DP50 digital camera (Olympus, Tokyo, Japan). Ultra-thin sections (70–80nm) were taken on a UCT ultramicrotome (Leica Microsystems) and mounted on formvar (Fluka) -coated single-slot copper grids. Sections were stained with uranyl acetate (Fluka) and lead citrate (Sigma) (Golinowski et al., 1996) and examined with a 268D Morgagni transmission electron microscope (FEI, Hillsboro, OR, USA) operating at 80kV. The images were taken with an Morada digital camera (Olympus SIS, Münster, Germany) at 10 Mpix resolution.
Preparation of fungal CF and CWE
CFs were obtained from sterile culture of P. indica on modified Aspergillus nidulans minimal medium (Varma et al., 1999). The Erlenmeyer flasks were closed with cotton stopper, incubated at 28 °C on a shaker at 200rpm. After 14 days, fungal mycelia and chlamydospores were separated from fungal exudates by medium filtration through a sterile sieve (0.22 μm pore size; Rotilabo, Karlsruhe, D). CWEs were prepared from fungal hyphae according to Vadassery et al. (2009) using the same growing protocol as described above.
Nematode infection and development assays
P. indica has been reported to affect root architecture and induce root growth (Varma et al., 1999). In order to relate nematode infection to root development, P. indica-induced effects were taken into account by estimating root length according to Jürgensen (2001), in which A. thaliana plants were cultivated in Petri dishes, root length was determined with a digital map measurer, and different root lengths were classified in five groups. For the infection assays of the present work, 12-day-old P. indica-colonized and control A. thaliana plants were used. Plants were inoculated as described above. Prior to or simultaneously with nematode inoculation, plants were inoculated with P. indica or treated with CF or CWE (Fig. 1). In the control treatments, plants were inoculated with H. schachtii J2s only. Nematode infection rate in each treatment was monitored during the first 3 days after inoculation. Fifteen days after nematode inoculation, the number of developed males and females was counted and both infection and development rates were calculated. All experiments were conducted in three independent replicates, each containing seven Petri dishes with five plants per Petri dish (total: 105 plants per treatment). Two months after nematode inoculation, the reproduction rate was analysed for each treatment. Therefore, 30 randomly selected cysts from each treatment were placed either in hatching funnel in order to count the number of hatched juveniles or they were crushed in order to count the number of produced eggs. For details of the time course, see Fig. 1.
Length of syncytia was measured as the lineal distance between the two farthest points of a syncytium. Therefore, nematode infection sites were marked at 1 dpi and the length of corresponding syncytia was measured after 10 days. Only syncytia associated with single female nematode being sufficiently translucent to be measured with the AxioVision 4.1 software (Zeiss, Hallerbergmoos, Germany) were selected.
Effects of fungal CF and CWE on mobile J2s
Approximately 70 freshly hatched J2s of H. schachtii were washed with sterile water and subsequently immersed either in prepared CF or CWE obtained from P. indica. As controls, Aspergillus-minimal medium and water were used respectively. The numbers of mobile and immobile J2s were counted at 10min, 30min, 1h, 2h, 6h, and 24h after incubation using an SZ2-ILST stereo microscope (Olympus). The experiment was performed in three independent replicates, with 210 J2s per treatment.
Nematode attraction assay
The nematode attraction assay was performed as described by Dalzell et al. (2011). Briefly, uniform cylindrical troughs (20mm long x 2.5mm deep) connecting cylindrical counting wells (diameter 8mm) at each end were constructed in 2% water agar. Same size circular agar plugs containing Arabidopsis root exudates of P. indica-infected and non-infected plants were cut from the culture medium. The plugs were cut exactly next to plant’s roots and transferred to assay Petri dishes with experimental wells. One plug with root exudates was put on one side of the cylindrical trough and the second plug was put on the opposite side of trough. One hundred H. schachtii J2s were placed in the middle of the 2cm trough. The Petri dishes (six for each treatment and each replicate) were covered and incubated at room temperature for 3h in the dark. The number of J2s that reached the experimental plugs with exudates was scored as attracted by the root exudates. The experiment was performed in three independent replicates with 600 J2s per treatment/per replicate (1800 in total for each treatment). The attraction rate of each exudate was expressed as percentage of total numbers of applied nematodes.
Statistical analysis
For all experiments, three independent replicates were performed and differences were analysed by one-way ANOVA. The data were checked for homogeneity of variance and P < 0.05 was used to determine significance.
Results
In order to study the effect of P. indica root colonization on the life cycle of H. schachtii, a strict experimental set up and temporal regime was implemented. Nematode inoculation was always conducted on 12-day-old A. thaliana plants. Nematode root infection and development were examined at 3 and 15 dpi, respectively. Nematode reproduction was analysed 2 months after inoculation. Fungal mycelia were inoculated on the tested plants at three different time points: 7 days before nematode inoculation on 5-day-old plants, 3 days before nematode inoculation on 9-day-old plants, and simultaneously with nematode inoculation on 12-day-old plants. Control plants were inoculated with J2s of H. schachtii only. With this experimental set up (Fig. 1), the following could be studied: (i) which developmental stages of H. schachtii were most affected by P. indica colonization; and (ii) how long the potentially nematode-antagonistic fungus had to interact with the host plant to impair the nematode parasitism most effectively.
P. indica root colonization
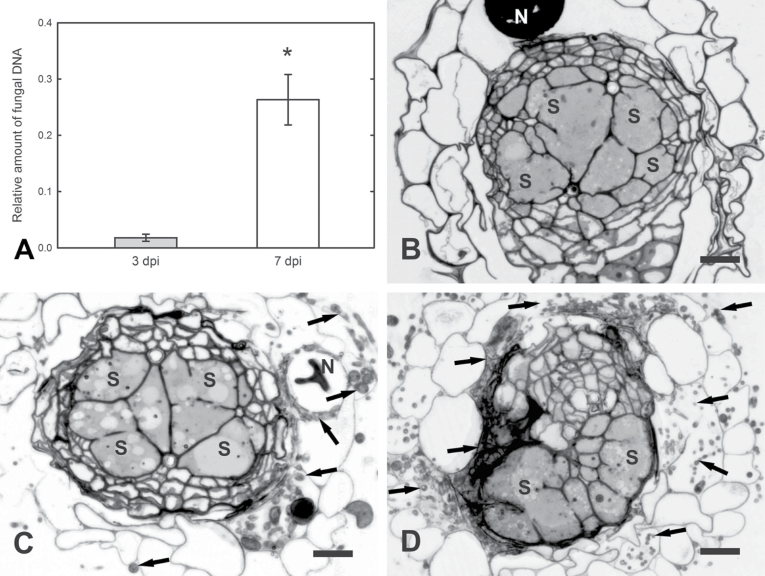
Colonization of Arabidopsis root by P. indica was studied with –7 and –3 treatments using light and transmission electron microscopy. Microscopic observation revealed that in nematode-non-infected roots, hyphae grew along the root epidermis (Fig. 2A and B) and invaded epidermal (Fig. 2C), cortical, and endodermal cells intracellularly. Along some root hairs and epidermal cells, an extracellular mucilaginous material was observed (Fig. 2B). Fungal hyphae were never able to invade the vascular cylinder cells, which remained non-colonized and retained functional protoplasts in vivid cells. Cells invaded by hyphae always contained necrotized protoplasts (Fig. 2B, C, E, and F). In order to quantify fungal root colonization, qPCR was performed using 12-day-old plants (the time point of nematode inoculation) from the –7 and –3 treatments. Results showed an increase of fungal DNA from the –3 to the –7 treatment, indicating a successful P. indica root colonization (Fig. 3A).
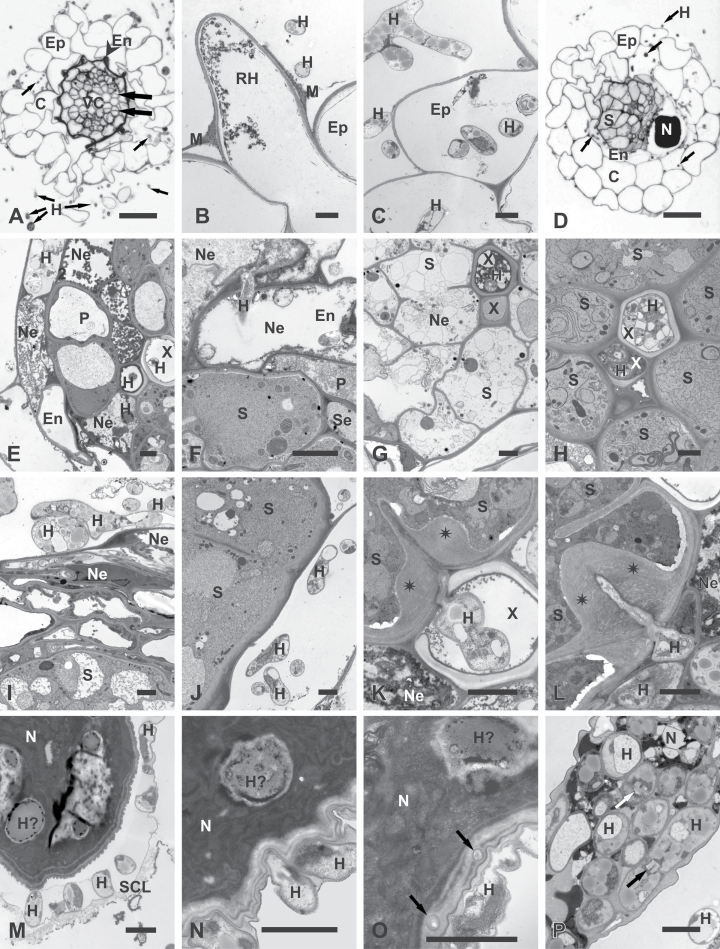
Fig. 2.
Light (A and D) and transmission electron microscopy (B, C, E–P) micrographs of Arabidopsis roots infected with P. indica only (A–C) or with P. indica and H. schachtii (D–P). (A–C) Cross-sections of roots infected with P. indica (17 dpi; equivalent to 10 days after nematode inoculation): (A) early stage of secondary thickening when cambial stripes had developed (broad arrows) by divisions of procambial cells and endodermis collapsed (arrowhead); fungal hyphae (small arrows) present outside and inside the root; (B) root-hair and epidermal cells with necrotized protoplasts and extracellular hyphae; (C) epidermal cells with necrotized protoplasts and extra- and intracellular hyphae. (D–L) Cross-sections through nematode-induced syncytia. (D–G) At 4 dpi in –7 treatment roots: (D) nematode and syncytium developed inside root infected with hyphae (arrows); (E) hyphae inside necrotized cells of endodermis and the vascular cylinder; some hyphae have entered the lumen of xylem vessels; (F) hyphae inside necrotized endodermal cells next to alive and non-infected syncytium; (G) necrotized syncytium next to vessel invaded with fungal hyphae. (H and I) At 10 dpi in –3 treatment roots: (H) syncytium with regular cytoplasm next to vessel invaded with fungal hyphae; (I) Well-developed network of hyphae separated from syncytium by a layer of cells. (J–L) At 10 dpi in –7 treatment roots: (J) hyphae next to syncytial elements containing regular cytoplasm. (K) hypha growing inside vessel lumen had attempted to penetrate syncytial cell wall that responded with formation of pronounced cell-wall thickening (asterisks); (L) hypha penetrating syncytial cell wall induced formation of pronounced cell-wall thickening (asterisks). (M–P) Nematode bodies and fungal hyphae. (M, O, and P) At 10 dpi in –3 treatment roots: (M) hyphae breaking a subcrystalline layer; (O) possible infection hyphae (arrows) penetrating the cuticle; (P) hyphae inside deteriorated nematode corpse. (N) At 10 dpi in –7 treatment roots: hyphae in direct contact with the cuticle of a juvenile. Arrows indicate septae typical for Sebacinaceae. C, Cortical parenchyma; En, endodermis; Ep, epidermis; H, hypha; H?, hypha-like structure; M, mucilage; N, nematode; Ne, necroses; P, pericycle; RH, root hair; SCL, subcrystalline layer; Se, sieve tube; S, syncytium; X, xylem vessel. Bars: 20 μm (A and D) and 2 μm (B, C, and E–P).
Fig. 3.
Quantitative analysis of Arabidopsis root colonization of 12-day-old Arabidopsis plants by P. indica (the time point of H. schachtii inoculation). (A) Fungal DNA abundance in roots from the –3 and –7 treatments in relation to amount of plant DNA; values are mean ± SE (n = 3); * indicates significant difference (t-test, P < 0.05). (B–D) Light microscopy micrographs of syncytia (10 dpi) from P. indica-non-colonized roots and syncytia (10 dpi) in –3 and –7 P. indica-colonized roots, respectively. N, nematode; S, syncytium; arrows, hyphae. Bars: 20 μm.
Cross-sections of root samples containing nematode-feeding sites showed that fungal hyphae penetrated into the vascular cylinder (Fig. 2D) when its cells were previously destroyed by the intracellular migration of the J2s (Fig. 2E). In these cases, hyphae were able even to enter the lumen of xylem vessels (Fig. 2E, G, H, and K). In few such cases, syncytia were electron translucent, indicating necrotized protoplasts next to colonized vessels (Fig. 2G), while most neighbouring syncytia contained electron-dense and well-preserved cytoplasms (Fig. 2H and K). Necrotized syncytia with electron-translucent cytoplasm were also found next to vessels without hyphae (data not shown). Thus, it is hard to speculate if occlusion of vessel lumen by hyphae may lead to feeding site collapse, especially as necrotizing syncytia were observed in all experimental time points.
The hyphae of P. indica were never found inside nematode-induced syncytia (Fig. 2F, H–L), not even inside those containing collapsed cytoplasm (Fig. 2G). Very rare fungal attempts to invade syncytia or living vascular cylinder cells were observed to be successfully circumvented by massive depositions of cell-wall material along penetration sites (Fig. 2K and L). These depositions were similar in structure and appearance to the regular syncytial walls and they differed from the electron-translucent layers of callose-like material deposited locally around inserted nematode stylet or next to the nematode’s head during initial stages of syncytium development described by Sobczak et al. (1999). Colonization of P. indica around nematode-induced syncytia increased over the time as observed on microscope sections (Fig. 3C and D). The structure and organization of syncytia appeared not to be affected by fungal root colonization (Fig. 3B–D). Apart from plant cell colonization, hyphae of P. indica attempted to penetrate the bodies of sedentary juveniles at both tested time points (Fig. 2M–P). However, it could not be determined whether the hyphae attacked dead juveniles (as few juveniles die during development when their syncytia necrotize) or whether they were able to parasitize alive juveniles and cause their death. In most examined juveniles, the hyphae were separated from the nematode bodies by a subcrystalline layer secreted by the nematode cuticle. This layer was supposed to play a protective role against soil-borne nematode pathogens (Zunke, 1985). Only in a few cases were hyphae able to overcome or break the subcrystalline layers to get into direct contact with the nematodes’ bodies (Fig. 2M). There they tightly adhered to the cuticle, filling even small ridges on the cuticle surface (Fig. 2N and O). In a few cases, small structures similar in appearance to hyphae were found in the nematode cuticle (Fig. 2O) and there was only a single probe that found hyphae equivocally present inside a strongly degraded nematode corpse (Fig. 2P).
P. indica colonization affects H. schachtii infection and development
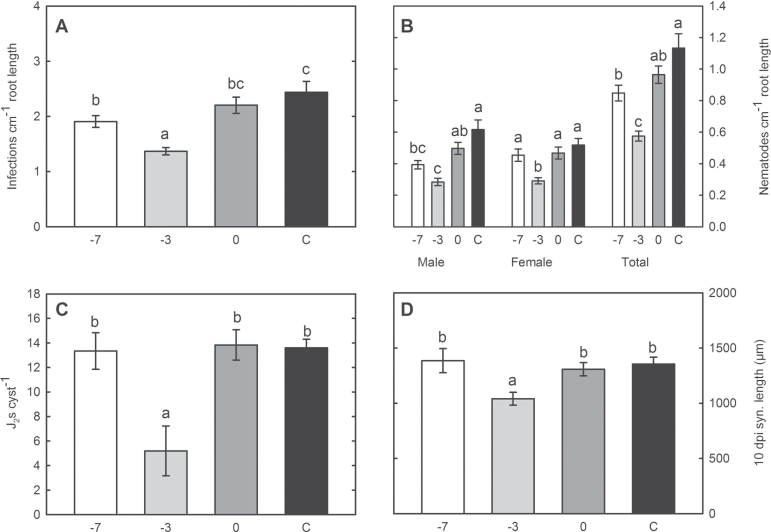
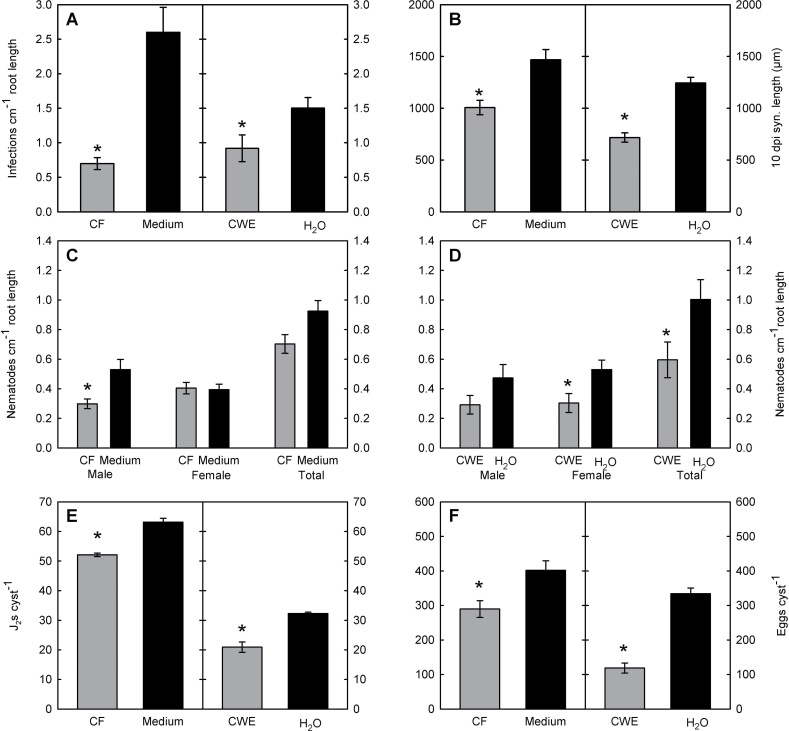
The microscopic studies clearly showed that hyphae of P. indica never penetrated nematode-feeding cells. The high abundance of fungal hyphae encompassing root cells and later the syncytia may nevertheless affect H. schachtii infection, development and reproduction. Thus, the impact of root colonization by P. indica on the infection of H. schachtii was studied at 3 dpi. Under the applied sterile conditions, the majority of the mobile J2s localized and infected roots within this time span. The obtained results showed a significant reduction of nematode infection sites in the –7 and –3 treatments as compared to the control (Fig. 4A). Thereafter, the development of the nematodes was studied at 15 dpi in P. indica-colonized roots. In the –7 treatment, the number of males as well as the total number of nematodes was significantly reduced when compared to fungus-non-colonized plants, whereas the number of females remained unaffected. In the –3 treatment, a significant reduction of both males and females in P. indica-colonized plants was observed (Fig. 4B). This result correlates with the strong decrease in the infection rate observed in the same treatments (Fig. 4A); however, nematode development was more strongly affected by P. indica colonization than nematode infection. In the 0 treatment, nematode development was not significantly affected. Finally, the nematode reproduction efficiency in P. indica-colonized roots was studied. Therefore, the number of J2s hatching from single cyst from each treatment was counted and used as an indication of offspring vitality. The number of J2s hatching from cysts collected from –3 plants was significantly reduced, whereas the –7 and 0 treatments did not show any statistically significant differences, in comparison to the control (Fig. 4C).
Fig. 4.
Nematode infection assays in –7, –3, and 0 P. indica-treated and control plants. (A) Number of nematode infection sites per cm of root length at 3 dpi. (B) Number of developed nematodes per cm root length at 15 dpi. (C) Number of second-stage juveniles hatched from single cysts at 60–75 dpi. (D) Average length of syncytia associated with female nematodes at 10 dpi. Values are mean ± SE (n = 3); different letters indicates significant difference (Tukey test, P < 0.05).
Fungal proliferation around nematode-induced syncytia may affect the developing nematode by a hyphal network mechanically restricting syncytium expansion or chemically by the exudation of fungal compounds that may affect enzymic processes of plant cells. Since nematode development has been shown to be related to syncytium expansion (Golinowski et al., 1996; Grundler et al., 1998; Wieczorek et al., 2006), the lengths of similarly developed syncytia associated with female juveniles were studied at 10 dpi. The results showed that syncytia established in –3 roots were significantly shorter when compared to all other treatments (Fig. 4D). The effect of the –3 treatment on syncytium enlargement makes a sole mechanical restriction of cell expansion improbable. In this case, the smallest syncytia would be expected in roots of –7 P. indica-treated plants due to the higher abundance and dense network of the fungal hyphae (Fig. 3C and D). This indicates that a distinct chemical interaction between the fungus, the plant, and/or the nematode induced by potential fungal effectors may have significant effects on the life cycle of H. schachtii.
Application of P. indica CF and CWE reduces nematode development and reproduction
In order to test the hypothesis of a chemical nature of fungus–plant–nematode interaction, the effect of fungal-derived compounds on the life cycle of H. schachtii was studied. Previously it has been shown that fungal exudates and cell-wall components have an effect on nematode development (Hallmann and Sikora, 1996). Accordingly, CF and CWE of P. indica were produced and applied on roots prior nematode inoculation. Since the strongest effect of P. indica plant-colonization on nematode development was observed in the –3 treatment (Fig. 4), CF and CWE were applied on Arabidopsis plants 3 days before H. schachtii inoculation. The effects of CF and CWE root treatment on the nematodes were always compared to root treatments with medium and sterile water, respectively. The obtained results showed first a significantly reduced infection rate at 3 dpi in both treatments (Fig. 5A) and both fungal extracts caused significantly shorter syncytia (Fig. 5B). Further, the application of the CF affected male nematode development (Fig. 5C) while the application of the CWE affected the total number of nematodes, especially the number of developing females (Fig. 5D). Finally, offspring vitality measured as a number of J2s hatching from cysts developed on CF- or CWE-treated roots was significantly lowered compared to control plants (Fig. 5E). In order to test if total offspring production is affected by root treatment with fungal CF and CWE, the numbers of eggs per cyst was counted in crushed mature cysts. Again, the number of eggs per cyst developed on CF- or CWE-treated plants was decreased (Fig. 5F). Finally, the effects of CF and CWE on nematode development were compared. Syncytium length as well as numbers of eggs and J2s per cyst were significantly stronger affected by CWE than CF (one-way ANOVA, data not shown).
Fig. 5.
Effect of P. indica culture filtrate (CF) and cell-wall extract (CWE) on (A) nematode infection at 3 dpi; (B) length of syncytia at 10 dpi; (C and D) nematode development at 15 dpi; (E) number of juveniles hatched from single cysts at 60–75 dpi; and (F) number of eggs in single cysts at 60–75 dpi. Plants were treated 3 days before nematode inoculation either with CF (50 μl) or CWE (50 μl) obtained from P. indica liquid culture. Control plants were treated with medium or water, respectively. Values are mean ± SE (n = 3); * indicates significant difference (t-test, P < 0.05).
Fungal exudates and fungus-triggered root exudates affect migration of juveniles
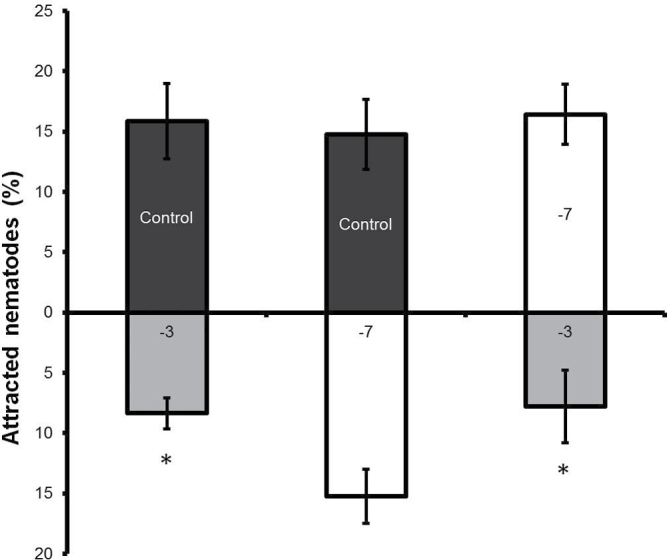
Apart from nematode infection and development, P. indica may alter the composition of root exudates that are known to be essential for J2s to localize and identify their hosts (Grundler et al., 1991). A reduced nematode penetration of roots colonized by fungal endophytes was reported before and it was suggested that fungal metabolites might possess a nematistatic potential (Hallmann and Sikora, 1996; Dababat and Sikora, 2007b; Vu et al., 2005). Thus, attraction assays with root exudates collected from plants colonized by the fungus at two different co-cultivation times (–3 and –7 treatments) and from control plants were performed implementing the procedure described by Dalzell et al. (2011). Nematode juveniles were significantly less attracted by root exudates of the –3 treatment than by those collected from the –7 treatment and the control (no fungus) plants (Fig. 6).
Fig. 6.
Nematode attraction assay towards root exudates obtained from –3 and –7 treatment A. thaliana plants. Control plants were mock-treated. Values are mean ± SE (n = 3); * indicates significant difference (t-test, P < 0.05).
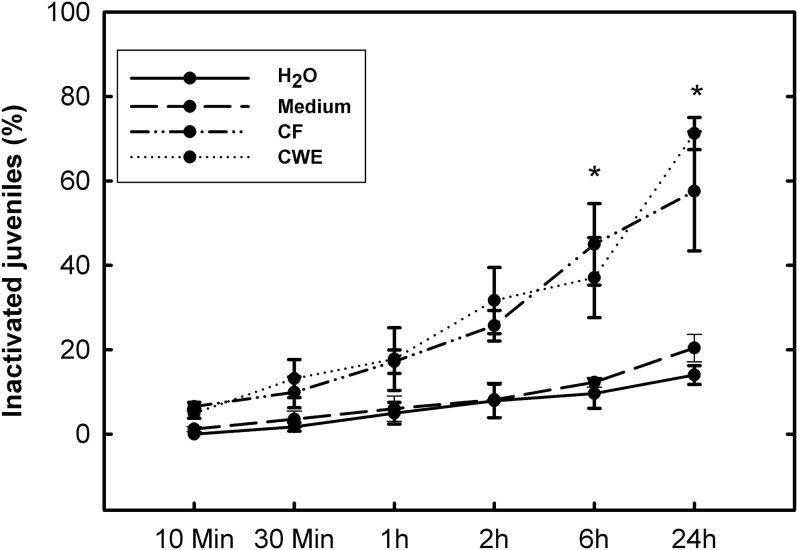
Further, the direct effects of P. indica CF and CWE on the mobility of H. schachtii J2s were tested. Both, CF and CWE were able to immobilize migrating J2s already 2h after exposure, and the percentage of immobile J2s steadily increased (Fig. 7); 24h after exposure to CF or CWE, about 60 and 70%, respectively, of the tested J2s were immobilized, whereas less than 20% of the J2s were motionless in the control treatments.
Fig. 7.
Nematode second-stage juvenile inactivation assay. Juveniles were exposed to culture filtrate (CF) and cell-wall extracts (CWE) of P. indica, as well as water or medium as control treatments for 24h. The percentage of immobilized second-stage juveniles was calculated. Values are mean ± SE (n = 3); * indicates significant difference (LSD test, P < 0.05).
Discussion
International concerns over the excessive use of harmful pesticides have given a particular push to search for natural microbial pesticides as inoculants (Paulitz and Bélanger, 2001). Beside well-established agricultural methods such as crop rotation, biological pest control based on the application of antagonists is a promising alternative to expensive and toxic nematicides (Sikora, 1992). The use of beneficial micro-organisms such as arbuscular mycorrhizal fungi (AMF), rhizobacteria, endophytic bacteria, and endophytic fungi for the control of plant-parasitic nematodes has been studied before (Dababat and Sikora, 2007a, b; Mendoza, 2008; Le et al., 2009; Vos et al., 2011, 2012). In these works, the antagonists were shown to affect nematode penetration and life cycle in the host plant. These effects were triggered either by direct competition, microbe-derived antibiotics, or induced systemic resistance (Sikora, 1992; Kerry, 2000; Sikora et al., 2007). The current work studied the potentials of the endophytic fungus P. indica as an antagonistic organism to battle against the plant-parasitic beet cyst nematode, H. schachtii.
The first step during plant–nematode interaction is stimulation of hatching and attraction of mobile J2s towards host roots. Active compounds from culture filtrates of different endophytic fungi were described to have nematicidal or nematistatic potentials (Mani and Sethi, 1984a, b; Cayrol et al., 1989; Cayrol and Djian, 1990; Hallmann and Sikora, 1996; Anke and Sterner, 1997; Chen et al., 2000; Meyer et al., 2000; Köpcke et al., 2001). This nematode-antagonistic activity may be explained by production of secondary fungal metabolites and enzymes such as chitinases that feature toxicity against plant-parasitic nematodes (Shinya et al., 2008). Thus, the CF and CWE of P. indica studied in the present work may contain a cocktail of nematicidal compounds that significantly reduced J2 hatching and vitality that may consequently affect nematode infection.
Except for the direct nematicidal activity of fungus-derived compounds, endophytic fungi are able to produce large amounts of toxic chemicals in vitro (Vu, 2005). Thus, they may also secrete such compounds to the surrounding environment when growing in planta. Several studies have elucidated that fungal endophytes may alter chemical properties of root exudates or may stimulate plants to produce chemicals or hormones which repel or disturb nematode attraction (Diez and Dusenbury, 1989; Vu, 2005; Shahasi et al., 2006; Dababat and Sikora, 2007a; Le et al., 2009). Le et al. (2009) demonstrated that the reduction in migration and penetration of Meloidogyne graminicola juveniles towards the Fusarium moniliforme-treated roots was based on the induced systemic resistance evoked by fungal colonization of the root. Similarly, repellent effect of root exudates from tomato treated with the non-pathogenic endophytic Fusarium oxysporum 162 on Meloidogyne incognita was reported (Dababat and Sikora, 2007b). Root exudates from tomato plants colonized by the AMF fungus Glomus mosseae reduced nematode penetration (Vos et al., 2011). The present work determined a decreased attractiveness for the infective J2s of exudates from roots colonized by P. indica. However, since the plants were not grown in a split-root system, this evidence cannot be referred to plant defence. Recently, Jacobs et al. (2011) described four different colonization phases during the P. indica–plant interaction: (1) extracellular (approx. 1 dpi); (2) biotrophic (less than 3 dpi); (3) cell death associated (approx. 7 dpi); and (4) fungal reproduction (approx. 14 dpi). The present work only observed effects on J2 attraction when nematode inoculation occurred during the biotrophic colonization phase of P. indica (–3 treatment). However, exudates collected from roots in the cell death-associated colonization phase (–7 treatment) caused no changes in attractiveness for J2s compared to exudates from non-colonized control roots. In the –7 treatment, either P. indica may not affect root exudates anymore or the induced changes have no anti-nematode effect.
The present results showed that P. indica root colonization affects J2 infection especially during the biotrophic phase. In this phase, the expression of MYB51, which is involved in the biosynthesis of antimicrobial indole glucosinolates (Clay et al., 2009), is induced in roots of P. indica-treated plants (Jacobs et al., 2011). The intracellularly migrating J2s do cause severe cell damage and thus may have to cope with glucosinolate-based plant defence in a compatible plant– nematode interaction. Salicylic acid-mediated signalling may also be involved in the A. thaliana–P. indica–H. schachtii interaction since this hormone was shown to have a significant inhibitory effect on H. schachtii (Wubben et al., 2008), and root inoculation with P. indica (3 dpi) upregulated expression of CBP60g and SID2, markers of the salicylic acid-mediated signalling pathway (Jacobs et al., 2011).
Apart from the infection, nematode development and reproduction were significantly affected by P. indica colonization in the –3 treatment. On the one hand, reduced J2 vitality and nematode infection ability lead to a decline in the number of developing adults. On the other hand, the development rate after 14 days was relatively more strongly affected than the infection rate in the first 3 days in the –3 P. indica-treated plants. These results indicate that not only infection and migration of J2s but also later stages of nematode development are affected by P. indica. Accordingly, syncytium size was reduced in –3 treatment plants. The current analysis suggests that mechanical barriers may play a minor role in syncytium size reduction. Microscopic observation showed that hyphae of P. indica were not able to penetrate and thus irritate syncytial protoplast. Although the microscopic analysis showed the evidence of vessel colonization by P. indica hyphae following nematode infection, a clear relationship with the state of syncytial protoplasts could not be found. Syncytia with electron-translucent and necrotizing protoplasts were found next to vessels with hyphae as well as next to vessels without hyphae inside their lumen. There are also too few data for statistical evaluation of the possible role of fungal parasitism and hyphae penetration into nematode bodies, and it was not clear if alive or dead nematodes were successfully infected. Therefore, nematode as well as syncytium development appear to be primarily affected by fungus-derived chemical compounds. Accordingly, development and reproduction of H. schachtii as well as syncytium expansion were significantly affected when only CF and even more when only CWE were added onto the roots and no direct fungus–root–nematode interaction occurred. The composition of both extracts is currently unknown. CWE may contain active chitinases that were demonstrated to coincide with the development of resistance against the root-knot nematode M. incognita in AMF-colonized roots (Li et al., 2006).
Along the fungal root colonization, the biotrophic phase of the direct P. indica–Arabidopsis interaction (3 dpi) is characterized by a systemic fungus-mediated suppression of flg22-triggered callose deposition in all parts of root (Jacobs et al., 2011). Microscopic studies showed that, in A. thaliana, callose is deposited and degraded in a development-dependent manner along syncytial cell walls of H. schachtii. In the initial syncytial cell, callose-like material is deposited locally at cell walls along the inserted nematode stylet and nematode head (Sobczak et al., 1999). In young syncytia, a specific temporal callose deposition along plasmodesmata was observed that results in their symplasmic isolation during the first days of development (Hofmann and Grundler, 2006; Hofmann et al., 2010). Thus, the ability of P. indica to abolish callose deposition during the biotrophic phase may affect juvenile migration and stylet insertion as well as syncytium induction and expansion. Accordingly, the size of syncytia in –3 treated roots was significantly reduced.
Summarizing, these results show that the inoculation with H. schachtii during the biotrophic colonization stage of P. indica (–3 treatment) led to a significant reduction in the number of nematode infection sites and disturbed nematode development. Potential mechanical barriers formed by the hyphae hindering J2 migration or syncytium expansion may play a secondary and rather minor role. Hence, the data suggest that fungal-derived chemicals, exudates, and cell-wall compounds cause the major inhibitory effects on the development of H. schachtii in Arabidopsis roots. The presented data introduce P. indica as a new nematode-antagonistic fungus. They improve the understanding on the interaction between different organisms and provide essential information for applied agricultural and pest management practices. Future identification of P. indica-derived compounds with anti-nematode activity could present a significant progress for developing new agriculturally beneficial and environmentally friendly agents.
Acknowledgements
The authors thank Ralf Oelmüller (Friedrich-Schiller-University Jena, Germany) for providing P. indica and help with cultivating the fungus.
References
- Anke H, Sterner O. 1997. Nematicidal metabolites from higher fungi. Current Organic Chemistry 1, 427–440. [Google Scholar]
- Böckenhoff A, Prior DAM, Grundler FMW, Oparka KJ. 1996. Induction of phloem unloading in Arabidopsis thaliana roots by the parasitic nematode Heterodera schachtii . Plant Physiology 112, 1421–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayrol JC, Djian C. 1990. Study of the toxicity of Fusarium roseum var. arthrosporioides to the nematode Meloidogyne arenaria . Comptes-rendus de l’Academie d’Agriculture de France 76, 121–129. [Google Scholar]
- Cayrol JC, Djian C, Pijarowski L. 1989. Study of the nematocidal properties of the culture filtrate of the nematophagous fungus Paecilomyces liliacinus . Revue Nèmatologie 12, 331–336. [Google Scholar]
- Chen SY, Dickson DW, Mitchell DJ. 2000. Viability of Heterodera glycines exposed to fungal filtrates. Journal of Nematology 32, 190–197. [PMC free article] [PubMed] [Google Scholar]
- Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM. 2009. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dababat AA, Sikora RA. 2007a. Induced resistance by the mutualistic endophyte, Fusarium oxysporum strain 162 toward Meloidogyne incognita on tomato. Biocontrol Science and Technology 17, 969–975. [Google Scholar]
- Dababat AA, Sikora RA. 2007b. Influence of the mutualistic endophyte Fusarium oxysporum 162 on Meloidogyne incognita attraction and invasion. Nematology 9, 771–776. [Google Scholar]
- Dalzell JJ, Kerr R, Corbett MD, Fleming CC, Maule AG. 2011. Novel bioassays to examine the host-finding ability of plant-parasitic nematodes. Journal of Nematology 13, 211–220. [Google Scholar]
- Deshmukh SD, Kogel KH. 2007. Piriformospora indica protects barley from root rot caused by Fusarium graminearum. . Journal of Plant Diseases and Protection 114, 263–268. [Google Scholar]
- Diez JA, Dusenbery DB. 1989. Repellent of root-knot nematodes from exudates of host roots. Journal of Chemical Ecology 15, 2445–2455. [DOI] [PubMed] [Google Scholar]
- Elsen A, Gervacio D, Swennen R, De Waele D. 2008. AMF-induced biocontrol against plant parasitic nematodes in Musa sp.: a systemic effect. Mycorrhiza 18, 251–256. [DOI] [PubMed] [Google Scholar]
- Farkya S, Baldi A, Kumar V, Datta V, Mehra R, Gupta N, Jain A, Srivastava AK, Bisaria VS. 2010. Impact of symbiotic fungi on production of secondary metabolites by plant cell culture. Asia-Pacific Journal of Molecular Biology and Biotechnology 18, 51–53. [Google Scholar]
- Golinowski W, Grundler FMW, Sobczak M. 1996. Changes in the structure of Arabidopsis thaliana during female development of the plant-parasitic nematode Heterodera schachtii . Protoplasma 194, 103–116. [Google Scholar]
- Grundler FMW, Schnibbe L, Wyss U. 1991. In vitro studies on the behaviour of second-stage juveniles of Heterodera schachtii (Nematoda: Heteroderidae) in response to host plant root exudates. Parasitology 103, 149–155. [Google Scholar]
- Grundler FMW, Sobczak M, Golinowski W. 1998. Formation of wall openings in root cells of Arabidopsis thaliana following infection by the plant-parasitic nematode Heterodera schachtii . European Journal of Plant Pathology 104, 545–551. [Google Scholar]
- Hallmann J, Davies KG, Sikora R. 2009. Biological control using microbial pathogens, endophytes and antagonists. In: Perry RN, Moens M, Starr JL, eds, Root-knot nematodes. Wallingford: Centre for Agricultural Bioscience International, pp 380–411. [Google Scholar]
- Hallmann J, Sikora R. 1996. Toxicity of fungal endophyte secondary metabolites to plant parasitic nematodes and soil-borne plant pathogenic fungi. European Journal of Plant Pathology 102, 155–162. [Google Scholar]
- Hofmann J, Grundler FMW. 2006. Females and males of root-parasitic cyst nematodes induce different symplasmic connections between their syncytial feeding cells and the phloem in Arabidopsis thaliana . Plant Physiology and Biochemistry 44, 430–433. [DOI] [PubMed] [Google Scholar]
- Hofmann J, Youssef-Banora M, de Almeida-Engler J, Grundler FMW. 2010. The role of callose deposition along plasmodesmata in nematode feeding sites. Molecular Plant–Microbe Interactions 23, 549–557. [DOI] [PubMed] [Google Scholar]
- Jacobs S, Zechmann B, Molitor A, Trujillo M, Petutschnig E, Lipka V, Kogel KH, Schäfer P. 2011. Broad-spectrum suppression of innate immunity is required for colonization of Arabidopsis roots by the fungus Piriformospora indica . Plant Physiology 156, 726–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgensen K. 2001. Untersuchungen zum Assimilat- und Wassertransfer in der Interaktion zwischen Arabidopsis thaliana und Heterodera schachtii . PhD thesis. Kiel, Germany: Christian-Albrechts Universität. [Google Scholar]
- Jung C, Wyss U. 1999. New approaches to control plant parasitic nematodes. Applied Microbiology and Biotechnology 51, 439–446. [Google Scholar]
- Kerry BR. 2000. Rhizosphere interactions and the exploitation of microbial agents for the biological control of plant-parasitic nematodes. Annual Review of Phytopathology 38, 423–441. [DOI] [PubMed] [Google Scholar]
- Köpcke B, Wolf D, Anke H, Sterner O. 2001. New natural products with nematicidal activity from fungi. British Mycological Society International Symposium, Bioactive Fungal Metabolites – Impact and Exploitation, UW. Swansea, UK, 22–27 April 2001, p 72 (Abstract).
- Kumari R, Pham GH, Prasad R, et al. 2004. Piriformospora indica: fungus of the millennium. In: Podila G, Varma A, eds, Basic research and applications: mycorrhizae. Microbiology series. New York: IK International-India; pp 259–295. [Google Scholar]
- Le TH, Padgham LJ, Sikora RA. 2009. Biological control of the rice root-knot nematode Meloidogyne graminicola on rice, using endophytic and rhizosphere fungi. International Journal of Pest Management 55, 31–36. [Google Scholar]
- Li HY, Yang GD, Shu HR, Yang YT, Ye BX, Nishida I, Zheng CC. 2006. Colonization by the arbuscular mycorrhizal fungus Glomus versiforme induces a defense response against the root-knot nematode Meloidogyne incognita in grapevine (Vitis amurensis Rupr.), which includes transcriptional activation of the class III chitinase gene VCH3. Plant Cell Physiology 47, 154–163. [DOI] [PubMed] [Google Scholar]
- Mani A, Sethi CL. 1984a. Effect of culture filtrates of Fusarium oxysporum f. sp. cicero and Fusarium solani on hatching and juvenile mobility of Meloidogyne incognita . Nematropica 14, 139–144. [Google Scholar]
- Mani A, Sethi CL. 1984b. Some characteristics of culture filtrate of Fusarium solani toxic to Meloidogyne incognita . Nematropica 14, 121–129. [Google Scholar]
- Mendoza AR. 2008. Interrelationships between microbial antagonists having divergent modes-of-action and their influence on biological control of plant-parasitic nematodes. PhD thesis. Bonn, Germany: University of Bonn. [Google Scholar]
- Meyer SLF, Massoud SI, Chitwood DJ, Roberts DP. 2000. Evaluation of Trichoderma virens and Burkholderia cepacia for antagonistic activity against root-knot nematode, Meloidogyne incognita . Nematology 2, 871–879. [Google Scholar]
- Paulitz TC, Bélanger RR. 2001. Biological control in greenhouse systems. Annual Review of Phytopathology 39, 303–308. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative CT method. Nature Protocols 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Shahasi YA, Dubois T, Coyne D, Gold CS, Labuschagne N, Viljoen A. 2006. Effect of endophytic Fusarium oxysporum on host preference of Radopholus similis to tissue culture banana plants. Journal of Nematology 38, 455–460. [PMC free article] [PubMed] [Google Scholar]
- Shinya R, Aiuchi D, Kushida A, Tani M, Kuramochi K, Koike M. 2008. Effect of fungal culture filtrates of Verticillium lecanii (Lecanicillium spp.) hybrid strains on Heterodera glycines eggs and juveniles. Journal of Invertebrate Pathology 97, 291–297. [DOI] [PubMed] [Google Scholar]
- Sijmons PC, Grundler FMW, von Mende S, Burrows PR, Wyss U. 1991. Arabidopsis thaliana as a new model host for plant-parasitic nematodes. The Plant Journal 1, 245–254. [Google Scholar]
- Sikora RA. 1992. Management of the antagonistic potential in agricultural ecosystems for the biological control of plant-parasitic nematodes. Annual Review of Phytopathology 30, 245–270. [Google Scholar]
- Sikora RA, Schäfer K, Dababat AA. 2007. Modes of action associated with microbially induced in planta suppression of plant-parasitic nematodes. Australasian Plant Pathology 36, 124–134. [Google Scholar]
- Sobczak M, Golinowski W, Grundler FMW. 1999. Ultrastructure of feeding plugs and feeding tubes formed by Heterodera schachtii . Nematology 1, 363–374. [Google Scholar]
- Vadassery J, Ranf S, Drzewiecki C, Mithofer A, Mazars C, Scheel D, Lee J, Oelmuller R. 2009. A cell wall extract from the endophytic fungus Piriformospora indica promotes growth of Arabidopsis seedlings and induces intracellular calcium elevation in roots. The Plant Journal 59, 193–206. [DOI] [PubMed] [Google Scholar]
- Varma A, Verma S, Sahay N, Bütehorn B, Franken PH. 1999. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Applied and Environmental Microbiology 65, 2741–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos C, Claerhout S, Mkandawire R, Panis B, De Waele D, Elsen A. 2011. Arbuscular mycorrhizal fungi reduce root-knot nematode penetration through altered root exudation of their host. Plant and Soil 354, 335–345. [Google Scholar]
- Vos C, Geerinckx K, Mkandawire R, Panis B, De Waele D, Elsen A. 2012. Arbuscular mycorrhizal fungi affect both penetration and further life stage development of root-knot nematodes in tomato. Mycorrhiza 22, 157–163. [DOI] [PubMed] [Google Scholar]
- Vu TT. 2005. Modes of action of non-pathogenic Fusarium oxysporum endophytes for bio-enhancement of banana toward Radopholus similis . PhD thesis. Bonn, Germany: University of Bonn. [Google Scholar]
- Waller F, Achatz B, Baltruschat H, et al. 2005. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proceedings of the National Academy of Sciences, USA 102, 13386–13391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller F, Mukherjee K, Deshmukh SD, Achatz B, Sharma M, Schäfer P, Kogel KH. 2008. Systemic and local modulation of plant responses by Piriformospora indica and related Sebacinales species. Journal of Plant Physiology 165, 60–70. [DOI] [PubMed] [Google Scholar]
- Wieczorek K, Golecki B, Gerdes L, et al. 2006. Expansins are involved in the formation of nematode-induced syncytia in roots of Arabidopsis thaliana . The Plant Journal 48, 98–112. [DOI] [PubMed] [Google Scholar]
- Wubben MJ, Jin J, Baum TJ. 2008. Cyst nematode parasitism of Arabidopsis thaliana is inhibited by salicylic acid (SA) and elicits uncoupled SA-independent pathogenesis-related gene expression in roots. Molecular Plant–Microbe Interactions 21, 424–432. [DOI] [PubMed] [Google Scholar]
- Zuccaro A, Basiewicz M, Zurawska M, Biedenkopf D, Kogel KH. 2009. Karyotype analysis, genome organization and stable genetic transformation of the root colonizing fungus Piriformospora indica . Fungal Genetics and Biology 46, 543–550. [DOI] [PubMed] [Google Scholar]
- Zunke U. 1985. Zur Bildung der subkristallinen Schicht bei Heterodera schachtii unter aseptischen Bedingungen. Nematologica 31, 117–120. [Google Scholar]