Abstract
Critical responses to developmental or environmental stimuli are mediated by different transcription factors, including members of the ERF, bZIP, MYB, MYC, and WRKY families. Of these, MYB genes play roles in many developmental processes. The overexpression of one MYB gene, MYBH, significantly increased hypocotyl elongation in Arabidopsis thaliana plants grown in the light, and the expression of this gene increased markedly in the dark. The MYBH protein contains a conserved motif, R/KLFGV, which was implicated in transcriptional repression. Interestingly, the gibberellin biosynthesis inhibitor paclobutrazol blocked the increase in hypocotyl elongation in seedlings that overexpressed MYBH. Moreover, the function of MYBH was dependent on phytochrome-interacting factor (PIF) proteins. Taken together, these results suggest that hypocotyl elongation is regulated by a delicate and efficient mechanism in which MYBH expression is triggered by challenging environmental conditions such as darkness, leading to an increase in PIF accumulation and subsequent enhanced auxin biosynthesis. These results indicate that MYBH is one of the molecular components that regulate hypocotyl elongation in response to darkness.
Key words: Arabidopsis, auxin, hypocotyl elongation, MYBH, PIF, photomorphogenesis.
Introduction
Photomorphogenesis, which is the light-mediated regulation of plant development, integrates many genetic and environmental factors. Light-grown seedlings exhibit short hypocotyls, whereas dark-grown seedlings exhibit long hypocotyls. During plant growth, the intensity and characteristics of light determine the rate and extent of cell elongation, and thus these factors directly affect hypocotyl length. Numerous studies have identified the key factors that trigger photomorphogenesis (for reviews, see Chory, 1993; Deng, 1994; McNellis and Deng, 1995). The hy1, hy2, hy3/phyB, and hy6 mutations are associated with phytochromes, whereas the cry1 (hy4) mutation is associated with a blue light receptor (Koornneef et al., 1980; Chory et al., 1989; Parks and Quail, 1991; Ahmad and Cashmore, 1993; Reed et al., 1993). In contrast, Constitutive Photomorphogenic/De-etiolated/Fusca (COP/DET/FUS) proteins play negative roles in photomorphogenesis (Wei and Deng, 1999). COP1 encodes a RING-type E3 ubiquitin ligase that mediates the ubiquitination of positive regulators of photomorphogenesis in the dark, thereby targeting these regulators for degradation (Yi and Deng, 2005).
Plant photomorphogenesis is regulated by various hormone signalling pathways. In plants overexpressing auxin biosynthetic genes, hypocotyl elongation was found to be enhanced because of elevated auxin levels (Zhao et al., 2002). Furthermore, the expression of several auxin-inducible genes during hypocotyl elongation has been observed in Arabidopsis thaliana and Glycine max (soybean) (McClure and Guilfoyle, 1989; Li et al., 1991; Gil and Green, 1997). Recently, the overexpression of A. thaliana SMALL AUXIN UP RNA63 was shown to promote hypocotyl elongation (Chae et al., 2012). Additionally, gibberellic acid (GA) appears to promote skotomorphogenic growth and repress photomorphogenesis. Thus, reductions in endogenous levels of GA cause a light-grown phenotype in seedlings that are grown in the dark (Alabadi et al., 2004). The same phenomenon was also observed in a ga1 mutant, which cannot perform GA biosynthesis (Alabadi et al., 2004; Achard et al., 2007). Recently, GA responses were shown to be repressed by the DELLA protein, which interferes with the function of basic helix–loop–helix transcription factors called phytochrome-interacting factors (PIFs) (De Lucas et al., 2008; Feng et al., 2008). PIFs are degraded via proteasome-dependent pathways in response to interactions with light-activated phytochrome B (Bauer et al., 2004; Park et al., 2004; Shen et al., 2005). Thus, PIFs appear to play crucial roles in the integration of light and GA signalling, thereby contributing to the fine-tuning of plant development in response to various signals. In contrast, cytokinins have been found to promote de-etiolation in the absence of light (Chory et al., 1991). Moreover, an amp mutant, which expresses high levels of cytokinins, showed different de-etiolation phenotypes in the dark (Chin-Atkins et al., 1996). These observations indicate that hypocotyl elongation must be optimally modulated by both positive and negative regulators in response to environmental challenges.
Various transcription factors, including members of the ERF, bZIP, MYB, MYC, and WRKY families, are involved in regulating gene expression in response to developmental or environmental stimuli. The MYB protein family controls gene expression by binding to DNA via MYB domain repeat sequences. MYB proteins are associated with a diverse array of cellular responses, including plant secondary metabolism, as well as biotic and abiotic stress tolerance (Jin et al., 2000; Singh et al., 2002; Vom Endt et al., 2002; Taki et al., 2005; Agarwal et al., 2006; Cheng et al., 2009; Mandaokar and Browse, 2009). In particular, MYB proteins play positive or negative roles in the production of enzymes involved in the biosynthesis of phenylpropanoids (Legay et al., 2007; Bomal et al., 2008), flavonoids (Grotewold, 2005), and benzenoids (Verdonk et al., 2005). Although more than 100 R2R3-MYBs have been identified in Arabidopsis, the function of many MYB proteins remains largely unknown (Yanhui et al., 2006). Therefore, this study aimed to identify the MYB gene that plays a role in plant growth and development by using an ectopic expression approach. We showed that the MYB hypocotyl elongation-related gene, MYBH, is involved in the positive regulation of dark-induced hypocotyl elongation in Arabidopsis. However, a T-DNA knockout mutant of MYBH (mybh) showed no significant phenotypic differences in hypocotyl elongation. MYBH promoter activity in the hypocotyls was strong in the dark, which is consistent with the role of MYBH in regulating dark-induced hypocotyl elongation. Furthermore, MYBH increased the accumulation of PIFs, thereby leading to increased biosynthesis of auxin. Our results suggest that a delicate and efficient mechanism regulates hypocotyl elongation and show that MYBH may be one of the molecular components that regulate hypocotyl elongation in response to the dark.
Materials and methods
Plant materials and growth conditions
A. thaliana ecotype Columbia (Col-0) plants were used in this study. The seeds were surface-sterilized and sown in normal Murashige and Skoog (MS) agar medium supplemented with 2% sucrose. After 3 d of stratification at 4 °C, the seeds were allowed to germinate at 23±1°C under normal light conditions, namely a 16h light/8h dark cycle (white light: 200 µmol m–2 s–1). The T-DNA insertion mutant line AT5G47390, which is termed mybh (GK-783B02: NASC ID N365026), was obtained from the Nottingham Arabidopsis Stock Centre (http://arabidopsis.info). The presence of the insertion was confirmed by PCR using a combination of four primers: MYBH-F (5′-ATGACTCGTCGATGTTCTCACTGC-3′), MYBH-R (5′-GC GTGTATCACGCTTTTG-3′), GABI-F (5′-CGCCAGGGTTTTC CCAGTCACGACG-3′), and GABI-R (5′-GAAGGCGGGAAAC GACAATCTG-3′). In the mybh mutant line, the T-DNA was inserted into the first exon of AT5G47390.
Gene constructs
Transgenic Arabidopsis plants (Col-0) expressing full-length AT5G47390 (MYBH) cDNA were generated. Full-length MYBH cDNA was amplified via PCR and cloned into pBI121, which contains the cauliflower mosaic virus (CaMV) 35S promoter. To obtain transgenic overexpression of MYBH, Arabidopsis plants were transformed with Agrobacterium tumefaciens strain GV3101 using the floral dip method. Homozygous T4 transgenic plants were used for all of the experiments. For the construction of the MYBHpro::GUS plasmids, the MYBH promoter region was amplified by performing PCR and cloned using the pCR®8/GW/TOPO® TA Cloning® kit (Invitrogen). Additional information about the Gateway site-specific cloning protocols is provided online by the manufacturer (http://www.invitrogen.com/). The MYBHpro::GUS clones were created with LR Clonase reactions (Invitrogen) using MYBHpro/TOPO and the destination vector pMDC162.
Histochemical β-glucuronidase (GUS) staining
MYBHpro::GUS transgenic seedlings were grown on MS medium containing 2% sucrose and cultivated under normal light or dark conditions for 7 d. To observe GUS activity in the transgenic plants, whole seedlings were treated with GUS staining buffer [0.1M NaPO4 (pH 7.0), 10mM EDTA, 0.5mM ferricyanide, 0.5mM ferrocyanide, 1.9mM X-glucuronide, and 0.1% Triton X-100] and incubated at 37°C overnight. GUS-stained seedlings were cleared overnight in 100% ethanol to remove their chlorophyll.
RNA gel blot analysis
Col-0 and transgenic plants were grown on MS medium (2% sucrose) in a growth chamber for 7 d. The seedlings were then exposed to the treatment conditions (normal light or continuous dark). Total RNA was purified by using the aurintricarboxylic acid and lithium chloride method (Lee et al., 2002). Total RNA (20 µg) was separated by performing electrophoresis on 1.5% (w/v) agarose gels that contained formaldehyde and then transferred onto a HybondTM-XL membrane (Amersham Biosciences) and left for over 24h before being subjected to UV cross-linking. Radiolabeled probes were prepared using a random primer DNA Labeling System (Invitrogen). After pre-hybridization of the membrane for 1h at 65 °C, hybridization was performed with 32P-labelled probes for over 16h. The RNA blot was then washed twice in 1× SSC, 0.1% SDS and once more in 0.1× SSC, 0.1% SDS at 42 °C. The RNA blot was then scanned using a Multiplex Bio-Imaging System (Fujifilm FLA-7000).
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from whole seedlings using TRI Reagent (MRC Inc.). Briefly, 1 µg of total RNA was subjected to cDNA synthesis using oligo(dT) reverse transcriptase (Promega) and an RNase inhibitor (Intron Biotechnology). qRT-PCR was carried out in 20 µl reactions that contained 10ng of diluted cDNA, 10 µl of 2× iQSYBR Green Supermix (Bio-Rad), and 0.2mM gene-specific primers (Supplementary Table S1 at JXB online). The relative expression level of each transcript was analysed using Gene Expression Analysis for the iCycleriQ Real-Time PCR Detection System (Bio-Rad). Transcript levels were normalized to the expression of ACTIN7. Each experiment was repeated at least three times.
Plant hormone analysis
Arabidopsis plants were grown on MS medium for 8 d and then approximately 500mg (fresh weight) of each sample (Col-0, MYBH-OX, and mybh) was collected. Whole seedlings were frozen and homogenized with liquid nitrogen. Plant hormones were extracted at 4 °C with 3ml of 80% methanol that contained 1% acetic acid. The extraction was repeated twice with a 1h incubation in the first extraction and a 10min incubation in the second extraction. The supernatant was collected after centrifugation at 3000g for 10min and then filtered with a 3ml capacity RESERVOIR-2 FRITS (Varian). The filtrates were evaporated in vacuo using a centrifuge evaporator (SpeedVac). The dried sample was dissolved in 1ml methanol, which was subsequently removed by evaporation. Phytohormones were quantified by liquid chromatography-tandem mass spectrometry by the Growth Regulation Research Group at RIKEN Plant Science Center. GA and indole-3-acetic acid (IAA) were analysed as described previously (Yoshimoto et al., 2009).
Subcellular localization of MYBH
The CaMV35S::MYBH:GFP construct was used for the subcellular localization of MYBH in onion epidermal cells. The plasmid DNA (5 µg per experiment) was precipitated onto gold microparticles. Onion (Allium cepa) epidermal cells were grown on MS agar prior to transfection by particle bombardment (Bio-Rad, http://www.bio-rad.com/). After bombardment, the cells were placed in the dark at 23 °C for 18–48h. Green fluorescent protein (GFP) fluorescence was visualized via confocal lase- scanning fluorescence microscopy (LSM 5 Exciter; Carl-Zeiss, http://www.zeiss.com/).
Statistical analysis
Statistical analysis was performed by one-way analysis of variance (Duncan’s test) at a 95% confidence level. Differences were considered statistically significant when P <0.05.
Accession numbers
Sequence data for this article can be found in the Arabidopsis Genome Initiative databases under the following Arabidopsis Information Resource accession numbers: MYBH (AT5G47390), PIF4 (AT2G43010), PIF5 (AT3G59060), HY5 (AT5G11260), CAB (AT1G29910), EXP3 (AT2G37640), YUCCA8 (AT4G28720), and SAUR-like (AT2G21220 and AT2G45210).
Results
Enhanced hypocotyl elongation in Arabidopsis MYBH-OX plants
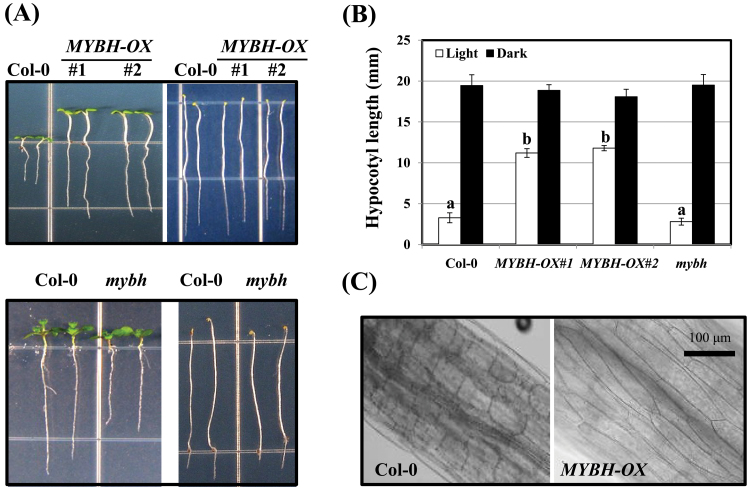
Although many MYB genes that play important roles in various cellular processes have been identified, many of them have not yet been characterized at the molecular level. Therefore, we decided to study the functions of MYB genes with respect to growth performance and stress tolerance. As most MYB genes exist in family groupings (Dubos et al., 2010), we thought that their knockout mutants may not show significant alterations in growth or stress tolerance because of redundancy. Therefore, we attempted to ectopically express various MYB genes in Arabidopsis and then recovered the MYBH gene that was involved in hypocotyl elongation. MYBH-OX plants showed significantly increased hypocotyl elongation under the light growth condition compared with wild-type plants (Fig. 1).
Fig. 1.
Enhanced hypocotyl elongation in MYBH-OX seedlings. Hypocotyl elongation phenotype (A) and length quantification (B) of wild-type seedlings (Col-0), MYBH-OX (#1 or #2), and mybh at 7 d after germination under light or in the dark. In (A), the left and right panels show seedlings that were grown in the light and dark, respectively. Three independent experiments were conducted (n=100). One-way analysis of variance (Duncan’s test) was conducted to indicate significant differences (P <0.05) in hypocotyl length between Col-0, MYBH-OX (#1 or 2), and mybh. Columns labelled above with the letter a are significantly different (P <0.05) from those labelled with b. Vertical bars represent standard error (n=300). (C) Hypocotyl cells in wild-type (Col-0) and MYBH-OX (#1) seedlings. Seven-d-old seedlings were grown under normal conditions and used for these photos. The samples were visualized using a microscope (LSM 5 Exciter, Carl-Zeiss). (This figure is available in colour at JXB online.)
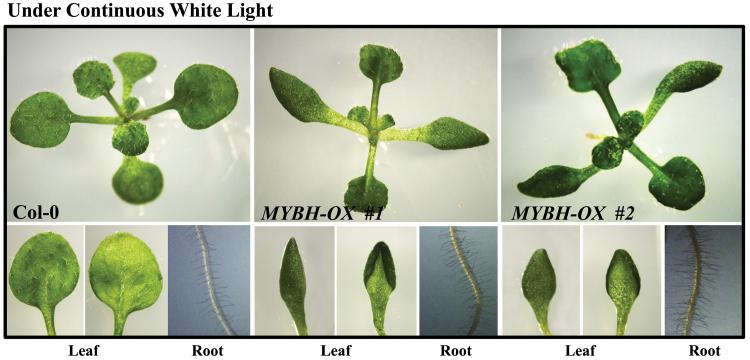
To understand the function of MYBH in hypocotyl elongation, a mybh knockout mutant (GK-783B02) was obtained for our experimental analysis. However, no significant differences in hypocotyl elongation were detected between the mybh mutant and Col-0 under the light or dark continuous conditions (Fig. 1A, B). In contrast, substantial differences in root growth were observed in the mybh line, and this defect could be recovered by the recombinant expression of wild-type MYBH (Fig. 1A and Supplementary Fig. S1 at JXB online). Additionally, the MYBH-OX seedlings exhibited longer hypocotyl cells than did Col-0 seedlings (Fig. 1C), and the MYBH-OX plants exhibited altered phenotypes when grown on MS medium under continuous light (Fig. 2). The MYBH-OX plants had darker leaves than the Col-0 plants. Moreover, leaf curling and increased root hair number were observed only in the transgenic plants (Fig. 2).
Fig. 2.
Phenotypic changes in MYBH-OX seedlings grown under continuous light. Col-0 and two lines of MYBH-OX (#1 and #2) seedlings were allowed to germinate on MS medium (2% sucrose) under continuous light for 14 d prior to being transferred to soil. The root samples were visualized using a microscope. The leaves of MYBH-OX (#1 and #2) seedlings were darker in colour, more curled, and had increased root hair numbers compared with those of Col-0. (This figure is available in colour at JXB online.)
A schematic representation of MYBH, including its putative functional domains, is shown in Supplementary Fig. S2 at JXB online. MYBH encodes a MYB-like transcription factor; thus MYBH was expected to localize to the nucleus. MYBH localization was assessed using a construct containing a fusion between MYBH and the GFP reporter gene. The CaMV35S::MYBH:GFP construct was transiently expressed in onion epidermis and stably transformed into Arabidopsis. As predicted, the MYBH–GFP fusion protein localized to the nucleus in both the onion and Arabidopsis (Supplementary Fig. S3 at JXB online).
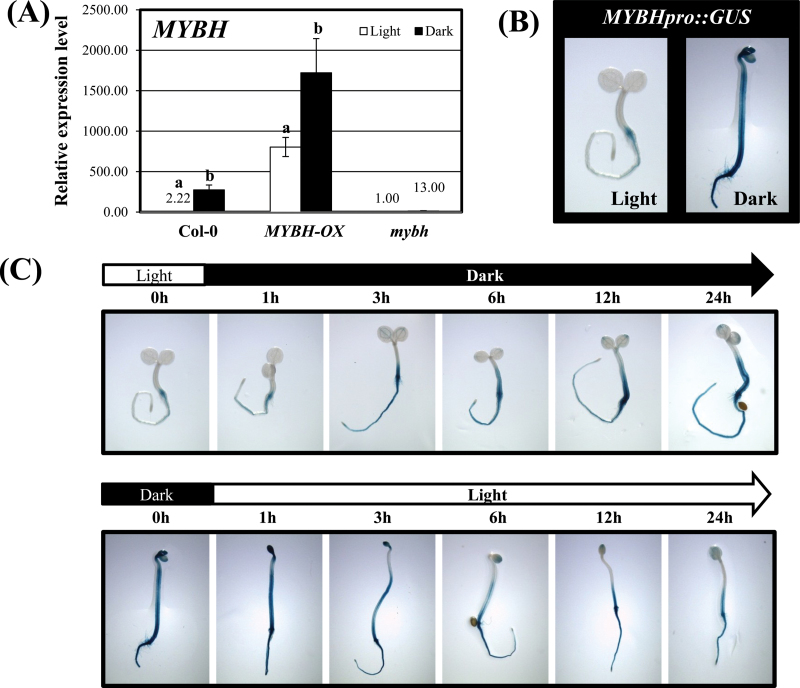
MYBH promoter activity is regulated by light
As expected, MYBH transcript levels were increased in MYBH-OX seedlings; however, for both the MYBH-OX and Col-0 seedlings, the highest transcript levels were observed in plants grown in continuous dark conditions (Fig. 3A). These results suggested the existence of post-transcriptional regulatory mechanisms that stabilize MYBH transcript levels. As altered hypocotyl elongation was observed in MYBH-OX plants grown under light conditions, the effect of light on promoter activity was examined by fusing ~1kb of the sequence located upstream of the MYBH start codon to the GUS reporter gene. Surprisingly, GUS was only expressed at low levels in the light, whereas its expression increased strongly in the dark (Fig. 3B and Supplementary Fig. S4 at JXB online). GUS expression became apparent 1h after MYBHpro::GUS seedlings were transferred from the light to the dark (Fig. 3C). Moreover, when dark-grown MYBHpro::GUS seedlings were exposed to light, GUS expression decreased gradually (Fig. 3C and Supplementary Fig. S4).
Fig. 3.
The promoter activity of MYBH is regulated by light. (A) qRT-PCR was performed to determine MYBH transcript levels in wild-type (Col-0), MYBH-OX, and mybh plants that were grown in the dark or light. Three independent experiments were conducted. The error bars represent standard deviations. (B) Transgenic plants that harboured MYBHpro::GUS were grown on MS medium under normal light or continuous darkness for 3 d prior to histochemical GUS staining. (C) The MYBHpro::GUS seeds were sown on MS medium under light (top panel) or continuous darkness (bottom panel). After 3 d, the seedlings were transferred from light to dark (top panel) or from dark to light (bottom panel) for the indicated times. The MYBHpro::GUS transgenic seedlings were then collected for histochemical GUS staining at 0, 1, 3, 6, 12, and 24h after exposure. Three independent experiments were conducted. (This figure is available in colour at JXB online.)
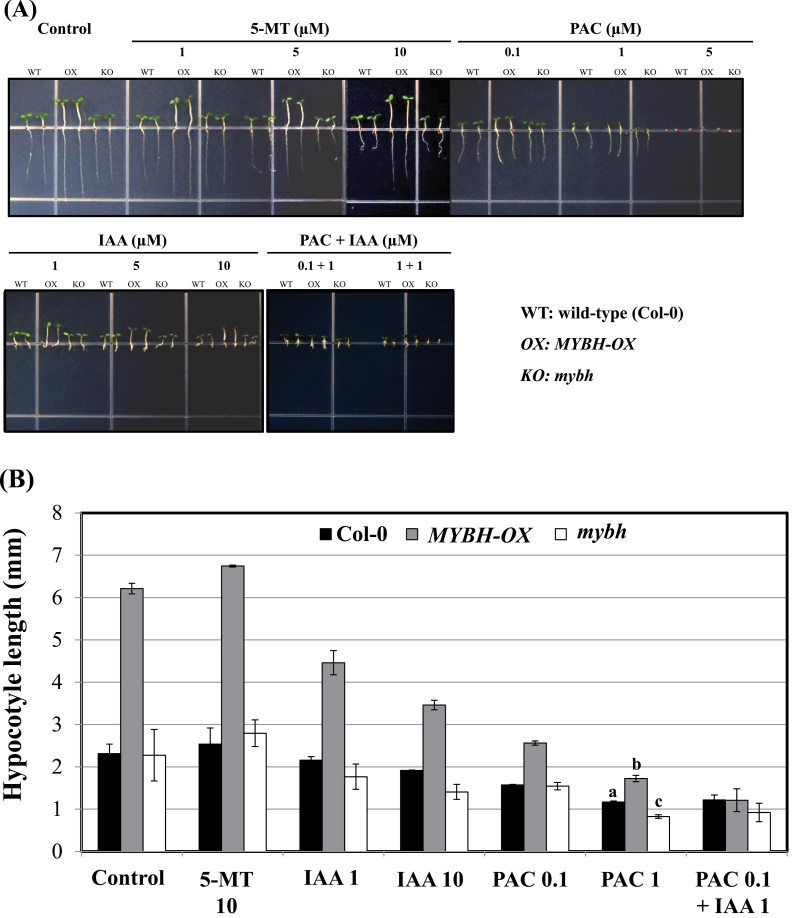
Hypocotyl elongation is suppressed by inhibition of GA biosynthesis in MYBH-OX plants
The presence of long hypocotyls in various Arabidopsis mutants revealed that phytohormones are important for the determination of this phenotype. In the present study, several inhibitors of hormone biosynthesis or activity were used to monitor the recovery of normal hypocotyl elongation in MYBH-OX seedlings. The GA biosynthesis inhibitor paclobutrazol (PAC) effectively restored hypocotyl length in MYBH-OX seedlings that were grown in the light (Fig. 4). In contrast, the Trp analogue 5-methyltryptophan (5-MT) was not able to suppress hypocotyl elongation in the MYBH-OX seedlings (Fig. 4). However, root development was strongly inhibited in MYBH-OX seedlings in response to high concentrations of 5-MT (Supplementary Fig. S5 at JXB online), indicating that the shoot and root tissues exhibit different sensitivities to 5-MT. The reason for this is not clearly understood. The mybh mutants were also observed to be highly sensitive to PAC and IAA (Fig. 4).
Fig. 4.
Hypocotyl elongation of MYBH-OX seedlings in response to 5-MT, PAC, or IAA. Hypocotyl phenotype (A) and length quantification (B) of wild-type (Col-0), MYBH-OX, and mybh seedlings grown on MS medium that was supplemented with the indicated concentrations of 5-MT, PAC, or IAA. 5-MT did not affect hypocotyl elongation of MYBH-OX, while PAC and IAA showed inhibition hypocotyl elongation in all experimental samples. Three independent experiments were conducted. The letters a, b, and c above the columns represent statistically significant differences (P <0.05). Vertical bars show the standard error (n=30). (This figure is available in colour at JXB online.)
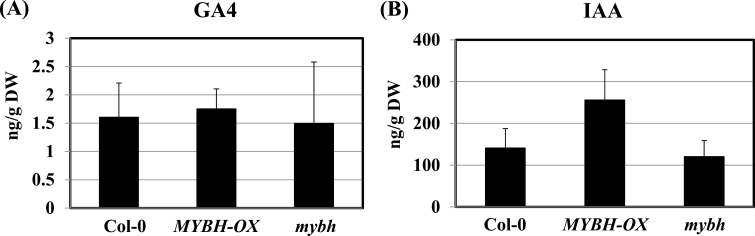
The sensitivity of MYBH-OX seedlings to PAC could be due to alterations in their GA levels. Therefore, hormone levels were determined in whole seedlings of Col-0, MYBH-OX, and mybh that were grown in MS medium for 8 d under normal light condition. Compared with Col-0 seedlings, MYBH-OX plants showed slightly elevated levels of auxin (IAA) but not GA (Fig. 5). The mybh mutants exhibited slightly decreased levels of auxin relative to Col-0. However, the MYBH-OX and mybh mutant seedlings did not show altered levels of the bioactive form GA4 (Fig. 5A).
Fig. 5.
Endogenous auxin and gibberellin (GA4) accumulation in Col-0, MYBH-OX, and mybh seedlings. Wild-type (Col-0), MYBH-OX, and mybh seedlings were grown on MS medium for 8 d under normal light condition and used for the extraction and quantification of GA4 (A) and IAA (B). No significant differences were observed. Vertical bars show the standard error (n=3).
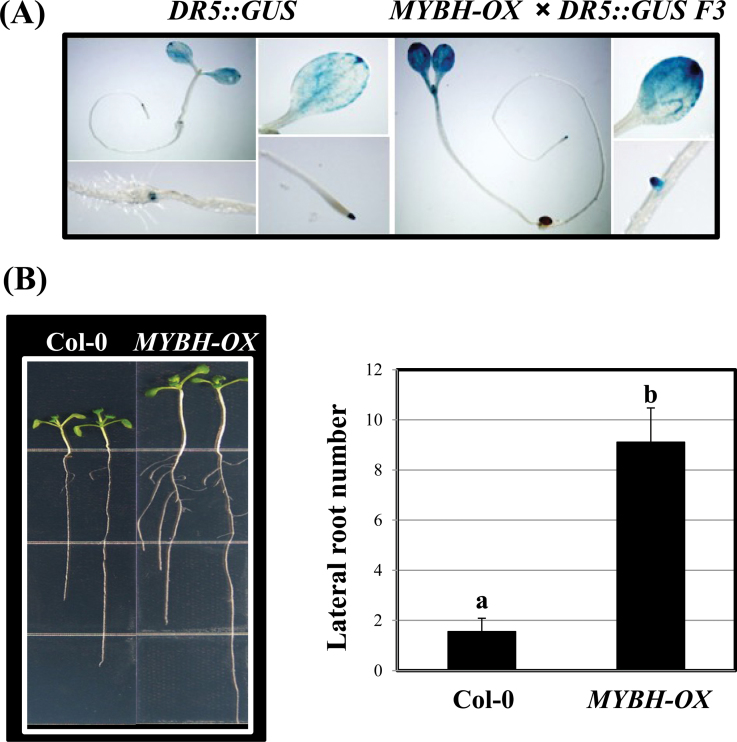
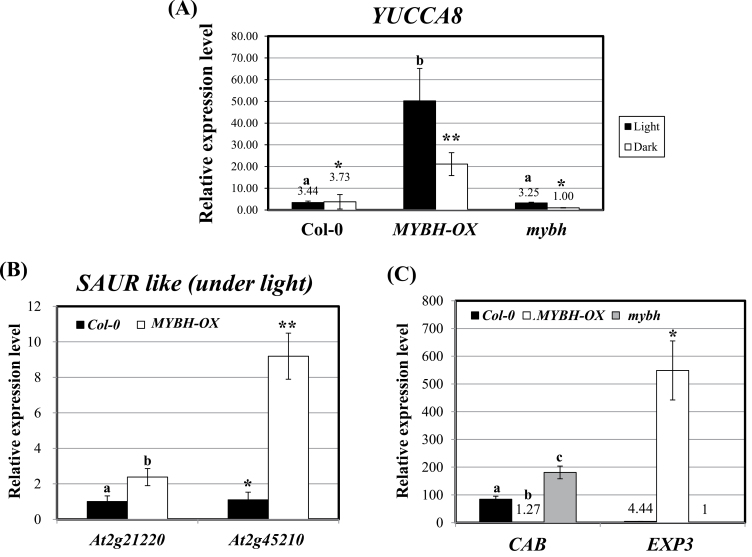
To examine the auxin sensitivity of the MYBH-OX seedlings, MYBH-OX/DR5::GUS F3 progeny were obtained by crossing MYBH-OX with DR5::GUS plants that harboured a reporter system responsive to auxin levels. As shown in Fig. 6A, GUS expression that was driven by the DR5 promoter was stronger in the MYBH-OX background than in the wild-type plants, which confirmed the result shown in Fig. 5B. Additionally, the MYBH-OX seedlings also produced more lateral roots than the wild-type seedlings (Fig. 6B), and the enhanced lateral root development was more obvious when the MYBH-OX seedlings were grown on MS medium that was supplemented with higher concentrations of sucrose (Supplementary Fig. S6 at JXB online). Furthermore, qRT-PCR was used to examine whether auxin-responsive genes or auxin biosynthetic genes were altered in the MYBH-OX seedlings. The transcript level of the auxin biosynthetic gene YUCCA8 was increased in the MYBH-OX seedlings under both the dark and light conditions (Fig. 7A). In addition, other auxin-responsive marker genes such as the SAUR (small auxin responsive) genes were upregulated in the MYBH-OX seedlings, which could explain their increased auxin levels (Fig. 7B).
Fig. 6.
Increased auxin sensitivity in MYBH-OX seedlings. (A) Histochemical GUS staining of DR5::GUS and DR5::GUS×MYBH-OX F3 seedlings. (B) Wild-type (Col-0) and MYBH-OX seedlings were grown on MS medium for phenotype observation and counting of the lateral roots. Three independent experiments were conducted. Columns labelled above with the letter a are significantly different (P <0.05) from those labelled with b. Vertical bars show the standard error (n=30). (This figure is available in colour at JXB online.)
Fig. 7.
Expression level of genes related to auxin and light signalling in MYBH-OX plants. qRT-PCR was performed to evaluate the level of expression of several genes: YUCCA8 (A), SAUR-like (B) and CAB and EXP3 (C). Seven-d-old seedlings of wild-type (Col-0), MYBH-OX, and mybh grown in light or continuous darkness were used for RNA extraction and qRT-PCR. The experiments were performed three times with 25 cycles of PCR amplification. Values are expressed as the means of three replicates. Columns labelled with the letter a are significantly different (P <0.05) from those labelled with b; columns labelled with a single asterisk are significantly different (P <0.05) from those labelled with a double asterisk. Vertical bars show the standard error (n=3).
CHLOROPHYLL-BINDING PROTEIN (CAB) and EXPANSIN are up- and downregulated, respectively, in mybh plants
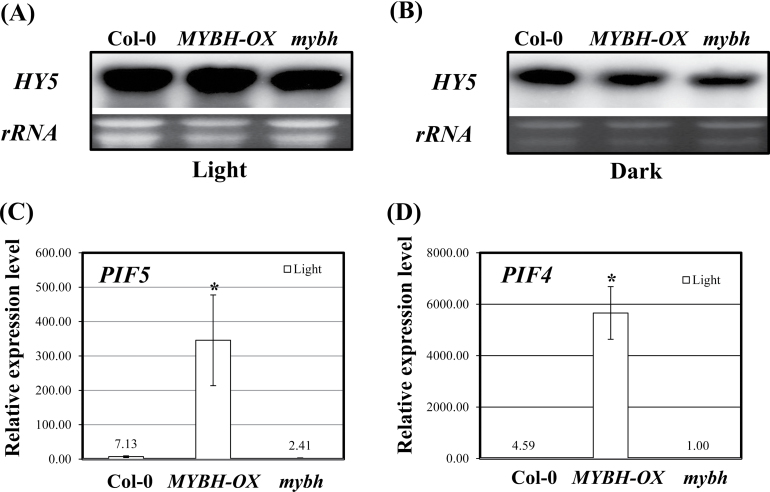
Although the mybh mutant did not exhibit shorter hypocotyls than Col-0, the expression of several genes differed among Col-0, MYBH-OX, and mybh. qRT-PCR was used to examine the transcript levels of the CAB and EXPANSIN3 genes in these same plants. Transcription of CAB, which is involved in plant photomorphogenesis, was upregulated in the mybh mutant and downregulated in the MYBH-OX plants (Fig. 7C). Otherwise, the MYBH-OX seedlings showed elevated EXPANSIN3 transcript levels, which may well explain the increased hypocotyl elongation that was detected in these plants (Fig. 7C). MYBH contains a MYB domain that could function in the transcriptional regulation of HY5, which is key player in the mediation of light signalling in plants. Thus, transcription of HY5 in the Col-0, mybh, and MYBH-OX plants was assessed via Northern blotting. However, no significant difference in HY5 expression was observed in these plants in response to light or dark conditions (Fig. 8A, B).
Fig. 8.
Expression level of HY5, PIF5, and PIF4 in MYBH-OX plants. Northern blotting and qRT-PCR were performed to evaluate the level of expression of several genes: HY5 (A and B), PIF5 (C) and PIF4 (D). Samples were collected from 7-d-old wild-type (Col-0), MYBH-OX and mybh seedlings for total RNA preparation. The experiments were performed three times with 25 cycles of PCR amplification. Values are expressed as the means of three replicates. Columns labelled with an asterisk are significantly different (P <0.05) from those without an asterisk. Vertical bars show the standard error (n=3).
PIF4 or PIF5 is required for increased hypocotyl elongation in MYBH-OX plants under light conditions
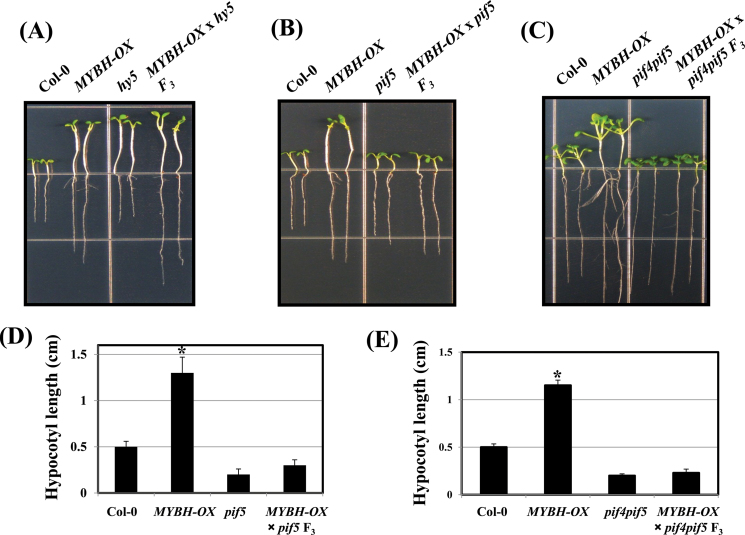
PIFs are key players in the regulation of skotomorphogenesis in plants; therefore, qRT-PCR was used to examine PIF transcript levels in the Col-0, mybh, and MYBH-OX plants. PIF4 and PIF5 expression levels were upregulated in the MYBH-OX plants (Fig. 8C, D). To determine whether MYBH is involved in the PIF-dependent light signalling pathway, MYBH-OX plants were crossed with pif5, and pif4pif5 mutants. The MYBH-OX/pif5 F3 and MYBH-OX/pif4pif5 F3 seedlings exhibited inhibition of hypocotyl elongation in the light, suggesting that the function of MYBH is PIF dependent (Fig. 9B–E). We also generated MYBH-OX/hy5 F3 seedlings and found that hypocotyl elongation was not additive (Fig. 9A).
Fig. 9.
Hypocotyl elongation phenotypes of F3 progeny of MYBH-OX×hy5, MYBH-OX×pif5, and MYBH-OX×pif4pif5. The DNA for genotyping was obtained from F3 progeny seedlings of MYBH-OX×hy5 (A), MYBH-OX × pif5 (B), and MYBH-OX × pif4pif5 (C). After germination under light, 7-d-old seedlings from Col-0, MYBH-OX, and each homozygous mutant were selected for quantification of hypocotyl elongation (D, E). Three independent experiments were conducted. Columns labelled with an asterisk are significantly different (P <0.05) from those without an asterisk. Vertical bars show the standard error (n=30). (This figure is available in colour at JXB online.)
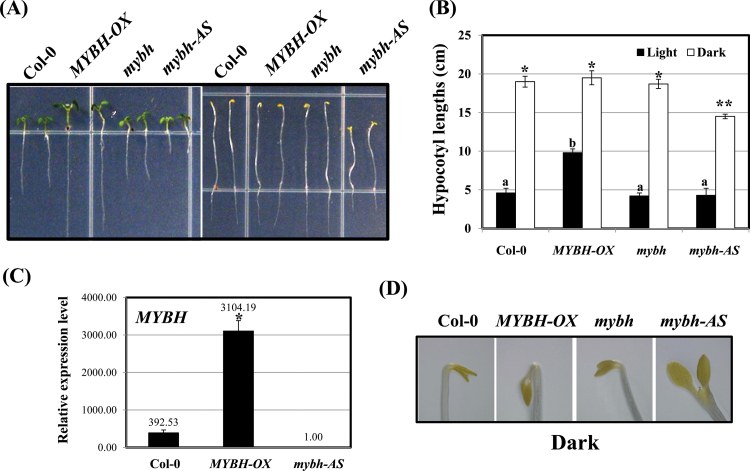
As the phenotype of the mybh mutant seedlings was not significantly different from that of Col-0 plants, mybh antisense (mybh-AS) lines were also generated. Although the MYBH transcript was not detected in most of the antisense lines, truncated bands were observed in several lines (Supplementary Fig. S7 at JXB online). Under light conditions, the Col-0 and mybh-AS seedlings exhibited similar hypocotyl lengths (Fig. 10A, B). However, the mybh-AS lines developed shorter primary roots than those of the Col-0 plants (Fig. 10A). In contrast, when grown in the dark, the hypocotyls of the mybh-AS plants were significantly shorter than those of the Col-0 plants (Fig. 10A, B). On the other hand, cotyledon structures were observed in Col-0, mybh-AS, mybh, and MYBH-OX seedlings after germination in the continuous dark condition. While the MYBH-OX seedlings had a folded apical hook, the apical hook of the mybh-AS seedlings was fully open, indicating that MYBH plays a crucial role in light signalling (Fig. 10D). As shown in Supplementary Fig. S8 at JXB online, the MYBH gene has some close homologues in Arabidopsis; thus, effects on the expression of these genes in the mybh-AS plants may explain the phenotypic differences between mybh-AS and mybh plants.
Fig. 10.
mybh-AS plants exhibit shorter roots than wild-type seedlings in the light. Wild-type (Col-0), MYBH-OX, and mybh-AS seedlings were germinated on MS medium under light or continuous darkness for 7 d. The hypocotyl elongation phenotypes (A) and quantification (B) of each line of seedlings were compared with Col-0. Three independent experiments were conducted. Columns labelled above with the letter a are significantly different (P <0.05) from those labelled with b; columns labelled above with a single asterisk are significantly different (P <0.05) from those labelled with a double asterisk. Vertical bars show the standard error (n=100). (C) The expression level of MYBH was determined by qRT-PCR. The column labelled above with an asterisk is significantly different (P <0.05) from those without an asterisk. Vertical bars show the standard error (n=3). (D) The cotyledons of wild-type (Col-0), MYBH-OX, mybh, and mybh-AS seedlings that were grown in the dark for 7 d are shown. (This figure is available in colour at JXB online.)
Discussion
In this study, the molecular mechanisms that underlie increased hypocotyl elongation due to MYBH overexpression were characterized. Three lines of evidence were presented that indicate MYBH involvement in the regulation of skotomorphogenesis. First, the elongation of hypocotyls was enhanced in MYBH-OX plants. Increased hypocotyl elongation is a known phenotype of plants that are grown in the dark. However, although no significant differences in hypocotyl length were observed between the Col-0 plants and mybh mutants, the mutant plants exhibited much shorter roots and smaller leaves than the Col-0 plants (Fig. 1), indicating that MYBH may play an additional crucial role in the light, although this possibility was not examined in the current study. Secondly, using a GUS reporter system and qRT-PCR, we observed increased MYBH expression during growth in the dark. Stronger GUS expression was observed in the hypocotyls, and positive staining was detected in the leaves and roots (Fig. 3), indicating that MYBH is expressed locally and is important for hypocotyl elongation. Moreover, MYBH promoter activity was reduced rapidly upon exposure to light (Fig. 3B). It remains unclear whether the reduction in promoter activity was due to repression by the light or activation by the dark. Thirdly, the MYBH-OX plants exhibited reduced expression of CAB, which is a marker of photomorphogenesis (Fig. 7C). The fact that the mybh-AS seedlings developed much shorter hypocotyls in the dark suggests that the function of MYBH can be replaced by homologous genes, as the expression of these genes may be downregulated in the mybh-AS seedlings (Fig. 10).
GA has been implicated in cell expansion in the hypocotyl (De Lucas et al., 2008). Because the hypocotyl lengths of the MYBH-OX plants were comparable to those of the Col-0 seedlings in the presence of PAC, we thought that MYBH-OX plants may have increased levels of GA. However, the levels of active GA4 were not different in the MYBH-OX plants and Col-0 plants that were grown under normal conditions (Fig. 5A). Instead, the auxin levels were slightly enhanced in the MYBH-OX plants, and altered auxin sensitivity in the MYBH-OX plants was detected in the DR5::GUS×MYBH-OX F3 seedlings (Fig. 6A). Moreover, the MYBH-OX plants produced more lateral roots than the Col-0 plants during normal growth, suggesting that overexpression of the MYBH gene led to increased auxin biosynthesis (Figs 5B and 6B). This notion was further supported by the observation that the auxin biosynthetic gene (YUCCA8) and auxin marker genes (SAURs) were upregulated in the MYBH-OX plants (Fig. 7A, B).
PIF transcription factors function in promoting skotomorphogenic growth in the dark by controlling downstream gene expression (Leivar et al., 2008). MYBH expression was enhanced in the dark, which suggests that MYBH might function in a PIF-dependent manner in hypocotyl elongation. Moreover, PIF4 and PIF5 transcript levels were significantly elevated in the MYBH-OX plants, which led us to examine the role of PIFs in the enhancement of hypocotyl elongation in the MYBH-OX seedlings (Fig. 8C, D). As illustrated in Fig. 9, MYBH appears to require PIF proteins to enhance hypocotyl elongation. PIF4 is known to play an essential role in high temperature-mediated morphological adaptation (Koini et al., 2009), and binds to genes that are responsible for auxin biosynthesis, such as YUCCA8 and CYP79B2 (Franklin et al., 2011; Sun et al., 2012). An increase in accumulation of YUCCA8 transcripts was also observed in the MYBH-OX plants (Fig. 7A). Thus, it appears that the increased auxin levels were due to the increased accumulation of PIF4 in the MYBH-OX seedlings, which led to increased hypocotyl elongation (Supplementary Fig. S9 at JXB online). Recently, the Arabidopsis MYB gene REVEILLE 1 (RVE1) was shown to be involved in auxin biosynthesis and to promote hypocotyl elongation (Rawat et al., 2009). RVE1 was shown to mediate cell elongation independently of PIF4 and PIF5. Therefore, although auxin biosynthesis is regulated by both RVE1 and MYBH, their effects on hypocotyl elongation clearly occur through different mechanisms. Recently, it was also suggested that PIF4 and/or PIF5 may regulate hypocotyl elongation via control of the auxin signalling pathway (Nozue et al., 2011). It was shown that pif4pif5 mutants are less sensitive to auxin in terms of the growth inhibition response to high auxin concentrations, whereas plants that overexpress PIF5 are more sensitive. Because exogenous auxin does not recover the short-hypocotyl phenotype of the pif4pif5 mutants, PIF5 and/or PIF4 do not seem to regulate auxin levels (Nozue et al., 2011). It was speculated in these studies that PIF5 and/or PIF4 may transcriptionally regulate one or more master regulator(s) of auxin sensitivity. However, the precise roles of auxin- or GA-regulated molecular components of hypocotyl elongation have not been established.
The auxin-related phenotype of the MYBH-OX plants observed in this study could be due to the altered levels of PIF4 and/or PIF5 interfering with the auxin signalling pathway (Supplementary Fig. S9). Compared with Col-0 plants, the MYBH-OX seedlings did not show significant differences in expression of the bZIP transcription factor HY5. This result indicates that enhanced hypocotyl elongation is not due to a reduction in HY5 activity. It appears that EXPANSIN is also involved in the enhanced hypocotyl elongation of MYBH-OX plants. EXP3 expression was upregulated in the MYBH-OX seedlings and reduced in the mybh plants, while the photomorphogenesis marker gene CAB showed the opposite pattern of gene expression. The fine modulation of hypocotyl elongation in response to light is very important for seedling development. When the amount of light is insufficient, seedlings must continue to grow by elongating their hypocotyls. Otherwise, they will remain in the shade and be unable to perform photosynthesis. Therefore, the proper regulation of hypocotyl elongation requires a very delicate and efficient mechanism that must be responsive to changes in environmental conditions. The findings of this study support the possibility that the regulation of hypocotyl elongation may be achieved via a complex web of molecular components that regulate auxin levels.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Complementation assay.
Supplementary Fig. S2. Schematic of the MYBH gene and protein.
Supplementary Fig. S3. Subcellular localization of MYBH.
Supplementary Fig. S4. Light-dependent activity of the MYBH promoter.
Supplementary Fig. S5. Effect of the auxin biosynthesis inhibitor 5-methyltryptophan on MYBH-OX seedling growth.
Supplementary Fig. S6. Increase in lateral root number of MYBH-OX in response to sucrose.
Supplementary Fig. S7. MYBH transcript levels in Col-0, MYBH-OX, mybh-AS, and mybh plants.
Supplementary Fig. S8. MYBH homologues in A. thaliana.
Supplementary Fig. S9. Working model of MYBH activity during dark-induced hypocotyl elongation.
Supplementary Table S1. Sequences of the primers used in this study.
Acknowledgements
This work was supported by a grant from the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (to Hojoung Lee, 2012; grant #2012-112068-3) and by a grant from the Korea Research Foundation (to Hojoung Lee, grant # 2009-0065693).
Glossary
Abbreviations:
- 5-MT
Trp analogue 5-methyltryptophan
- GA
gibberellic acid
- GFP
green fluorescent protein
- GUS
β-glucuronidase
- IAA
indole-3-acetic acid
- MS
Murashige and Skoog
- PAC
paclobutrazol
- PIF
phytochrome-interacting factor
- qRT-PCR
quantitative reverse transcriptase-PCR.
References
- Achard P, Liao L, Jiang C, Desnos T, Bartlett J, Fu X, Harberd NP. 2007. DELLAs contribute to plant photomorphogenesis. Plant Physiology 143, 1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal M, Hao Y, Kapoor A, Dong CH, Fujii H, Zheng X, Zhu JK. 2006. A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. Journal of Biological Chemistry 281, 37636–37645. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Cashmore AR. 1993. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366, 162–166. [DOI] [PubMed] [Google Scholar]
- Alabadi D, Gil J, Blazquez MA, Garcia-Martinez JL. 2004. Gibberellins repress photomorphogenesis in darkness. Plant Physiology 134, 1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D, Viczian A, Kircher S, Nobis T, Nitschke R, Kunkel T, Panigrahi KC, Adam E, Fejes E, Schafer E. 2004. Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis . Plant Cell 16, 1433–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomal C, Bedon F, Caron S, et al. 2008. Involvement of Pinus taeda MYB1 and MYB8 in phenylpropanoid metabolism and secondary cell wall biogenesis: a comparative in planta analysis. Journal of Experimental Botany 59, 3925–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae K, Isaacs CG, Reeves PH, Maloney GS, Muday GK, Nagpal P, Reed JW. 2012. Arabidopsis SMALL AUXIN UP RNA63 promotes hypocotyl and stamen filament elongation. The Plant Journal 71, 684–697. [DOI] [PubMed] [Google Scholar]
- Cheng H, Song S, Xiao L, So HM, Cheng Z, Xie D, Peng J. 2009. Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in Arabidopsis . PLoS Genetics 5, 440–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin-Atkins AN, Craig S, Hocart CH, Dennis ES, Chaudhury AM. 1996. Increased endogenous cytokinin in the Arabidopsis amp1 mutant corresponds with de-etiolation responses. Planta 198, 549–556. [DOI] [PubMed] [Google Scholar]
- Chory J, Aguilar N, Peto CA. 1991. The phenotype of Arabidopsis thaliana det1 mutants suggests a role for cytokinins in greening. Symposia of the Society for Experimental Biology 45, 21–29. [PubMed] [Google Scholar]
- Chory J, Peto CA, Ashbaugh M, Saganich R, Pratt L, Ausubel F. 1989. Different roles for phytochrome in etiolated and green plants deduced from characterization of Arabidopsis thaliana mutants. Plant Cell 1, 867–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J. 1993. Out of darkness: mutants reveal pathways controlling light-regulated development in plants. Trends in Genetics 9, 167–172. [DOI] [PubMed] [Google Scholar]
- De Lucas M, Daviere JM, Rodriguez-Falcon M, Pontin M, Iglesias-Pedraz JM, Lorrain S, Fankhauser C, Blazquez MA, Titarenko E, Prat S. 2008. A molecular framework for light and gibberellin control of cell elongation. Nature 451, 480–484. [DOI] [PubMed] [Google Scholar]
- Deng XW. 1994. Fresh view of light signal transduction in plants. Cell 76, 423–426. [DOI] [PubMed] [Google Scholar]
- Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. 2010. MYB transcription factors in Arabidopsis . Trends in Plant Science 15, 573–81. [DOI] [PubMed] [Google Scholar]
- Feng S, Martinez C, Gusmaroli G, et al. 2008. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451, 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA, Lee SH, Patel D, et al. 2011. PHYTOCHROME-INTERACTING FACTOR 4 (PIF4) regulates auxin biosynthesis at high temperature. Proceedings of the National Academy of Sciences, USA 108, 20231–20235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil P, Green PJ. 1997. Regulatory activity exerted by the SAUR-AC1 promoter region in transgenic plants. Plant Molecular Biology 34, 803–808. [DOI] [PubMed] [Google Scholar]
- Grotewold E. 2005. Plant metabolic diversity: a regulatory perspective. Trends in Plant Science 10, 57–62. [DOI] [PubMed] [Google Scholar]
- Jin H, Cominelli E, Bailey P, Parr A, Mehrtens F, Jones J, Tonelli C, Weisshaar B, Martin C. 2000. Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis . EMBO Journal 19, 6150–6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koini MA, Alvey L, Allen T, Tilley CA, Harberd NP, Whitelam GC, Franklin KA. 2009. High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Current Biology 19, 408–413. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Rolff E, Spruit CJP. 1980. Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L.) Heynh. Z Pflanzenphysiol Journal 100, 147–160. [Google Scholar]
- Lee H, Yan G, Masaru O, Liming X, Becky S, Jian-Kang Z. 2002. LOS2, a genetic locus required for cold-responsive gene transcription encodes a bi-functional enolase. EMBO Journal 21, 2692–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legay S, Lacombe E, Goicoechea M, Brie`re C, Se′guin A, Mackay J, Grima-Pettenati J. 2007. Molecular characterization of EgMYB1, a putative transcriptional repressor of the lignin biosynthetic pathway. Plant Science 173, 542–549. [Google Scholar]
- Leivar P, Monte E, Oka Y, Liu T, Carle C, Castillon A, Huq E, Quail PH. 2008. Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Current Biology 18, 1815–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hagen G, Guilfoyle TJ. 1991. An auxin-responsive promoter is differentially induced by auxin gradients during tropisms. Plant Cell 3, 1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandaokar A, Browse J. 2009. MYB108 acts together with MYB24 to regulate jasmonate-mediated stamen maturation in Arabidopsis . Plant Physiology 149, 851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure BA, Guilfoyle T. 1989. Rapid redistribution of auxin-regulated RNAs during gravitropism. Science 243, 91–93. [DOI] [PubMed] [Google Scholar]
- McNellis TW, Deng XW. 1995. Light control of seedling morphogenetic pattern. Plant Cell 7, 1749–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozue K, Harmer SL, Maloof JN. 2011. Genomic analysis of circadian clock-, light-, and growth-correlated genes reveals PHYTOCHROME-INTERACTING FACTOR5 as a modulator of auxin signaling in Arabidopsis . Plant Physiology 156, 357–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Kim J, Lee Y, Shin J, Oh E, Chung WI, Liu JR, Choi G. 2004. Degradation of phytochrome interacting factor 3 in phytochrome-mediated light signaling. Plant and Cell Physiology 45, 968–975. [DOI] [PubMed] [Google Scholar]
- Parks BM, Quail PH. 1991. Phytochrome-deficient hy1 and hy2 long hypocotyl mutants of Arabidopsis are defective in phytochrome chromophore biosynthesis. Plant Cell 3, 1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat R, Schwartz J, Jones MA, Sairanen I, Cheng Y, Andersson CR, Zhao Y, Ljung K, Harmer SL. 2009. REVEILLE1, a Myb-like transcription factor, integrates the circadian clock and auxin pathways. Proceedings of the National Academy of Sciences, USA 106, 16883–16888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. 1993. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5, 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Moon J, Huq E. 2005. PIF1 is regulated by light mediated degradation through the ubiquitin-26S proteasome pathway to optimize photomorphogenesis of seedlings in Arabidopsis . The Plant Journal 44, 1023–1035. [DOI] [PubMed] [Google Scholar]
- Singh KB, Foley RC, Õnate-Sánchez L. 2002. Transcription factors in plant defense and stress responses. Current Opinion in Plant Biology 5, 430–436. [DOI] [PubMed] [Google Scholar]
- Sun J, Qi L, Li Y, Chu J, Li C. 2012. PIF4-mediated activation of YUCCA8 expression integrates temperature into the auxin pathway in regulating Arabidopsis hypocotyl growth. PLoS Genetics 8, e1002594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki N, Sasaki-Sekimoto Y, Obayashi T, et al. 2005. 12-Oxophytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis . Plant Physiology 139, 1268–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdonk JC, Haring MA, van Tunen AJ, Schuurink RC. 2005. ODORANT1 regulates fragrance biosynthesis in petunia flowers. Plant Cell 17, 1612–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vom Endt D, Kijne JW, Memelink J. 2002. Transcription factors controlling plant secondary metabolism: what regulates the regulators? Phytochemistry 61, 107–114. [DOI] [PubMed] [Google Scholar]
- Wei N, Deng XW. 1999. Making sense of the COP9 signalosome: a regulatory protein complex conserved from Arabidopsis to human. Trends in Genetics 15, 98–103. [DOI] [PubMed] [Google Scholar]
- Yanhui C, Xiaoyuan Y, Kun H, et al. 2006. The MYB transcription factor superfamily of Arabidopsis: expression analysis and phylogenetic comparison with the rice MYB family. Plant Molecular Biology 60, 107–124. [DOI] [PubMed] [Google Scholar]
- Yi C, Deng XW. 2005. COP1-from plant photomorphogenesis to mammalian tumorigenesis. Trends in Cell Biology 15, 618–625. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Jikumaru Y, Kamiya Y, Kusano M, Consonni C, Panstruga R, Ohsumi Y, Shirasu K. 2009. Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis . Plant Cell 21, 2914–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Hull AK, Gupta NR, Goss KA, Alonso J, Ecker JR, Normanly J, Chory J, Celenza JL. 2002. Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes and Development 16, 3100–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.