Abstract
Background
Nerve transfers are an effective means of restoring control to paralyzed somatic muscle groups and recently shown to be effective in denervated detrusor muscle in a canine model.
Objective
A cadaveric project was performed to examine the anatomic feasibility of transferring femoral muscular nerve branches to vesical branches of the pelvic nerve as a method of potentially restoring innervation to control the detrusor muscle in humans using transfer of somatic nerves.
Methods
Twenty cadavers were dissected bilaterally to expose pelvic and femoral muscular nerve branches. Ease of access and ability to transfer the nerves were assessed, as were nerve cross sectional areas.
Results
The pelvic nerve was accessed at the base of the bladder, inferior to the ureter and accompanied by inferior vesical vessels. Muscular branches of the femoral nerve to the vastus medialis and intermedius muscles (L3, 4 origins) were followed distally for 17.4 ± 0.8 cm. Two muscle branches were split from the femoral nerve trunk, and tunneled inferior to the inguinal ligament. One was moved medially towards the base of the bladder and linked to the ipsilateral pelvic nerve. The second was tunneled superior to the bladder and linked to the contralateral pelvic nerve. The cross sectional area of the pelvic nerve vesical branch was 2.60 ± 0.169 mm2 (mean ± SEM), and the femoral nerve branches at the suggested transection site was 4.40 ± 0.41 mm2.
Conclusion
Use of femoral nerve muscular branches from the vastus medialis and intermedius muscles for heterotopic nerve transfer of bilateral pelvic nerves is surgically feasible, based on anatomical location and cross sectional areas.
Keywords: bladder, femoral nerve, nerve transfer, incontinence
Introduction
In the United States, of the 236,000 to 327,000 individuals affected by spinal cord injury (SCI), 52% are considered paraplegic and 47% as quadriplegic. Bladder and bowel dysfunction is common, leads to significant impairment in quality of life and often results in social isolation.2 In a survey study of 681 SCI patients, regaining bladder and bowel function was of shared importance to both paraplegics and quadriplegics and even rated more important than recovering ambulation in paraplegics.1 Our long-term goals are to develop surgical approaches to reinnervate the urinary bladder so the patients with cervical and thoracic spinal cord injuries or developmental defects can regain control of bladder emptying.
The idea of restoring urinary control via nerve transfer has been pursued for over a century. Unilateral S1 and L7 nerve transfers to S2 and S3 roots in 3 dogs described in 1907 resulted in bladder and anal sphincter contraction with “Faradic stimulation” of the transferred nerves 4-6 months post-operatively.11 The concept has been pursued numerous times since then with varying degrees of success.6,7,20,27,30 There are several recent reports of successful somatic nerve transfers in animal models and in patients for restoration of bladder function.3,4,9,10,14,19-22,26,31 We found, using a canine model, that bladder reinnervation after sacral ventral root transection can be achieved by nerve transfer and surgical coaptation of coccygeal roots to the severed sacral roots, or transfer of genitofemoral nerves to the pelvic nerve as evidenced by increased bladder pressure upon functional electrical stimulation of these nerves after time had elapsed for reinnervation. In the majority of animals in each group, effective detrusor contractions were elicited with stimulation of the reinnervated pelvic nerve. Retrograde fluoro-gold tracing from the bladder confirmed the regrowth of axons from the spinal cord at L1,2 through the nerve repair sites to the bladder wall.4,21,22 Anterograde axonal tracing also confirmed regrowth of axons from the genitofemoral nerve into the bladder detrusor muscle after these heterotopic somatic nerve transfers.3
Similar heterotopic nerve transfer methods might be used in patients to restore bladder emptying. To do this, motor nerves need to be transferred to the pelvic nerve branches innervating the bladder. We are proposing to co-apt motor branches for nerve transfer to the pelvic nerves bilaterally. In cases of suprasacral lumbar lesions we propose using a branch of the genitofemoral or ilioinguinal nerve to the pelvic nerve, whereas in the case of cervical and thoracic spinal cord injury, distances are too great to bring normal volitional control from a supra lesional distribution. Instead, the femoral nerve was selected as it is easily accessible and might be artificially activated by a number of methods even if it is disconnected from the volitional areas of the brain in order to trigger contraction of the detrusor and bladder emptying. Thus, our objectives in this cadaveric pilot study are to explore the feasibility of transferring long muscular branches of the femoral nerve to the pelvic nerve using an anterior thigh and retroperitoneal approach. It is our long-term hope that surgical transfer of one or more of these nerve branches, as appropriate for the patient, to the pelvic nerve might serve as a surgical treatment for high spinal cord injury-induced incontinence.
Methods
Thirty-one pelvic and anterior thigh regions were dissected in 20 formalin fixed cadavers, thirteen females and seven males. Three cadavers were used solely for this project and were not pre-dissected by others. Nine of the cadavers dissected by others were used for non-lower extremity and non-pelvic anatomical studies. They were inspected to ensure that the following anatomy was intact, including pelvic floor and peripheral nerves of the anterior thigh. Any non-intact specimens were not included in this study. One-half of the included cadavers underwent hemisection to facilitate the pelvic nerve dissections.
Gross dissection techniques were used. In each cadaver, the dissection proceeded with: 1) an anterior pelvic approach to identify the vesical branches of the pelvic nerve, the bladder vasculature including the superior and inferior vesical vessels and the ureter and 2) an anterior thigh approach to identify the femoral nerve and its branches focusing on muscular branches to the vastus medialis and intermedialis muscles. Careful examination of the dissected fields was made.
The following measurements were performed using both a caliper (Mitutoyo Vernier Pointed Jay Caliper, model 536-121, Aurora, IL) and a flexible measuring tape with centimeter markings: 1) pertinent distances that could be used to locate the femoral muscular nerve branch to the vastus medialis in relationship to the inguinal ligament, the main trunk of the femoral nerve. These measurements are presented as mean ± SEM. The cross sectional areas of the nerves were determined by measuring the long and short radii with engineering calipers, then multiplying these two numbers together, and multiplying by Π. Thin cross sections were then collected by scalpel, placed onto microscope slides, and the cross sectional areas verified using a bright field dissecting microscope, a 5x objective, and image analysis software (Bioquant II, Nashville, TN).
Results
Identification of the pelvic nerve
Vesical branches of the pelvic nerve were identified passing to the posterolateral bladder wall with inferior vesical vessels (Figures 1 and 2). The vesicle trunk of the pelvic nerve could be tracked from the sacral plexus and pelvic ganglia to the bladder wall in each cadaver (Fig 1B-D). The vesical branch often passed over the ureter at the site of its entrance to the bladder wall in the cadavers (Fig 1C,D). Typically, a second branch could be followed from the pelvic ganglia to the vaginal or prostate regions (Fig 1B-D).
Figure 1.
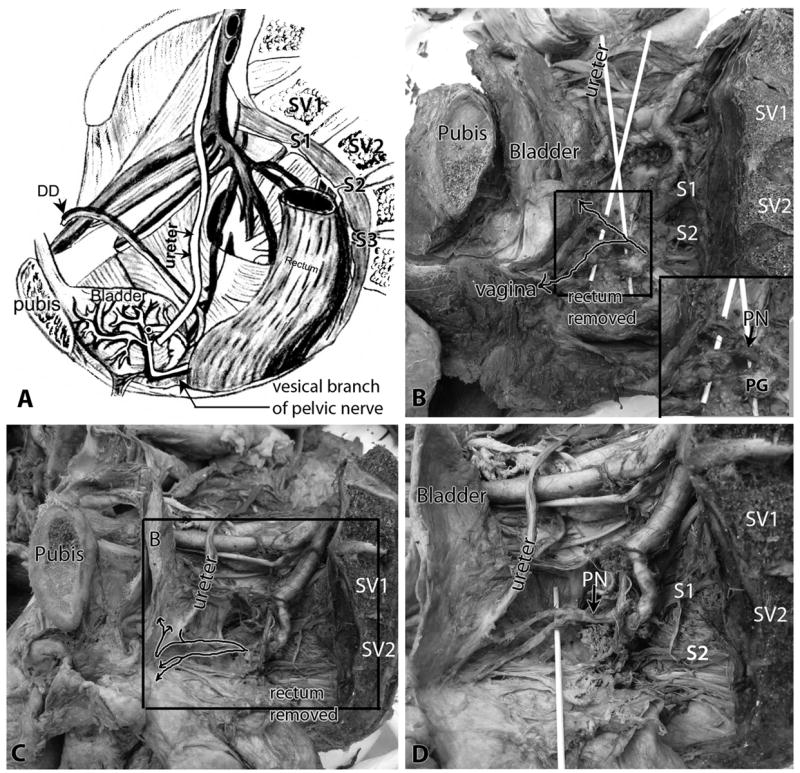
Identification of the pelvic nerve and its vesical branch to the bladder within the pelvic cavity. Lateral view, with pubis (pubic bone) and sacral vertebrae (SV) indicated as landmarks. A) Diagram of male pelvis showing the relationship of the vesical branch of the pelvic nerve to the bladder, ureter and ductus deferens (DD). B) Pelvic cavity of a female cadaver showing similar relationships. Arrows in the box indicate the path of the pelvic nerve (PN), shown passing over wooden sticks. One branch passes to the bladder (the vesical branch of the pelvic nerve) and a second passes to the vaginal area in this cadaver. The rectum is removed. Inset in B shows enlargement of boxed area, and shows the relationship of the pelvic nerve (PN) with the pelvic ganglia (PG). C) The pelvic cavity of a second cadaver showing similar relationships. Arrows in box indicate the path of the pelvic nerve, and shows one branch passing to the bladder (the vesical branch of the pelvic nerve) that then splits into a branch to the ureter and two branches to the detrusor wall. A second branch is shown passing to the vaginal area that splits into two branches. The rectum is removed. D) Enlargement of box shown in panel C, with a wooden stick now elevating the branches of the pelvic nerve. Abbreviations: DD = ductus deferens; PG = pelvic ganglia; PN = pelvic nerve; SV1 and SV2 = sacral vertebra 1 and 2; S1, S2 and S3 = sacral nerve roots 1,2, and 3.
Figure 2.
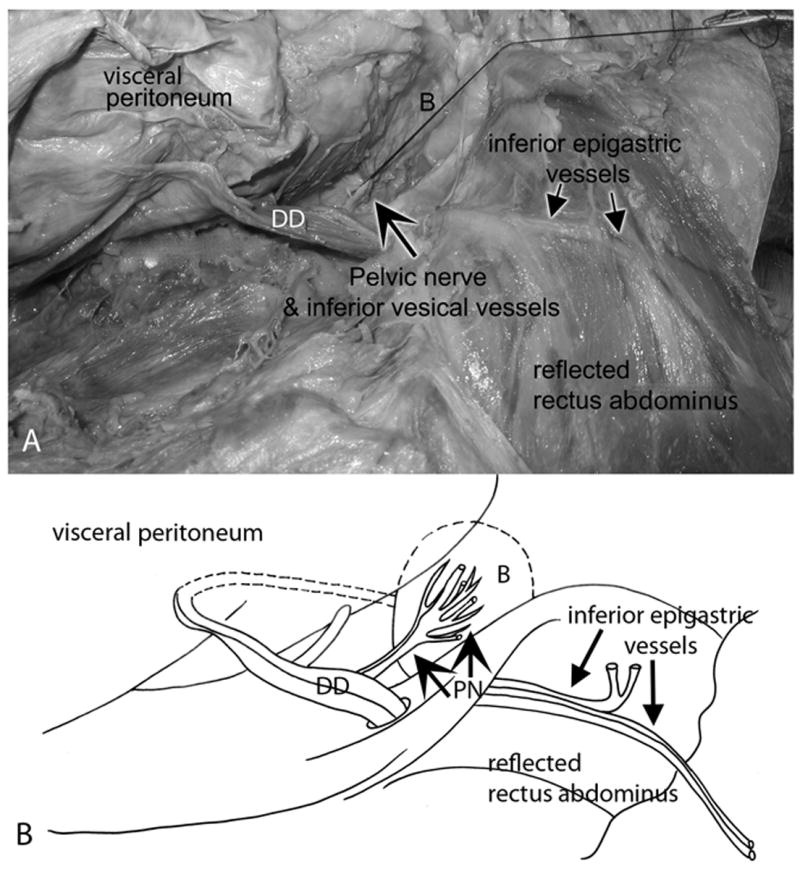
Relationship of the pelvic nerve to the inferior vesical vessels, ductus deferens and inferior epigastric vessels. Lateral view; legs are located on the right side of these panels. The parietal peritoneum was separated manually from the visceral peritoneum and viscera. C) The vesical branch of the pelvic nerve (large arrow) was identified at the base of the bladder (B) in the retroperitoneal space. A suture was tied around the pelvic nerve for identification purposes. The suture is shown pulled inferiorly over the pubic bone (right side of panel). Pertinent landmarks such as the inferior epigastric artery and vein, ductus deferens (DD), and the reflected rectus abdominis muscle are indicated. (B) Diagram of the same figure showing the same landmarks. PN = pelvic nerve.
The pelvic dissection involved a midline longitudinal laparotomy, followed by an extraperitoneal approach to expose the bladder (Fig 2A) in its retroperitoneal position, immediately superoposterior to the pubic symphysis (Fig 1A-C, Fig 2B). Vesical branches of the pelvic nerve were identified on the posterolateral bladder wall bilaterally (Fig 2A,B). They were usually located adjacent to the inferior vesical artery and just distal to the ureterovesical junction. Either one large trunk of the pelvic nerve (most common finding), or two medium sized trunks were located at the proximal aspect of the lateral wall of the bladder, inferior to the ureter and adjacent to the inferior vesical artery and vein (Fig 1B,D, Fig 2A,B). In fact, we were able to use the inferior vesical vessels on the bladder as landmarks to locate the pelvic nerve. This large trunk of the pelvic nerve could be tracked superiorly on the bladder wall towards its superior apex in each cadaver. Figure 2 also shows the medial relationship of the pelvic nerve and inferior vesical vessels to the inferior epigastric vessels located on the anterior abdominal wall. Also note the relationship of the pelvic nerve to the ductus deferens in Figures 2 and Figure 3D-F).
Figure 3.
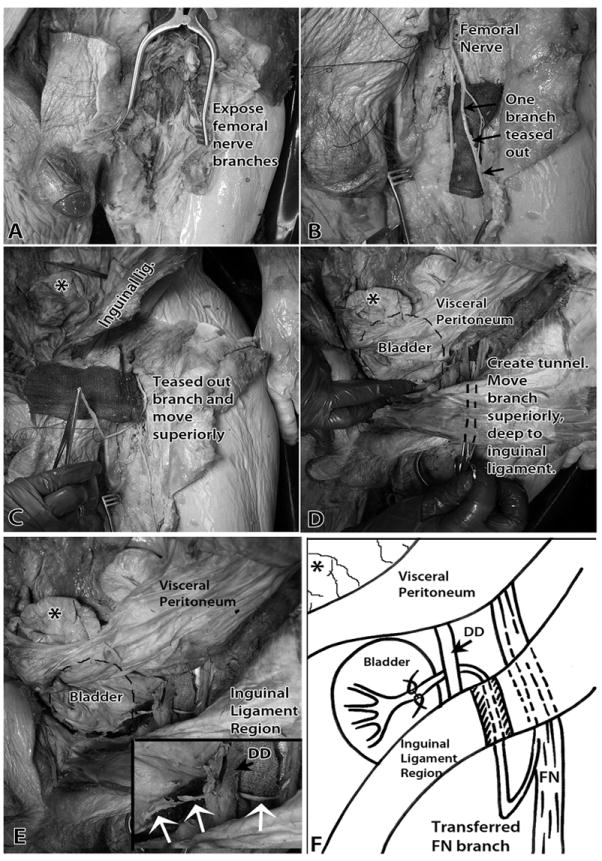
Transfer of a muscular branch of the left femoral nerve to the left (ipsilateral) pelvic nerve. A) The branches of the left femoral nerve in the left anterior thigh were exposed. B) Branches of left femoral nerve were cleared of fat. A branch to the vastus medialis muscle was teased out from the femoral nerve trunk. A surgical cloth was placed under the nerve branches to aid visualization. C) This muscular branch was moved superiorly in the subcutaneous space. An asterisk indicates a loop of gut. D) The cut branch was tunneled inferior to the inguinal ligament into the abdominal cavity’s retroperitoneal space. The location of the bladder is indicated with a dashed line. E) The cut muscular branch of the left femoral nerve had enough length to be easily anastomosed with the left pelvic nerve on the bladder wall. Inset shows higher magnification of transferred femoral nerve and femoral-pelvic nerve “reanastomosis” site (white arrows). F) Diagram of image shown in panel E and of surgical procedure. Abbreviations: DD = ductus deferens; FN = Femoral nerve. The asterisk indicates the same loop of gut in each panel, as demarcated first in panel C.
Identification and transfer of muscular branches of the femoral nerve to the pelvic nerve
The femoral nerve, its anterior division cutaneous branches, and its posterior division muscular branches were exposed in the upper anterior-medial thigh (Fig 3A). It was located immediately lateral to the femoral artery and the great saphenous vein. We then exposed more of the femoral nerve distally, following its branches inferiorly until long muscular branches (Fig 3B) that innervated the vastus medialis and vastus intermedius muscles could be identified.
We then separated the femoral nerve into its component fascicles. These branches were easily teased apart proximally from the main femoral nerve trunk (Fig 3B). The location of the branch site of these muscular branches from the main trunk of the femoral nerve was 13.7±0.71 cm inferior to the inguinal ligament. Muscular branches to the vastus medialis could be traced 17.4 ± 0.8 cm inferiorly into the muscle mass. We also identified 2-3 branches per cadaver to the vastus intermedius muscle that were of similar length (Fig 3B). These latter branches entered the anterior surface of the vastus intermedius muscle at about mid-thigh. The components of the femoral nerve sub serving the musculature of vastus medialis and vastus intermedius muscles were teased out from the remaining branches.
One of the muscular branches to the vastus medialis muscle was then transected and moved proximally (superiorly) and subcutaneously into the extraperitoneal space by moving it proximally to its site of entrance into the anterior thigh (Fig 3C), tunneling the nerve deep to the inguinal ligament, and then into the abdomen (Fig 3D). The pelvic nerve was located just a few centimeters within the retroperitoneal space on the lateral wall of the bladder at the site of entrance of the ureters (Figures 2 and 3E,F). We next tunneled the femoral nerve branch deep to the ductus deferens (indicated by an asterisk in inset in Fig 3E). Thus, the transferred femoral muscular nerve branch was more than sufficient in length for transfer to the pelvic nerve on the lateral wall of the bladder (Fig 3F).
Additionally, we were able to utilize another branch of the femoral nerve, a muscular branch to the vastus intermedius, in this case, for transfer to the contralateral pelvic nerve (Fig 4A-D). This branch was also moved superiorly on the thigh and below the inguinal ligament, and then into the retroperitoneal space of the abdomen (Fig 4A,C). We next moved this nerve superior to the bladder through the mesenteries (Fig 4A-D), before bringing it immediately adjacent to the trunk of the contralateral pelvic nerve at the base of the contralateral side of the bladder (Fig 4A-D).
Figure 4.
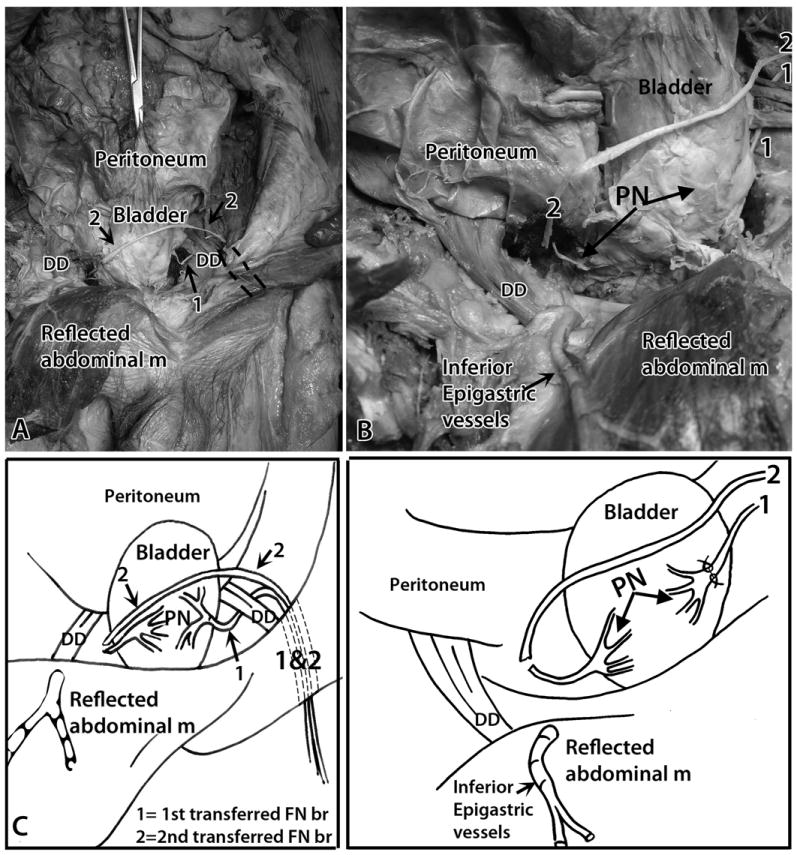
Transfer of a muscular branch of the left femoral nerve to the right (contralateral) pelvic nerve. Anterior view, similar to Figure 3. A) Another muscular branch of the femoral nerve was identified and tunneled inferior to the inguinal ligament into the abdominal cavity’s retroperitoneal space. Tunnel is indicated by dashed lines. 1 = first transferred nerve (ipsilateral transfer); 2 = second transferred nerve (contralateral transfer). The left femoral nerve branch is moved across the abdomen, superior to the bladder, to the contralateral (right) pelvic nerve. B) Similar but enlarged image as shown in panel A. The cut muscular branch of the left femoral nerve (indicated with a 2) was placed adjacent to the transected right pelvic nerve (PN, left arrow). C) A diagram of panel A. D) A diagram of panel B. Abbreviations: DD = ductus deferens; m = muscle; PN = pelvic nerve; 1 = first transferred nerve (ipsilateral transfer); 2 = second transferred nerve (contralateral transfer).
Cross sectional areas of the nerves
Finally, in order to determine if the femoral nerve branch was appropriate for surgical anastomosis to the pelvic nerve, we measured the cross sectional areas of each nerve. The cross sectional area of the pelvic nerve visceral branch was 2.60 ± 0.169 mm2. The cross sectional area of the femoral nerve muscular branch at 17.4 ± 0.8 cm distal to its division from the femoral nerve, our suggested transection site, was 4.40 ± 0.41 mm2. Thus, the chosen branches of the femoral nerve were more than large enough to be surgically coapted to the pelvic nerve at the site where it lies on the lateral wall of the bladder adjacent to the ureter’s entrance to the bladder, as diagramed in Figures 3F, 4C, and 5.
Figure 5.
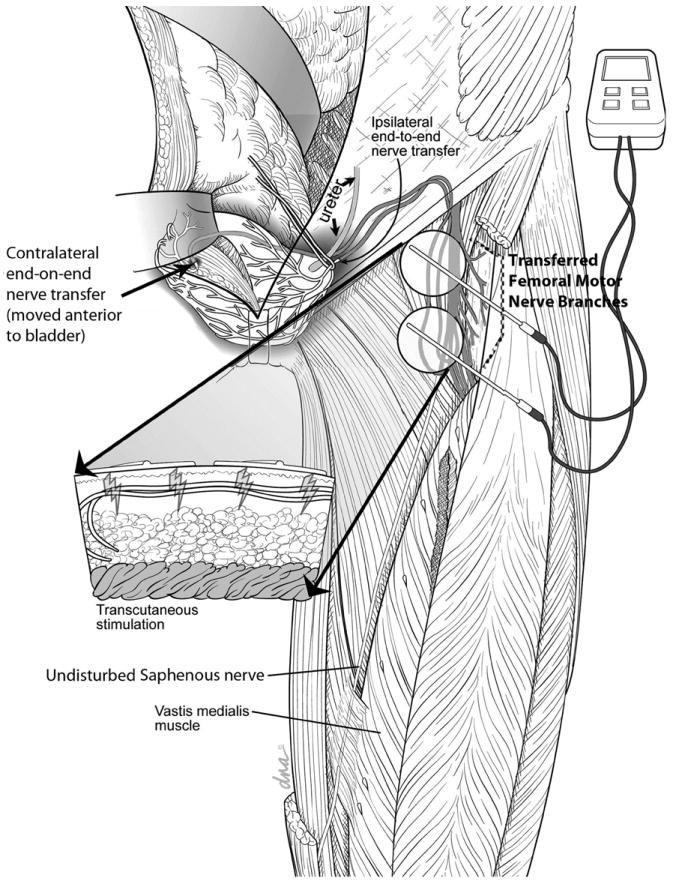
Illustration of the transfer of muscular braches of the left femoral nerve to both the left and right dominant branches of the pelvic nerves innervating the urinary bladder using an anterior pelvic extraperitoneal approach. The transferred nerves are passed through a subcutaneous tunnel on the left inner thigh to allow for functional electrical stimulation of the transferred nerves with standard transepidermal nerve stimulators and electrodes.
Discussion
Patients with neurogenic bladder dysfunction are at risk for a number of urologic complications. Current treatment methods for neurogenic bladder are focused on preventing these complications by replacing the bladder’s storage and/or emptying function rather than restoring normal function. In fact, this feature of SCI alone is one of the major limitations of a person’s participation in life’s activities – the primary objective of any good rehabilitation program.17 Restoration of normal bladder function requires regaining neurologic control over both storage and emptying functions. Historically, one of the most successful functional electrical stimulation (FES) treatments has been the Brindley Vocare sacral root stimulator.12 This treatment utilizes an implantable stimulating device, connected to leads that are surgically placed around the sacral (S) 2-4 nerve roots, bilaterally. Using varied stimulation patterns, activation of the stimulator produces micturition, defecation or in some cases even erection.25 Dorsal rhizotomies are performed to limit afferent stimulation leading to loss of bladder and rectal activity, which leads to loss of reflex defecation or loss of erectile function and ejaculation in men who had these responses before surgery. The need for the dorsal rhizotomy has been a major impediment to its acceptance in the United States.23 Alternatives proposed have included selective sacral rhizotomy, nerve rerouting procedures and stem cell therapies.8,18,24
The use of nerve transfer operations to restore neurological function in patients with deficits is not novel. Brachial plexus injuries have been treated with nerve transfers throughout the last century.16 Examples of successful nerve transfers include connecting the spinal accessory or intercostal nerves to the musculocutaneous nerve to restore biceps elbow flexion function, connecting sensory branches of the ulnar nerve to the median nerve to restore sensation in the thumb and index finger, and in spinal cord injury, connecting the medial forearm cutaneous nerve to lateral thigh cutaneous nerve to move sensation from above the level of injury to below the level of injury.5,15
In the current study, we were able to locate vesical branches of the pelvic nerve on the lateral bladder wall. Typically one, but sometimes two dominant trunks of the pelvic nerve were identified on each side of the bladder in each cadaver. These pelvic nerve trunks were associated with inferior vesical branches of the internal iliac vessels. While there were some additional other small nerves that also innervated the detrusor muscle, use of this main trunk will likely allow reinnervation of a large part of the detrusor wall. The proximity of the pelvic nerve to the inferior vesical branches of the internal iliac artery allowed easy identification of the pelvic nerve. These branches of the pelvic nerve appeared to be quite distinct from other elements of the pelvic plexus that innervate the pelvic floor and genital structures. Therefore, transection of this nerve should not interfere with erectile function should it be preserved in this population. The selected femoral muscular nerve branches (muscular branches to the vastus medialis and vastus intermedius) were long enough to be transferred to both the ipsilateral and contralateral pelvic nerves in their retroperitoneal positions. Lastly, cross sectional areas of each branch was sufficiently equivalent to allow surgical reanastomosis. In fact, even smaller nerve bundles could have been teased out from the femoral nerve for transfer, due to the small diameter of the pelvic nerve (<3 mm2).
One of the technical problems of bladder restoration using nerve transfers is that there are very few spinal cord injuries where a volitionally controlled donor nerve is in proximity to the sacral nerve roots. Xiao and colleagues developed a strategy to address this problem.29-32 While nerves below the level of injury are not under volitional control, they can be activated through the use of spinal reflexes, for example. Xiao re-engineered a common lumbar reflex, a cutaneous-muscular reflex, so that a lumbar afferent root (the dorsal root) is left intact, but its efferent root (the ventral root) was transferred to a sacral ventral root to reinnervate the bladder, thus creating a skin-CNS-bladder reflex.30 Studies of this concept by his group have spanned numerous animal models and have been taken to clinical trials.29,31,32 An outside group has confirmed this effect in a rat model.28 The procedure is now commonly practiced by this same group in China. Recently, a second group has utilized a very similar concept, but via a different reflex pathway, and have reported very good results in a clinical trial in China.13
A concern with the above intervention is the proximal site of implementation and resultant lack of specificity of the nerve transfer. The proximal site of implementation used in Xiao’s strategy (a lumbar to sacral ventral root transfer) is problematic in that the long distance from the spinal canal to the bladder wall may result in a loss of axons into sensory distributions, colonic innervation, somatic distributions and all other targets supplied by the chosen sacral nerve roots. Nerve transfers that are performed closer to the target organ are preferable for several reasons, including: shorter times are required for regeneration, fewer potential destinations for the transferred axons, and better ability to select a compatible donor axon source. Our strategy directs all candidate axons to the pelvic nerve trunk that is directly innervating the detrusor muscle, as shown in Figure 5. Recreating this degree of reinnervation using the transfer of ventral roots at the spinal level would require transection and reinnervation with roots from S2, S3, and S4. Our strategy of using a single coapted nerve to the target nerve should provide for a more robust reinnervation of the detrusor and avoid co-contraction of the external sphincter or other sacral innervated targets.
Because the lumbar spinal cord, even in isolation from other nervous system regions, retains an intact reflex arc, this procedure can be undertaken even in cervical spinal cord injuries as long as the lumbar spinal cord is not directly injured. We selected the femoral nerve for this study, as it has the added advantage of an easily accessible tendon trigger through the patellar tendon reflex or a potential cutaneo-muscular activation. Following regeneration, a somatic-autonomic reflex arc could be utilized to empty the bladder. Should this not be an effective method of activating the transferred nerve, a failsafe may be provided by direct trans epidermal nerve stimulation (TENS) activation of the transferred nerves subcutaneously to “turn on” bladder contraction. Also, the femoral nerve provides multiple branches to the vastus medialis and intermedialis, each of which are each sufficiently long for transfer to either the ipsilateral or contralateral pelvic nerves. Bilateral reinnervation of pelvic nerves from branches of one femoral nerve would provide a single trigger for symmetrical bilateral contraction.
Limitations of this study include its use in cadavers to date rather than in patients. However, we felt that feasibility of the method needed to be established prior to performing these surgeries in a patient population. A second limitation is the inability to determine axon counts in the nerves due to fixation methods used for the cadavers and due to the long time period between fixation and dissection because most of the cadavers were either used for or slated for medical educational purposes.
Conclusion
We demonstrated in cadavers that the transfer of femoral motor branches to vesical branches of the pelvic nerve is anatomically feasible. The next step is to determine whether functional and/or reflex stimulation of the femoral to pelvic nerve branches will result in detrusor contractions sufficient to empty the bladder. If so, this procedure opens the door to the restoration of volitional voiding in patients with decentralized bladders.
Acknowledgments
This publication was made possible by Grant Number 1R01NS070267 from NIH-NINDS to M.R.R. and M.F.B. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH-NINDS. We would also like to thank the Department of Anatomy and Cell Biology of Temple University for allowing access to the cadavers used in this study. The drawing in Figure 1 was created by Dr. Mary F. Barbe and drawings in Figures 2-4 were prepared and provided by Professor Susan B. Fecho at Barton College. Figure 5 was prepared and provided by David and Alex Baker, DNA Illustrations, Inc. of Asheville, North Carolina (www.dnaillustrations.com). These artists own the copyrights to these drawings.
References
- 1.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous: Spinal Cord Injury Facts and Figures at a Glance, in 1717 6th Avenue South, SRC 515, Birmingham, AL 35233-7330. Vol. 2012 National Spinal cord Statistical Center, University of Alabama; Birmingham: 2012. [Google Scholar]
- 3.Barbe MF, Ruggieri MR., Sr Innervation of parasympathetic postganglionic neurons and bladder detrusor muscle directly after sacral root transection and repair using nerve transfer. Neurourology & Urodynamics. 2011 doi: 10.1002/nau.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braverman AS, Doumanian LR, Ruggieri MR., Sr M2 and M3 muscarinic receptor activation of urinary bladder contractile signal transduction. II. Denervated rat bladder. J Pharmacol Exp Ther. 2006;316:875–880. doi: 10.1124/jpet.105.094961. [DOI] [PubMed] [Google Scholar]
- 5.Brown JM, Yee A, Mackinnon SE, Brown JM, Yee A, Mackinnon SE. Distal median to ulnar nerve transfers to restore ulnar motor and sensory function within the hand: technical nuances. Neurosurgery. 2009;65:966–977. doi: 10.1227/01.NEU.0000358951.64043.73. discussion 977-968. [DOI] [PubMed] [Google Scholar]
- 6.Browne EZ, Snyder CC. Intercosto-sacral neural anastomosis. Surgical Forum. 1971;22:474–476. [PubMed] [Google Scholar]
- 7.Carlsson CA, Sundin T. Reconstruction of efferent pathways to the urinary bladder in a paraplegic child. Review of Surgery. 1967;24:73–76. [PubMed] [Google Scholar]
- 8.Egon G, Barat M, Colombel P, Visentin C, Isambert JL, Guerin J. Implantation of anterior sacral root stimulators combined with posterior sacral rhizotomy in spinal injury patients. World Journal of Urology. 1998;16:342–349. doi: 10.1007/s003450050078. [DOI] [PubMed] [Google Scholar]
- 9.Girdler BJ, Turzewski C, Feber K, Nantau W, Gonzalez J, de Benito J, et al. One year clinical outcomes with lumbar to sacral nerve rerouting in spinal bifida. Journal of Urology. 2009;181:310. doi: 10.1016/j.juro.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 10.Johnston L. Human spinal cord injury: new and emerging approaches to treatment. Spinal Cord. 2001;39:609–613. doi: 10.1038/sj.sc.3101220. [DOI] [PubMed] [Google Scholar]
- 11.Kilvington B. Report C. An investigation on the regeneration of nerves, with regard to surgical treatment of certain paralysis. British Medical Journal. 1907;1:988–990. doi: 10.1136/bmj.1.2417.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirkham AP, Knight SL, Craggs MD, Casey AT, Shah PJ, Kirkham APS, et al. Neuromodulation through sacral nerve roots 2 to 4 with a Finetech-Brindley sacral posterior and anterior root stimulator. Spinal Cord. 2002;40:272–281. doi: 10.1038/sj.sc.3101278. [DOI] [PubMed] [Google Scholar]
- 13.Lin H, Hou C, Zhen X, Xu Z, Lin H, Hou C, et al. Clinical study of reconstructed bladder innervation below the level of spinal cord injury to produce urination by Achilles tendon-to-bladder reflex contractions. Journal of Neurosurgery Spine. 2009;10:452–457. doi: 10.3171/2009.1.SPINE08540. [DOI] [PubMed] [Google Scholar]
- 14.Livshits A, Catz A, Folman Y, Witz M, Livshits V, Baskov A, et al. Reinnervation of the neurogenic bladder in the late period of the spinal cord trauma. Spinal Cord. 2004;42:211–217. doi: 10.1038/sj.sc.3101574. [DOI] [PubMed] [Google Scholar]
- 15.Louie G, Mackinnon SE, Dellon AL, Patterson GA, Hunter DA. Medial antebrachial cutaneous--lateral femoral cutaneous neurotization in restoration of sensation to pressure-bearing areas in a paraplegic: a four-year follow-up. Annals of Plastic Surgery. 1987;19:572–576. doi: 10.1097/00000637-198712000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Lurje A. Concerning Surgical Treatment of Traumatic Injury to the Upper Division of the Brachial Plexus (Erb’s Type) Annals of Surgery. 1948;127:317–326. [PMC free article] [PubMed] [Google Scholar]
- 17.Migliorini CE, New PW, Tonge BJ. Quality of life in adults with spinal cord injury living in the community. Spinal Cord. 2011;49:365–370. doi: 10.1038/sc.2010.102. [DOI] [PubMed] [Google Scholar]
- 18.Ninkovic M, Stenzl A, Hess M, Feichtinger H, Schwabegger A, Colleselli K, et al. Functional urinary bladder wall substitute using a free innervated latissimus dorsi muscle flap. Plastic & Reconstructive Surgery. 1997;100:402–411. doi: 10.1097/00006534-199708000-00020. discussion 412-404. [DOI] [PubMed] [Google Scholar]
- 19.Patil A. Intercostal nerves to spinal nerve roots anastomosis (spinal cord bypass) and Harrington rod fusion in traumatic paraplegia--technical note. Acta Neurochirurgica. 1981;57:299–303. doi: 10.1007/BF01664846. [DOI] [PubMed] [Google Scholar]
- 20.Rao CR, Bruce AW, Lywood DW, Robertson DM. Reinnervation of the neurogenic bladder with somatic motor nerves. Investigative Urology. 1971;9:59–63. [PubMed] [Google Scholar]
- 21.Ruggieri MR, Braverman AS, D’Andrea L, Betz R, Barbe MF. Functional reinnervation of the canine bladder after spinal root transection and genitofemoral nerve transfer at one and three months after denervation. Journal of Neurotrauma. 2008;25:401–409. doi: 10.1089/neu.2007.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruggieri MR, Braverman AS, D’Andrea L, McCarthy J, Barbe MF. Functional reinnervation of the canine bladder after spinal root transection and immediate somatic nerve transfer. Journal of Neurotrauma. 2008;25:214–224. doi: 10.1089/neu.2007.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shellock FG. NeuroControl VOCARE Bladder System/Finetech-Brindley Bladder System (Vocare) Vol. 2011. Shellock R & D Services, Inc; 2011. MRIsafety.com. [Google Scholar]
- 24.Sidi AA, Becher EF, Reddy PK, Dykstra DD. Augmentation enterocystoplasty for the management of voiding dysfunction in spinal cord injury patients. Journal of Urology. 1990;143:83–85. doi: 10.1016/s0022-5347(17)39872-5. [DOI] [PubMed] [Google Scholar]
- 25.Steers WD, Wind TC, Jones EV, Edlich RF, Steers WD, Wind TC, et al. Functional electrical stimulation of bladder and bowel in spinal cord injury. Journal of Long-Term Effects of Medical Implants. 2002;12:189–199. [PubMed] [Google Scholar]
- 26.Trumble HC. Experimental reinnervation of the paralyzed bladder. Medical journal of Austrailia. 1935;1:118. [Google Scholar]
- 27.Vorstman B, Schlossberg S, Kass L. Investigations on urinary bladder reinnervation. Historical perspective and review. Urology. 1987;30:89–96. doi: 10.1016/0090-4295(87)90168-3. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Hou C, Jiang J, Li Q, Zhang F, Wang J, et al. Selection of the sacral nerve posterior roots to establish skin-CNS-bladder reflex pathway: an experimental study in rats. Microsurgery. 2007;27:118–124. doi: 10.1002/micr.20316. [DOI] [PubMed] [Google Scholar]
- 29.Xiao C-G, Du M-X, Dai C, Li B, Nitti VW, de Groat WC. An artificial somatic-central nervous system-autonomic reflex pathway for controllable micturition after spinal cord injury: preliminary results in 15 patients. Journal of Urology. 2003;170:1237–1241. doi: 10.1097/01.ju.0000080710.32964.d0. see comment. [DOI] [PubMed] [Google Scholar]
- 30.Xiao CG. Reinnervation for neurogenic bladder: historic review and introduction of a somatic-autonomic reflex pathway procedure for patients with spinal cord injury or spina bifida. Eur Urol. 2006;49:22–28. doi: 10.1016/j.eururo.2005.10.004. discussion 28-29. [DOI] [PubMed] [Google Scholar]
- 31.Xiao CG, de Groat WC, Godec CJ, Dai C, Xiao Q. “Skin-CNS-bladder” reflex pathway for micturition after spinal cord injury and its underlying mechanisms. J Urol. 1999;162:936–942. doi: 10.1097/00005392-199909010-00094. [DOI] [PubMed] [Google Scholar]
- 32.Xiao CG, Godec CJ. A possible new reflex pathway for micturition after spinal cord injury. Paraplegia. 1994;32:300–307. doi: 10.1038/sc.1994.52. [DOI] [PubMed] [Google Scholar]


