Introduction
After decades of epidemiologic and clinical research, the influence of overall diet on health and disease, particularly coronary heart disease (CHD), has become widely accepted.1 The pioneering ecological and metabolic studies of Keys and colleagues placed the focus on fat types, showing that populations consuming more saturated fatty acids (SFA) had higher blood cholesterol levels and associated higher rates of coronary heart disease (CHD),2 while individuals fed SFA had increased blood cholesterol levels, as opposed to the effects of monounsaturated (MUFA) or polyunsaturated fatty acids (PUFA). Cholesterol changes derived from switching fatty acid species in the diet were found to be predictable with specific equations.3-5 Subsequent epidemiologic studies confirmed that elevated serum cholesterol was a strong independent risk factor for CHD. This led to the formulation of the diet-heart hypothesis, whereby high intake of saturated fats leads to elevated cholesterol levels, which in turn promote atherosclerosis, coronary artery occlusion, and subsequent ischemic events.6 Though the diet-heart hypothesis shaped dietary guidelines towards reduction of all dietary fat, with a concomitant increase in carbohydrates, it proved futile in reducing the incidence of cardiovascular disease (CVD) in women.7 However, no randomized controlled trial (RCT) of a low-fat diet for effects on CVD events has been conducted in men. In the last three decades, the decreasing fat consumption as percentage of energy in the US population has done little to slow the increasing rates of obesity and type 2 diabetes mellitus (T2DM).8 It is now widely recognized that higher-fat diets can be beneficial if healthy fats are consumed, while high-carbohydrate diets (particularly those with high glycemic load) might be contributing to CHD9-12 and other negative health outcomes.12,13 As reviewed here, healthy, plant-based unsaturated fats are major components of widely consumed edible seeds, such as tree nuts and peanuts.
More important than isolated foods or nutrients, the effect of overall dietary patterns on disease risk has become a new approach in nutritional epidemiology.14 Plant-based dietary patterns, such as the “prudent” diet identified by Hu et al.15 contain high amounts of vegetables, legumes, whole grains, and fruit and moderate amounts of fish, poultry, and low-fat dairy products. This dietary pattern has been associated with lower risk of CVD16-20 and T2DM21 and is widely recommended for heart health.22 The traditional Mediterranean diet, which is akin to the prudent diet but has a higher total fat content in the form of olive oil and also alcohol (red wine) in moderation, has emerged as another dietary approach to chronic disease prevention, including CVD.23,24
Another potentially healthy dietary pattern is the so-called paleolithic diet, still consumed by hunter-gatherer societies worldwide and put into perspective by Eaton and Conner more than 25 years ago.25 The hunter-gatherer's diet is high in slow-release carbohydrate and fiber from plant foods, high in protein from lean wild animal meat and plant seeds, and low in fat, which is mostly unsaturated and derived from both wild game meat and seed sources, with contributions to total energy intake of approximately 45%, 35%, and 20%, respectively.25,26 Noticeably, of the plant-foods foraged by hunter-gatherers, an average 41% is made up of fruit and 26% comes from nuts and whole seeds,26 considerably higher than amounts consumed by modern humans, including former hunters-gatherers settled into a Westernized existence.27
The integral role of seeds in pre-agricultural diets is understandable given their high energy and nutrient density. Seeds are also particularly important in human nutrition because of their unique composition in bioactive compounds. Of note, in the last decade a large body of scientific evidence has been built on the beneficial effects of increasing consumption of plant seeds and derived products on various health outcomes, chiefly CVD, T2DM, and intermediate markers. The purpose of this review is to summarize the state of the evidence for the cardiovascular health effects of plant seeds, a food category bound to have a prominent place in health-promoting diets.
Data Sources and Selection of Studies on Plant Seeds and Cardiovascular Health
For this narrative review we conducted a comprehensive search of MEDLINE and EMBASE through December 2012 for English language descriptions of seed composition, reports of epidemiologic and clinical studies describing effects of seeds (whole grains, tree nuts and peanuts, pulses, cocoa and cocoa products such as chocolate, and coffee) on cardiometabolic outcomes, and the most recent reviews and meta-analyses of these studies. We also searched for reviews and meta-analyses of the effects of the main bioactive seed components on the same outcomes. Finally, we searched references cited in original studies and reviews identified, together with papers citing landmark clinical studies, reviews and meta-analyses, as provided by the publishers of individual articles in their websites. Data were examined for relevance, quality, and consistency and independently extracted by the two authors, who reached a consensus when in doubt about a specific citation. Given that, up to the time of submission, few controlled trials on the effects of seed consumption on clinical end points had been published, we have derived the core of scientific evidence from prospective cohort studies reporting disease outcomes and randomized clinical trials (RCTs) with intermediate end points, with a particular emphasis on meta-analyses of such studies.
Broad Definition of Seeds and their Purpose in Nature
Seeds are small embryonic plants enclosed in a coat, the product of the ripened ovule of flowering plants after pollination and the completion of the process of reproduction. Seeds are made of complex matrices in the outer layer and the germ, rich in minerals, vitamins, and bioactive phytochemicals that protect the plant's DNA from oxidative stress, thus facilitating the perpetuation of the species.28 On the other hand, the endosperm of seeds stores nutritive components to sustain the future seedling with a variable mixture of high-quality protein, complex carbohydrate, and fat depending on the type of seed. In this sense seeds are like eggs, which also contain nutrients to sustain the growth of bird and reptile embryos. For instance, seeds contain sizable amounts of phytosterols, important structural components of plant membranes that stabilize phospholipid bilayers just as the cholesterol that abounds in eggs does in animal cell membranes.29 Whole seeds provide a wide array of bioactive molecules likely to have significant health benefits.
Widely Consumed Seeds
Edible seeds having a significant contribution to human nutrition include grains (cereals), nuts, legumes, and cocoa and coffee beans. Human populations derive most of their energy needs from seeds or seed products, particularly grains (wheat, corn and rice) and legumes (soybeans). Seeds also provide most culinary oils and widely consumed beverages (coffee).
Grains (cereals) include wheat, oats, rye, rice, barley, millet, and corn.30 The foods originating from them range from highly nutrient-dense wholegrain breads and cereal foods such as oats, to less nutrient–dense white rice, white bread, pasta and noodles or refined grain products with high levels of added sugar, fat and/or sodium, such as many biscuits, pastries, and cakes.
Nuts are dry fruits with one seed in which the ovary wall becomes hard at maturity. The most popular edible tree nuts are almonds, walnuts, hazelnuts, and pistachios. Other common edible nuts are pine nuts, cashews, pecans, macadamias, and Brazil nuts.31 The consumer definition also includes peanuts, which botanically are legumes but are widely identified as part of the nuts food group. In addition, peanuts have a similar nutrient profile to tree nuts.32
Pulses are the seeds of leguminous plants contained within pods. Lentils, chickpeas, black-eyed peas, and a variety of dry beans, including pinto, kidney, navy, and fava beans, are the most common edible pulses.33 The term “pulses” excludes legumes used for oil extraction, such as soybeans and peanuts, and those harvested green, such as green peas and green beans, which are classified as vegetables. Pulses usually require cooking prior to consumption and are eaten untransformed, unlike soybeans, which are the basis for soy oil and various foods such as tofu and derived products, widely consumed in Asia and by vegetarian populations worldwide.
Cocoa beans are the seeds of the tropical tree Theobroma cacao. The main product derived from cocoa is chocolate, a variable combination of cocoa solids, cocoa butter, milk, and sugar.34 The roasted, ground seeds of the Coffea plant are used to brew coffee, which competes with tea as the most consumed beverage worldwide.
Grains, nuts, legumes, and cocoa products represent a sizable contribution to human energy consumption in various populations, while daily coffee drinking is customary for many individuals worldwide. Many other edible seeds that are sparingly consumed and/or contribute only marginally to energy intake will not be dealt with here: pepper, mustard, cumin, capers and other seasoning seeds; flax, hemp, pumpkin, sesame, and sunflower seeds; berries; and others like coconut.
Composition of Edible Seeds
The macro- and micronutrients, minerals, vitamins and phytochemicals in seeds reflect their role of nourishment and protection of the future seedling (Table 1 and Table 2). Edible seeds, particularly fat-rich nuts and cocoa products, are good sources of energy (Table 1). Brewed coffee is an exception because of the lack of macronutrients. The protein from seeds provides most essential amino acids, although some foods must be complemented in order to obtain significant amounts of all of them. For instance, the content of sulphur amino acids methionine and cysteine is marginal in most nuts38 and pulses,39 while grains contain sizable quantities,30 a reason why mixing the former with cereals suffices to meet human requirements. Again, except for coffee, seeds are rich in carbohydrate and fiber (Table 1). Most energy in nuts comes from fat, but fatty acids are mostly unsaturated. Some nuts, especially walnuts, contain sizable amounts of PUFA, including linoleic acid (18:2n-6) and α-linolenic acid (C18:3n-3), the principal plant n-3 fatty acid.31 Conversely, SFA abound in cocoa seeds, but the predominant species is stearic acid (C18:0), the consumption of which does not raise cholesterol as shorter-chain SFA do.40
Table 1.
Average Nutrient Composition of Selected Seeds and Seed Products
| Seeds | Energy (kJ) | Protein (g) | Carbohydrate (g) | Fiber (g) | Total fat (g) | SFA (g) | MUFA (g) | PUFA (g) |
|---|---|---|---|---|---|---|---|---|
| Whole grain flour | 465 to 1587 | 2.6 to 15.9 | 23.0 to 76.9 | 1.8 to 23.8 | 0.9 to 6.5 | 0.2 to 1.1 | 0.2 to 2 | 0.3 to 2.6 |
| Tree nuts and peanuts | 2200 to 3000 | 7.8 to 23.7 | 12.3 to 32.7 | 3.0 to 12.2 | 46.0 to 76.1 | 3.7 to 11.9 | 8.9 to 59.3 | 1.5 to 47.2 |
| Pulses (baked) | 352 to 687 | 4.8 to 9.0 | 15.6 to 27.4 | 5.4 to 10.5 | 0.2 to 2.6 | 0.04 to 0.27 | 0.02 to 0.58 | 0.1 to 1.2 |
| Dark chocolate | 2286 to 2504 | 4.9 to 7.8 | 45.9 to 61.2 | 7.0 to 10.9 | 31.3 to 42.6 | 18.5 to 24.5 | 9.5 to 12.8 | 1.1 to 1.3 |
| Coffee (per cup)* | 5 | 0.3 | 0 | 0 | 0.05 | 0.005 | 0.036 | 0.002 |
Data for raw products and 100 g serving size, except where specified.
SFA indicates saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids.
One cup is made from about 5 g ground coffee.
Source: US Department of Agriculture Nutrient Data Base.35
Table 2.
Average Composition of Seeds and Seed Products in Selected Micronutrients, Minerals and Phytochemicals
| Seeds | Folate (μg) | Tocopherols (mg) | Potassium (mg) | Calcium (mg) | Magnesium (mg) | Phytosterols (mg) | Polyphenols (mg) |
|---|---|---|---|---|---|---|---|
| Whole grain flour | 4 to 44 | 0.03 to 2.7 | 43 to 717 | 7 to 52 | 43 to 160 | 28 to 89 | 72 to 95 |
| Tree nuts and peanuts | 10 to 145 | 0.9 to 26.2 | 363 to 1042 | 16 to 264 | 118 to 1223 | 72 to 214 | 126 to 1816 |
| Pulses (baked) | 47 to 208 | 0.01 to 0.94 | 194 to 508 | 19 to 69 | 36 to 70 | 108 to 121 | 120 to 6500 |
| Dark chocolate* | 7 to 10 | 0.54 to 0.59 | 559 to 715 | 56 to 73 | 146 to 228 | 102 to 129 | 1105 to 1860 |
| Coffee (per cup)† | 5 | 0.04 | 116 | 5 | 7 | 0.2 | 267 |
Data for raw products and 100 g serving size, except where specified.
45% to 85% cacao solids.
One cup is made from about 5 g ground coffee.
Regarding micronutrients (Table 2), the folate content of seeds and seed products ranges from low to moderate, but frequent consumption is likely to contribute to adequate folate intake. The seed content of tocopherols (the main vitamin E compounds) is also low to moderate, except for nuts, which can be particularly rich in α-tocopherol, the principal vitamin E species. Regarding minerals, seeds are excellent sources of potassium (K+), magnesium (Mg++) (excluding coffee), and calcium (Ca++). Like all vegetables, unprocessed seeds have very low sodium content. Seeds contains enough phytosterols to build the cell membranes of the future seedling,29 giving them the highest content of these bioactive molecules among natural plant foods. Finally, all seeds contain bioactive polyphenols, water-soluble pigments capable of quenching oxygen-derived free radicals.41 As such, these compounds defend seeds against oxidative stress and radiation injury, pathogenic micro-organisms and insects, besides conferring organoleptic characteristics (odor and flavor). Polyphenols are structurally diverse and the amounts and predominant species contained in different seeds and seed products are quite variable, a detailed description being beyond the scope of this review.42 Although the evidence remains inconclusive, polyphenols are believed to be involved in the cardiovascular benefit associated with consumption of seeds, particularly cocoa products and coffee, and of other polyphenol-rich foods, such as tea and red wine.43
Because many components of whole seeds and derived products such as macronutrient, micronutrient, and non-nutrient substances (Table 1 and Table 2) have been linked to reduced risk of CVD and related metabolic disturbances, seeds can be considered natural health capsules, where the synergistic interaction of their many bioactive constituents may all favorably influence human physiology.44 Indeed, in recent years evidence has accumulated that increased consumption of whole seeds or derived products is beneficial for heart health independently of the background diet.
Review of Evidence on Edible Seeds and their Products
Numerous epidemiologic studies have examined consumption of edible seeds in relation to risk of CVD, T2DM, or mortality, and meta-analyses summarize the observational evidence. Meta-analyses have also been published on the effects of these foods on blood cholesterol, glycemic control, blood pressure, or vascular function from small, usually short-term, RCTs. Table 3 lists the latest meta-analyses and their main results.45-61
Table 3.
Effect of Seed Consumption on CVD Outcomes Based on Selected Meta-analyses of Findings of Epidemiologic Studies and Randomized Clinical Trials
| Author (year) (reference) | Type of study | n | Population | Follow-up | Outcomes | Effect of increased seed consumption* (numbers between brackets are 95% confidence intervals) |
|---|---|---|---|---|---|---|
| Whole grains | ||||||
| Ye (2012) (45) | Meta-analysis of 8 cohort studies | 298,592 | Generally healthy | 6 to 14 y | Incident CVD | RR=0.79 (0.74-0.85) |
| Meta-analysis of 6 cohort studies | 288,319 | Generally healthy | 6 to 12 y | Incident T2DM | RR=0.74 (0.69-0.80) | |
| Meta-analysis of 21 RCTs | Not available | Healthy or patients with HC or hypertension | 4 to 16 wk | Lipid profile | Δ Total cholesterol: –0.83 mmol/L (–1.24 to –0.42) Δ LDL-cholesterol: –0.72 mmol/L (–1.34 to –0.11) |
|
| Glycemic control | Δ Fasting glucose: –0.93 mmol/L (–1.65 to –0.21) | |||||
| de Munter (2007) (46) | Meta-analysis of 6 cohort studies | 286,125 | Generally healthy populations | 6 to 18 y | Incident T2DM | 21% (13%-28%) risk reduction for a 2-serving/d increment |
| Nuts | ||||||
| Kris-Etherton (2008) (47) | Pooled analysis of 4 U.S. prospective studies | 135,604 | CVD-free men and women | 6 to 17 y | Incident CHD | RR=0.65 (0.47-0.89) |
| Banel & Hu (2009) (48) | Meta-analysis of 13 walnut RCTs | 365 | Subjects with normal lipids or HC | 4 to 24 wk | Lipid profile | Δ Total cholesterol: –0.27 mmol/L (–0.38 to –0.15) Δ LDL-cholesterol: –0.24 mmol/L (–0.34 to –0.14) |
| Sabaté (2010) (49) | Pooled analysis of 27 nut RCTs | 583 | Subjects with normal lipids or HC | 3 to 8 wk | Lipid profile | Δ Total cholesterol: –0.28 mmol/L (–0.37 to –0.20) Δ LDL-cholesterol: –0.26 mmol/L (–0.34 to –0.19) |
| Pulses | ||||||
| Bazzano (2011) (50) | Meta-analysis of 10 RCTs | 268 | Subjects with normal lipids or HC | 3 to 8 wk | Lipid profile | Δ Total cholesterol: –0.31 mmol/L (–0.42 to –0.19) Δ LDL-cholesterol: –0.21 mmol/L (–0.30 to –0.12) Δ Triglycerides: –1.67 mmmol/l (–3.63 to 0) (Results as SMD)† |
| Sievenpiper (2009) (51) | Meta-analysis of 11 RCTs of pulses alone | 253 | Subjects with T2DM, normoglycemia or HC | 1 to 96 wk | Glycemic control | Fasting glucose: –0.82 (–1.36 to –0.27) Fasting insulin: –0.49 (–0.93 to –0.04) |
| Meta-analysis of 19 RCTs of pulses in low-GI diets Meta-analysis of 11RCTs of pulses in high-fiber diets |
72 641 |
Subjects with type-1 diabetes, T2DM or normoglycemia Subjects with type-1 diabetes, T2DM or normoglycemia |
2 to 52 wk 1.4 to 156 wk |
HbA1c: –0.28 (–0.42 to –0.14) Fasting glucose: –0.32 (–0.49 to –0.15) HbA1c: –0.27 (–0.45 to –0.09) |
||
| Chocolate | ||||||
| Buitrago-Lopez (2011) (52) | Meta-analysis of 5 prospective studies | 60,455 | CVD-free men and women (patients with prior CHD in one study) | 8 to 16 y | Incident CVD | RR=0.63 (0.44-0.90) |
| Stroke | RR=0.71 (0.52-0.98) | |||||
| Larsson (2012) (53) | Meta-analysis of 5 prospective studies | 131,3455 | CVD-free men and women (patients with prior CHD in one study) | 8 to 16 y | Stroke | RR=0.81 (0.73-0.90) |
| Tokede (2011) (54) | Meta-analysis of 10 RCTs | 320 | Healthy subjects or subjects with CVD risk factors | 2 to 12 wk | Lipid profile | Δ Total cholesterol: –0.16 mmol/L (–0.30 to –0.02) Δ LDL-cholesterol: –0.15 mmol/L (–0.27 to –0.03) |
| Hooper (2012) (55) | Meta-analysis of 21 RCTs reporting lipid outcomes | 290 | Healthy subjects or subjects with CVD risk factors | 2 to 26 wk | Lipid profile | Δ LDL-cholesterol: –0.07 mmol/L (–0.14 to –0.0) Δ HDL-cholesterol: 0.03 mmol/L (0.0-0.06) |
| Meta-analysis of 6 RCTs reporting glycemic control | 91 | Healthy subjects or subjects with CVD risk factors | 2 to 26 wk | Glycemic control | Δ Insulin: –2.65 μU/mL (–4.65 to –0.65) Δ HOMA-IR: –0.67 (–0.98 to –0.36) |
|
| Meta-analysis of 22 RCTs reporting blood pressure | 918 | Healthy subjects or subjects with CVD risk factors | 2 to 18 wk | Blood pressure | Δ DBP: –1.60 mm Hg (–2.77 to –0.43) | |
| Meta-analysis of 11 acute RCTs on postprandial FMD | 373 | Healthy subjects or subjects with CVD risk factors | 90 to 150 min | FMD | Δ FMD: 3.19% (2.04-4.33) | |
| Meta-analysis of 11 chronic RCTs reporting FMD | 382 | Healthy subjects or subjects with CVD risk factors | 2 to 18 wk | FMD | Δ FMD: 1.34% (1.00-1.68) | |
| Ried (2012) (56) | Meta-analysis of 20 RCTs | 865 | Healthy subjects or subjects with CVD risk factors | 2 to 18 wk | Blood pressure | Δ SBP: –2.77 mm Hg (–−4.72 to –0.82) Δ DBP: –2.30 mm Hg (–3.46 to –0.93) |
| Coffee | ||||||
| Wu (2009) (57) | Meta-analysis of 21 cohort studies | 407,806 | Generally healthy | 4 to 32 y | Incident CHD | RR=0.82 (0.73-0.92) in women RR=0.87 (0.80-0.86) in men and women followed ≤ 10 y |
| Larsson (2011) (58) | Meta-analysis of 11 cohort studies | 479,689 | Generally healthy | 2 to 20.8 y | Incident stroke | RR=0.86 (0.78-0.94) for 2 cups of coffee/d RR=0.83 (0.74-0.92) for 3-4 cups/d RR=0.87 (0.77-0.97) for 6 cups/d RR=0.93 (0.79-1.08) for 8 cups/d |
| Mostofsky (2012) (59) | Meta-analysis of 5 cohort studies | 140,220 | Generally healthy or patients with myocardial infarction | 8 to 35 y | Incident congestive heart failure | RR=0.90 (0.82-0.99) for 3 to 4 cups/d RR=0.89 (0.81-0.99) for 4 to 5 cups/d |
| Huxley (2009) (60) | Meta-analysis of 18 cohort studies | 457,922 | Generally healthy | 2.6 to 20 y | Incident T2DM | 7% (9%-5%) risk reduction for each additional cup of coffee/d 25% (18%-31%) risk reduction for consumption of 3 to 4 cups of coffee/d |
| Meta-analysis of 6 cohort studies | 225,516 | Generally healthy | 8.4 to 12 y | Incident T2DM | RR=0.64 (0.54-0.77) for 3 to 4 cups/d of decaffeinated coffee | |
| Zhang (2011) (61) | Meta-analysis of 6 cohort studies | 172,567 | Generally healthy | 6.4 to 33 y | Incident hypertension | Habitual coffee consumption of >3 cups/d was not associated with an increased risk of hypertension compared with <1 cup/d Slightly elevated risk associated with light-to-moderate consumption of 1 to 3 cups/d (RR for comparison of 3 with 0 cups/d: 1.07 (0.97-1.20) |
CVD indicates cardiovascular disease; T2DM, type 2 diabetes mellitus; CHD, coronary heart disease; RR, relative risk; CI, 95% confidence interval; RCT, randomized controlled trial; HC, hypercholesterolemia; SMD, standardized mean difference; GI, glycemic index; HOMA-IR, insulin resistance index; FMD, flow-mediated dilatation; DBP, diastolic blood pressure; SBP, systolic blood pressure..
Risk ratios in meta-analyses of epidemiologic studies, usually adjusted for multiple confounders, are for highest versus lowest categories of consumption or increased servings/d, as specified. Outcome changes (Δ) are means for average doses of seeds or seed products in seed diets compared to control diets in meta-analyses of RCTs; only statistically significant changes are shown. Values between brackets are 95% confidence intervals.
SMDs are interpreted as follows: <0.4, small effect size; 0.4–0.7, moderate effect size; and >0.7, large effect size.
Whole grains
Prior to the Industrial Revolution, all cereals were stone-milled and contained the entire constituents of the grain kernel.62 The advent of industrialized roller milling at the end of the 19th century significantly changed the nutritional quality of milled grain. In the 1970's Burkitt and Trowell pioneered the link between chronic disease and refined grain by observing that Africans who consumed large quantities of whole plant foods had a lower prevalence of chronic diseases than Western countries where grain products are highly processed.63 Whole grains are comprised of germ, bran, and endosperm. In contrast, refining removes both the germ and bran along with fiber, vitamins, minerals, phenolic compounds, and phytochemicals.30,64 The unique constellation of constituents is thought to confer the beneficial effects of whole grains on chronic disease risk via mechanisms shared by all seeds (see section on Mechanisms for Beneficial Effects of Seeds).
A meta-analysis by Mellen et al.65 of seven prospective cohorts found a 21% lower risk of CVD for greatest compared with lowest whole grain intake. A more recent meta-analysis of 45 prospective cohort studies45 showed that, compared with participants who rarely or never consumed whole grains, those reporting an average intake of 48–80 g/d (3–5 serving/d) had a 21% lower risk of CVD and a 26% lower risk of T2DM (Table 3). Based on data from 21 short-term RCTs, this meta-analysis also found that whole grain interventions significantly reduced total and LDL-cholesterol and fasting glucose. de Munter et al.46 conducted a meta-analysis of six prospective cohort studies and found a 21% reduction of T2DM risk for two servings/d increment in whole grain intake. The inverse association was stronger for bran than for germ, and there was no independent association for germ intake after adjustment for bran intake. These results provide some evidence to support independent benefits of bran, although it is often difficult to tease out the effects of the individual components of a whole food.
Nuts (tree nuts and peanuts)
Over the last two decades four large prospective studies in the U.S. have reported on incident CHD in relation to frequency of nut consumption (including peanuts and peanut butter). As summarized in a pooled analysis,47 the results have consistently shown protection from CHD in participants who ate nuts at least twice per week compared with those who never or rarely consumed nuts (Table 3). Frequent nut consumption also relates inversely to total mortality, as suggested by recent reports from a large Dutch cohort66 and the Nurses’ Health Study (NHS).67 As reviewed,68 in the Physician's Health Study (PHS) nut consumption was related to lower rates of sudden cardiac death, but not of stroke, heart failure, or atrial fibrillation; two other studies reported that nut and peanut intake was associated with a reduced risk of T2DM in women, but not in men.
Over 50 short-term RCTs have compared the effects of nut-enriched and nut-free diets on blood lipids and lipoproteins. A meta-analysis of 13 walnut feeding studies48 and a recent pooled analysis of 25 RCTs using various nuts49 indicate a consistent cholesterol-lowering effect (Table 3). In the pooled analysis LDL-cholesterol was reduced by 7.4% for an average consumption of 67 g (2.4 oz) of nuts depending on nut dose and baseline LDL-cholesterol, and was similar by gender and age group and independent of the type of nut tested.49 Acute feeding studies indicate that nuts reduce postprandial glucose responses when consumed with foods having a high glycemic index (GI), which suggests that they may be useful in diabetic control.69 Importantly, recent evidence from the PREDIMED (PREvención con DIeta MEDiterránea) intervention trial in Spanish subjects at high cardiovascular risk showed that a Mediterranean diet with one daily serving of mixed nuts reduced incident T2DM by 52% (95% confidence interval [CI], 4%-76%) after 4 years compared with a control diet.70 Limited evidence from RCTs suggests that nuts, particularly walnuts, have beneficial effects on blood pressure and endothelial function.71 Nut feeding studies have also documented reduced circulating concentrations of inflammatory cytokines but no consistent changes of C-reactive protein.72 Finally, in spite of the high energy density of nuts, there is no evidence that their frequent consumption promotes obesity, probably because of a prominent satiating effect.68 In the PREDIMED trial, the Mediterranean diet supplemented with nuts significantly reduced the prevalence of metabolic syndrome compared with the control diet after 1-year follow- up, mainly by reducing waist circumference.73
Recently, the final results of the landmark PREDIMED RCT have been published showing for the first time a reduction of incident CVD after long-term consumption of a diet enriched in seeds.74 The PREDIMED study was a multicenter, nutrition intervention, primary prevention trial conducted in Spain wherein nearly 7500 participants at high cardiovascular risk, but with no cardiovascular disease at enrollment, were randomly assigned to one of three diets: a Mediterranean diet supplemented with daily doses of 30 g of mixed nuts (15 g walnuts, 7.5 g almonds, and 7.5 g hazelnuts), a Mediterranean diet supplemented with extra-virgin olive oil (1 liter per week), or a control diet (advice to reduce dietary fat). After a median follow-up of 4.8 years, the participants in the two Mediterranean diet groups showed a 30% reduction in CVD events (myocardial infarction, stroke or cardiovascular death) compared with the control diet. The nut diet was also associated with a significant 49% reduction in risk of stroke. Of note, the interventions were intended to improve the overall dietary pattern, but the major between-group differences in food intake were for the supplemental items. Thus, nuts were probably responsible for most of the observed benefits in the Mediterranean diet with nuts group. The results of the PREDIMED trial74 show the full potential of nuts and other healthy foods such as extra-virgin olive oil to improve cardiovascular health.
Pulses
Two studies have examined the association between pulse consumption and risk of CVD.75,76 In the First National Health and Nutrition Examination Survey (NHANES) Epidemiologic Follow-up Study, frequency of pulse plus peanut and peanut butter consumption was inversely associated with incidence of CHD and CVD during a 19-y follow-up. Legume consumption ≥4/wk compared with <1/wk was associated with a 22% lower risk of CHD (adjusted risk ratio [RR] 0.78; 95% CI, 0.68-0.90) and an 11% lower risk of CVD (adjusted RR 0.89; 95% CI, 0.80-0.98).75 A case-control study conducted in Costa Rica, where dry beans are a staple, compared bean intake in 2119 survivors of a first acute myocardial infarction (MI) with matched population controls. Compared with non-consumers, one serving of beans/d was inversely associated with MI, with an adjusted odds ratio (OR) of 0.62 (95% CI, 0.45-0.88).76 In another report on that study's control population, substituting one serving of beans for one serving of white rice was associated with a 35% (95% CI, 15%-50%) lower risk of having the metabolic syndrome, related mainly to a decreased frequency of low HDL-cholesterol and high fasting glucose.77 Similar findings were reported from the NHANES 1999-2002; compared to non-consumers, bean consumers had significantly lower body weight, smaller waist circumference, and lower systolic blood pressure.78 The risk of T2DM associated with pulse consumption has not been specifically assessed in epidemiologic studies. However, pulses are an important component of dietary patterns clearly related to a lower T2DM risk, such as low-GI, high-fiber, and Mediterranean diets.21
Studies dating back to the 1980s showed that daily servings of pulses had a beneficial effect on the lipid profile. A recent updated meta-analysis of ten RCTs evaluating the effects of pulse consumption on cholesterol levels indicates that a pulse-rich diet significantly decreases total cholesterol and LDL-cholesterol, with non-significant increases in HDL-cholesterol and reductions in triglycerides (Table 3).50 A meta-analysis on glucose metabolism indicates that pulses, alone or in low-GI or high-fiber diets, modestly improve medium- to longer-term glycemic control in subjects with or without T2DM.51 Acute feeding studies indicate that pulses, like nuts, reduce postprandial glucose responses compared with higher-GI foods,79 further suggesting a beneficial impact on diabetic control.
Cocoa and chocolate
Six recent cohort studies have reported on incident CVD in relation to frequency of chocolate consumption (Table 3).52,53 Larsson et al.53 reported lower stroke incidence associated with chocolate consumption and performed another meta-analysis that confirmed its protective effect. Two of the cohort studies included in the first meta-analysis on CVD outcomes52 also reported that blood pressure was significantly lower in the highest category of chocolate consumption.80,81 A prospective study evaluated the association between chocolate consumption and T2DM and reported a beneficial effect.82 A common problem is that different types of chocolate (dark vs. milk vs. white) were not distinguished in epidemiological studies. Even though the composition of chocolate is highly variable and diverges significantly from that of the original seed, it is the cocoa product most widely consumed.34 For this reason, most epidemiological studies analyzed consumption of any type of chocolate as an exposure variable.
A number of small RCTs assessing the short-term effects of dark chocolate or other cocoa products on serum lipids, blood pressure, or other intermediate markers of CVD have been published in the last decade (Table 3).54-56 Some used isolated flavanols, a subclass of flavonoid phenolics believed to be the principal bioactive components of cocoa.83 The effect sizes on lipids and blood pressure differ among meta-analyses, though the direction of the effects is consistently beneficial. These findings need to be confirmed in larger, longer, well-controlled feeding studies. On the other hand, a meta-analysis55 indicates a clear benefit of cocoa products on insulin resistance. Also, endothelial function was improved in both acute and chronic studies without evidence of heterogeneity between trials. Subgroup analyses indicated stronger benefit in participants at high risk of CVD than in healthy subjects for many outcomes.55 There is also RCT evidence that acute consumption of dark chocolate induces coronary vasodilation.84 Finally, studies support the additional anti-atherogenic effects of cocoa products, such as reduction of oxidative stress, inflammation, and platelet aggregation.83,85 Importantly, there is a negligible risk of weight gain in spite of the high energy content of chocolate, probably related to high satiation.85
Coffee
Coffee has been extensively studied in relation to various diseases, including CVD and T2DM. In Wu et al.'s57 meta-analysis of 21 prospective cohort studies on coffee and risk of CHD, there was no overall increased risk with greater consumption. Among women, but not men, there was an indication of an inverse association for moderate amount of coffee consumption (2-4 cups/d) and risk of CHD (Table 3). In a meta-analysis of coffee consumption and risk of stroke, compared with non-consumers, consumption of 1–6 cups/d was inversely associated with risk of stroke, with the strongest association (17% lower risk) for 3–4 cups/day.58 Mostofsky et al.59 conducted a meta-analysis of five prospective studies of coffee and heart failure and observed the lowest risk for consumption of 4 cups/d. These meta-analyses suggest a J-shaped relationship between coffee consumption and incidence of CHD, stroke, and heart failure, with modest benefit associated with moderate consumption (3-4 cups/d).
In 2005, van Dam and Hu conducted a meta-analysis of nine cohort studies of coffee consumption and risk of T2DM, including 193,473 participants and 8394 incident cases. The RR of T2DM was 0.65 (95% CI, 0.54-0.78) for the highest (6 or 7 cups/d) and 0.72 (95% CI, 0.62-0.83) for the second highest (4-6 cups per day) category of coffee consumption compared with the lowest consumption category (0 or 2 cups/d).86 In 2009, Huxley et al.60 updated the meta-analysis with 18 similar cohort studies. Overall, each additional cup of coffee/d was associated with a 7% reduction in incidence of T2DM after adjustment for potential confounders. Risk reduction was 27% for consumers of 3 to 4 cups of total coffee/d and 36% for a similar consumption of decaffeinated coffee.
Habitual consumption of unfiltered coffee, such as French pressed and boiled coffee, has been shown to increase total and LDL-cholesterol concentrations due to high concentrations of the cholesterol-raising compound cafestol.87 Paper-filtered or instant coffees do not appear to have cholesterol-raising effects. In addition, acute caffeine intake raises blood pressure, but habitual consumption has much smaller or no effects in normotensive or hypertensive individuals.88.89 Moreover, large prospective studies showed no or a modest inverse association between habitual coffee intake and long-term risk of hypertension,61 likely related to developing tolerance. Furthermore, potential harmful effects of caffeine on blood pressure may be counterbalanced by the beneficial effects of other components, such as polyphenols and minerals.
Several prospective studies have suggested a modest inverse association between coffee and risk of total and cause-specific mortality. In the AARP Diet and Health Study,90 adjusted RRs for death among men who drank coffee as compared with nondrinkers were 0.99 for drinking less than 1 cup/d; 0.94 for 1 cup; 0.90 for 2 or 3 cups; 0.88 for 4 or 5 cups; and 0.90 for 6 or more cups of coffee/d (P<0.001 for trend); the corresponding RRs among women were 1.01, 0.95, 0.87, 0.84, and 0.85 (P<0.001 for trend). Inverse associations were observed for deaths due to CVD, respiratory disease, diabetes, and infections, but not for deaths due to cancer. Similar results were observed in two other large cohort studies, the Health Professionals Follow-up Study and the NHS.91 Taken together, large prospective cohort studies have demonstrated that higher consumption of coffee is also associated with a moderately lower mortality risk.
Mechanisms for Beneficial Effects of Seeds
The nutrient density in the complex matrices of seeds probably explains the lower CVD risk observed with higher seed consumption. Many of these food constituents synergistically interact to beneficially affect various metabolic pathways intermediate in CVD and T2DM risk (Figure). We will briefly summarize the scientific evidence on the bioactivity of known seed components to provide a mechanistic frame for their cardioprotective benefits.
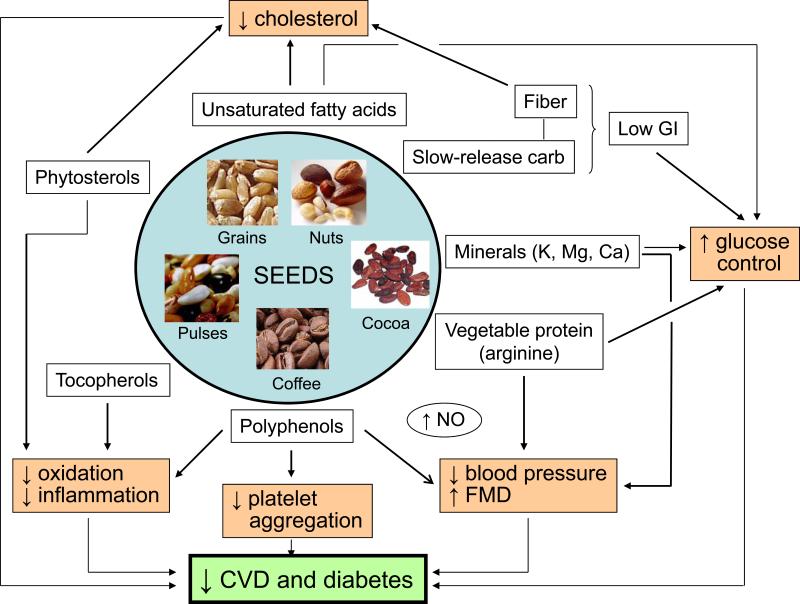
Figure.
Consumption of seeds improves cardiovascular health due to their unique composition in bioactive nutrients and phytochemicals and a complex synergy among them for effects on metabolic and vascular physiology pathways. The main known nutrients of seeds are represented together with their principal biological targets (long arrow connections). The net effects on intermediate markers of cardiovascular risk that have been demonstrated for most seed classes in clinical trials are cholesterol-lowering, improved glycemic control, decreased blood pressure, improved vasomotion, reduced platelet aggregation, and antioxidant and antiinflammatory actions. The overall result is reduced CVD and/or T2DM, as suggested for all seeds in observational cohort studies and observed for nuts in clinical trials. See text for details. Abbreviations: carb, carbohydrate, GI, glycemic index; K, potassium; Mg, magnesium; Ca, calcium; NO, nitrous oxide; FMD, flow-mediated vasodilation.
Fatty acids
Among seeds, nuts and chocolate have the highest total fat content (Table 1). However, except for chocolate (rich in stearic acid), seeds have a low SFA content and most fat is made up of unsaturated fatty acids, with variable proportions of MUFA and essential PUFA, such as linoleic and α-linolenic acids, the latter being particularly abundant in walnuts.32 While not as much investigated as marine n-3 fatty acids, in observational studies higher exposure to α-linolenic acid has been associated with a moderately lower risk of CVD.92 It has been known for decades that SFA intake raises blood cholesterol, while replacing SFA with MUFA or PUFA decreases blood cholesterol,3-5,93 with parallel effects on CVD risk.1,24 As mentioned, stearic acid, the predominant fatty acid in cocoa products, does not raise blood cholesterol.40,93 On the other hand, substituting SFA with unsaturated fatty acids, either MUFA or PUFA, improves insulin sensitivity and likely reduces the risk of T2DM (reviewed in94). Thus, by way of salutary effects on lipid and glucose metabolism, the fat fraction of seeds could help reduce the risk of CVD and T2DM.
Carbohydrate and fiber
Except for coffee as beverage, which contains very low amounts of macronutrients, whole seeds are important sources of slow-release carbohydrate and dietary fiber, a non-absorbable nutrient that has received much attention in nutritional epidemiology. There are two main types of dietary fiber: metabolically inert insoluble fiber and bioactive soluble or viscous (gel-forming) fiber, which has cholesterol-lowering properties and reduces postprandial glucose responses after carbohydrate-rich meals.95 With the exception of oat and barley, rich in soluble beta-glucans, whole grains contain little soluble fiber but are rich in insoluble fiber (bran), while the main sources of soluble fiber in the usual diet are fruit and vegetables. Observational studies have consistently shown that increased intake of dietary fiber, usually from cereals rather than fruit and vegetables, is associated with reduced incidence of CVD and T2DM.45,96 The reason for this apparent contradiction is that, in epidemiological studies, insoluble fiber cannot easily be dissociated from whole-grain foods, which provide many other bioactive phytochemicals associated with beneficial health effects, as discussed here. Dietary fiber may also reduce CVD and T2DM risk through blood pressure reduction, improvement of insulin sensitivity, changes in secretion of various gut hormones that may act as satiety factors, and anti-inflammatory effects.97
Protein
Seeds are important sources of protein. In large observational studies, increased consumption of plant protein as opposed to animal protein has been consistently associated with a moderate reduction in incident CHD .98-100 A mediating factor in protection from CVD by plant protein might be an inverse association with blood pressure.101 The OmniHeart trial also demonstrated that partial substitution of carbohydrate with protein (about half from plant sources) lowered blood pressure and improved the lipid profile in pre-hypertensive and hypertensive subjects.102 Again, it is difficult to dissociate the benefit from the vegetable protein per se from synergizing effects of other bioactive constituents in the protein-containing foods. Some amino acids in seeds may be particularly relevant to CVD protection, such as L-arginine, which is the substrate for endothelium-derived nitric oxide (NO) synthesis, a principal regulator of vascular tone and blood pressure.103 L-arginine supplementation improves endothelial function in clinical trials and reduces blood pressure in experimental animal models of hypertension.104 The L-arginine content of seeds probably contributes to the beneficial vasomotor effects of diets or meals enriched with nuts71 and cocoa products.83 For instance, in a clinical trial in hypercholesterolemic subjects, a diet containing walnuts (18% of the total energy) improved endothelial function in comparison with a Mediterranean diet, and it was estimated that walnut intake increased dietary L-arginine up to 1.4 g/d,105 which is close to supplement doses showing vasomotor effects in clinical studies.104
Sterols
Like all plant foods, seeds are cholesterol-free, but their fatty fraction contains sizeable amounts of chemically related non-cholesterol sterols known as plant sterols or phytosterols, non-nutritive components that play important structural roles in membranes.29 Relevant for human physiology, phytosterols interfere with cholesterol absorption in the intestinal lumen and thus possess cholesterol-lowering properties.106 Phytosterols are established non-pharmacological agents that are useful adjuncts to a healthy diet for helping lower serum total and LDL-cholesterol, resulting in average reductions of 10% at the usually recommended doses of 2 g/d,107 an effect additive to that of statins.108 However, at doses up to 2 g/d, the effect of phytosterols is dose-related;107 doses as low as 400 mg/day, easily achieved with frequent intake of various seed products (Table 2), were shown to significantly influence cholesterol metabolism and reduce LDL-cholesterol by an average 5% in moderately hypercholesterolemic individuals.109 There is also consistent experimental evidence that phytosterols possess anti-inflammatory properties, though clinical studies have proven inconclusive.110
Minerals
Seeds contain little sodium but are rich in K+, Mg++, and Ca++. All three are critical in cellular metabolism and many physiologic processes, of which blood pressure regulation has been most studied.111 Whereas high sodium (salt) intake is associated with significantly increased risk of stroke and total CVD,112 intake of non-sodium minerals generally has the opposite effect. A meta-analysis of prospective studies relating dietary Ca++ intake with risk of CVD showed non-significant reductions of 8% for incident CHD and 14% for incident stroke comparing the highest to the lowest level of dietary Ca++ intake.113 Two similar meta-analyses have shown a significant inverse association between dietary K+ intake and risk of stroke, with a decrease in risk of 21% for every 1.64 g/d114 or 11% for every 1.0 g/d115 increase in dietary K+ intake. The latter dose of K+ can be obtained from combining seed servings in the usual diet (Table 2). The association of dietary K+ with lower CHD risk, however, is only suggestive.114 Concerning dietary Mg++ intake, a meta-analysis of prospective studies by Larsson et al.116 shows a modest but significant inverse association with stroke risk, with an 8% reduction for an increment of 100 mg/d in intake. Hypertension is the main risk factor for cerebrovascular disease; thus the stronger beneficial effect of reducing sodium and increasing non-sodium mineral intake from dietary sources on stroke risk than CHD risk is ascribable to reductions of blood pressure.111 Indeed, changes in dietary mineral intake like those derived from increased seed consumption are believed to be instrumental in the substantial blood pressure-lowering effect of the Dietary Approaches to Stop Hypertension (DASH) diet.117
Regarding dietary minerals and T2DM, limited evidence from prospective studies suggests that Ca++ intake relates inversely to risk.118 Observational studies more strongly support an independent protective role of Mg++ intake against T2DM, with a dose-response effect translating into a 14% risk reduction for each 100 mg/d increment in Mg++ intake.119 This dose of Mg++ is easily obtained with feasible daily servings of seeds and seed products (Table 2). Dietary K+ could also play a preventive role against T2DM, as suggested by findings from the NHS after a 6-year follow-up;120 to our knowledge, there have been no further studies examining this association. Increasing intake of non-sodium minerals influences the risk of T2DM through their critical participation in intracellular processes related to glucose homeostasis, such as a cofactor role for enzymes involved in glucose transport, enhanced insulin signaling, and modulation of glucose-induced insulin secretion, which all lead to increased insulin sensitivity.119,121 Besides blood pressure reduction,117 increased intake of K+, Mg++, and Ca++ in the DASH dietary pattern is also believed to underlie its effect of improving insulin sensitivity,122 an example of multi-purpose nutrient synergy.
Tocopherols
Among seeds, nuts are particularly rich in vitamin E compounds, mainly α-tocopherol, a well-known antioxidant that, together with vitamin C, carotenoids, and selenium, contributes to the body's defense against reactive oxygen species. α-Tocopherol, the biologically most active and most frequently studied form of vitamin E, is a lipid-soluble molecule transported in blood in lipoprotein fractions that acts as a peroxyl radical scavenger in lipid environments, preventing lipid peroxidation in lipoproteins and membranes. By reducing LDL oxidation or other mechanisms, vitamin E also displays anti-inflammatory properties, inhibits platelet aggregation, and enhances NO bioavailability.123 This helps explain the beneficial health effects of lifelong high intake of vitamin E. Thus, a large meta-analysis of 15 cohort studies reported that vitamin E intake was associated with a reduced CHD risk, with RR=0.76 (95% CI, 0.63-0.89) for the top tertile compared with the bottom tertile of intake.124 However, large-scale RCTs have shown an unexpected lack of efficacy for different doses of supplemental vitamin E or other antioxidants against CVD.125 A likely reason for the discrepancy between prospective studies and RCTs is that the latter usually enrolled patients with prior CHD or at high risk of CVD; established atherosclerotic damage is unlikely to regress with antioxidant treatment. Likewise, there is no evidence from RCTs that vitamin E helps prevent or manage T2DM.121 Hence, the use of vitamin E or other antioxidant vitamin supplements for CVD prevention is discouraged, while the recommendation stands for a healthy diet, rich in food sources of antioxidant nutrients such as fruits and vegetables, including seeds.22
Polyphenols
Phenolic compounds are strong antioxidants ubiquitous in plants. They are structurally diverse, belonging to several families such as benzoic acid derivatives, flavonoids, proanthocyanidins, stilbenes, coumarins, lignans, and lignins.41,126 Several thousand molecules with structural features of polyphenols (one or more phenolic rings with a variable number of hydroxyl groups) have been isolated from higher plants, and nearly 500 have been identified in various plant-based foods and beverages.42 Seeds, particularly nuts, pulses, and cocoa products, are particularly rich in polyphenols (Table 2), but they are present mostly in the outer layers and many are heat-labile. Thus peeling or roasting nuts, decortication of cereals, or dehulling of pulses results in sizeable losses, as do boiling, frying, and microwave heating.126,127
Polyphenols are strong antioxidants, but evidence is increasing of their pleiotropic effects, i.e., flavanols, a type of phenolic abundant in cocoa and black tea, increase the availability of NO, which may explain their favorable vascular effects.128 In an acute study in heart-transplant recipients, flavanol-rich chocolate induced coronary vasodilation and a decrease in platelet adhesion, concomitant with a reduction in measures of oxidative stress in comparison with a similar product devoid of flavanols.84 Some flavonoids have been shown to inhibit NF-kB, a critical pro-inflammatory protein that enhances the expression of cytokines and cell adhesion molecules.129 However, the precise molecular mechanisms underlying these beneficial effects remain elusive.
Mounting evidence from prospective studies indicates that higher intake of polyphenol-rich foods in general and of flavonoid-rich foods in particular is associated with protection from CVD, although potential publication bias and marked heterogeneity in results across studies43,130 render the evidence inconclusive. The abundance of polyphenols in dark chocolate and coffee is thought to be responsible for the CVD benefit observed in high consumers.52,53,57-60,80-83,86,87 A meta-analysis of 113 interventional studies of nearly 6000 subjects given different cocoa products or their pure phenolic extracts55 demonstrated improvement in various intermediate markers of cardiovascular health, including blood pressure, endothelial function, blood lipids, and insulin sensitivity. Several of the intervention studies reviewed55 were conducted with pure flavonoids and had effects similar to those elicited by the parent foods, which suggests that the benefit is at least partly due to these antioxidant molecules. However, no long-term RCTs have evaluated the effects of flavonoids or other phenolic compounds on clinical cardiovascular endpoints.
As shown in part in Figure, most bioactive seed components synergize to affect multiple metabolic and vascular physiology pathways leading to protection from CVD and T2DM. For the sake of simplicity, the scheme cannot include all the reported effects of particular seed molecules. For instance, besides their proven antioxidant, vascular, and antiplatelet effects, polyphenols have been shown to lower LDL-cholesterol and improve insulin sensitivity in RCTs.55 The complex bioactivity and multi-functionality of whole seed components supports their important role in a heart-healthy diet.
Implications for dietary recommendations
The current dietary strategies for CVD prevention focus primarily on the role of individual macronutrients (e.g., fat, carbohydrates, or protein). Although reduction in percentage of calories from dietary fat intake has been commonly recommended, long-term RCTs have provided no good evidence that reducing dietary fat per se can reduce CVD risk.1,7 Likewise, carbohydrate restriction alone is unlikely to have appreciable long-term impact on CVD. It is now recognized that over-focus on individual nutrients is counterproductive, because it misses the complexities of foods and overall eating patterns. A simpler and more “friendly” food-based dietary approach should be easier to communicate to the public and to implement in dietary practice. The past decade has seen an expansion of epidemiologic and clinical research on the roles of seeds in the prevention of CVD, revolutionizing our thinking about the biological mechanisms that link dietary factors and CVD. Some foods that were thought unhealthy on the basis of fat content (e.g., nuts) have become important in lowering serum cholesterol and controlling weight.68 The PREDIMED trial has convincingly demonstrated that a Mediterranean diet supplemented with nuts significantly reduces hard CVD endpoints.74 In addition, there is compelling evidence that increasing consumption of whole grains and legumes confers cardioprotective benefits. Therefore, dietary recommendations should encourage substitution of whole grains and legumes for refined grains as part of a healthy diet. Whole grains, nuts, and legumes (but not dark chocolate or coffee due to insufficient evidence at the time) were included in the American Heart Association report setting goals for health promotion and disease reduction for 2020.131 As reviewed here, current evidence suggests that moderate consumption of coffee and cocoa products can be incorporated into a healthy dietary pattern. The evidence base assembled in this review provides further support for the plant-based dietary patterns recommended by dietary guidelines.132
Conclusions
There is substantial evidence that increased consumption of seeds, including whole grains, nuts, legumes, cocoa products, and coffee, is associated with lower risk of CVD and T2DM or a significant reduction in CVD risk factors such as serum cholesterol or blood pressure. Seeds are made of complex matrices in the outer layer and the germ, rich in minerals (K+, Mg++, and Ca++, but little sodium), vitamins (particularly tocopherols and folic acid), phytosterols, polyphenols, and other bioactive phytochemicals, where, among other functions, they serve to protect the plant's DNA from oxidative stress and thus help perpetuate the species. On the other hand, the endosperm of seeds contains nutritive components to sustain the growth of the embryo, with a variable mixture of essential amino acids and fatty acids. While some seed components (soluble fiber, unsaturated fatty acids, phytosterols) have the potential to reduce blood cholesterol, the whole seed provides a wide array of bioactive molecules likely to have synergistic effects on health outcomes. However, separating the effects of different components of seeds in epidemiologic and clinical studies is exceedingly challenging. There is some evidence that a single component such as fiber may not be as beneficial when it is not consumed as a whole grain.133 Therefore, dietary recommendations should embrace a wide array of seeds as part of a plant-based dietary pattern, instead of putting too much emphasis on individual nutrients.
Acknowledgments
Funding Sources: Dr. Ros’ research is supported by grants PS09/01292 and PI12/01231 from Fondo de Investigaciones Sanitarias and CIBERobn, both from Instituto de Salud Carlos III, Spain. Dr. Hu's research is supported by NIH grant HL60712. CIBERobn is an initiative of Instituto de Salud Carlos III, Spain.
Footnotes
The two authors contributed to this work equally.
Conflict of Interest Disclosures: Dr. Ros and Dr. Hu have received grants from the California Walnut Commission, Sacramento, CA, and Dr. Ros is a non-paid member of its Scientific Advisory Committee.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA. 2002;288:2569–2578. doi: 10.1001/jama.288.20.2569. [DOI] [PubMed] [Google Scholar]
- 2.Keys A. Coronary heart disease in Seven Countries. Circulation. 1970;41(supl. 1):1–211. [PubMed] [Google Scholar]
- 3.Keys A, Anderson JT, Grande F. Prediction of serum-cholesterol responses of man to changes in fats in the diet. Lancet. 1957;2:959–966. doi: 10.1016/s0140-6736(57)91998-0. [DOI] [PubMed] [Google Scholar]
- 4.Hegsted DM, Ausman LM, Johnson JA, Dallal GE. Dietary fat and serum lipids: an evaluation of the experimental data. Am J Clin Nutr. 1993;57:875–883. doi: 10.1093/ajcn/57.6.875. [DOI] [PubMed] [Google Scholar]
- 5.Clarke R, Frost C, Collins R, Appleby P, Peto R. Dietary lipids and blood cholesterol: quantitative meta-analysis of metabolic ward studies. BMJ. 1997;314:112–117. doi: 10.1136/bmj.314.7074.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon T. The diet-heart idea: outline of a history. Am J Epidemiol. 1988;127:220–225. doi: 10.1093/oxfordjournals.aje.a114798. [DOI] [PubMed] [Google Scholar]
- 7.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, Kuller LH, LaCroix AZ, Langer RD, Lasser NL, Lewis CE, Limacher MC, Margolis KL, Mysiw WJ, Ockene JK, Parker LM, Perri MG, Phillips L, Prentice RL, Robbins J, Rossouw JE, Sarto GE, Schatz IJ, Snetselaar LG, Stevens VJ, Tinker LF, Trevisan M, Vitolins MZ, Anderson GL, Assaf AR, Bassford T, Beresford SA, Black HR, Brunner RL, Brzyski RG, Caan B, Chlebowski RT, Gass M, Granek I, Greenland P, Hays J, Heber D, Heiss G, Hendrix SL, Hubbell FA, Johnson KC, Kotchen JM. Low-fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 8.Willett WC, Leibel RL. Dietary fat is not a major determinant of body fat. Am J Med. 2002;113(Suppl):9B, 47S–59S. doi: 10.1016/s0002-9343(01)00992-5. [DOI] [PubMed] [Google Scholar]
- 9.Halton TL, Willett WC, Liu S, Hu FB, Franz M, Sampson L, Hennekens CH, Manson JE. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006;355:1991–2002. doi: 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- 10.Beulens JWJ, de Bruijne LM, Stolk RP, Peeters PH, Bots ML, Grobbee DE, van der Schouw YT. High dietary glycemic load and glycemic index increase risk of cardiovascular disease among middle-aged women: a population-based follow-up study. J Am Coll Cardiol. 2007;50:14–21. doi: 10.1016/j.jacc.2007.02.068. [DOI] [PubMed] [Google Scholar]
- 11.Jakobsen MU, Dethlefsen C, Joensen AM, Stegger J, Tjønneland A, Schmidt EB, Overvad K. Intake of carbohydrates compared with intake of saturated fatty acids and risk of myocardial infarction: importance of the glycemic index. Am J Clin Nutr. 2010;91:1764–1768. doi: 10.3945/ajcn.2009.29099. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287:2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 13.Halton TL, Liu S, Manson JE, Hu FB. Low-carbohydrate-diet score and risk of type 2 diabetes in women. Am J Clin Nutr. 2008;87:339–346. doi: 10.1093/ajcn/87.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69:243–249. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 16.Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72:912–921. doi: 10.1093/ajcn/72.4.912. [DOI] [PubMed] [Google Scholar]
- 17.Fung TT, Willett WC, Stampfer MJ, Manson JE, Hu FB. Dietary patterns and the risk of coronary heart disease in women. Arch Intern Med. 2001;161:1857–1862. doi: 10.1001/archinte.161.15.1857. [DOI] [PubMed] [Google Scholar]
- 18.Diehr P, Beresford SA. The relation of dietary patterns to future survival, health, and cardiovascular events in older adults. J Clin Epidemiol. 2003;56:1224–1235. doi: 10.1016/s0895-4356(03)00202-6. [DOI] [PubMed] [Google Scholar]
- 19.Nettleton JA, Polak JF, Tracy R, Burke GL, Jacobs DR., Jr Dietary patterns and incident cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2009;90:647–654. doi: 10.3945/ajcn.2009.27597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odegaard AO, Koh WP, Yuan JM, Gross MD, Pereira MA. Western-style fast food intake and cardiometabolic risk in an Eastern country. Circulation. 2012;126:182–188. doi: 10.1161/CIRCULATIONAHA.111.084004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salas-Salvadó J, Martinez-González MA, Bulló M, Ros E. The role of diet in the prevention of type 2 diabetes. Nutr Metab Cardiovasc Dis. 2011;21:B32–B48. doi: 10.1016/j.numecd.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J. Diet and Lifestyle Recommendations Revision 2006: A Scientific Statement From the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 23.Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92:1189–1196. doi: 10.3945/ajcn.2010.29673. [DOI] [PubMed] [Google Scholar]
- 24.Mente A, Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659–669. doi: 10.1001/archinternmed.2009.38. [DOI] [PubMed] [Google Scholar]
- 25.Eaton SB, Konner M. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med. 1985;312:283–289. doi: 10.1056/NEJM198501313120505. [DOI] [PubMed] [Google Scholar]
- 26.Cordain L, Miller JB, Eaton SB, Mann N, Holt SHA, Speth JD. Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. Am J Clin Nutr. 2000;71:682–692. doi: 10.1093/ajcn/71.3.682. [DOI] [PubMed] [Google Scholar]
- 27.Lee A. Transition of Australian Aboriginal diet and nutritional health. World Rev Nutr Diet. 1996;79:1–52. [PubMed] [Google Scholar]
- 28.Raven PH, Evert RF, Eichhorn SE. Biology of plants. 7th ed. WH Freeman and Co; New York, NY: 2005. [Google Scholar]
- 29.Hartmann MA. Plant sterols and the membrane environment. Trends Plant Sci. 1998;3:170–175. [Google Scholar]
- 30.Marquart L, Slavin JL, Fulcher RG, editors. Whole-grain foods in health and disease. American Association of Cereal Chemists; St Paul, MN: 2002. [Google Scholar]
- 31.Sabaté J, Salas-Salvadó J, Ros E. Nuts, nutrition and health outcomes. Br J Nutr. 2006;96(Suppl. 2):S1–S102. doi: 10.1017/bjn20061857. [DOI] [PubMed] [Google Scholar]
- 32.Ros E, Mataix J. Fatty acid composition of nuts. Implications for cardiovascular health. Br J Nutr. 2006;96(Suppl. 2):S29–S35. doi: 10.1017/bjn20061861. [DOI] [PubMed] [Google Scholar]
- 33.Rochefort S, Panozzo J. Phytochemicals for health. The role of pulses. J Agric Food Chem. 2007;55:7981–7994. doi: 10.1021/jf071704w. [DOI] [PubMed] [Google Scholar]
- 34.Bloom C. All about chocolate: the ultimate resource for the world's favorite food. Macmillan; New York, NY: 1998. [Google Scholar]
- 35. [20 September 2012]; http://ndb.nal.usda.gov/ndb/foods/list.
- 36. [26 September 2012]; http://www.fineli.fi.
- 37. [25 September 2012]; http://www.phenol-explorer.eu.
- 38.Brufau G, Boatella J, Rafecas M. Nuts: source of energy and macronutrients. Br J Nutr. 2006;96(Suppl. 2):S24–S28. doi: 10.1017/bjn20061860. [DOI] [PubMed] [Google Scholar]
- 39.Boye J, Zare F, Pletch A. Pulse proteins: processing, characterization, functional properties and applications in food and feed. Food Res Int. 2011;43:414–431. [Google Scholar]
- 40.Hunter JE, Zhang J, Kris-Etherton PM. Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: a systematic review. Am J Clin Nutr. 2010;91:46–63. doi: 10.3945/ajcn.2009.27661. [DOI] [PubMed] [Google Scholar]
- 41.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perez-Jimenez J, Neveu V, Vos F, Scalbert A. Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: an application of the phenol-explorer database. J Agric Food Chem. 2010;58:4959–4969. doi: 10.1021/jf100128b. [DOI] [PubMed] [Google Scholar]
- 43.Hollman PC, Cassidy A, Comte B, Heinonen M, Richelle M, Richling E, Serafini M, Scalbert A, Sies H, Vidry S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J Nutr. 2011;141:989S–1009S. doi: 10.3945/jn.110.131490. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs DR, Jr, Gross MD, Tapsell LC. Food synergy: an operational concept for understanding nutrition. Am J Clin Nutr. 2009;89(suppl):1543S–1548S. doi: 10.3945/ajcn.2009.26736B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye EQ, Chacko SA, Chou EL, Kugizaki M, Liu S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J Nutr. 2012;142:1304–1313. doi: 10.3945/jn.111.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Munter JS, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med. 2007;4:e261. doi: 10.1371/journal.pmed.0040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kris-Etherton PM, Hu F, Ros E, Sabaté J. The role of tree nuts and peanuts in the prevention of coronary heart disease: multiple potential mechanisms. J Nutr. 2008;138:1746S–1751S. doi: 10.1093/jn/138.9.1746S. [DOI] [PubMed] [Google Scholar]
- 48.Banel DK, Hu FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am J Clin Nutr. 2009;90:56–63. doi: 10.3945/ajcn.2009.27457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sabaté J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med. 2010;170:821–827. doi: 10.1001/archinternmed.2010.79. [DOI] [PubMed] [Google Scholar]
- 50.Bazzano LA, Thompson AM, Tees MT, Nguyen Ch, Winham DM. Non-soy legume consumption lowers cholesterol levels: A meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2011;21:94–103. doi: 10.1016/j.numecd.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sievenpiper JL, Kendall CW, Esfahani A, Wong JM, Carleton AJ, Jiang HY, Bazinet RP, Vidgen E, Jenkins DJ. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia. 2009;52:1479–1495. doi: 10.1007/s00125-009-1395-7. [DOI] [PubMed] [Google Scholar]
- 52.Buitrago-Lopez A, Sanderson J, Johnson L, Warnakula S, Wood A, Di Angelantonio E, Franco OH. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ. 2011;343:d4488. doi: 10.1136/bmj.d4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larsson SC, Virtamo J, Wolf A. Chocolate consumption and risk of stroke: A prospective cohort of men and meta-analysis. Neurology. 2012;79:1223–1229. doi: 10.1212/WNL.0b013e31826aacfa. [DOI] [PubMed] [Google Scholar]
- 54.Tokede OA, Gaziano JM, Djoussé L. Effects of cocoa products/dark chocolate on serum lipids: A meta-analysis. Eur J Clin Nutr. 2011;65:879–886. doi: 10.1038/ejcn.2011.64. [DOI] [PubMed] [Google Scholar]
- 55.Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr. 2012;95:740–751. doi: 10.3945/ajcn.111.023457. [DOI] [PubMed] [Google Scholar]
- 56.Ried K, Sullivan TR, Fakler P, Frank OR, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2012;8:CD008893. doi: 10.1002/14651858.CD008893.pub2. [DOI] [PubMed] [Google Scholar]
- 57.Wu JN, Ho SC, Zhou C, Ling WH, Chen WQ, Wang CL, Chen YM. Coffee consumption and risk of coronary heart diseases: a meta-analysis of 21 prospective cohort studies. Int J Cardiol. 2009;137:216–225. doi: 10.1016/j.ijcard.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 58.Larsson SC, Orsini N. Coffee consumption and risk of stroke: a dose-response meta-analysis of prospective studies. Am J Epidemiol. 2011;174:993–1001. doi: 10.1093/aje/kwr226. [DOI] [PubMed] [Google Scholar]
- 59.Mostofsky E, Rice MS, Levitan EB, Mittleman MA. Habitual coffee consumption and risk of heart failure: a dose-response meta-analysis. Circ Heart Fail. 2012;5:401–405. doi: 10.1161/CIRCHEARTFAILURE.112.967299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huxley R, Lee CM, Barzi F, Timmermeister L, Czernichow S, Perkovic V, Grobbee DE, Batty D, Woodward M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009;169:2053–2063. doi: 10.1001/archinternmed.2009.439. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Z, Hu G, Caballero B, Appel L, Chen L. Habitual coffee consumption and risk of hypertension: a systematic review and meta-analysis of prospective observational studies. Am J Clin Nutr. 2011;93:1212–1219. doi: 10.3945/ajcn.110.004044. [DOI] [PubMed] [Google Scholar]
- 62.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O'Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 63.Burkitt D, Trowell H. Refined carbohydrate foods and disease: some implications of dietary fibre. Academic Press; London: 1975. [Google Scholar]
- 64.Malik VS, Hu FB. Dietary prevention of atherosclerosis: go with whole grains. Am J Clin Nutr. 2007;85:1444–1445. doi: 10.1093/ajcn/85.6.1444. [DOI] [PubMed] [Google Scholar]
- 65.Mellen PB, Walsh TF, Herrington DM. Whole grain intake and cardiovascular disease: a meta-analysis. Nutr Metab Cardiovasc Dis. 2008;18:283–290. doi: 10.1016/j.numecd.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 66.van den Brandt PA. The impact of a Mediterranean diet and healthy lifestyle on premature mortality in men and women. Am J Clin Nutr. 2011;94:913–920. doi: 10.3945/ajcn.110.008250. [DOI] [PubMed] [Google Scholar]
- 67.Baer HJ, Glynn RJ, Hu FB, Hankinson SE, Willett WC, Colditz GA, Stampfer M, Rosner B. Risk factors for mortality in the Nurses’ Health Study: A competing risks Analysis. Am J Epidemiol. 2011;173:319–329. doi: 10.1093/aje/kwq368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ros E, Tapsell LC, Sabaté J. Nuts and berries for heart health. Cur Atheroscler Rep. 2010;12:397–406. doi: 10.1007/s11883-010-0132-5. [DOI] [PubMed] [Google Scholar]
- 69.Kendall CWC, Josse AR, Esfahani A, Jenkins DJA. Nuts, metabolic syndrome and diabetes. Br J Nutr. 2010;104:465–473. doi: 10.1017/S0007114510001546. [DOI] [PubMed] [Google Scholar]
- 70.Salas-Salvadó J, Bulló M, Babio N, Martínez-González MA, Ibarrola N, Basora J, Estruch R, Covas MI, Corella D, Arós F, Ros E, the PREDIMED Study investigators Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-REUS dietary intervention trial. Diabetes Care. 2011;34:14–19. doi: 10.2337/dc10-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Casas-Agustench P, López-Uriarte P, Ros E, Bulló M, Salas-Salvadó J. Nuts, hypertension and endothelial function. Nutr Metab Cardiovasc Dis. 2011;21:S21–S33. doi: 10.1016/j.numecd.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 72.Ros E. Nuts and novel biomarkers of cardiovascular disease. Am J Clin Nutr. 2009;89(suppl):1649S–1656S. doi: 10.3945/ajcn.2009.26736R. [DOI] [PubMed] [Google Scholar]
- 73.Salas-Salvadó J, Fernández-Ballart J, Ros E, Martínez-González MA, Fitó M, Estruch R, Corella D, Fiol M, Gómez-Gracia E, Arós F, Flores G, Lapetra J, Lamuela-Raventós R, Ruiz-Gutiérrez V, Bulló M, Covas MI, on behalf of the PREDIMED Study Investigators Effect of the Mediterranean diet supplemented with nuts on metabolic syndrome status. One-year results of the PREDIMED randomized trial. Arch Intern Med. 2008;168:2449–2458. doi: 10.1001/archinte.168.22.2449. [DOI] [PubMed] [Google Scholar]
- 74.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Martínez-González MA, the PREDIMED Study Investigators Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 75.Bazzano LA, He J, Ogden LG, Loria C, Vupputuri S, Myers L, Whelton PK. Legume consumption and risk of coronary heart disease in US men and women: NHANES I Epidemiologic Follow-up Study. Arch Intern Med. 2001;161:2573–2578. doi: 10.1001/archinte.161.21.2573. [DOI] [PubMed] [Google Scholar]
- 76.Kabagambe EK, Baylin A, Ruiz-Narvarez E, Siles X, Campos H. Decreased consumption of dried mature beans is positively associated with urbanization and nonfatal acute myocardial infarction. J Nutr. 2005;135:1770–1775. doi: 10.1093/jn/135.7.1770. [DOI] [PubMed] [Google Scholar]
- 77.Mattei J, Hu FB, Campos H. A higher ratio of beans to white rice is associated with lower cardiometabolic risk factors in Costa Rican adults. Am J Clin Nutr. 2011;94:869–786. doi: 10.3945/ajcn.111.013219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Papanikolaou Y, Fulgoni VL III. Bean consumption is associated with greater nutrient intake, reduced systolic blood pressure, lower body weight, and a smaller waist circumference in adults: Results from the National Health and Nutrition Examination Survey 1999-2002. J Am Coll Nutr. 2008;27:569–576. doi: 10.1080/07315724.2008.10719740. [DOI] [PubMed] [Google Scholar]
- 79.Hutchins AM, Winham DM, Thompson SV. Phaseolus beans: impact on glycaemic response and chronic disease risk in human subjects. Br J Nutr. 2012;108:S52–S65. doi: 10.1017/S0007114512000761. [DOI] [PubMed] [Google Scholar]
- 80.Buijsse B, Feskens EJ, Kok FJ, Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality: the Zutphen Elderly Study. Arch Intern Med. 2006;166:411–417. doi: 10.1001/archinte.166.4.411. [DOI] [PubMed] [Google Scholar]
- 81.Buijsse B, Weikert C, Drogan D, Bergmann M, Boeing H. Chocolate consumption in relation to blood pressure and risk of cardiovascular disease in German adults. Eur Heart J. 2010;31:1616–1623. doi: 10.1093/eurheartj/ehq068. [DOI] [PubMed] [Google Scholar]
- 82.Oba S, Nagata C, Nakamura K, Fujii K, Kawachi T, Takatsuka N, Shimizu H. Consumption of coffee, green tea, oolong tea, black tea, chocolate snacks and the caffeine content in relation to risk of diabetes in Japanese men and women. Br J Nutr. 2010;103:453–459. doi: 10.1017/S0007114509991966. [DOI] [PubMed] [Google Scholar]
- 83.Corti R, Flammer AJ, Hollenberg NK, Luscher TF. Cocoa and cardiovascular health. Circulation. 2009;119:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.827022. [DOI] [PubMed] [Google Scholar]
- 84.Flammer AJ, Hermann F, Sudano I, Spieker L, Hermann M, Cooper KA, Serafini M, Luscher TF, Ruschitzka F, Noll G, Corti R. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation. 2007;116:2376–2382. doi: 10.1161/CIRCULATIONAHA.107.713867. [DOI] [PubMed] [Google Scholar]
- 85.Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal. 2011;15:2779–2811. doi: 10.1089/ars.2010.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes: a systematic review. JAMA. 2005;294:97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]
- 87.van Dam RM. Coffee consumption and risk of type 2 diabetes, cardiovascular diseases, and cancer. Appl Physiol Nutr Metab. 2008;33:1269–1283. doi: 10.1139/H08-120. [DOI] [PubMed] [Google Scholar]
- 88.Mesas AE, Leon-Muñoz LM, Rodriguez-Artalejo F, Lopez-Garcia E. The effect of coffee on blood pressure and cardiovascular disease in hypertensive individuals: a systematic review and meta-analysis. Am J Clin Nutr. 2011;94:1113–1126. doi: 10.3945/ajcn.111.016667. [DOI] [PubMed] [Google Scholar]
- 89.Winkelmayer WC, Stampfer MJ, Willett WC, Curhan GC. Habitual caffeine intake and the risk of hypertension in women. JAMA. 2005;294:2330–2335. doi: 10.1001/jama.294.18.2330. [DOI] [PubMed] [Google Scholar]
- 90.Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R. Association of coffee drinking with total and cause-specific mortality. N Engl J Med. 2012;366:1891–1904. doi: 10.1056/NEJMoa1112010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lopez-Garcia E, van Dam RM, Li TY, Rodriguez-Artalejo F, Hu FB. The relationship of coffee consumption with mortality. Ann Intern Med. 2008;148:904–914. doi: 10.7326/0003-4819-148-12-200806170-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pan A, Chen M, Chowdhury R, Wu JHY, Sun Q, Campos H, Mozaffarian D, Hu FB. α-Linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr. 2012;96:1262–1273. doi: 10.3945/ajcn.112.044040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mensink RP, Zock PL, Kester ADM, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL-cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 94.Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009;48:44–51. doi: 10.1016/j.plipres.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jenkins DJ, Kendall CW, Axelsen M, Augustin LS, Vuksan V. Viscous and nonviscous fibres, nonabsorbable and low glycaemic index carbohydrates, blood lipids and coronary heart disease. Curr Opin Lipidol. 2000;11:49–56. doi: 10.1097/00041433-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 96.Pereira MA, O'Reilly E, Augustsson K, Fraser GE, Goldbourt U, Heitmann BL, Hallmans G, Knekt P, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Ascherio A. Dietary fiber and risk of coronary heart disease. A pooled analysis of cohort studies. Arch Intern Med. 2004;164:370–376. doi: 10.1001/archinte.164.4.370. [DOI] [PubMed] [Google Scholar]
- 97.Weickert MO, Pfeiffer AFH. Metabolic effects of dietary fiber consumption and prevention of diabetes. J Nutr. 2008;138:439–442. doi: 10.1093/jn/138.3.439. [DOI] [PubMed] [Google Scholar]
- 98.Keleman LE, Kushi L, Jacobs DR, Jr, Cerhan J. Associations of dietary protein with disease and mortality in a prospective study of postmenopausal women. Am J Epidemiol. 2005;161:239–249. doi: 10.1093/aje/kwi038. [DOI] [PubMed] [Google Scholar]
- 99.Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, Hu FB. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006;355:1991–2002. doi: 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- 100.Preis SR, Stampfer MJ, Spiegelman D, Willett WC, Rimm EB. Dietary protein and risk of ischemic heart disease in middle-aged men. Am J Clin Nutr. 2010;92:1265–1272. doi: 10.3945/ajcn.2010.29626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Altorf-van der Kuil W, Engberink MF, Brink EJ, van Baak MA, Bakker SJ, Navis G, van't Veer P, Geleijnse JM. Dietary protein and blood pressure: a systematic review. PLoS One. 2010;5:e12102. doi: 10.1371/journal.pone.0012102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, 3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, Charleston J, McCarron P, Bishop LM, the OmniHeart Collaborative Research Group Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 103.Moncada S, Higgs A. The L-arginine–nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 104.Gokce N. L-arginine and hypertension. J Nutr. 2004;134:2807S–2811S. doi: 10.1093/jn/134.10.2807S. [DOI] [PubMed] [Google Scholar]
- 105.Ros E, Núñez I, Pérez-Heras A, Serra M, Gilabert R, Casals E, Deulofeu R. A walnut diet improves endothelial function in hypercholesterolemic subjects. A randomized crossover trial. Circulation. 2004;109:1609–1614. doi: 10.1161/01.CIR.0000124477.91474.FF. [DOI] [PubMed] [Google Scholar]
- 106.Ostlund RE., Jr Phytosterols in human nutrition. Annu Rev Nutr. 2002;22:533–549. doi: 10.1146/annurev.nutr.22.020702.075220. [DOI] [PubMed] [Google Scholar]
- 107.Demonty I, Ras RT, van der Knaap HC, Guus SMJE, Duchateau GSMJE, Meijer L, Zock PL, Geleijnse JM, Trautwein EA. Continuous dose–response relationship of the LDL-cholesterol lowering effect of phytosterol intake. J Nutr. 2009;139:271–284. doi: 10.3945/jn.108.095125. [DOI] [PubMed] [Google Scholar]
- 108.Scholle JM, Baker WL, Talati R, Coleman CI. The effect of adding plant sterols or stanols to statin therapy in hypercholesterolemic patients: Systematic review and meta-analysis. J Am Coll Nutr. 2009;28:517–524. doi: 10.1080/07315724.2009.10719784. [DOI] [PubMed] [Google Scholar]
- 109.Racette SB, Lin X, Lefevre M, Spearie CA, Most MM, Ma L, Ostlund RE., Jr Dose effects of dietary phytosterols on cholesterol metabolism: a controlled feeding study. Am J Clin Nutr. 2010;91:32–38. doi: 10.3945/ajcn.2009.28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Othman RA, Moghadasian MH. Beyond cholesterol-lowering effects of plant sterols: clinical and experimental evidence of anti-inflammatory properties. Nutr Rev. 2011;69:371–382. doi: 10.1111/j.1753-4887.2011.00399.x. [DOI] [PubMed] [Google Scholar]
- 111.Karppanen H, Karppanen P, Mervaala E. Why and how to implement sodium, potassium, calcium, and magnesium changes in food items and diets? J Hum Hypertens. 2005;19:S10–S19. doi: 10.1038/sj.jhh.1001955. [DOI] [PubMed] [Google Scholar]
- 112.Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang L, Manson JE, Sesso HD. Calcium intake and risk of cardiovascular disease: a review of prospective studies and randomized clinical trials. Am J Cardiovasc Drugs. 2012;12:105–116. doi: 10.2165/11595400-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.D'Elia L, Barba G, Cappuccio FP, Strazzullo P. Potassium intake, stroke, and cardiovascular disease. A meta-analysis of prospective studies. J Am Coll Cardiol. 2011;57:1210–1219. doi: 10.1016/j.jacc.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 115.Larsson SC, Orsini N, Wolk A. Dietary potassium intake and risk of stroke. A dose–response meta-analysis of prospective studies. Stroke. 2011;42:2746–2750. doi: 10.1161/STROKEAHA.111.622142. [DOI] [PubMed] [Google Scholar]
- 116.Larsson SC, Orsini N, Wolk A. Dietary magnesium intake and risk of stroke: a meta-analysis of prospective studies. Am J Clin Nutr. 2012;95:362–366. doi: 10.3945/ajcn.111.022376. [DOI] [PubMed] [Google Scholar]
- 117.Akita S, Sacks FM, Svetkey LP, Conlin PR, Kimura G. DASH-Sodium Trial Collaborative Research Group. Effects of the Dietary Approaches to Stop Hypertension (DASH) diet on the pressure-natriuresis relationship. Hypertension. 2003;42:8–13. doi: 10.1161/01.HYP.0000074668.08704.6E. [DOI] [PubMed] [Google Scholar]
- 118.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and metaanalysis. J Clin Endocrinol Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dong JY, Xun P, He K, Qin LQ. Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Diabetes Care. 2011;34:2116–2122. doi: 10.2337/dc11-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Colditz GA, Manson JE, Stampfer MJ, Rosner B, Willett WC, Speizer FE. Diet and risk of clinical diabetes in women. Am J Clin Nutr. 1992;55:1018–1023. doi: 10.1093/ajcn/55.5.1018. [DOI] [PubMed] [Google Scholar]
- 121.Martini LA, Catania AS, Ferreira SR. Role of vitamins and minerals in prevention and management of type 2 diabetes mellitus. Nutr Rev. 2010;68:341–354. doi: 10.1111/j.1753-4887.2010.00296.x. [DOI] [PubMed] [Google Scholar]
- 122.Ard J D, Grambow SC, Liu D, Slentz CA, Kraus W E, Svetkey LP. The effect of the PREMIER intervention on insulin sensitivity. Diabetes Care. 2004;27:340–347. doi: 10.2337/diacare.27.2.340. [DOI] [PubMed] [Google Scholar]
- 123.Traber MG. Vitamin E regulatory mechanisms. Annu Rev Nutr. 2007;27:347–362. doi: 10.1146/annurev.nutr.27.061406.093819. [DOI] [PubMed] [Google Scholar]
- 124.Ye Z, Song H. Antioxidant vitamins intake and the risk of coronary heart disease: meta-analysis of cohort studies. Eur J Cardiovasc Prev Rehabil. 2008;15:26–34. doi: 10.1097/HJR.0b013e3282f11f95. [DOI] [PubMed] [Google Scholar]
- 125.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361:2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 126.Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 127.Vinson JA, Cai Y. Nuts, especially walnuts, have both antioxidant quantity and efficacy and exhibit significant potential health benefits. Food Funct. 2012;3:134–140. doi: 10.1039/c2fo10152a. [DOI] [PubMed] [Google Scholar]
- 128.Schewe T, Steffen Y, Sies H. How do dietary flavanols improve vascular function? A position paper. Arch Biochem Biophys. 2008;476:102–106. doi: 10.1016/j.abb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 129.Sies H. Polyphenols and health: update and perspectives. Arch Biochem Biophys. 2010;501:2–5. doi: 10.1016/j.abb.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 130.Heiss C, Carl L, Keen CL, Kelm M. Flavanols and cardiovascular disease prevention. Eur Heart J. 2010;31:2583–2592. doi: 10.1093/eurheartj/ehq332. [DOI] [PubMed] [Google Scholar]
- 131.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW. Rosamond WD; American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 132.U.S. Department of Health and Human Services. U. S. Department of Agriculture . Dietary guidelines for Americans, 2010. 7th ed. U.S. Government Printing Office; Washington, DC: 2010. [Google Scholar]
- 133.Flight I, Clifton P. Cereal grains and legumes in the prevention of coronary heart disease and stroke: a review of the literature. Eur J Clin Nutr. 2006;60:1145–1159. doi: 10.1038/sj.ejcn.1602435. [DOI] [PubMed] [Google Scholar]