Summary
The ANHL01P1 trial was undertaken to determine pharmacokinetics and safety following the addition of rituximab to French-American-British/ /Lymphome Malins de Burkitt (FAB/LMB96) chemotherapy in 41 children and adolescents with Stage III/IV mature B-cell lymphoma/leukaemia. Patients received rituximab (375mg/m2) days -2 and 0 of two induction cycles and day 0 of two consolidation cycles. Highest peak levels were achieved following the second dose of each induction cycle (299±19 and 384±25 µg/ml (Group-B); 245±31 and 321±32 µg/ml (Group-C)) with sustained troughs and t½ of 26–29 days. Rituximab can be safely added to FAB chemotherapy with high early rituximab peak/trough levels and a long t½..
Keywords: non-Hodgkin lymphoma, rituximab, pharmacokinetics, pediatric, CD20
Introduction
The introduction of the anti-CD20 monoclonal antibody rituximab significantly improved outcomes in adult patients with aggressive B-cell non-Hodgkin lymphoma (B-NHL) (Coiffier, et al 2002). Children with B-NHL generally present with aggressive disease, nearly uniformly expressing CD20 (Perkins, et al 2003). The efficacy of rituximab in paediatric B-NHL continues to be investigated in clinical trials (Goldman, et al 2012, Meinhardt, et al 2010). Children and adolescents with B-NHL frequently present with advanced stage disease and high tumour burden. Pre-clinical in vivo modelling and clinical data in adults suggests that tumour burden has an inverse relationship with serum rituximab concentration, suggesting “dose dense” rituximab dosing may be beneficial to better saturate CD20 receptors in high tumour burden states (Dayde, et al 2009, Jager, et al 2012). The Children’s Oncology Group (COG) ANHL01P1 (Rituximab, rasburicase, and combination chemotherapy in treating young patients with newly diagnosed advanced B-cell leukaemia or lymphoma) trial investigated the safety and pharmacokinetics of adding dose-dense rituximab to chemotherapy in children and adolescents with newly-diagnosed B-NHL. The results contained in this report represent the single largest cohort of rituximab pharmacokinetics data in children with mature B-NHL to date.
Methods
General
The ANHL01P1 pilot trial investigated the addition of rituximab to a French, American, British (FAB)/lymphoma malignancy B-cell (LMB) 96 chemotherapy backbone in children and adolescents with newly diagnosed B-NHL. The trial was open to all COG centres in the United States, Canada, Australia and New Zealand. The protocol was approved by each respective institutional review board (IRB). Parents or patients over 18 years of age signed an IRB-approved informed consent before study enrollment. Patients were stratified as intermediate-risk Group-B or high-risk Group-C, as previously described (Cairo, et al 2007, Cairo, et al 2012, Patte, et al 2007). An initial sub-pilot opened in June, 2004 in which rituximab was not initiated until the second induction cycle. The pilot portion of the study opened in September, 2005 and had a planned final closure in October, 2006. The COG independent Data and Safety Monitoring Committee reviewed safety reports and interim analyses every 6 months.
Eligibility
Patients under 30 years of age with newly-diagnosed de-novo mature B-NHL classified by the Revised European-American Lymphoma (REAL) criteria, including diffuse large B-cell lymphoma, primary mediastinal large B-cell lymphoma, Burkitt lymphoma, and high-grade B-cell Burkitt-like lymphoma, were eligible. Patients with St. Jude Stages III/IV were eligible. CD20 positive immunohistochemistry was required. Pathology was centrally reviewed. Central nervous system disease was defined as any cerebral spinal fluid blasts on diagnostic lumbar puncture and/or isolated intracerebral mass, cranial nerve palsy, clinical spinal cord compression and parameningeal extension. Patients with known congenital or acquired immunodeficiency or prior organ transplant were ineligible. Carriers of hepatitis B were eligible, but carefully monitored for reactivation. Bilateral bone marrow aspirate and diagnostic lumbar puncture were required prior to study entry. Anatomic imaging (computerized tomography and/or ultrasound) was required at diagnosis.
Treatment
Chemotherapy
The chemotherapy backbones for Group-B and C patients were similar to those reported for the B4 and C1 arms of the FAB/LMB96 trial, respectively (Cairo, et al 2007, Cairo, et al 2012, Patte, et al 2007). The FAB/LMB96 trial initially employed a 48-h infusion of doxorubicin during each induction cycle, but was amended midway to reduce the infusion time to 6 h due to unacceptable rates of grade III/IV mucositis (Patte, et al 2007). The current trial empirically reduced the doxorubicin infusion time to 30–60 min.
Immunotherapy
Rituximab was administered at the standard dose of 375 mg/m2. Patients were pre-medicated with acetaminophen and diphenhydramine prior to each dose. Rituximab, supplied by Genentech through the Cancer Therapy Evaluation Program, National Cancer Institute, was diluted in normal saline at a concentration of 1 mg/ml. The first infusion of rituximab utilized a rate of 0.5 mg/kg/h for the first hour with gradually increased infusion rate (every 30 min) by patient tolerance. Blood pressure, pulse, respiratory rate and temperature were monitored every 15 min. If tolerated, subsequent infusions were begun at a rate of 1 mg/kg/h. If infusion-related events occurred, the infusion was temporarily slowed or stopped and the subsequent rate was halved, then increased every 30 min as tolerated. During cyclophosphamide, vincristine, prednisone, adriamycin and methotrexate (COPADM) induction cycles, rituximab was administered 48 h prior (day –2) and repeated on the day of chemotherapy administration (day 0). During consolidation cycles (cytarabine/high dose methotrexate [CYM] or cytarabine/etoposide [CYVE]), rituximab was administered just prior to chemotherapy administration (day 0). In the initial sub-pilot, rituximab administration began with the second induction cycle (4 total doses). In the pilot study, rituximab was given beginning the first induction cycle (6 total doses).
Rituximab pharmacokinetics
Rituximab levels were measured prior to any antibody infusion, during COPADM1 (pilot only) and COPADM2 (pilot and sub-pilot) induction cycles (peak 30 min prior to dose and trough 1–2 h following dose) and following consolidation cycles (1, 3, 6 and 9 months after completion of the last dose). Rituximab was measured by enzyme-linked immunoassay with polyclonal goat anti-rituximab antibody as the capture reagent and goat anti-mouse IgG-conjugated to horseradish peroxidase as the detection reagent (detection limit of 0.5 µg/ml)(Maloney, et al 1997). Rituximab terminal half-life (t½) was calculated if at least 3 time points after the last dose were measurable in an individual subject.
Statistics
Rituximab levels are expressed as means and standard errors (SE) at each of the time points measured. Rituximab levels were compared between Group-B and Group-C patients and between patients with normal or elevated (≥2 × upper limit of normal [ULN]) lactate dehydrogenase (LDH) at selected time points during induction with t-tests, using the Holm method to adjust p-values for multiple comparisons. P-values less than 0.05 were considered to be statistically significant.
Results
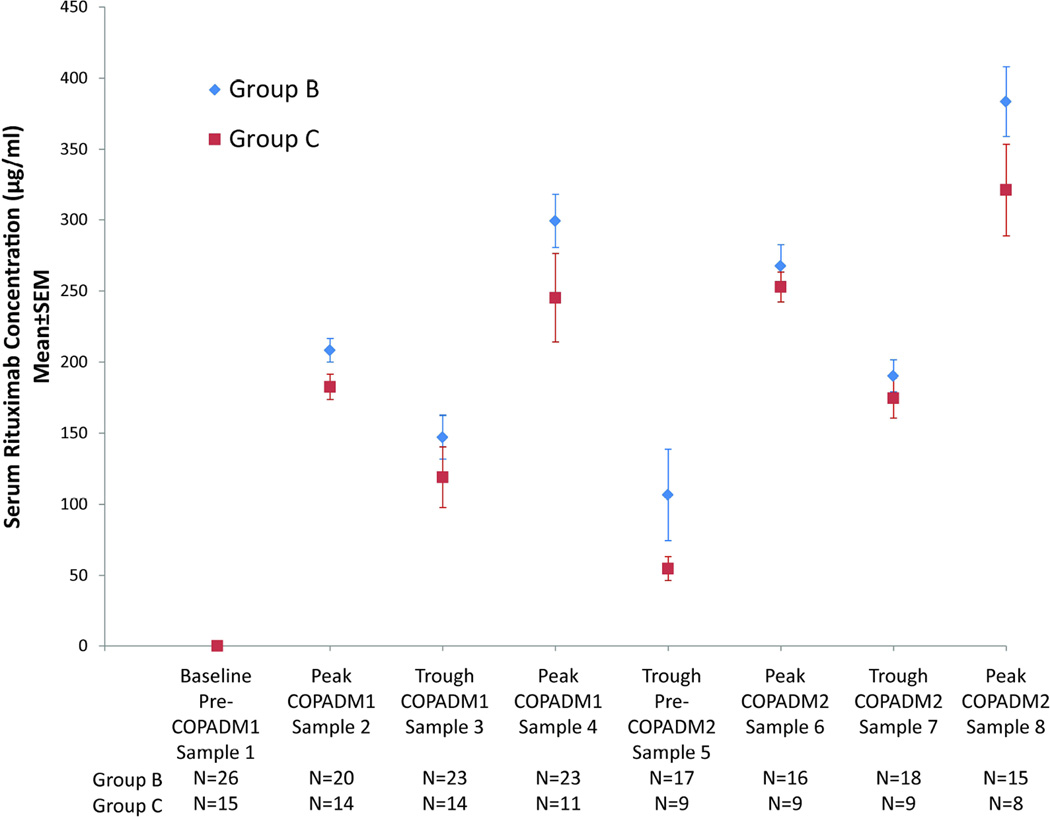
We previously reported the safety and efficacy of rituximab with FAB96 Group-B and Group-C chemotherapy (Cairo, et al 2010, Goldman, et al 2012). There were 26 Group-B and 15 Group-C patients with evaluable rituximab levels assayed. On average, within each induction cycle, the highest peak rituximab concentration was reached following the second infusion, with peak rituximab levels following the second dose in Induction 1 and 2 of 299±19 and 384±25 µg/ml (Group-B) and 245±31 and 321±32 µg/ml (Group-C) (Fig. 1A).
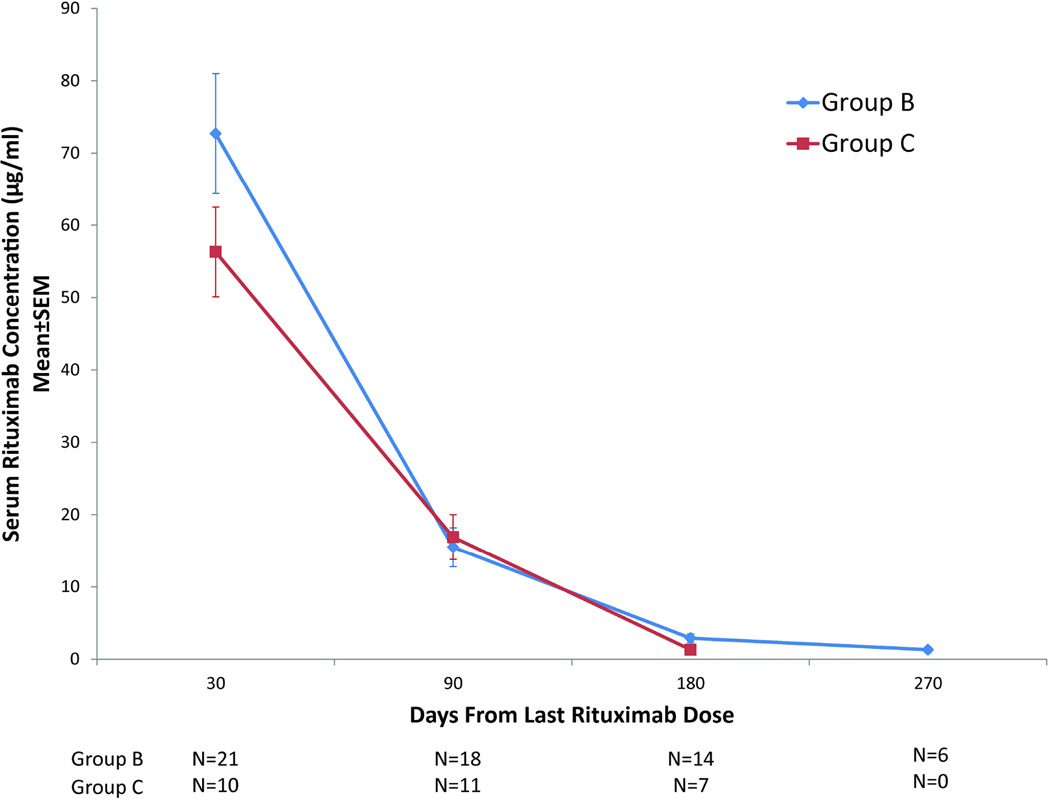
Figure 1. Serum rituximab concentrations in patients receiving chemoimmunotherapy.
(A) Graphical representation of mean ± standard error of the mean (SEM) serum rituximab concentration during induction cycles 1 (COPADM1) and 2 (COPADM2) in Group-B and Group-C patients. (B) Graphical representation of mean ± SEM serum rituximab concentrations from the last administered rituximab dose in Group-B and Group-C patients. COPADM: cyclophosphamide, vincristine, prednisone, adriamycin and methotrexate
Rituximab was detectable at each trough time point in all samples assayed. Three weeks after the first induction cycle, patients had sustained trough levels of 107±32 µg/ml (Group-B) and 55±8 µg/ml (Group-C). Eleven Group-B and 4 Group-C subjects had at least 3 evaluable samples after the last dose of rituximab for determination of rituximab t½. The mean±standard error t½ was 29±7 days for Group-B and 26±3 days for Group-C subjects (Fig. 1B). The median time to undetectable serum rituximab concentration was 9 months after the last dose of rituximab.
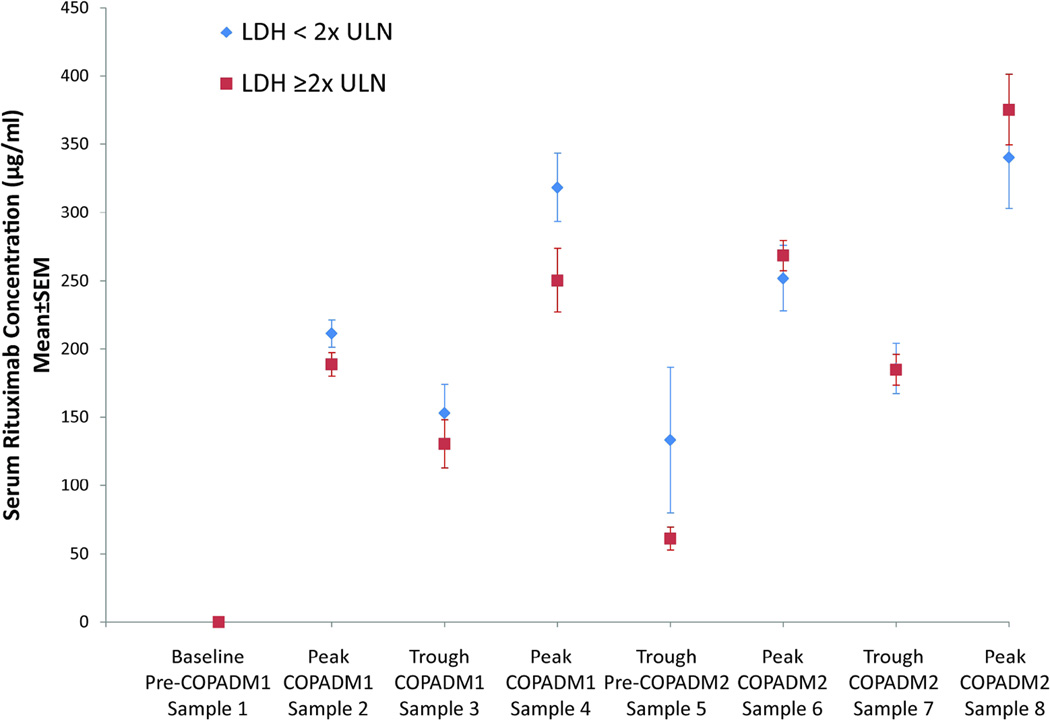
Group-C patients demonstrated lower peak and trough rituximab levels than Group-B patients. A graphical analysis was performed to examine the potential dependency of age and pre-treatment LDH levels on the above pharmacokinetics parameters. Patients with a pre-treatment LDH ≥2xULN were noted to have lower peak and trough levels during Induction 1 and 3 weeks following the last Induction 1 rituximab dose (Fig. 2A). Group-B children (<13 years) tended to exhibit higher peak concentrations, similar trough levels and shorter t½ than adolescents (≥13 years) (Fig. 2B).
Figure 2. Variability in serum rituximab concentrations based on patient age and lactate dehydrogenase.
(A) Graphical representation of mean ± standard error of the mean (SEM) serum rituximab concentration during induction cycles 1 (COPADM1) and 2 (COPADM2) with patients stratified by lactate dehydrogenase (LDH) < or ≥2 × upper limit of normal (ULN). (B) Graphical representation of mean ± SEM serum rituximab concentration during induction cycles 1 (COPADM1) and 2 (COPADM2) with patients stratified by age < vs. ≥13 years.
Discussion
Similar to adult studies demonstrating mean peak rituximab levels in the range of 90–250 µg/ml after a dose of 375 mg/m2, the pharmacokinetic analysis demonstrated increasing rituximab levels following serial rituximab infusions with the highest peak levels achieved after the second of two doses in each cycle and a median terminal rituximab t½ of 29 and 26 days for Group-B and –C, respectively (Tobinai, et al 1998). Two prior rituximab pharmacokinetics studies in paediatric patients with non-malignant disorders noted an age-dependent difference in rituximab pharmacokinetics (Bennett, et al 2006, Pranzatelli, et al 2010). In comparison, we had too few patients to demonstrate a significant age-dependent difference in rituximab pharmacokinetics. However, younger children in Group-B had non-significantly higher peak levels than adolescents, probably due to dosing rituximab by surface area, which administers a higher per kilogram dose to younger children. This, however, is balanced by a higher rate of clearance in children thereby resulting in similar trough values. Despite higher peak rituximab levels, no added toxicity was encountered in younger patients on the study (Goldman, et al 2012). There were not enough patients in the age ≥13 years category with pharmacokinetics data to compare by age within Group-C. Group-C patients demonstrated a trend toward lower rituximab levels, compared to Group-B. High pre-treatment LDH patients were also noted to trend (non-significantly) toward lower rituximab levels during the first induction cycle compared to patients with low LDH. These findings may be related to increased tumour burden in patients with more advanced stage disease. However, there were too few patients to reach statistical significance. Tumour burden was inversely correlated with serum rituximab concentrations and response in a recently reported murine model (Dayde, et al 2009). Similar findings have been reported in the clinical setting in adults with low-grade lymphomas treated with rituximab, where variability in serum rituximab concentrations were associated with tumour burden and therapeutic response (Jager, et al 2012).Overall, high early peak (245–354 µg/ml) rituximab levels were achieved with sustained troughs (54–190 µg/ml) prior to subsequent chemotherapy cycles utilizing this dose-dense approach in paediatric patients with advanced mature B-NHL. Despite a trend towards higher peak values, increased rituximab clearance in children without excess toxicity supports the continued use of body surface area-based rituximab dosing.
Acknowledgements
Funding was provided by The Division of Cancer Treatment, National Cancer Institute, and National Institutes of Health, Department of Health and Human Services (COG) (CA98543-09 and CA98413-09), Pediatric Cancer Research Foundation and the Doris Duke Charitable Foundation
Footnotes
Author contributions: MJB analysed the results and wrote the paper; SG designed and performed the research, analysed the results and wrote the paper; LS analysed the data and wrote the paper; SP analysed the data and critically reviewed the paper; BS analysed the data and critically reviewed the paper; TGG analysed the data and critically reviewed the paper; WS analysed the data and critically reviewed the paper; LH performed the research, analysed the data and wrote the paper; MG analysed the data; and LG-R analysed the data and critically reviewed the paper; and MSC designed and performed the research, analysed the results and wrote the paper.
Competing interests: T.G. has been a consultant in the last 12 months for Genentech/Roche, Boehringer Ingelheim Pharma GmbH & Co., and Janssen Biotech, Inc.
All other authors declare no conflict of interest.
References
- Bennett CM, Rogers ZR, Kinnamon DD, Bussel JB, Mahoney DH, Abshire TC, Sawaf H, Moore TB, Loh ML, Glader BE, McCarthy MC, Mueller BU, Olson TA, Lorenzana AN, Mentzer WC, Buchanan GR, Feldman HA, Neufeld EJ. Prospective phase 1/2 study of rituximab in childhood and adolescent chronic immune thrombocytopenic purpura. Blood. 2006;107:2639–2642. doi: 10.1182/blood-2005-08-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo M, Lynch J, Harrison L, Perkins S, Shiramizu B, Gross T, Sanger W, Goldman S. Safety, kinetics, and outcome following rituximab (R) in combination with FAB chemotherapy in children and adolescents (C+A) with stage III/IV (Group B) and BM+/CNS+ (Group C) mature B-NHL: A Children's Oncology Group report. J Clin Oncol. 2010;28(suppl) (abstract 9536) [Google Scholar]
- Cairo MS, Gerrard M, Sposto R, Auperin A, Pinkerton CR, Michon J, Weston C, Perkins SL, Raphael M, McCarthy K, Patte C. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007;109:2736–2743. doi: 10.1182/blood-2006-07-036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo MS, Sposto R, Gerrard M, Auperin A, Goldman SC, Harrison L, Pinkerton R, Raphael M, McCarthy K, Perkins SL, Patte C. Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (>/= 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature B-cell non-Hodgkin's lymphoma: results of the FAB LMB 96 study. J Clin Oncol. 2012;30:387–393. doi: 10.1200/JCO.2010.33.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- Dayde D, Ternant D, Ohresser M, Lerondel S, Pesnel S, Watier H, Le Pape A, Bardos P, Paintaud G, Cartron G. Tumor burden influences exposure and response to rituximab: pharmacokinetic-pharmacodynamic modeling using a syngeneic bioluminescent murine model expressing human CD20. Blood. 2009;113:3765–3772. doi: 10.1182/blood-2008-08-175125. [DOI] [PubMed] [Google Scholar]
- Goldman S, Smith L, Anderson JR, Perkins S, Harrison L, Geyer MB, Gross TG, Weinstein H, Bergeron S, Shiramizu B, Sanger W, Barth M, Zhi J, Cairo MS. Rituximab and FAB/LMB 96 chemotherapy in children with Stage III/IV B-cell non-Hodgkin lymphoma: a Children's Oncology Group report. Leukemia. 2012;27:1174–1177. doi: 10.1038/leu.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager U, Fridrik M, Zeitlinger M, Heintel D, Hopfinger G, Burgstaller S, Mannhalter C, Oberaigner W, Porpaczy E, Skrabs C, Einberger C, Drach J, Raderer M, Gaiger A, Putman M, Greil R. Rituximab serum concentrations during immuno-chemotherapy of follicular lymphoma correlate with patient gender, bone marrow infiltration and clinical response. Haematologica. 2012;97:1431–1438. doi: 10.3324/haematol.2011.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, Janakiraman N, Foon KA, Liles TM, Dallaire BK, Wey K, Royston I, Davis T, Levy R. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood. 1997;90:2188–2195. [PubMed] [Google Scholar]
- Meinhardt A, Burkhardt B, Zimmermann M, Borkhardt A, Kontny U, Klingebiel T, Berthold F, Janka-Schaub G, Klein C, Kabickova E, Klapper W, Attarbaschi A, Schrappe M, Reiter A. Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin's lymphoma and Burkitt leukemia. J Clin Oncol. 2010;28:3115–3121. doi: 10.1200/JCO.2009.26.6791. [DOI] [PubMed] [Google Scholar]
- Patte C, Auperin A, Gerrard M, Michon J, Pinkerton R, Sposto R, Weston C, Raphael M, Perkins SL, McCarthy K, Cairo MS. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood. 2007;109:2773–2780. doi: 10.1182/blood-2006-07-036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins SL, Lones MA, Davenport V, Cairo MS. B-Cell non-Hodgkin's lymphoma in children and adolescents: surface antigen expression and clinical implications for future targeted bioimmune therapy: a children's cancer group report. Clin Adv Hematol Oncol. 2003;1:314–317. [PubMed] [Google Scholar]
- Pranzatelli MR, Tate ED, Verhulst SJ, Bertolone SJ, Bhatla D, Granger M, Lebowizc J, Lockhart SK, Wiley JM. Pediatric dosing of rituximab revisited: serum concentrations in opsoclonus-myoclonus syndrome. J Pediatr Hematol Oncol. 2010;32:e167–e172. doi: 10.1097/MPH.0b013e3181cf0726. [DOI] [PubMed] [Google Scholar]
- Tobinai K, Kobayashi Y, Narabayashi M, Ogura M, Kagami Y, Morishima Y, Ohtsu T, Igarashi T, Sasaki Y, Kinoshita T, Murate T. Feasibility and pharmacokinetic study of a chimeric anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab) in relapsed B-cell lymphoma. The IDEC-C2B8 Study Group. Ann Oncol. 1998;9:527–534. doi: 10.1023/a:1008265313133. [DOI] [PubMed] [Google Scholar]