Abstract
Background
The lymphatic vascular system regulates tissue fluid homeostasis and plays important roles in immune surveillance, inflammation and cancer metastasis. However, the molecular mechanisms involved in the regulation of lymphangiogenesis remain incompletely characterized. Objective: We aimed to identify new pathways involved in the promotion of skin lymphangiogenesis.
Methods
We used a mouse embryonic stem cell-derived embryoid body vascular differentiation assay to investigate the effects of a selection of pharmacological agents with the potential to inhibit blood and/or lymphatic vessel formation. We also used a subcutaneous Matrigel assay to study candidate lymphangiogenesis factors as well as skin-specific transgenic mice.
Results
We found that compounds inhibiting the epidermal growth factor (EGF) receptor (EGFR) led to an impaired formation of lymphatic vessel-like structures. In vitro studies with human dermal lymphatic endothelial cells (LECs), that were found to express EGFR, revealed that EGF promotes lymphatic vessel formation. This effect was inhibited by an EGFR-blocking antibody and by low molecular weight inhibitors of either the EGFR or its associated tyrosine kinase. Incorporation of EGF into a mouse matrigel plug assay showed that EGF promotes enlargement of lymphatic vessels in the skin in vivo. Moreover, transgenic mice with skin-specific overexpression of amphiregulin, another agonistic ligand of the EGFR, displayed an enhanced size and density of lymphatic vessels in the skin.
Conclusion
These findings reveal that EGFR activation is involved in lymphatic remodeling and suggest that specific EGFR antagonists might be used to inhibit pathological lymphangiogenesis.
Introduction
The lymphatic vascular system plays an essential role in physiological fluid homeostasis. It is also involved in several pathological conditions, including inflammation and cancer metastasis [1]. In recent years, our understanding of how lymphatic endothelial cell (LEC) differentiation, growth and function are regulated has significantly increased [1]. This progress became possible based on the discovery of lymphatic endothelium-specific markers, namely podoplanin [2] and lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) [3], and on studies of lymphatic system development in genetic mouse models [1].
Vascular endothelial growth factor-C (VEGF-C) is the best characterized lymphangiogenic factor and predominantly activates VEGF receptor (VEGFR)-3. Under normal conditions, VEGFR-3 is expressed by LECs but not by the endothelial cells of blood vessels. Activation of VEGFR-3 promotes LEC proliferation and migration in vitro [4] and lymphatic vessel formation in vivo [5]. Lymphangiogenesis is also stimulated by VEGF-A [6–8]. Additional growth factors including fibroblast growth factor-2, hepatocyte growth factor, angiopoietin-1 and -2, and platelet-derived growth factor, have been shown to promote lymphangiogenic processes [9]. Because of the emerging role of the lymphatic vascular system in human diseases such as cancer metastasis, chronic inflammation, organ transplant rejection and hypertension [1], understanding and modulating lymphangiogenesis is of primary interest. The present study was aimed at unraveling novel mechanisms involved in the regulation of lymphatic vessel formation.
Materials and methods
Mouse embryonic stem cell culture, establishment and treatment of embryoid bodies (EBs)
Murine C57BL/6×129SvEv derived embryonic stem cells (mES cells; passage 3–12; kindly provided by N. Gale, Regeneron Pharmaceuticals, Tarrytown, NY, USA), were cultured on mitotically inactivated primary mouse embryonic fibroblasts (PMEFs, passage 2–5, Institute of Laboratory Animal Science, University of Zurich, Switzerland) in Dulbecco’s modified Eagle medium (Gibco, Eggenstein, Germany), supplemented with 18% fetal bovine serum (FBS; Gibco), 100 nM sodium pyruvate (Sigma-Aldrich, Buchs, Switzerland), MEM vitamins, 2 mM L-glutamine, streptomycin and penicillin (all from Gibco), 10 mM 2-mercaptoethanol and 2000 U/ml recombinant leukemia inhibitory factor (LIF; Chemicon International, Temecula, CA, USA). PMEFs and LIF were removed and mES cells were transferred to suspension culture for embryoid body (EB) formation as described [10, 11]. After 3 or 4 days, EBs of similar size were transferred into 12-well dishes (BD Bioscience, San Diego, CA, USA). This step is termed “initiation of the EBs” throughout the text. The EBs were cultured for 14 days and then incubated for 4 days with either 100 ng/ml human recombinant epidermal growth factor (EGF, BD Biosciences) or a mixture of 10 μM all-trans-retinoic acid (RA; Sigma-Aldrich), 0.5 mM 3′,5′-cyclic monophosphate (cAMP; Fluka, Buchs, Switzerland), and 200 ng/ml recombinant human VEGF-C (R&D Systems, Minneapolis, MN, USA). These agents were used alone or in combination with one of the following pharmacological agents (all from Sigma-Aldrich) added at 10 μM concentrations: 5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one (genistein); N4-(1-benzyl-1H-indazol-5-yl)-N6,N6-dimethyl-pyrido[3,4-d]pyrimidine-4,6-diamine (GW2974); 3-(2,4-dimethylpyrrol-5-yl)-methylidene-indolin-2-one (SU5416). Medium only and medium containing 0.1% dimethyl sulfoxide (DMSO) were used as negative vehicle controls. EBs were fixed in 20°C cold methanol for 10 minutes prior to analysis.
Immunofluorescence analysis of vessel development in EBs
EBs (n=9 per group) were stained with antibodies against mouse LYVE-1 (Angiobio, Del Mar, CA and R&D Systems), CD31 (BD Bioscience), and secondary antibodies labelled with Alexa-Fluor 488 or 594 (Molecular Probes, Eugene, OR, USA) as described [11]. Cell nuclei were stained with Hoechst bisbenzimide (Sigma-Aldrich). The stained samples were examined with a Zeiss Axiovert 200M microscope, images were captured with a Zeiss AxioCam-MRm (Carl Zeiss; Oberkochen, Germany) and the Axio Vision4.4 software (Zeiss). Adobe Photoshop CS3 (Adobe Systems, San Jose, CA, USA) was used for image overlay. Computer-assisted morphometric vessel analyses were performed using the IP-LAB software (Scanalytics; Fairfax, VA, USA). The lymphatic vessel area was determined relative to the total EB area. The vessel number per EB was quantified by manually counting all independent CD31+/LYVE-1+ structures present in an EB. Statistical analysis (unpaired Student’s t-test) was performed using Microsoft Excel 2003.
Proliferation, migration and tube formation of human lymphatic endothelial cells
Human dermal microvascular LECs were isolated from neonatal human foreskins by immunomagnetic purification as described [12]. Cells were cultured in endothelial basal medium (Cambrex, Verviers, Belgium) with 20% FBS, antibiotic antimycotic solution, L-glutamine (2 mmol/L), hydrocortisone (10 μg/ml), and N6,2′-O-dibutyryladenosine-3′,5′-cyclicmonophosphate sodium salt (25 μg/ml; all from Fluka, Buchs, Switzerland) at 37°C and 5% CO2 for up to 11 passages.
Cells were incubated with either 10 μM GW2974 (Sigma-Aldrich) or a vehicle control containing the same amount of DMSO. Recombinant human VEGF-A165 (20 ng/ml; kindly provided by the National Cancer Institute, Bethesda, MD, USA), or Pichia Pastoris-derived human VEGF-C (1.2 μg/ml; kindly provided by Dr. K. Ballmer-Hofer, Paul Scherrer Institute, Switzerland) were used as positive controls. The relatively high amounts of VEGF-C used are based on the fact that N-glycans added by Pichia Pastoris to proteins are of the oligomannose-type. Such glycans are bound by the mannose receptor expressed on lymphatic endothelial cells, likely leading to scavenging and endocytosis of VEGF-C and thus a lower availability of VEGF-C for binding to its receptor. In additional experiments, LECs were treated with 100 ng/ml human EGF with or without 10 μg/ml of recombinant humanized monoclonal antibody 2C4 (Pertuzumab, kindly provided by Roche Diagnostics GmbH, Mannheim, Germany), an EGFR blocking antibody (Calbiochem, Darmstadt, Germany) or mouse IgG (Santa Cruz Biotechnology, Heidelberg, Germany).
Proliferation, migration, and tube formation assays were performed as previously described [12]. For proliferation assays, LECs (1.25 to 1.5 × 103) were seeded into fibronectin (Chemicon International, Temecula, CA, USA)-coated 96-well plates. After 72 hours incubation with the agents mentioned above, cells were incubated with 5-methylumbelliferylheptanoate (MUH, Sigma-Aldrich) as described [13]. The intensity of fluorescence, which is proportional to the number of viable cells, was measured using a SpectraMaxGemini EM microplate reader (Bucher Biotec AG, Basel, Switzerland). Ten wells per incubation mixture were analyzed.
For migration assays, two parallel lines and one orthogonal line were scratched into confluent LEC cultures, removing LECs without damaging the fibronectin coating. Pictures of the two crosses per well were taken immediately after scratching (T0) and after 16 hours of incubation (T16) with a Zeiss AxioCam-MRm. The quantitative analysis of the non-closed area was performed using Photoshop C3. Three wells per incubation mixture were analyzed.
For tube formation assays, confluent LEC monolayers were overlaid with collagen type I gels (1 mg/ml; Cohesion, Palo Alto, CA, USA), that contained the studied compounds, as described [12]. Tube-like structure formation was evaluated for up to 20 hours. Three representative pictures were taken per well, and tube length was analyzed using the IP Lab software as described [12]. Three wells per incubation mixture were used. For all these assays, three independent experiments were performed. Statistical analyses were performed using the two-tailed unpaired Student’s t-test.
Immunoblotting
Confluent cultures of LECs were homogenized in lysis buffer [12]. Protein concentrations were determined using the NanoOrange Protein Quantification Kit (Molecular Probes, Eugene, OR, USA). The lysates (100 μg of total protein) were subjected to SDS-polyacrylamide gel electrophoresis using NuPAGE 10% BT gels, 1.0 mm, 12 wells. The proteins were transferred onto Trans-Blot Transfer Medium pure nitrocellulose membranes (BioRad, Hercules, CA, USA). A rabbit anti-human EGFR antibody (Abcam) and the ECL Plus Western Blotting Detection System (GE Healthcare, Buckinghamshire, UK) were used for immunostaining. Equal loading was confirmed using an antibody against β-actin (Sigma).
Quantitative Real-time PCR
A total of 1μg of RNA isolated from human dermal microvascular LECs was used to generate cDNA with the high capacity cDNA reverse transcriptase kit (Applied Biosystems). The genes’ of interest expression levels were analysed in a SYBR Green real-time reverse transcribed PCR reaction with the AB7900 HT Fast real-time PCR system machine (Life Technologies) in two different cell isolates in triplicates. The housekeeping gene Rplp0 served as an internal control. Represented are raw Ct values and the standard deviations were calculated for the two different cell isolates. The designed primers (Microsynth) of the genes of interest were: Rplp0 (fwd: 5′CAGATTGGCTACCCAACTGTT3′; rev: 5′GGGAAGGTGTAATCCGTCTCC3′), EGF (fwd: 5′TGTCCACGCAATGTGTCTGAA3′; rev: 5′CATTATCGGGTGAGGAACAACC3′), TGFalpha (fwd: 5′AGGTCCGAAAACACTGTGAGT3′; rev: 5′AGCAAGCGGTTCTTCCCTTC3′), AREG (fwd: 5′GAGCCGACTATGACTACTCAGA3′; rev: 5′TCACTTTCCGTCTTGTTTTGGG3′), HBEGF (fwd: 5′ATCGTGGGGCTTCTCATGTTT3′; rev: 5′TTAGTCATGCCCAACTTCACTTT3′), NRG1 (fwd: 5′AGAGCCTGTTAATGAAACTCGC3′; rev: 5′GTCCACTTCCAATCTGTTAGCA3′), NRG2 (fwd: 5′ACAGCGGAAGCAGATGCAC3′; rev: 5′GTTTCTCTCCTGATGACATGGTC3′), NRG3 (fwd: 5′ACGACGACATATTCCACAGAGC3′; rev: 5′CCGGTCAGGGTTTCGATCAC3′), NRG4 (fwd: 5′ATGCCAACAGATCACGAAGAG3′; rev: 5′AATGGGCTGGGAATAGTAGGT3′), ErbB4 (fwd: 5′GCAGATGCTACGGACCTTACG3′; rev: 5′GACACTGAGTAACACATGCTCC3′), ErbB3 (fwd: 5′GGTGATGGGGAACCTTGAGAT3′; rev: 5′CTGTCACTTCTCGAATCCACT3′), ErbB2 (fwd: 5′TGTGACTGCCTGTCCCTACAA3′; rev: 5′CCAGACCATAGCACACTCGG3′) and EGFR (fwd: 5′TTGCCGCAAAGTGTGTAACG3′; rev: 5′GTCACCCCTAAATGCCACCG3′).
VEGF-R2 and VEGF-R3 phosphorylation studies
LECs and porcine aortic endothelial (PAE) cells, which over-express VEGF-R3 (kindly provided by Dr. Lena Claesson-Welsh), were grown to subconfluency and then starved in endothelial basal medium with 2% FBS overnight. The cells were stimulated with either VEGF-C (1.2 μg/ml) or EGF (200 ng/ml) in the presence or absence of GW2974 (10 μM) for 20 min. VEGF-A (20ng/ml) added for 20 min served as a positive control for stimulation of VEGF-R2 phosphorylation. Cells were lysed using a lysis buffer containing phosphatase and protease inhibitors (10 mM Tris, 150 mM NaCl, 5mM EDTA, 1% Triton-X, 25 mM, NaF, 1mM PMSF, 1 mM Na3VO4, 10% glycerol, protein inhibitor mixture (complete mini EDTA-free, Roche Applied Science)). The cell lysates of LECs were directly analyzed for phosphorylated VEGF-R2 (using the rabbit anti-human Phospho-VEGF-R2 Tyr 1175 from Cell Signalling) by Western blot, stripped and then investigated for total VEGF-R2 (using the rabbit anti-human VEGF-R2 antibody from Cell Signalling) as described below.
The stimulated PAE cells were lysed and VEGF-R3 was isolated by immunoprecipitation using an antibody directed against VEGF-R3 (rabbit anti-human VEGF-R3, Santa Cruz) coupled to dynabeads (Invitrogen). For this, the cell lysates were incubated with the antibody-carrying dynabeads for 1h at 4°C. The dynabeads were extensively washed with cold Tris HCl (10 mM, pH 7.4). The protein was then released from the beads by heating for 10 min at 70°C in SDS-PAGE sample buffer. The supernatant was loaded onto SDS-PAGE gels (6%, 1.0 mm) and the separated proteins were transferred onto PVDF membranes (Immobilon-P, Millipore). Tyrosine phosphorylation of VEGF-R3 was analyzed using a mouse anti-human tyrosine phosphorylation antibody (Millipore). The blots were stripped by incubation in 62.5 mM Tris-HCl pH 6.8, 2% SDS, 100 mM β-mercaptoethanol at 50°C for 30 min, washed, blocked and reprobed with an antibody recognizing total VEGF-R3 (rabbit-anti-human VEGF-R3, Santa Cruz). For detection, the ECL system (high performance chemiluminescence, Amersham Pharmacia Biotech) was utilized.
In vivo Matrigel lymphangiogenesis assay
FVB wild-type mice (female, 9-weeks old, n=5 per group; CharlesRiver, Sulzbach, Germany) were subcutaneously injected with 500 μl of growth factor-reduced Matrigel (Becton Dickinson) to which 1 μg/ml of either VEGF-C or EGF (BD Biosciences), or an equal volume of PBS had been added. After 15 days, tissues were excised, embedded and frozen in optimal cutting temperature (OCT) compound (Sakura Finetek, Zoeterwoude, Netherlands). Immunofluorescence staining for mouse LYVE-1 (Angiobio) and CD31 (BD Pharmingen) was performed using secondary antibodies labeled with Alexa-Fluor 488 or 594 (Molecular Probes). Cell nuclei were counterstained with Hoechst bisbenzimide. The sections were recorded using a Zeiss Axioskop 2 mot plus and a Zeiss AxioCam-MRm. Vessel area, density (number of vessels/mm2) and size were evaluated with Photoshop C3. For statistical analyses, the two-tailed unpaired student’s t-test was used. The animal study was approved by the Veterinaeramt des Kantons Zuerich, Zuerich, Switzerland.
Immunohistochemistry
Dermal lymphatic endothelial cells were fixed in 4% paraformaldehyde (PFA) at 4°C and stained with rabbit anti-human EGFR antibody (Abcam, Cambridge, UK) and a secondary antibody conjugated with Alexa 594. Cell nuclei were counterstained with bisbenzimide. Human skin sections were fixed in acetone for 10 minutes. Double immunofluorescence staining for podoplanin (D2-40, Signet, Dedham, MA, USA) and EGFR (Abcam) was performed using secondary antibodies labeled with Alexa-Fluor 488 or 594 (Molecular Probes). Cell nuclei were counterstained with bisbenzimide or hematoxylin. Tail and ear skin paraffin sections from 7-days old keratin14-amphiregulin transgenic mice (K14-ARtg) [14] and from wildtype FVB mice were kept at 60°C for four hours, dewaxed in xylene, hydrated and processed for immunohistochemistry with a biotinylated goat anti-mouse LYVE-1 antibody (R&D Systems).
Microscopic examination and recording as well as lymphatic vessel quantification were performed as described for the matrigel plug assay. The percentage of the area covered by vessels, the vessel density and sectioned area (size) were evaluated with Photoshop C3. For statistical analyses, the two-tailed unpaired Student’s t-test was used.
Results
Blockade of the EGFR/ErbB2 pathway inhibits the formation of CD31+/LYVE-1+ vessel-like structures in mouse embryoid bodies
A previously established mouse embryoid body (EB) vascular differentiation assay [10, 11] was used to investigate the effects of selected low molecular weight pharmacological agents on the development of lymphatic vessel-like structures. These agents were genistein, an isoflavonoid derived from plants and inhibitor of various tyrosine kinases, GW2974 that primarily inhibits the EGFR/ErbB2 tyrosine kinase, and the inhibitor of the VEGF receptor-2 tyrosine kinase SU5416. These compounds were selected based on our recent findings that they might affect the blood vessel and/or lymphatic vessel development in Xenopus laevis tadpoles [15]. Treatment was started 14 days after initiation of the EB culture. Compounds were added alone or in combination with a mixture of all-trans-retinoic acid (RA), cAMP and VEGF-C, which we have previously shown to promote lymphatic differentiation and lymphatic vessel-like structure formation in EBs [11].
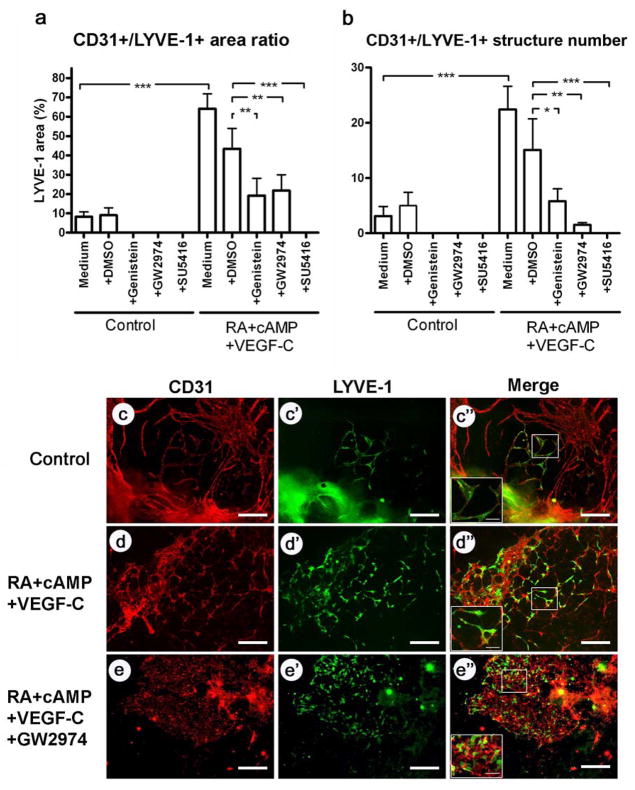
After four days, EBs incubated with RA, cAMP and VEGF-C showed an increased area and number of CD31+/LYVE-1+ vessel-like structures as compared to control EBs (Fig. 1a, b, c–d″). The CD31 and LYVE-1 double staining allowed to distinguish lymphatic endothelial cells from both, blood vascular endothelial cells (CD31+/LYVE-1−) and other LYVE-1-positive cells such as macrophages (CD31−/LYVE-1+) [16]. Incubation with SU5416, genistein or GW2974 significantly reduced the CD31+/LYVE-1+ area. These effects were seen whether the inhibitors were used alone or together with RA, cAMP and VEGF-C (Fig. 1a). All three compounds also reduced the number of CD31+/LYVE-1+ vessel-like structures when used alone or in combination with RA, cAMP and VEGF-C, (Fig. 1b). Addition of GW2974 to the RA/cAMP/VEGF-C mixture almost completely prevented the formation of CD31+/LYVE-1+ vessel-like structures, and only single CD31+/LYVE-1+ cells were detected (Fig. 1d–e″). All compounds, alone or in combination with RA/cAMP/VEGF-C, also slightly reduced the area and number of LYVE-1- vessel-like structures (data not shown).
Fig. 1. The EGFR/ErbB2 pathway is involved in the formation of CD31+/LYVE-1+ lymphatic vessel-like structures in mouse embryoid bodies.
An inhibitor of various tyrosine kinases (genistein), an inhibitor of the EGFR/ErbB2 tyrosine kinase (GW2974), and an inhibitor of the VEGF receptor-2 tyrosine kinase (SU5416) were added at 10 μM to the embryoid bodies either alone (control) or together with all-trans-retinoic acid (RA), cAMP and VEGF-C. After 4 days, the EBs were stained for CD31 (red; c, d, e) and LYVE-1 (green; c′, d′, e′). The overlays of the resulting images are shown in c″, d″, e″. The percentage of tissue covered by CD31+/LYVE-1+ vessels and their number were quantified (a, b). Data are expressed as mean values (n=9) + SEM; *p<0.05; **p<0.01; *** p<0.001. Scale bars: 100 μm.
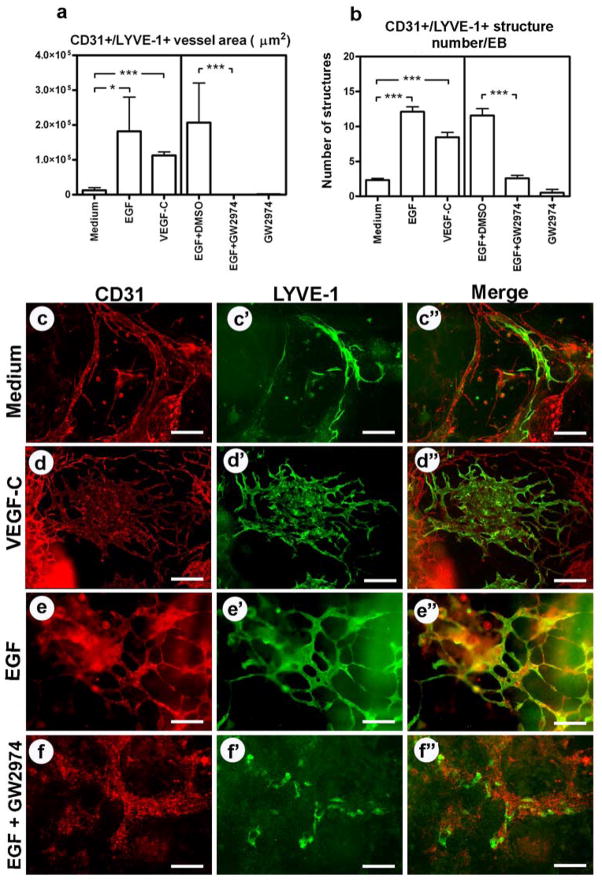
Because of the ability of the EGFR/ErbB2 tyrosine kinase inhibitors genistein and GW2974 to reduce the formation of CD31+/LYVE-1+ vessel-like structures in EBs, we investigated whether epidermal growth factor (EGF), an activating ligand of the EGFR/ErbB2, might promote vessel formation. Indeed, EBs exposed to EGF for 4 days showed a significant increase in the EB area occupied by CD31+/LYVE-1+ structures (Fig. 2a, c–c″, e–e″) and in the number of these structures (Fig. 2b, c–c″, e–e″). VEGF-C, used as a positive control, showed a similar effect (Fig. 2a, b, c–d″). The stimulatory effect of EGF was abolished by GW2974 (Fig. 2a, b, e–f″). Taken together, these data indicate a potential role of the EGFR/ErbB2 signalling pathway in the formation of lymphatic vessel-like structures.
Fig. 2. EGF promotes the formation of CD31+/LYVE-1+ lymphatic vessel-like structures in mouse embryoid bodies.
EGF (100 ng/ml) and GW2974 (10 μM) were added to embryoid bodies either alone or together. VEGF-C and medium alone served as positive and negative controls, respectively. After 4 days, the EBs were stained for CD31 (red; c, d, e, f) and LYVE-1 (green; c′, d′, e′, f′). The overlays of the resulting images are shown in c″, d″, e″, f″. The area covered by CD31+/LYVE-1+ vessels (a) and the number of vessel-like structures per embryoid body (b) were assessed. Data are expressed as mean values (n=9) + SEM; *p<0.05; **p<0.01; *** p<0.001. Scale bars: 50 μm.
EGFR/ErbB2 signalling promotes in vitro lymphangiogenesis by human LECs
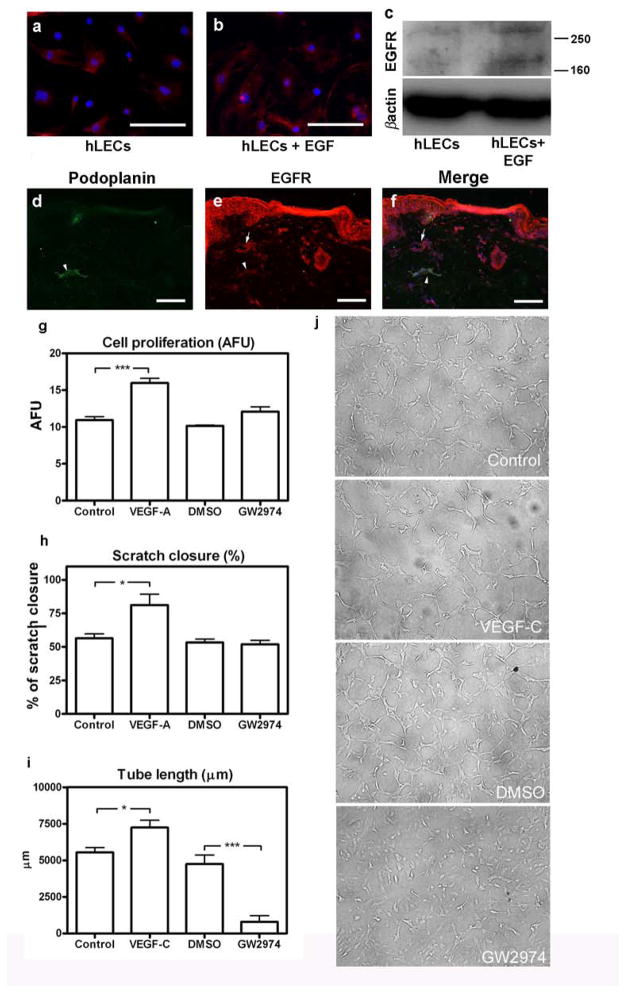
Immunofluorescence and Western blot analysis revealed that human LECs express the EGFR and that this expression was slightly enhanced after a 24h exposure to EGF (Fig. 3a, b, c). EGFR expression was also found in podoplanin-positive lymphatic vessels in human skin (Fig. 3d, e, f). To characterize the role of EGFR/ErbB2 signalling in more detail, we treated human LECs with GW2974 and performed in vitro proliferation, migration and tube formation assays. Migration was evaluated in an in vitro “scratch” wound healing assay [17]. We found that GW2974 did not inhibit LEC proliferation or migration/scratch closure (Fig. 3g, h), but that it potently inhibited tube formation (Fig. 3i, j). Tube formation of GW2974-treated LECs was significantly lower than of vehicle-treated controls.
Fig. 3. EGFR is expressed by human lymphatic endothelial cells and is involved in tube formation in vitro.
EGFR expression by cultured human LECs was assessed by immunofluorescence (a, b; red) and Western blot (c). EGFR appeared as two bands (ca. 170 and above 250 kDa), possibly representing the monomeric and dimeric receptor [40]. Lymphatic vessels in human skin expressing EGFR (red) and podoplanin (green) are indicated by arrow heads (d, e, f); blood vessels expressing only EGFR are indicated by arrows (e, f). Scale bars: 50 μm. Proliferation (g), migration/scratch closure (h) and tube formation (i, j) by human dermal LECs were assessed after GW2974 (10 μM) treatment. VEGF-A and -C served as positive controls, medium alone as negative control. Data are expressed as mean values (n=3) + SEM; *p<0.05; **p<0.01; *** p<0.001. AFU: absolute fluorescence units. Scale bars: 50 μm.
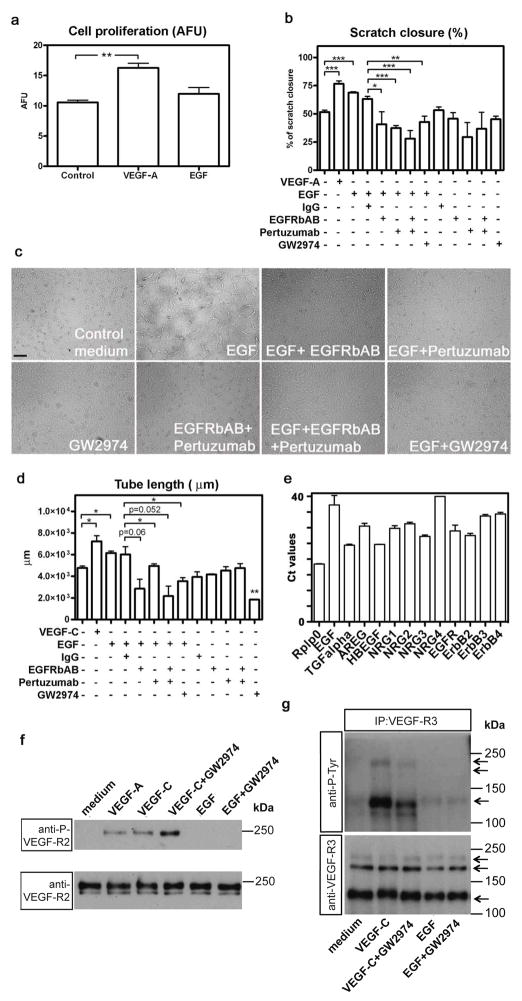
Next, we assessed the contribution of EGF and EGFR/ErbB2-signalling to LEC proliferation, migration and tube formation more specifically. LECs were treated either with EGF or with EGF combined with various inhibitors of EGFR/ErbB2 activation. As GW2974 is known to inhibit tyrosine phosphorylation not only of EGFR/ErbB-2, but also of ErbB-4 and probably other ErbB family members [18] antibodies specifically blocking either the EGFR (EGFRbAB) or its heterodimerization with ErbB2 (pertuzumab) were used as well. While EGF had no effect on proliferation (Fig. 4a), it stimulated migration/scratch closure and tube formation (Fig. 4b, c, d). The stimulating effects of EGF on cell migration/scratch closure were inhibited not only by GW2974 but also by the EGFRbAB and pertuzumab (Fig. 4b). In the absence of EGF, neither the blocking antibodies nor GW2974 affected LEC migration (Fig. 4b). The enhancing effect of EGF on LEC tube formation was also inhibited by the EGFRbAB and by pertuzumab, albeit to a lesser extent (Fig. 4c, d). GW2974 inhibited not only the EGF-induced tube formation, but also the basal tube formation observed under control conditions (Fig. 4c, d).
Fig. 4. EGF promotes human lymphatic endothelial cell migration and tube formation in vitro via EGFR/ErbB2.
The proliferation (a), migration (b) and tube formation (c, d) were investigated in human lymphatic endothelial cells treated with EGF (100 ng/ml) alone or EGF combined with various inhibitors of the EGFR/ErbB2 pathway. As GW2974 (used at 10 μM) might also inhibit other, related members of the EGFR/ErbB family, a specific EGFR blocking antibody (EGFRbAB; 10 μg/ml) as well as an antibody interfering with ErbB2 heterodimerisation (pertuzumab; 10 μg/ml) were used. VEGF-A (20 ng/ml) and VEGF-C (1.2 μg/ml) served as positive stimulatory controls and mouse IgG as a negative control for the antibody treatments. Data are expressed as mean values (n=3) + SEM; *p<0.05; **p<0.01; *** p<0.001. Scale bar: 50 μm. The expression levels (Ct values) of EGF, transforming growth factor-α (TGFalpha), amphiregulin (AREG), heparin-binding growth factor (HBEGF), neuregulin-1, -2, -3, -4 (NRG1, NRG2, NRG3, NRG4) as well as of the cognate receptors EGFR, ErbB2, ErbB3, ErbB4 were measured in human dermal lymphatic endothelial cells by qRT-PCR (e). Rplp0 (ribosomal protein) served and an internal control. High expression levels were found for TGFalpha, HBEGF and EGFR, and medium expression levels for AREG, NRG1, NRG2, NRG3 and ERBB2. However, ErbB3 and ErbB4 were expressed at very low levels and EGF and NRG4 were not expressed at all. LECs were treated with either VEGF-C (1.2 μg/ml) or EGF (200 ng/ml) with or without addition of GW2974 for 20 min (f). VEGF-A (20 ng/ml) was used as a positive control. Cell lysates were analyzed for VEGF-R2 phosphorylation by Western blot (anti-P-VEGF-R2). Total VEGF-R2 was stained as loading control (anti-VEGF-R2). VEGF-R2 migrates as two species with apparent molecular weights slightly below 250 kDa (compare [41]). VEGF-R2 was phosphorylated upon VEGF-A and VEGF-C, but not EGF treatment and this phosphorylation was unaffected by GW2974. Porcine aortic endothelial cells (PAE), which over-express human VEGF-R3, were treated in the same manner as the LECs (g). VEGF-R3 was immunoprecipitated from the cell lysates and analysed for Tyr phosphorylation by Western blot (anti-P-Tyr). Total VEGF-R3 was stained as a loading control (anti-VEGF-R3). As previously reported ([41]), VEGFR3 migrates as three species with apparent molecular weights of 195, 175 and 125 kDa. VEGF-R3 was phosphorylated upon VEGF-C, but not EGF treatment and this phosphorylation was only slightly inhibited by GW2974.
To find out whether growth factors secreted by the LECs may induce this basal tube formation in an autocrine manner, we investigated expression of various ErbB family members and of their ligands by qRT-PCR. We found that LECs expressed significant amounts of transforming growth factor α TGF-α, heparin-binding growth factor (HB-EGF), and EGFR, medium amounts of amphiregulin, neuregulin-1, -2, -3, and ErbB2 and very low amounts of ErbB3 and ErbB4 (Fig. 4e). These data suggest that there may be some autocrine regulation of tube formation by LECs, which is however limited to TGF-α HB-EGF and EGFR/ErbB2.
As VEGF-C is a well-known inducer of LEC tube formation, we wondered whether its receptors VEGF-R2 and VEGF-R3 might be involved in the LEC tube formation induced by EGF. However, EGF treatment did not induce phosphorylation of either the VEGF-R2 or the VEGF-R3 (Figure 4f, 4g). In contrast, both receptors were abundantly phosphorylated upon treatment of LECs with VEGF-C (Figure 4f, 4g). GW2974 had no major influence on phosphorylation of VEGF-R2 or VEGF-R3. These results demonstrate that neither activation of VEGF-R2 nor of VEGF-R3 are involved in the induction of LEC tube formation by EGF.
EGF promotes lymphangiogenesis in vivo
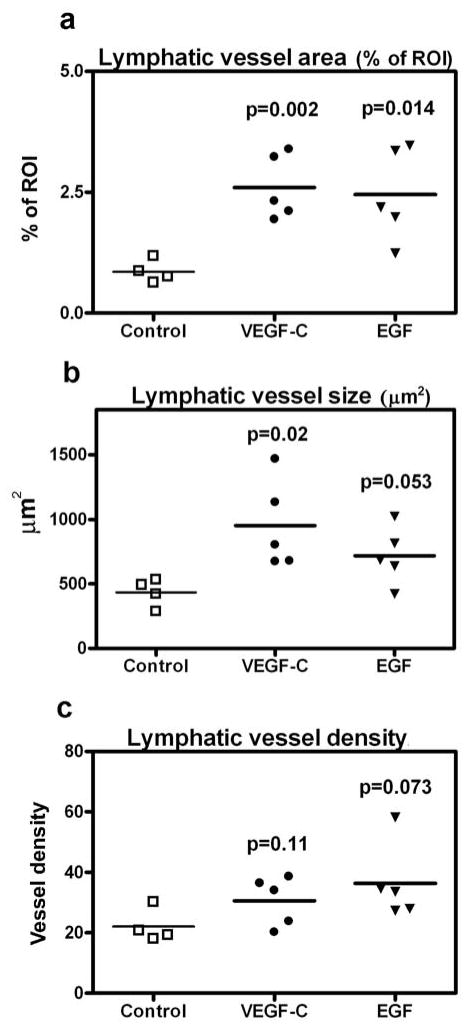
We next investigated whether EGF might also enhance lymphatic vessel formation in vivo. To this end, matrigel plugs containing EGF, VEGF-C or vehicle control were injected subcutaneously into the left inguinal area of wildtype FVB mice. After 2 weeks, the matrigel plugs and the associated skin were dissected and analyzed. In the skin that surrounded the matrigel plugs containing EGF, the increase in lymphatic vessel area was significant (p=0.014), whereas the increases in lymphatic vessel size (p=0.053) and density (p=0.073) did not reach statistical significance (Fig. 5a, b, c). In contrast, VEGF-C not only led to a significant increase in lymphatic vessel area but also of lymphatic vessel size (Fig. 5a, b, c).
Fig. 5. EGF promotes lymphangiogenesis in vivo.
Matrigel plugs containing either PBS (control), VEGF-C or EGF were injected subcutaneously into the left inguinal area of 9-weeks old FVB wildtype mice. After 2 weeks, the plug and the associated skin were dissected and stained for LYVE-1 and CD31. Lymphatic vessel area (a), size (b), and density (c) were quantified. Data are expressed as absolute values per matrigel plug; bars represent the median values.
Transgenic overexpression of amphiregulin in mouse skin promotes lymphangiogenesis in vivo
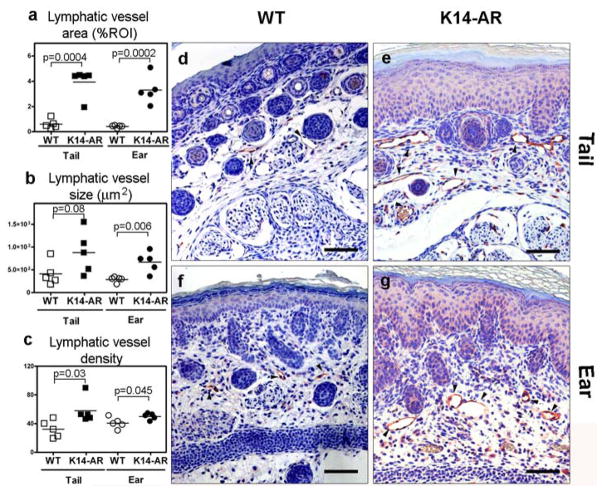
Similar to EGF, amphiregulin is a specific ligand for EGFR/ErbB2. Transgenic mice which express amphiregulin under the control of the keratin (K)14 promoter in the skin develop a chronic, psoriasis-like skin inflammation [14]. We investigated whether chronic transgenic overexpression of amphiregulin in mouse skin might also promote cutaneous lymphangiogenesis. Computer-assisted morphometric analyses of LYVE-1 stains of the tail and ear skin of K14/amphiregulin transgenic mice (Fig. 6e, g) and of age-matched wildtype mice (Fig. 6d, f) revealed that the lymphatic vessel area (Fig. 6a) and the average lymphatic vessel size (Fig. 6b) were significantly and strongly increased in the ear skin and also in the tail skin of seven-days old transgenic mice. The lymphatic vessel density (Fig. 6c) was also significantly increased, albeit to a lesser extent.
Fig. 6. Transgenic overexpression of amphiregulin in mouse skin promotes lymphangiogenesis.
Keratin14-amphiregulin (K14-AR) transgenic mice and age-matched wildtype mice were studied at 7 days of age. Paraffin-embedded sections of ear and tail skin were stained for LYVE-1 (red). The lymphatic vessel area (a), size (b), and density (c) were assessed by computer-assisted morphometric image analysis. Representative microscopic images are shown. Lymphatic vessels are highlighted by arrowheads (d, e, f, g). Data are expressed as absolute values per animal. Bars represent mean values.
Discussion
In a search for novel pathways involved in the regulation of lymphatic vessel growth, we have investigated several low molecular weight compounds for their effects on vascular development in mouse embryonic stem cell-derived embryoid bodies (EBs). These compounds were chosen based on the results of a recent study in which we investigated a library of 1,280 compounds with well-defined pathway specificities with regard to their effects on blood vessel and lymphatic vessel development in Xenopus laevis tadpoles [15]. Among the compounds with effects on vascular development, the VEGFR2 tyrosine kinase inhibitor SU5416 potently inhibited blood vessel formation, whereas GW2974, an EGFR/ErbB2 tyrosine kinase inhibitor, showed some inhibitory activity on lymphatic vessel development. The tyrosine kinase inhibitor genistein had shown effects on both blood and lymphatic vessel formation [15].
Our findings reveal that in mouse EBs, genistein, GW2974 and SU5416 potently reduced both lymphatic vessel area and the number of lymphatic vessel-like structures. All three compounds were also tested in combination with a mixture of retinoic acid (RA), cAMP and VEGF-C, which we recently found to promote the development of lymphatic vessel-like structures in EBs [11]. In this experimental setting, genistein reduced the number of CD31+/LYVE-1+ vessel-like structures in EBs, but GW2974 and SU5416 showed the strongest inhibitory effects. GW2974 almost completely prevented the formation of lymphatic vessel-like structures so that only single CD31+/LYVE-1+ cells were observed, even in the presence of RA/cAMP/VEGF-C. These results indicated that EGF receptor (EGFR/ErbB2) signalling might be involved in regulation of lymphatic vessel formation. Since low molecular weight tyrosine kinase inhibitors are often not fully specific for a given tyrosine kinase, we investigated the specific involvement of EGFR/ErbB2 by applying its natural ligand EGF to EBs. Our findings that incubation with EGF potently induced formation of lymphatic vessel-like structures and that this effect was abolished by GW2974 strongly support a major role of EGFR/ErbB2 signalling in lymphatic vessel formation.
EGFR family proteins play important roles in the regulation of growth, proliferation and differentiation of various cell types [19]. Mice lacking the EGFR (ErbB1) die before implantation or at mid to late gestation (depending on the genetic background), due to placental malformation and severe defects of epithelial cells [20]. EGFR activation might also regulate angiogenesis both directly [21] and indirectly [22]. Indeed, EGFR ligands such as EGF and TGF-α are potent pro-angiogenic factors [23], in part via upregulation of VEGF-A expression in epithelial cells [24]. Previously, EGFR was found to be expressed by quiescent and by tumor-associated blood vascular endothelial cells [25, 26]. Our study reveals that EGFR is also expressed by human dermal lymphatic endothelial cells in vitro and in vivo. This further supports the concept that the effects of EGF on lymphatic vessels are mediated, at least in part, by direct EGFR activation. Previous studies have implied that EGF might promote the proliferation of cultured bovine [27] and mouse mesenteric LECs [28]. However, we did not observe any effect of EGF or of the EGFR inhibitor GW2974 on the in vitro proliferation of human dermal LECs. These differing results may be due to different culture conditions, different tissues of origin of the LECs, or to species-specific differences in the response of lymphatic endothelium to growth factors. However, our in vitro studies revealed that migration and tube formation by human LECs were enhanced by EGF and abolished by GW2974. This effect was mediated by activation of EGFR/ErbB-2, as (i) it was blocked by antibodies specifically interfering with binding and activation of the EGFR/ErbB-2, (ii) LECs were shown not to express other receptors of the ErbB family, and (iii) neither EGF nor GW2974 had any impact on phosphorylation of the VEGF receptors VEGF-R2 and VEGF-R3. This leads us to conclude that rather than increasing the number of LECs, EGFR/ErbB activation specifically enhances remodelling and enlargement of lymphatic vessels.
Our study is the first to reveal that activation of the EGFR promotes formation and remodelling of lymphatic vessels in vivo. We have used two mouse models to investigate the impact of EGF on cutaneous lymphatic vessels. Implantation of an EGF-containing matrigel plug resulted in lymphatic vessels with a larger surface area in the surrounding skin after 14 days. The matrigel plug assay is a widely used, well-established (lymph)-angiogenic assay. However, implantation also leads to an inflammatory response, with possible confounding effects. Thus, we also used a second mouse model with chronic overexpression of the EGFR ligand amphiregulin by transgenic keratinocytes in the epidermis. We initially studied K14/amphiregulin mice at an age of three weeks and found dramatic enlargement of the cutaneous lymphatic vasculature (data not shown). However, at this time point, the mice had already developed a strong, psoriasis-like skin inflammation in agreement with the published phenotype [14]. Because psoriasis skin lesions in humans and psoriasis-like skin lesions in mice are associated with massive lymphatic hyperplasia [29], we decided to study the vasculature of younger, one week-old K14-amphiregulin transgenic mice, in which the massive inflammation had not yet developed. These mice displayed only slightly more dermal lymphatic vessels, which were however larger than the lymphatic vessels of their wild-type littermates. Together, these results clearly indicate an important role of EGFR signalling in remodeling of lymphatic vessels in vivo.
The effect of EGF on lymphatic endothelial cells might be both, direct and indirect. It has been shown that EGFR/ErbB2 signalling leads to the expression of angiogenic growth factors, such as VEGF-A in keratinocytes and cancer cells [22, 24, 30, 31], and we have previously shown that VEGF-A might promote in vitro and in vivo lymphangiogenesis [7, 8]. However, the effect of EGF on the migration and tube formation of cultured human dermal LECs was most likely direct since these cells express EGFR and no other cell type was present. Moreover, the levels of VEGF-A and VEGF-C in LEC cultures treated with EGF were below detection limit as evaluated by ELISA (data not shown).
Small molecule inhibitors targeting the EGFR such as erlotinib and EGFR-blocking antibodies such as cetuximab are used for cancer treatment [32, 33]. Inhibition of tumor associated-angiogenesis by EGFR blockade has been reported [34, 35]. Based on the data presented here, it may be speculated that EGFR blockade also leads to changes in the lymphatic vessel architecture. Indeed, patients undergoing EGFR blocking treatment often develop acute skin rash and inflammation [33, 36] as well as peripheral edema [37–39]. Some of these symptoms may be due to impaired lymphatic function. It would be of great interest to study the effects of EGFR inhibition on human lymph vessels in tissue samples from skin and tumors of patients under anti-EGFR therapy. It could be that EGF promotes morphological changes that optimize lymphatic vessel function. It can thus be speculated that local application of EGF could be beneficial to restore lymphatic vessel function and thus limit skin inflammation and lymphedema. On the other side, inhibitors of EGFR/ErbB2 signalling could be candidates for limiting pathological lymphangiogenesis as encountered in various diseases such as organ transplant rejection and metastatic spread of skin tumors.
Acknowledgments
This work was supported by National Institutes of Health grant CA69184, Swiss National Science Foundation grants 3100A0-108207, 31003A_130627 and 310030B_147087, Commission of the European Communities grant LSHC-CT-2005-518178, Advanced European Research Council grant LYVICAM, a Leducq Foundation Transatlantic Network of Excellence grant, Oncosuisse and Krebsliga Zurich (to M.D.).
Footnotes
Conflict of Interests
The authors declare no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol. 2006;126:2167–2177. doi: 10.1038/sj.jid.5700464. [DOI] [PubMed] [Google Scholar]
- 2.Breiteneder-Geleff S, Soleiman A, Horvat R, Amann G, Kowalski H, Kerjaschki D. Podoplanin--a specific marker for lymphatic endothelium expressed in angiosarcoma. Verh Dtsch Ges Pathol. 1999;83:270–275. [PubMed] [Google Scholar]
- 3.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman J, Rutkowski JM, Shields JD, Pasquier MC, Cui Y, Schmokel HG, et al. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. Faseb J. 2007;21:1003–1012. doi: 10.1096/fj.06-6656com. [DOI] [PubMed] [Google Scholar]
- 5.Veikkola T, Jussila L, Makinen T, Karpanen T, Jeltsch M, Petrova TV, et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. Embo J. 2001;20:1223–1231. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjorndahl MA, Cao R, Burton JB, Brakenhielm E, Religa P, Galter D, et al. Vascular endothelial growth factor-a promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res. 2005;65:9261–9268. doi: 10.1158/0008-5472.CAN-04-2345. [DOI] [PubMed] [Google Scholar]
- 7.Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong YK, Lange-Asschenfeldt B, Velasco P, Hirakawa S, Kunstfeld R, Brown LF, et al. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. Faseb J. 2004;18:1111–1113. doi: 10.1096/fj.03-1179fje. [DOI] [PubMed] [Google Scholar]
- 9.Cueni LN, Detmar M. The lymphatic system in health and disease. Lymphat Res Biol. 2008;6:109–122. doi: 10.1089/lrb.2008.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liersch R, Nay F, Lu L, Detmar M. Induction of lymphatic endothelial cell differentiation in embryoid bodies. Blood. 2006;107:1214–1216. doi: 10.1182/blood-2005-08-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marino D, Dabouras V, Brandli AW, Detmar M. A role for all-trans-retinoic acid in the early steps of lymphatic vasculature development. J Vasc Res. 2010;48:236–251. doi: 10.1159/000320620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kajiya K, Hirakawa S, Ma B, Drinnenberg I, Detmar M. Hepatocyte growth factor promotes lymphatic vessel formation and function. Embo J. 2005;24:2885–2895. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Detmar M, Tenorio S, Hettmannsperger U, Ruszczak Z, Orfanos CE. Cytokine regulation of proliferation and ICAM-1 expression of human dermal microvascular endothelial cells in vitro. J Invest Dermatol. 1992;98:147–153. doi: 10.1111/1523-1747.ep12555746. [DOI] [PubMed] [Google Scholar]
- 14.Cook PW, Piepkorn M, Clegg CH, Plowman GD, DeMay JM, Brown JR, et al. Transgenic expression of the human amphiregulin gene induces a psoriasis-like phenotype. J Clin Invest. 1997;100:2286–2294. doi: 10.1172/JCI119766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalin RE, Banziger-Tobler NE, Detmar M, Brandli AW. An in vivo chemical library screen in Xenopus tadpoles reveals novel pathways involved in angiogenesis and lymphangiogenesis. Blood. 2009;114:1110–1122. doi: 10.1182/blood-2009-03-211771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannig M, Figulla HR, Sauer H, Wartenberg M. Control of leucocyte differentiation from embryonic stem cells upon vasculogenesis and confrontation with tumour tissue. J Cell Mol Med. 2010;14:303–312. doi: 10.1111/j.1582-4934.2008.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gebaeck T, Schulz MM, Koumoutsakos P, Detmar M. TScratch: a novel and simple software tool for automated analysis of monolayer wound healing assays. Biotechniques. 2009;46:265–274. doi: 10.2144/000113083. [DOI] [PubMed] [Google Scholar]
- 18.Brignola PS, Lackey K, Kadwell SH, Hoffman C, Horne E, Carter HL, et al. Comparison of the biochemical and kinetic properties of the type 1 receptor tyrosine kinase intracellular domains. Demonstration of differential sensitivity to kinase inhibitors. J Biol Chem. 2002;277:1576–1585. doi: 10.1074/jbc.M105907200. [DOI] [PubMed] [Google Scholar]
- 19.Riese DJ, 2nd, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays. 1998;20:41–48. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 20.Wong RW. Transgenic and knock-out mice for deciphering the roles of EGFR ligands. Cell Mol Life Sci. 2003;60:113–118. doi: 10.1007/s000180300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker CH, Kedar D, McCarty MF, Tsan R, Weber KL, Bucana CD, et al. Blockade of epidermal growth factor receptor signaling on tumor cells and tumor-associated endothelial cells for therapy of human carcinomas. Am J Pathol. 2002;161:929–938. doi: 10.1016/S0002-9440(10)64253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar R, Yarmand-Bagheri R. The role of HER2 in angiogenesis. Semin Oncol. 2001;28:27–32. doi: 10.1016/s0093-7754(01)90279-9. [DOI] [PubMed] [Google Scholar]
- 23.Schreiber AB, Winkler ME, Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986;232:1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- 24.Detmar M, Brown LF, Claffey KP, Yeo KT, Kocher O, Jackman RW, et al. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med. 1994;180:1141–1146. doi: 10.1084/jem.180.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amin DN, Hida K, Bielenberg DR, Klagsbrun M. Tumor endothelial cells express epidermal growth factor receptor (EGFR) but not ErbB3 and are responsive to EGF and to EGFR kinase inhibitors. Cancer Res. 2006;66:2173–2180. doi: 10.1158/0008-5472.CAN-05-3387. [DOI] [PubMed] [Google Scholar]
- 26.Sini P, Wyder L, Schnell C, O’Reilly T, Littlewood A, Brandt R, et al. The antitumor and antiangiogenic activity of vascular endothelial growth factor receptor inhibition is potentiated by ErbB1 blockade. Clin Cancer Res. 2005;11:4521–4532. doi: 10.1158/1078-0432.CCR-04-1954. [DOI] [PubMed] [Google Scholar]
- 27.Liu NF, He QL. The regulatory effects of cytokines on lymphatic angiogenesis. Lymphology. 1997;30:3–12. [PubMed] [Google Scholar]
- 28.Rebhun RB, Langley RR, Yokoi K, Fan D, Gershenwald JE, Fidler IJ. Targeting receptor tyrosine kinase on lymphatic endothelial cells for the therapy of colon cancer lymph node metastasis. Neoplasia. 2006;8:747–757. doi: 10.1593/neo.06322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunstfeld R, Hirakawa S, Hong YK, Schacht V, Lange-Asschenfeldt B, Velasco P, et al. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104:1048–1057. doi: 10.1182/blood-2003-08-2964. [DOI] [PubMed] [Google Scholar]
- 30.Dvorak HF, Detmar M, Claffey KP, Nagy JA, van de Water L, Senger DR. Vascular permeability factor/vascular endothelial growth factor: an important mediator of angiogenesis in malignancy and inflammation. Int Arch Allergy Immunol. 1995;107:233–235. doi: 10.1159/000236988. [DOI] [PubMed] [Google Scholar]
- 31.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A. 2009;106:3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herbst RS, Sandler A. Bevacizumab and erlotinib: a promising new approach to the treatment of advanced NSCLC. Oncologist. 2008;13:1166–1176. doi: 10.1634/theoncologist.2008-0108. [DOI] [PubMed] [Google Scholar]
- 33.Tsimboukis S, Merikas I, Karapanagiotou EM, Saif MW, Syrigos KN. Erlotinib-induced skin rash in patients with non-small-cell lung cancer: pathogenesis, clinical significance, and management. Clin Lung Cancer. 2009;10:106–111. doi: 10.3816/CLC.2009.n.013. [DOI] [PubMed] [Google Scholar]
- 34.Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, et al. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- 35.Perrotte P, Matsumoto T, Inoue K, Kuniyasu H, Eve BY, Hicklin DJ, et al. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 1999;5:257–265. [PubMed] [Google Scholar]
- 36.Perez-Soler R, Saltz L. Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol. 2005;23:5235–5246. doi: 10.1200/JCO.2005.00.6916. [DOI] [PubMed] [Google Scholar]
- 37.Barbaud A, Granel F, Waton J, Poreaux C. How to manage hypersensitivity reactions to biological agents? Eur J Dermatol. 2011;21:667–674. doi: 10.1684/ejd.2011.1468. [DOI] [PubMed] [Google Scholar]
- 38.Settle SH, Washington K, Lind C, Itzkowitz S, Fiske WH, Burdick JS, et al. Chronic treatment of Menetrier’s disease with Erbitux: clinical efficacy and insight into pathophysiology. Clin Gastroenterol Hepatol. 2005;3:654–659. doi: 10.1016/s1542-3565(05)00368-x. [DOI] [PubMed] [Google Scholar]
- 39.Von Minckwitz G, Jonat W, Fasching P, du Bois A, Kleeberg U, Luck HJ, et al. A multicentre phase II study on gefitinib in taxane- and anthracycline-pretreated metastatic breast cancer. Breast Cancer Res Treat. 2005;89:165–172. doi: 10.1007/s10549-004-1720-2. [DOI] [PubMed] [Google Scholar]
- 40.Adamczyk KA, Klein-Scory S, Tehrani MM, Warnken U, Schmiegel W, Schnolzer M, et al. Characterization of soluble and exosomal forms of the EGFR released from pancreatic cancer cells. Life Sci. 2011;89:304–312. doi: 10.1016/j.lfs.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 41.Nilsson I, Bahram F, Li X, Gualandi L, Koch S, Jarvius M, et al. VEGF receptor 2/−3 heterodimers detected in situ by proximity ligation on angiogenic sprouts. Embo J. 2010;29:1377–1388. doi: 10.1038/emboj.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]