Abstract
OBJECTIVES
Determine if simvastatin impairs exercise training adaptations.
BACKGROUND
Statins are commonly prescribed in combination with therapeutic lifestyle changes, including exercise, to reduce cardiovascular disease risk in patients with the metabolic syndrome. Statin use has been linked to skeletal muscle myopathy and impaired mitochondrial function, but it is unclear whether statin use alters adaptations to exercise training.
METHODS
We examined the effects of simvastatin on changes in cardiorespiratory fitness and skeletal muscle mitochondrial content in response to aerobic exercise training. Sedentary overweight or obese adults with at least 2 metabolic syndrome risk factors (defined according to National Cholesterol Education Panel Adult Treatment Panel III criteria) were randomized to 12 weeks of aerobic exercise training or to exercise in combination with simvastatin (40 mg per day). The primary outcomes were cardiorespiratory fitness and skeletal muscle (vastus lateralis) mitochondrial content (citrate synthase enzyme activity).
RESULTS
Thirty-seven participants (exercise plus statins; n=18; exercise only; n=19) completed the study. Cardiorespiratory fitness increased by 10% (P<0.05) in response to exercise training alone, but was blunted by the addition of simvastatin resulting in only a 1.5% increase (P<0.005 for group by time interaction). Similarly, skeletal muscle citrate synthase activity increased by 13% in the exercise only group (P <0.05), but decreased by 4.5% in the simvastatin plus exercise group (P<0.05 for group by time interaction).
CONCLUSION
Simvastatin attenuates increases in cardiorespiratory fitness and skeletal muscle mitochondrial content when combined with exercise training in overweight or obese patients at risk of the metabolic syndrome.
Keywords: statin, obesity, metabolic syndrome, aerobic fitness, skeletal muscle mitochondria
Introduction
The metabolic syndrome is a cluster of inter-related factors, including insulin resistance, central adiposity, hypertension, and dyslipidemia, that are associated with increased risk of cardiovascular disease, stroke, type 2 diabetes, and early death (1, 2). Obesity and a sedentary lifestyle are closely linked to the metabolic syndrome. Currently, over 70% of adults in the United States are overweight or obese while 98% do not meet current physical activity guidelines (3). An estimated 23% have the metabolic syndrome (4).
Therapeutic lifestyle changes, including exercise, are the first line of treatment for patients with the metabolic syndrome. The health benefits of exercise have been widely described, the most notable of which is an increase in cardiorespiratory fitness. Importantly, cardiorespiratory fitness has been identified as the strongest independent predictor of both all-cause and cardiovascular disease mortality in nearly every population in which it has been examined (5–7).
Statins, a class of hydroxymethylglutaryl-coenzyme A reductase inhibitors that lower low-density lipoprotein cholesterol (LDL), are commonly prescribed to patients with the metabolic syndrome or those with multiple cardiovascular disease risk factors when lifestyle changes fail to achieve LDL targets to reduce the risk of coronary heart disease morbidity and mortality. Indeed, statins are the most widely prescribed drug in the United States and around the world. Many patients are advised to continue daily exercise when statin therapy is initiated. In recent years, there has been a growing movement to begin prescribing statins to low-risk patients and to all patients over the age of 50 for the primary prevention of cardiovascular disease (8) making the case for statins to be used in primary prevention. This concept is gaining momentum as inexpensive generic statins have become available.
Although reports from pharmaceutical trials indicate that statins are generally well-tolerated, statins have been linked to skeletal muscle cramping, pain, myalgia and in rare cases rhabdomyolysis (9). Statins are poorly tolerated among elite athletes (10) and may increase susceptibility to muscle damage during exercise (11, 12). Although the mechanisms are poorly understood, some statins (simva-, atorva-, fluva-) have been shown to reduce skeletal muscle mitochondrial content and oxidative capacity in humans (13–16). In rodents, atorvastatin lowers running capacity (17, 18) and impairs exercise-mediated mitochondrial adaptations in skeletal muscle (18). Despite the potential public health implications, studies examining the benefits and risks of combining statins and exercise in humans are limited.
This randomized, controlled trial was designed to compare the effects of exercise training to those of simvastatin in combination with exercise on changes in cardiorespiratory fitness and skeletal muscle citrate synthase activity, a marker of skeletal muscle mitochondrial content, in previously sedentary, overweight or obese patients with at least 2 metabolic syndrome risk factors.
METHODS
PARTICIPANTS
Volunteers were recruited through advertisements and word-of-mouth and underwent a thorough medical screening to determine eligibility. Volunteers were eligible if they were between 25 and 59 years of age, overweight or obese [body mass index (BMI) 26 to 39 kilograms of body weight per height in meters squared], sedentary (no more than 30 minutes of structured physical activity per week during the previous 6 months), weight stable (change in body weight of no more than 5% during the previous 3 months), and had at least 2 of the 5 metabolic syndrome risk factors as defined by the National Cholesterol Education Program’s Adult Treatment Panel III. Exclusion criteria included smoking, the use of statins or other medications or supplements that affect lipid profiles or body weight (e.g., fibric acids, bile acid sequestrants, nicotinic acids, fish oil), changes in the use or dose of other medications or supplements during the previous 3 months, diagnosis of chronic diseases including cardiovascular disease, diabetes mellitus, other metabolic diseases (e.g., thyroid), cancer, HIV or AIDS, positive graded exercise stress test, or musculoskeletal or other problems that result in an inability to walk on a treadmill. The study was approved by the Health Sciences Institutional Review Board at the University of Missouri. All volunteers provided written informed consent.
STUDY DESIGN
We used a block-randomized design to assign eligible participants to a 12 week supervised aerobic exercise training program or to the exercise program in combination with daily simvastatin use. Group assignment was stratified according to age, gender, and BMI.
The supervised exercise training program began with 30 minutes of treadmill walking or jogging at 60–75% of heart rate reserve (equivalent to approximately 60–75% of VO2peak) on 3 days during the first week and on 5 days during the second week, where 60% of heart rate reserve = [(peak heart rate during treadmill test – resting heart rate) X 0.60] + resting heart rate. During the remaining 12 weeks, participants completed 45 minutes of treadmill walking or jogging at 60–75% of heart rate reserve 5 days per week. Exercise intensity was monitored via Polar heart rate monitors as previously described (19). Adherence was calculated as the number of exercise sessions completed divided by the number of sessions prescribed. Exercise sessions were performed in a fitness facility on the MU campus under close supervision by study staff.
Participants assigned to the combination group participated in the exercise training program and were given 40 mg simvastatin per day (20).
ASSESSMENTS
Assessments were completed at baseline and at the end of the 12 week intervention. Body weight, height, and waist circumference were measured, and body composition was determined using a QDR-4500A dual X-ray absorptiometry (Hologic, Shelby Township, MI). Blood pressure was measured using a mercury sphygmomanometer following 10 minutes of seated rest.
Blood samples were collected after a 12 hour overnight fast. Fasting glucose was determined using the glucose oxidase method. Fasting insulin was measured by enzyme-linked immunosorbant assays. Total cholesterol, HDL, LDL and triglycerides were measured by immunocalorimetric assays by a commercial labororatory. Low density lipoprotein cholesterol (LDL) was calculated using the Friedewald equation (21).
On the same day, biopsies (50–100 mg) were obtained from the vastus lateralis muscle using the modified Bergstrom needle technique (22). Skeletal muscle samples were immediately cleaned of visible connective and adipose tissue and snap frozen in liquid nitrogen. Citrate synthase, a marker of skeletal muscle mitochondrial content (23), was measured by spectrophotometry (24) . Quantification of mitochondrial oxidative phosphorylation proteins was determined by immunoblotting (25) using the MitoProfile Total OXPHOS antibody (Abcam) (26). Insufficient tissue volume precluded the analysis of a small subset of samples. Thus, baseline and post-intervention skeletal muscle citrate synthase activity and mitochondrial oxidative phosphorylation protein content are presented on samples from 13 patients in the simvastatin plus exercise group and 17 in the exercise group.
A three-day dietary control period preceded the blood collection and muscle biopsy visits. Participants were given a food diary and instructed to follow habitual food intake patterns while recording the type, timing, and amount of food and beverage consumed for the three days preceding the pre-intervention blood collection and muscle biopsy. Participants were later given a copy of their food diary and instructed to replicate the amount, timing, and type of food and beverage consumed for three days prior to the post-intervention blood collection and muscle biopsy.
Expired gases were analyzed by a metabolic cart (TrueOne 2400, Parvo Medics, Salt Lake City, UT) during a ramped treadmill test (Bruce protocol) (19) to determine cardiorespiratory fitness (defined as peak oxygen consumption; VO2peak). Resting and peak heart rate were determined by electrocardiography. VO2peak was obtained when participants reached volitional exhaustion and met at least 3 of the following criteria: 1) respiratory exchange ratio ≥ 1.10, 2) peak heart rate within 10 beats of age predicted maximum, 3) rating of perceived exertion ≥ 18, or 4) plateau in oxygen consumption despite increase in workload (19).
STATISTICAL ANALYSIS
The main effects of time (baseline versus post-intervention), treatment (exercise alone versus exercise plus statin), and time by treatment interactions (between-group differences in change from baseline) were tested using 2-way repeated measures analysis of variance. Where significant main effects were found, posthoc tests were performed with least significant difference to identify specific pairwise differences. All statistical analyses were performed with SPSS-19. Statistical significance was set at P<0.05. Data in figures are shown as means±SE; data in Table 1 are shown as means±SD.
Table 1.
Subject Characteristics
| Ex (n=18) |
St + Ex (n=19) |
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Age (yrs) | 43.8 ± 12.9 | 42.5 ± 9.6 | ||
| Sex | 7 male; 11 female | 6 male; 13 female | ||
| Weight (kg) | 97.9 ± 18.4 | 96.23 ± 18.3* † | 98.2 ± 19.8 | 98.9 ± 21.4 |
| BMI (kg·m−1) | 33.9 ± 4.6 | 33.3 ± 4.6 | 33.9 ± 4.6 | 34.2 ± 5.1 |
| Body fat (%) | 39.3 ± 6.3 | 38.5 ± 6.4† | 40.3 ± 6.5 | 39.7 ± 6.3 |
| Fat Mass (kg) | 38.0 ± 8.5 | 36.6 ± 8.0† | 39.7 ± 11.6 | 39.4 ± 11.9 |
| Lean Body Mass (kg) | 58.1 ± 12.6 | 57.8 ± 13.2 | 55.5 ± 12.7 | 56.7 ± 13.6 * ‡ |
| Fasting glucose (mmol/l) | 4.86 ± 0.53 | 4.80 ± 0.57 | 4.95 ± 0.35 | 5.04 ± 0.45 |
| Triacylglycerol (mg/dl) | 142.2 ± 91.1 | 127.9 ± 81.4 | 124.4 ± 71.3 | 94.2 ± 45.5 |
| Total cholesterol (mg/dl) | 190.7 ± 50.7 | 193.3 ± 54.7 | 203 ± 51.0 | 144.6 ± 25.8 * § |
| LDL (mg/dl) | 122.7 ± 37.1 | 125.6 ± 43.8 | 147.9 ± 55.2 | 90.9 ± 31.2 * § |
| HDL (mg/dl) | 44.2 ± 9.6 | 47.11 ± 13.2 | 45.8 ± 12.1 | 45.6 ± 12.5 |
Subject characteristics before (Pre) and after (Post) 12 weeks of supervised aerobic exercise training (Ex) or combination exercise plus statin therapy (St+Ex). Data are expressed as Means ± SD.
P<0.05 for between group difference in change from baseline.
P<0.05,
P<0.01, and
P<0.001 for within group change from baseline.
BMI: body mass index; LDL: low density lipoprotein cholesterol; HDL: high density lipoprotein cholesterol.
RESULTS
STUDY PARTICIPANTS
Forty-one eligible volunteers were randomized to the exercise alone (n=21) or exercise plus statin (n=20) groups. All participants were statin-naïve. Three participants withdrew from the exercise group; one due to time constraints, one because of a desire to lose weight, and one because of a foot injury occurring outside of the intervention, One participant was released from the exercise plus statin group due to complications unrelated to the intervention. Thirty-seven participants (13 males and 24 females) completed the study. Cardiorespiratory fitness and blood variables are available from 18 participants in the exercise only group and 19 participants from the exercise plus statin group. Skeletal muscle citrate synthase activity data is available in a subset of participants (12 statin plus exercise subjects and 17 exercise only subjects).
At baseline, there were no differences between the groups for any of the outcome variables measured (Table 1).
ADHERENCE
There were no group differences in adherence to the exercise program, with participants in the exercise only group completing 95±2% of prescribed exercise sessions and participants in the statins plus exercise group completing 95±1% of prescribed sessions. Medication adherence was not quantified but was monitored by asking participants if they had any problems taking the medication. In addition, cholesterol was uniformly lowered in the statin group providing evidence that mediation adherence was more than adequate.
EFFECTS OF INTERVENTION ON ANTHROPOMETRIC OUTCOMES
At 12 weeks, body weight decreased significantly in the exercise group (Table 1; P<0.01for change within group) but not the exercise plus statin group (P<0.01 for between-group difference in change from baseline). Similarly, there was a significant decrease in fat mass in the exercise group (P<0.05). In the exercise plus statin group, the decrease in fat mass approached significance (P=0.056). Lean body mass increased significantly in the exercise plus statin group only (P<0.05 for with-in group change from baseline; P<0.05 for difference in between-group change from baseline).
EFFECTS OF INTERVENTION ON LIPID PROFILES
Lipid profiles are shown in Table 1. Total cholesterol decreased by 29% (P<0.001 for within-group change from baseline), and LDL decreased by 38% (P<0.001) in the exercise plus statin group. There were no significant changes in total cholesterol or LDL in the exercise group (P<0.001 for between-group differences in change from baseline). HDL did not change significantly in either group.
EFFECTS OF INTERVENTION ON CARDIORESPIRATORY FITNESS
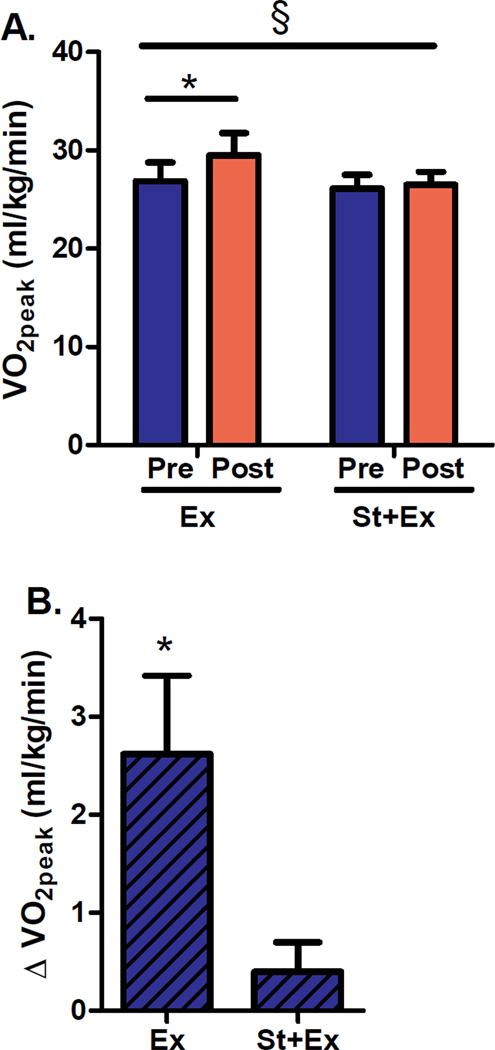
Simvastatin significantly attenuated increases in cardiorespiratory fitness (VO2peak, expressed as milliliters of oxygen consumed per kilogram of body weight per minute), in response to the exercise training program (P<0.005 for between-group difference in change from baseline; Figure 1A). Cardiorespiratory fitness, increased by 10% in response to exercise training alone (P<0.005 for change from baseline) but did not increase significantly in the group assigned to combined exercise plus statin therapy (Figure 1B).
Figure 1. Cardiorespiratory fitness (VO2peak).
(A) VO2peak before (Pre) and after (Post) 12 weeks of supervised aerobic exercise training (Ex) or combination exercise plus statin therapy (St+Ex). (B) VO2peak presented as within group change (Δ) from baseline. Data are expressed as means±SE. * P<0.005 for within-group change from baseline. § P<0.005 for between-group difference in change from baseline.
Because total body mass and fat mass decreased significantly in the exercise group and lean mass increased in the exercise plus statin group, we also compared changes in cardiorespiratory fitness expressed as absolute VO2peak (total liters of oxygen consumed per minute), VO2peak relative to lean body mass (milliliters of oxygen consumed per kilogram of lean body mass per minute), treadmill time to exhaustion (seconds), as well as peak workload (metabolic equivalents; METS). Regardless of how the data were expressed, cardiorespiratory fitness increased significantly in response to exercise training alone but not in response to exercise plus statin (P<0.005 for between group difference in change from baseline), indicating that simvastatin significantly attenuated exercise-mediated increases in cardiorespiratory fitness.
EFFECTS OF INTERVENTION ON SKELETAL MUSCLE CITRATE SYNTHASE ACTIVITY
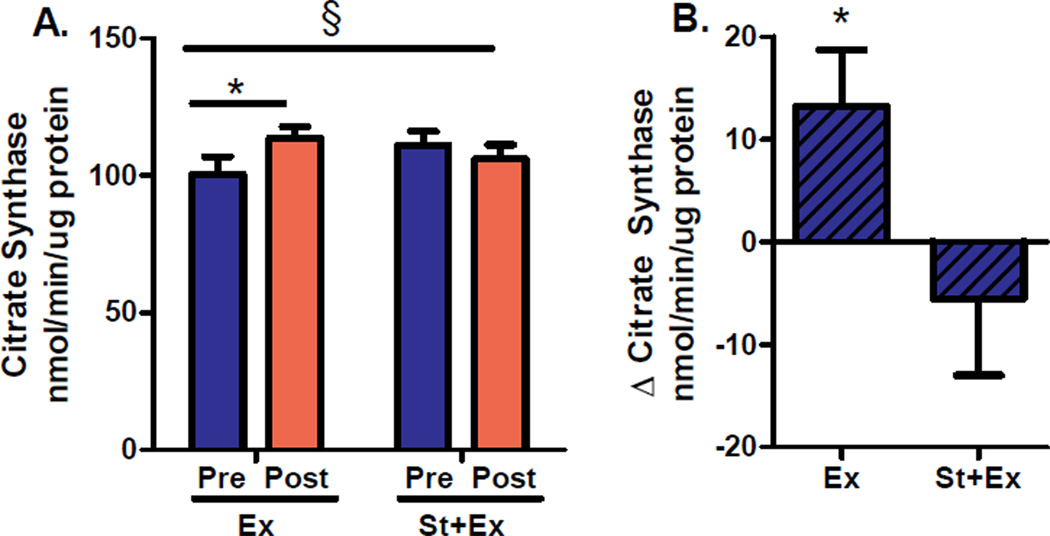
Simvastatin prevented exercise training induced increases in skeletal muscle citrate synthase activity, a marker of mitochondrial content (P<0.05 for between-group difference in change from baseline; Figure 2). Skeletal muscle citrate synthase activity increased by 13% in the exercise only group (P<0.05 for change from baseline) and decreased by 4.5% in the exercise plus statin group (not significant for change from baseline; Figure 2). Similar patterns were observed in the protein content of skeletal muscle mitochondrial complexes I, II, III, and IV (data not shown), providing further evidence that statins minimized or negated responses to exercise training.
Figure 2. Citrate synthase activity, a marker of skeletal muscle mitochondrial content.
(A) Citrate synthase activity before (Pre) and after (Post) 12 weeks of supervised aerobic exercise training (Ex) or combination exercise plus statin therapy (St+Ex). (B) Citrate synthase activity presented as within group change (Δ) from baseline. Data are expressed as means±SE. * P<0.05 for within-group change from baseline. § P<0.05 for between-group difference in change from baseline.
DISCUSSION
In this trial, simvastatin abated improvements in cardiorespiratory fitness and skeletal muscle citrate synthase activity, a marker of mitochondrial content, following 12 weeks of aerobic exercise training in overweight and obese volunteers at risk for the metabolic syndrome. The results have direct clinical ramifications as patients at risk for the metabolic syndrome are commonly prescribed statins to lower blood lipids and at the same time advised to exercise to improve fitness, both of which are independently proven to lower cardiovascular disease risk.
During exercise, skeletal muscle energy flux and mitochondrial respiration are increased to provide ATP for muscle contractions. Exercise also stimulates transcriptional responses that if repeated over time, promote mitochondrial biogenesis (increase in number or content) and increase mitochondrial oxidative capacity (improved function). These adaptations, which lead to greater capacity for skeletal muscle oxygen consumption, are a key component of exercise-mediated improvements in cardiorespiratory fitness. Our findings suggest that simvastatin may mitigate improvements in fitness in response to exercise training by impairing increases in skeletal muscle mitochondrial content and function. In support of these data, physiologic doses of simvastatin disrupt mitochondrial respiration, increase oxidative stress, and activate mitochondrial apoptotic pathways in isolated skeletal muscle fibers (27). Similar observations have been reported in studies of muscle fibers taken from patients using statins (28), and high dose simvastatin (80 mg per day) has been shown to decrease skeletal muscle mitochondrial content in the absence of exercise (29, 30). Statins have also been shown to reduce skeletal muscle force production (31), running capacity (17, 18) and voluntary running volume (31) in rodents. Collectively, these data indicate that statins may induce mitochondrial oxidative stress which activates pathways of apoptosis or autophagy, mitigating increases in mitochondrial content and oxidative capacity in response to exercise training.
It should be mentioned that a placebo was not given to participants in the exercise only group. Thus, participants were aware of their group assignment, introducing the possibility of a ‘placebo effect’. However, we do not think a placebo effect was the cause of our outcomes when our data are considered in light of accumulating evidence that statins can cause undesirable effects on skeletal muscle mitochondrial function ((13–18, 27–30, 32). One of the primary strengths of this trial is the robust agreement between changes in functional (cardiorespiratory fitness) and biochemical (skeletal muscle citrate synthase) outcomes in response to the interventions. To our knowledge, this is the first randomized controlled clinical trial directly comparing the effects of exercise training to exercise plus statins on changes in both functional and biochemical outcomes in previously statin naïve patients.
Therapeutic options which minimize the adverse effects of LDL lowering therapies on adaptions to exercise training are warranted. Emerging evidence indicates that some statins (e.g., pravastatin) may be less prone to disturbing skeletal muscle mitochondrial content or function than others (33). Alternatively, coenzyme Q10 supplementation or commencing exercise training prior to initiating statin therapy may lessen some of the untoward effects of statins (17, 31, 34). However, these findings are not always consistent (29), and many therapeutic alternatives are in the early stages of investigation, indicating that further research is needed in this area.
Statins are widely prescribed in combination with exercise to lower risk of cardiovascular disease morbidity and mortality. Every 1 millimole per liter reduction in LDL is associated with a 10–20% reduction in risk of cardiovascular events (35, 36) and all-cause mortality (36), while every 1 MET (3.5 milliters of oxygen per kilogram of body weight per minute) increase in fitness is associated with an 18% reduction in cardiovascular disease mortality (37) and an 11–50% reduction in all-cause mortality (7, 37, 38). As cardiorespiratory fitness increases, the predictive value of LDL on coronary heart disease mortality is significantly attenuated in men (39). In a large, prospective study of dyslipidaemic veterans, both fitness and statin use were independently associated with low mortality, with the lowest risk of mortality observed in highly fit patients taking statins (40). Notably, patients in the highest quartile of fitness had a 60–70% reduction in all-cause mortality relative to patients in the lowest quartile of fitness, irrespective of statin use, and the low-fit patients taking statins had a higher risk of mortality than highly fit patients not taking statins. Collectively, these data indicate that maintaining or improving cardiorespiratory fitness may mitigate some of the negative health consequences of elevated LDL. However, we are unaware of randomized, placebo-controlled trials directly comparing the long-term cardio-protective effects of exercise alone to statins plus exercise. Until such studies are undertaken, the relative importance of improving fitness and lowering LDL in moderating risk of cardiovascular events and death should be carefully weighed in the clinical setting.
In conclusion, simvastatin attenuates increases in cardiorespiratory fitness and skeletal muscle mitochondrial content associated with exercise training in previously sedentary, overweight or obese patients at risk of the metabolic syndrome. Given the strong independent cardio-protective effects of increasing cardiorespiratory fitness or lowering LDL, the benefits and risks of each should be carefully considered when choosing treatment modalities.
Acknowledgements
We thank Charla Jay and Peggy Nigh for technical assistance, Drs. R. Scott Rector, and Tom Thomas for consultation, and Drs. Adam Whaley-Connell, Nicholas Szary, and Abhishek Choudhary for providing medical coverage. Funding provided by MU Research Board Grant (JPT), Veterans Affairs Career Development Award (JPT), and American Heart Association Midwest Affiliate Clinical Research Award - #09CRP2260136 (JPT), and NIH grant T32 AR048523 (CRM). This work was also supported with resources and the use of facilities at the Harry S Truman Memorial Veterans Hospital in Columbia, MO.
Selected Abbreviations
- BMI
body mass index
- LDL
low density lipoprotein cholesterol
- HDL
high density lipoprotein cholesterol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There is no relationship with industry on this project.
References
- 1.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 2.Shaw DI, Hall WL, Williams CM. Metabolic syndrome: what is it and what are the implications? Proc Nutr Soc. 2005;64:349–357. doi: 10.1079/pns2005442. [DOI] [PubMed] [Google Scholar]
- 3.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Medicine and science in sports and exercise. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. Jama. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 5.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. The New England journal of medicine. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 6.Kokkinos P, Myers J, Kokkinos JP, et al. Exercise capacity and mortality in black and white men. Circulation. 2008;117:614–622. doi: 10.1161/CIRCULATIONAHA.107.734764. [DOI] [PubMed] [Google Scholar]
- 7.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. Jama. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 8.Lim GB. Vascular disease: Even low-risk individuals can benefit from statin therapy. Nature reviews Cardiology. 2012;9:371. doi: 10.1038/nrcardio.2012.79. [DOI] [PubMed] [Google Scholar]
- 9.Sinzinger H, Wolfram R, Peskar BA. Muscular side effects of statins. J Cardiovasc Pharmacol. 2002;40:163–171. doi: 10.1097/00005344-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Sinzinger H, O'rady J. Professional athletes suffering from familial hypercholesterolaemia rarely tolerate statin treatment because of muscular problems. British journal of clinical pharmacology. 2004;57:525–528. doi: 10.1111/j.1365-2125.2004.02044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kearns AK, Bilbie CL, Clarkson PM, et al. The creatine kinase response to eccentric exercise with atorvastatin 10 mg or 80 mg. Atherosclerosis. 2008;200:121–125. doi: 10.1016/j.atherosclerosis.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 12.Parker BA, Augeri AL, Capizzi JA, et al. Effect of statins on creatine kinase levels before and after a marathon run. The American journal of cardiology. 2012;109:282–287. doi: 10.1016/j.amjcard.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 13.Sirvent P, Mercier J, Vassort G, Lacampagne A. Simvastatin triggers mitochondria-induced Ca2+ signaling alteration in skeletal muscle. Biochem Biophys Res Commun. 2005;329:1067–1075. doi: 10.1016/j.bbrc.2005.02.070. [DOI] [PubMed] [Google Scholar]
- 14.Dirks AJ, Jones KM. Statin-induced apoptosis and skeletal myopathy. Am J Physiol Cell Physiol. 2006;291:C1208–C1212. doi: 10.1152/ajpcell.00226.2006. [DOI] [PubMed] [Google Scholar]
- 15.Sirvent P, Bordenave S, Vermaelen M, et al. Simvastatin induces impairment in skeletal muscle while heart is protected. Biochem Biophys Res Commun. 2005;338:1426–1434. doi: 10.1016/j.bbrc.2005.10.108. [DOI] [PubMed] [Google Scholar]
- 16.Wu JS, Buettner C, Smithline H, Ngo LH, Greenman RL. Evaluation of skeletal muscle during calf exercise by 31-phosphorus magnetic resonance spectroscopy in patients on statin medications. Muscle & nerve. 2011;43:76–81. doi: 10.1002/mus.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muraki A, Miyashita K, Mitsuishi M, Tamaki M, Tanaka K, Itoh H. Coenzyme Q10 reverses mitochondrial dysfunction in atorvastatin-treated mice and increases exercise endurance. J Appl Physiol. 2012;113:479–486. doi: 10.1152/japplphysiol.01362.2011. [DOI] [PubMed] [Google Scholar]
- 18.Bouitbir J, Charles AL, Rasseneur L, et al. Atorvastatin treatment reduces exercise capacities in rats: involvement of mitochondrial impairments and oxidative stress. J Appl Physiol. 2011;111:1477–1483. doi: 10.1152/japplphysiol.00107.2011. [DOI] [PubMed] [Google Scholar]
- 19.Thomas TR, Warner SO, Dellsperger KC, et al. Exercise and the metabolic syndrome with weight regain. J Appl Physiol. 2010;109:3–10. doi: 10.1152/japplphysiol.01361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunninghake DB, Ballantyne CM, Maccubbin DL, Shah AK, Gumbiner B, Mitchel YB. Comparative effects of simvastatin and atorvastatin in hypercholesterolemic patients with characteristics of metabolic syndrome. Clin Ther. 2003;25:1670–1686. doi: 10.1016/s0149-2918(03)80162-5. [DOI] [PubMed] [Google Scholar]
- 21.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972;18:499–502. [PubMed] [Google Scholar]
- 22.Sheldon RD, Roseguini BT, Thyfault JP, Crist BD, Laughlin MH, Newcomer SC. Acute impact of intermittent pneumatic leg compression frequency on limb hemodynamics, vascular function, and skeletal muscle gene expression in humans. J Appl Physiol. 2012;112:2099–2109. doi: 10.1152/japplphysiol.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen S, Nielsen J, Hansen CN, et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. The Journal of physiology. 2012;590:3349–3360. doi: 10.1113/jphysiol.2012.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rector RS, Uptergrove GM, Borengasser SJ, et al. Changes in skeletal muscle mitochondria in response to the development of type 2 diabetes or prevention by daily wheel running in hyperphagic OLETF rats. Am J Physiol Endocrinol Metab. 2010;298:E1179–E1187. doi: 10.1152/ajpendo.00703.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iglay HB, Thyfault JP, Apolzan JW, Campbell WW. Resistance training and dietary protein: effects on glucose tolerance and contents of skeletal muscle insulin signaling proteins in older persons. The American journal of clinical nutrition. 2007;85:1005–1013. doi: 10.1093/ajcn/85.4.1005. [DOI] [PubMed] [Google Scholar]
- 26.Rector RS, Uptergrove GM, Morris EM, et al. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. American journal of physiology Gastrointestinal and liver physiology. 2011;300:G874–G883. doi: 10.1152/ajpgi.00510.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwak HB, Thalacker-Mercer A, Anderson EJ, et al. Simvastatin impairs ADP-stimulated respiration and increases mitochondrial oxidative stress in primary human skeletal myotubes. Free radical biology & medicine. 2012;52:198–207. doi: 10.1016/j.freeradbiomed.2011.10.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sirvent P, Fabre O, Bordenave S, et al. Muscle mitochondrial metabolism and calcium signaling impairment in patients treated with statins. Toxicology and applied pharmacology. 2012;259:263–268. doi: 10.1016/j.taap.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Paiva H, Thelen KM, Van Coster R, et al. High-dose statins and skeletal muscle metabolism in humans: a randomized, controlled trial. Clinical pharmacology and therapeutics. 2005;78:60–68. doi: 10.1016/j.clpt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Schick BA, Laaksonen R, Frohlich JJ, et al. Decreased skeletal muscle mitochondrial DNA in patients treated with high-dose simvastatin. Clinical pharmacology and therapeutics. 2007;81:650–653. doi: 10.1038/sj.clpt.6100124. [DOI] [PubMed] [Google Scholar]
- 31.Meador BM, Huey KA. Statin-associated changes in skeletal muscle function and stress response after novel or accustomed exercise. Muscle & nerve. 2011;44:882–889. doi: 10.1002/mus.22236. [DOI] [PubMed] [Google Scholar]
- 32.Bouitbir J, Charles AL, Echaniz-Laguna A, et al. Opposite effects of statins on mitochondria of cardiac and skeletal muscles: a 'mitohormesis' mechanism involving reactive oxygen species and PGC-1. European heart journal. 2012;33:1397–1407. doi: 10.1093/eurheartj/ehr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufmann P, Torok M, Zahno A, Waldhauser KM, Brecht K, Krahenbuhl S. Toxicity of statins on rat skeletal muscle mitochondria. Cell Mol Life Sci. 2006;63:2415–2425. doi: 10.1007/s00018-006-6235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouitbir J, Daussin F, Charles AL, et al. Mitochondria of trained skeletal muscle are protected from deleterious effects of statins. Muscle & nerve. 2012;46:367–373. doi: 10.1002/mus.23309. [DOI] [PubMed] [Google Scholar]
- 35.Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barlow CE, Defina LF, Radford NB, et al. Cardiorespiratory fitness and long-term survival in "low-risk" adults. Journal of the American Heart Association. 2012;1:e001354. doi: 10.1161/JAHA.112.001354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blair SN, Kohl HW, 3rd, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. Jama. 1995;273:1093–1098. [PubMed] [Google Scholar]
- 39.Farrell SW, Finley CE, Grundy SM. Cardiorespiratory Fitness, LDL Cholesterol, and CHD Mortality in Men. Medicine and science in sports and exercise. 2012;44:2132–2137. doi: 10.1249/MSS.0b013e31826524be. [DOI] [PubMed] [Google Scholar]
- 40.Kokkinos PF, Faselis C, Myers J, Panagiotakos D, Doumas M. Interactive effects of fitness and statin treatment on mortality risk in veterans with dyslipidaemia: a cohort study. Lancet. 2012 doi: 10.1016/S0140-6736(12)61426-3. [DOI] [PubMed] [Google Scholar]