Abstract
Background
As a consequence of the substantial rise in the prescription of opioids for the treatment of chronic noncancer pain, greater attention has been paid to the factors that may be associated with an increased risk for prescription opioid misuse. Recently, a growing number of studies have shown that patients with high levels of catastrophizing are at increased risk for prescription opioid misuse.
Objective
The primary objective of this study was to examine the variables that might underlie the association between catastrophizing and risk for prescription opioid misuse in patients with chronic pain.
Methods
Patients with chronic musculoskeletal pain (n = 115) were asked to complete the SOAPP-R, a validated self-report questionnaire designed to identify patients at risk for prescription opioid misuse. Patients were also asked to complete self-report measures of pain intensity, catastrophizing, anxiety, and depression.
Results
Consistent with previous research, we found that catastrophizing was associated with an increased risk for prescription opioid misuse. Results also revealed that the association between catastrophizing and risk for opioid misuse was partially mediated by patients’ levels of anxiety. Follow-up analyses, however, indicated that catastrophizing remained a significant ‘unique’ predictor of risk for opioid misuse even when controlling for patients’ levels of pain severity, anxiety and depressive symptoms.
Discussion
Discussion addresses the factors that might place patients with high levels of catastrophizing at increased risk for prescription opioid misuse. The implications of our findings for the management of patients considered for opioid therapy are also discussed.
Keywords: Prescription opioid misuse, catastrophizing, anxiety, depression, chronic pain
1. INTRODUCTION
Over the past decade, there has been a substantial rise in the use of opioids for the treatment of chronic noncancer pain. Despite the potential benefits of opioid therapy, long-term opioid use may lead to a number of adverse outcomes, including prescription opioid misuse and addiction (Ballantyne, 2010; Banta-Green et al., 2009a; Compton et al., 2008; Edlund, 2011; Jamison et al., 2010; Morasco et al., 2013; Sullivan et al., 2010). Prescription opioid misuse, which broadly refers to the use of opioids in a manner other than prescribed, has become a significant concern for clinicians prescribing opioids (Banta-Green et al., 2009b; Compton et al., 2008; Jamison et al., 2011; Sehgal et al., 2012). Because of these concerns, many investigators have turned their attention to the factors that may be associated with an increased risk for prescription opioid misuse in patients with chronic pain.
A number of demographic and background variables have been found to be associated with an increased risk for prescription opioid misuse in patients with chronic pain, including young age and history of substance abuse (Edlund et al., 2007a; Michna et al., 2004; Ives et al., 2006; Morasco et al., 2008, 2013; Schieffer et al., 2005). Pain-related variables, such as self-reports of pain severity, have also been found to be associated with an increased risk for prescription opioid misuse, with patients reporting high levels of pain being at greater risk for opioid misuse than patients reporting low levels of pain (Adams et al., 2004; Grattan et al., 2012; Jamison et al., 2009; Morasco et al., 2013). In a recent study, it has also been found that patients with high levels of experimental pain sensitivity (i.e., hyperalgesic patients) are at greater risk for prescription opioid misuse than patients with low levels of pain sensitivity (Edwards et al., 2011a).
Associations have also been found between psychological factors and risk for prescription opioid misuse. For example, several studies have found that patients with psychiatric disorders are at greater risk for prescription opioid misuse (Dersh et al., 2008; Grattan et al., 2012; Turk et al., 2008; Wasan et al., 2007). Patients scoring high on measures of negative affect such as anxiety (Edlund et al., 2007b; Morasco et al., 2013; Schieffer et al., 2005; Wasan et al., 2007; Wilsey et al., 2008) and depression (Edlund et al., 2007a; Grattan et al., 2012; Morasco et al., 2013; Wasan et al., 2007) have also been found to be at increased risk for prescription opioid misuse. Finally, an increasing number of studies have shown that patients high in pain catastrophizing, a negative and pessimistic orientation toward pain, are at increased risk for prescription opioid misuse (Edwards et al., 2011a; Ferrari et al., 2012; Jamison et al., 2009; Morasco et al., 2013). Patients who are high in catastrophizing tend to ruminate about pain, to magnify the threat value of pain, and to experience feelings of helplessness when in pain (Edwards et al., 2006; Keefe et al., 2000; Sullivan et al., 2001). In a recent study conducted among patients with chronic pain, Morasco et al. (2013) found that pain catastrophizing was associated with an increased risk for prescription opioid misuse even after controlling for patients’ demographic variables, substance use disorder (SUD) status, and depressive symptoms.
To date, little is known on the specific mechanisms by which catastrophizing may lead to an increased risk for prescription opioid misuse. One possibility is that patients with high levels of catastrophizing are at increased risk for prescription opioid misuse because they experience high levels of clinical pain. Another possibility is that patients with high levels of catastrophizing are at increased risk for prescription opioid misuse due to heightened basal pain sensitivity, or alterations in central pain processing. Finally, it is possible that high catastrophizers are at increased risk for prescription opioid misuse due to high levels of negative affect. Past research has shown that catastrophizing is associated with heightened levels of pain severity (for a review, see Sullivan et al., 2001), pain sensitivity (for a review, see Quartana et al., 2009), and negative affect (for a review, see Edwards et al., 2011b).
The primary purpose of the present study was to examine the mechanisms that might underlie the association between catastrophizing and risk for prescription opioid misuse in patients with chronic pain. In this study, a sample of patients with chronic musculoskeletal pain were asked to complete the SOAPP-R (Butler et al., 2008), a self-report questionnaire designed to identify patients at risk for prescription opioid misuse. Analyses examined the potential role of patients’ pain severity, pain sensitivity, and negative affect as mediators of the association between catastrophizing and risk for prescription opioid misuse. Follow-up analyses examined the unique (i.e, independent) influence of catastrophizing on risk for prescription opioid misuse.
2. METHODS
2.1. Participants
Participants were 115 patients recruited from the Pain Management Center at Brigham and Women’s Hospital (BWH). Patients with a diagnosis of spinal pain, with or without radicular symptoms, and who had been experiencing pain for at least 6 months were invited to participate. Patients were excluded if they had a diagnosis of cancer or other malignant disease, or had cognitive limitations that precluded providing self-report data. Patients were also excluded if they had any active substance use disorder (SUD). Patients with an active SUD were excluded given current clinical practice guidelines and principles at the BWH Pain Center regarding the management of patients with an active SUD. Patients with an active SUD are generally referred to a local addiction treatment facility before undergoing pain treatment at the Pain Center, and before being eligible for study participation.
2.2. Procedure and measures
All procedures were approved by the Partners Institutional Review Board at BWH. Upon arrival at the laboratory, participants signed a consent form, provided demographic information, and reported whether or not they were currently taking any prescription opioid medication. Patients’ reports of medication were verified by a research assistant after the study session using the electronic medical record system. In addition to providing demographic and medication use information, participants were asked to complete self-report questionnaires (see below) prior to undergoing a series of standardized psychophysical pain testing procedures.
2.2.1. Screener and Opioid Assessment for Patients with Pain-Revised
The SOAPP-R (Butler et al., 2008) is a 24-item screening questionnaire validated for patients with chronic pain, and designed to assess patients’ risk for prescription opioid misuse. SOAPP-R items are rated from 0 (never) to 4 (very often) (e.g., How often have you felt consumed by the need to get pain medication?). The SOAPP-R has been shown to have good reliability and predictive validity. The SOAPP-R has been shown to be a significant predictor of prescription opioid misuse outcomes derived on the basis of other instruments, such as the Prescription Drug Use Questionnaire (PDUQ) and the Prescription Opioid Therapy Questionnaire (POTQ) (Butler et al., 2008, 2009). Multi-center prospective studies have also shown that the SOAPP-R is a significant predictor of patients who will actually turn out to misuse opioid medication, as measured by physicians’ ratings of opioid misuse or by urine toxicology screens (Akbik et al., 2006; Butler et al., 2004, 2008, 2009).
2.2.2. Brief Pain Inventory
The Brief Pain Inventory (BPI; Tan et al., 2004) was used as a measure of pain severity associated with patients’ musculoskeletal pain condition. On the BPI, patients are asked to rate their current level of pain on a numeric rating scale (NRS) with the endpoints 0 (no pain) and 10 (extreme pain). Patients are also asked to rate the degree to which pain interferes with their physical and emotional functioning on a NRS, with the endpoints 0 (does not interfere) and 10 (completely interferes). The BPI has been shown to be a reliable and valid measure of pain severity and pain interference among patients with chronic pain (Jamison et al., 2009; Tan et al., 2004; Wasan et al., 2009).
2.2.3. Pain Anxiety Symptoms Scale
The Pain Anxiety Symptoms Scale (PASS; McCracken et al., 2002) was used as a measure of pain-related anxiety. The PASS is a 20-item self-report questionnaire in which participants make ratings about anxiety on a six-point Likert scale ranging from 0 (never) to 5 (always). The PASS has been shown to be a reliable and valid measure of pain-related anxiety in patients with chronic pain (McCracken et al., 1992, 2002; Roelofs et al., 2004).
2.2.4. Beck Depression Inventory
The Beck Depression Inventory (BDI-II; Beck et al., 1996) was used as a measure of depressive symptomatology. The BDI consists of 21 items describing various symptoms of depression, and respondents choose statements that describe how they have been feeling over the past two weeks. Responses are summed to yield an overall index of depressive symptoms. The BDI has been shown to be a reliable and valid index of depressive symptoms in patients with pain (Poole et al., 2006; Sullivan et al., 2003; Vowles et al., 2004).
2.2.5. Pain Catastrophizing Scale
The Pain Catastrophizing Scale (PCS; Sullivan et al., 1995) was used as a measure of catastrophic thinking about pain. The PCS contains 13 items describing different thoughts and feelings that individuals may experience when they are in pain. Participants are asked to reflect on past painful experiences and to indicate the degree to which they experienced each of 13 thoughts or feelings when experiencing pain, on a 5-point scale from (0) not at all to (4) all the time. Responses are summed, and higher scores reflect higher levels of pain catastrophizing. Several studies in patients with pain have supported the reliability and the validity of the PCS as a measure of catastrophic thinking (Edwards et al., 2006; Keefe et al., 2003; Peters et al., 2005; Sullivan et al., 2001). In patients with pain, high scores on the PCS have been found to be associated with a wide range of negative pain-related outcomes, including heightened pain severity (Edwards et al., 2006; Sullivan et al., 2001), post-surgical pain intensity (Khan et al., 2009; Sullivan et al., 2009), and pain-related disability (Edwards et al., 2011b; Keefe et al., 2000; Sullivan et al., 2001).
2.2.6. Pain sensitivity
Pain sensitivity was assessed using quantitative sensory testing (QST). In the laboratory, QST typically involves the administration of calibrated noxious stimuli and the assessment of participants’ pain responses using a visual analogue scale (VAS). QST methods are commonly used to assess inter-individual differences in somatosensory function and pain sensitivity (Arendt-Nielsen et al., 2009; Edwards et al., 2005a; Fillingim and Lautenbacher, 2004; Greenspan et al., 2011; Yarnitsky and Pud, 2004).
During the QST session, patients were seated comfortably in a reclining chair for approximately 30 minutes while they underwent brief thermal pain threshold assessment. Thermal stimuli were delivered using a contact thermode (Medoc Advanced Medical Systems, Ramat Yishai, Israel). Thermal assessment included sampling of warmth and cool thresholds, followed by heat pain thresholds (HPThs) and cold pain thresholds (CPThs). Consistent with previous studies (Edwards et al., 2008, 2011a; Fillingim et al., 2004), thermal stimuli were delivered on the ventral forearm using an ascending method of limits, with a rate of temperature change of .5°C/Sec. Patients were instructed to verbally report when the thermal stimulus first became painful (i.e., pain threshold). Patients’ pain thresholds were recorded by the experimenter, who was sat next to patients throughout the QST session.
2.3. Data reduction and analysis
Descriptive data for continuous variables were analyzed using Independent samples t-tests, and descriptive data for categorical variables were analyzed using chi-square tests.
For the purposes of the present study, thermal pain thresholds (TPThs) were used as an index of pain sensitivity. To create this index, HPThs and CPThs were standardized to produce a normal distribution, and CPThs were reversed because CPThs and HPThs are opposite in their directionality (i.e., lower HPThs represent greater pain sensitivity, and higher CPThs represent greater pain sensitivity). CPThs and HPThs were then averaged to derive a composite index of thermal pain thresholds (TPThs), with lower scores on this index reflecting greater pain sensitivity (Diatchenko et al., 2005; Edwards et al., 2011a).
Associations between measures of pain severity (BPI), pain sensitivity (TPThs), negative affect (PASS, BDI), catastrophizing (PCS), and risk for opioid misuse (SOAPP-R) were assessed using Pearson correlations. The potential mediators of the association between catastrophizing and risk for opioid misuse were examined based on the procedure described by Baron and Kenny (1986). First, variables that showed significant zero-order correlations, both with the PCS and SOAPP-R, were considered as potential mediators. A series of multiple regression analyses were then conducted to determine whether preconditions for mediation were met, separately for each potential mediator. When preconditions for mediation were met, a hierarchichal multiple regression analysis and a Sobel test were conducted to test the significance of the mediation effect. Mediation was supported if the association between the predictor (i.e., PCS) and the dependent variable (i.e., SOAPP-R) was no longer significant after controlling for the potential mediator (full mediation), or if the association between PCS and SOAPP-R was significantly decreased (partial mediation). Following these mediational analyses, a hierarchical multiple regression analysis was conducted to examine the unique (i.e, independent) influence of catastrophizing on risk for opioid misuse (SOAPP-R).
3. RESULTS
3.1. Descriptive statistics
Descriptive statistics for all study measures are presented in Table 1, separately for men and women. Analyses revealed no significant sex differences in age, self-reported pain severity (BPI), pain interference (BPI), pain sensitivity (TPThs), pain-related anxiety (PASS), depression (BDI), catastrophizing (PCS), or risk for prescription opioid misuse (SOAPP-R) (all p’s > .05). Men and women did not differ significantly in the use of opioids, Χ2 (1) = .38, ns.
Table 1.
Descriptive data for study measures
| Men (n=48) |
Women (n=67) |
p | |
|---|---|---|---|
| Opioid status | 52.1 % | 46.3 % | .54 |
| Age | 46.0 (11.6) | 48.3 (10.3) | .25 |
| Pain interference (BPI) | 3.1 (1.6) | 3.7 (2.0) | .06 |
| Pain severity (BPI) | 4.9 (2.0) | 5.7 (2.1) | .06 |
| Pain sensitivity (TPThs) | 0.22 (0.8) | −0.10 (0.9) | .06 |
| Pain catastrophizing (PCS) | 20.9 (10.0) | 24.1 (12.7) | .15 |
| Depression (BDI) | 12.7 (9.3) | 14.6 (8.9) | .27 |
| Anxiety (PASS) | 38.9 (20.7) | 41.8 (17.3) | .42 |
| Risk for opioid misuse (SOAPP-R) | 20.4 (8.6) | 19.6 (10.9) | .69 |
Note. Opioid status refers to the % of patients currently taking opioids
Independent samples t-tests were conducted to examine differences between patients who were taking opioids and patients who were not taking opioids on study variables. Results indicated that patients who were taking opioids reported significantly higher levels of pain than patients who were not taking opioids, t (113) = −2.9, p < .05. These two groups, however, did not differ significantly on any other study variable (i.e., age, TPThs, BPI, PASS, BDI, PCS, SOAPP-R) (all p’s > .05).
3.2. Correlations among measures
Table 2 shows the correlations between measures of pain severity (BPI), pain sensitivity (TPThs), negative affect (PASS, BDI), and catastrophizing (PCS). The BPI was significantly correlated with the PASS (r = .43, p < .01), BDI (r = .39, p < .01), and PCS (r = .55, p < .01). Significant inter-correlations were also found between the PASS, BDI, and PCS (all p’s < .01).
Table 2.
Correlations among measures
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1. BPI-PS | - | −.02 | .43** | .39** | .55** | .19* |
| 2. TPThs | - | −.13 | −.16 | −.19* | −.19* | |
| 3. PASS | - | .47** | .69** | .44** | ||
| 4. BDI | - | .51** | .34** | |||
| 5. PCS | - | .45** | ||||
| 6. SOAPP-R | - |
Note. BPI-PS, Brief Pain Inventory-Pain severity index; TPThs, Thermal Pain thresholds; PASS. Pain Anxiety Symptoms Scale; BDI, Beck Depression Inventory; PCS, Pain Catastrophizing Scale; SOAPP-R. Screener and Opioid Assessment for Pain Patients-Revised
p < .05
p < .01
Correlational analyses revealed a significant negative correlation between thermal pain thresholds (TPThs) and the PCS (r = −.19, p < .05). In other words, higher TPThs were associated with lower levels of catastrophizing. TPThs were not significantly correlated with the BPI, PASS or BDI.
Table 2 also shows the correlations between measures of pain, negative affect, and risk for opioid misuse (SOAPP-R). Analyses revealed a significant positive correlation between the BPI and SOAPP-R (r = .19, p < .05), and a significant negative correlation between TPThs and the SOAPP-R (r = −.19, p < .05). The SOAPP-R was significantly correlated with the PASS (r = .44, p < .01), BDI (r = .34, p < .01), and PCS (r = .45, p < .01).
3.3. Mediators of the association between catastrophizing and risk for opioid misuse
The potential mediating role of pain severity (BPI), pain sensitivity (TPThs), negative affect (PASS, BDI) in the association between catastrophizing and risk for opioid misuse was examined using a series of hierarchical multiple regression analyses, as proposed by Baron and Kenny (1986). For these analyses, patients’ age, gender, and opioid status were used as covariates because prior research has indicated that these variables may be associated with an increased risk for prescription opioid misuse (Chabal et al., 1997; Edwards et al., 2011a; Ives et al., 2006; Michna et al., 2004). Given that the BPI and TPThs were not significantly associated with either the dependent variable (i.e., SOAPP-R) or the predictor (i.e., PCS) after adjusting for these covariates, preconditions for further mediation testing were not met and these variables were no longer considered as potential mediators (Baron and Kenny, 1986; Holmbeck, 1997).
Table 3 presents the results of hierarchical multiple regression analyses examining the role of pain-related anxiety as mediator of the association between catastrophizing and risk for opioid misuse. In the first regression analysis (regression 1a), the PCS accounted for 21 % of the variance in SOAPP-R scores after controlling for patients’ sex, age, and opioid status, Fchange (1, 110) = 29.4, p < .01. In the second regression analysis (regression 1b), the PASS accounted for 19 % of the variance in SOAPP-R scores after controlling for patients’ sex, age, and opioid status, Fchange (1, 110) = 26.1, p < .01. The PCS was entered in the last step of the analysis and accounted for 5 % of the variance in SOAPP-R scores, Fchange (1, 109) = 7.0, p < .05. After controlling for the PASS, the contribution of PCS to the prediction of SOAPP-R scores remained significant, but decreased from 21 % (Beta = .47) to 5 % (Beta = .31), suggesting a potential partial mediation effect of the PASS on the association between PCS and SOAPP-R (see Figure 1). Results of a Sobel test revealed that the partial mediation effect of the PASS was significant, Z = 2.1, p < .05.
Table 3.
Hierarchical multiple regression analyses examining the role of anxiety as mediator of the association between catastrophizing and risk for opioid misuse
| B | R2 change | F change | |
|---|---|---|---|
| Regression 1a: Catastrophizing predicting risk for opioid misuse (SOAPP-R) | |||
| Step 1 | 01 | .41 | |
| Sex | .03 | ||
| Age | −.06 | ||
| Opioid | .07 | ||
| Step 2 | |||
| PCS | .47 | .21 | 29.4** |
| Regression 1b: The mediating role of anxiety | |||
| Step 1 | .01 | .41 | |
| Sex | .03 | ||
| Age | −.06 | ||
| Opioid | .07 | ||
| Step 2 | |||
| PASS | .44 | .19 | 26.1** |
| Step 3 | |||
| PCS | .31 | .05 | 7.0* |
Note. Opioid, Opioid status; PASS. Pain Anxiety Symptoms Scale; PCS, Pain Catastrophizing Scale; SOAPP-R, Screener and Opioid Assessment for Patients with Pain-Revised
p < .05
p < .01
Figure 1.
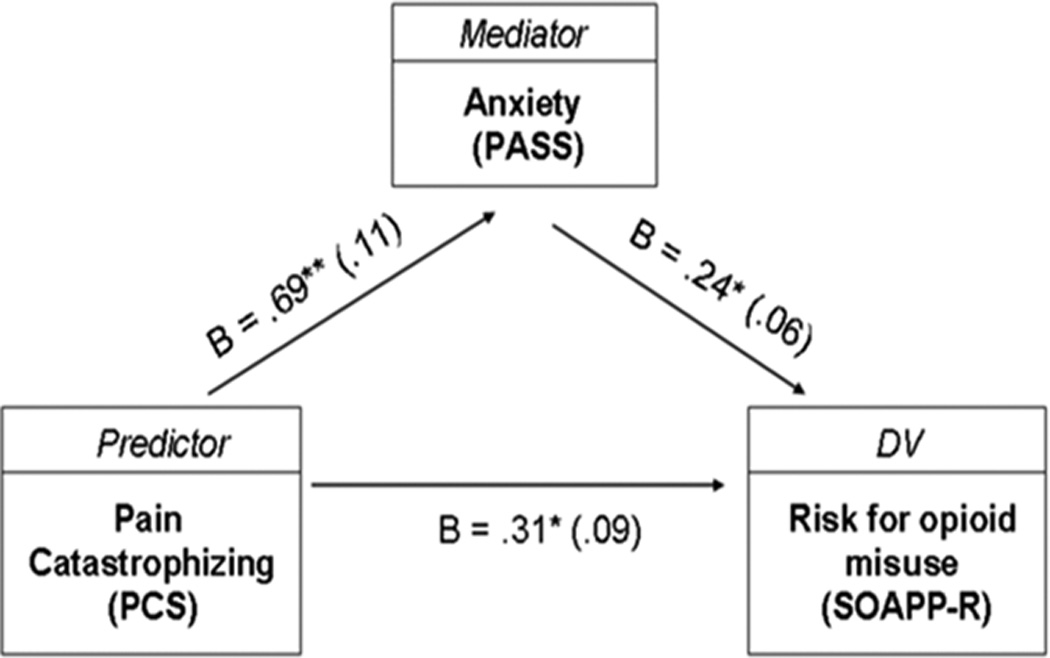
Partial mediation effect of anxiety on the association between catastrophizing and risk for prescription opioid misuse
As shown in Table 4, hierarchical multiple regression analyses were also conducted to examine the role of depression as mediator of the association between catastrophizing and risk for opioid misuse. In the first regression analysis (regression 1a), the PCS accounted for 21 % of the variance in SOAPP-R scores after controlling for patients’ sex, age, and opioid status, Fchange (1, 110) = 29.4, p < .01. In the second regression analysis (regression 1b), the BDI accounted for 11 % of the variance in SOAPP-R scores after controlling for patients’ sex, age, and opioid status, Fchange (1, 110) = 14.2, p < .01. The PCS was entered in the last step of the analysis and accounted for 10 % of the variance in SOAPP-R scores, Fchange (1, 109) = 15.6, p < .01. After controlling for the BDI, the contribution of PCS to the prediction of SOAPP-R scores remained significant, but decreased from 21 % (Beta = .47) to 10 % (Beta = .39), suggesting a potential partial mediation effect of the BDI in the association between PCS and SOAPP-R. Results of a Sobel test, however, revealed that the partial mediation effect of the BDI was not significant, Z = 1.4, ns.
Table 4.
Hierarchical multiple regression analyses examining the role of depression as mediator of the association between catastrophizing and risk for opioid misuse
| B | R2 change | F change | |
|---|---|---|---|
| Regression 1a: Catastrophizing predicting risk for opioid misuse (SOAPP-R) | |||
| Step 1 | .01 | .41 | |
| Sex | .03 | ||
| Age | −.06 | ||
| Opioid | .07 | ||
| Step 2 | |||
| PCS | .47 | .21 | 29.4** |
| Regression 1b: The mediating role of depressive symptoms | |||
| Step 1 | .01 | .41 | |
| Sex | .03 | ||
| Age | −.06 | ||
| Opioid | .07 | ||
| Step 2 | |||
| BDI | .34 | .11 | 14.2** |
| Step 3 | |||
| PCS | .39 | .10 | 15.6** |
Note. Opioid, Opioid status; BDI, Beck Depression Inventory; PCS, Pain Catastrophizing Scale; SOAPP-R; Screener and Opioid Assessment for Patients with Pain-Revised
p < .05
p < .01
3.4. Hierarchical multiple regression analysis examining the unique influence of catastrophizing on risk for opioid misuse
Table 5 shows the results of a hierarchical multiple regression analysis examining the unique (i.e., independent) influence of catastrophizing on risk for opioid misuse. In this analysis, patients’ sex, age, and opioid status were entered as covariates in the first step of the analysis but failed to contribute significantly to the prediction of SOAPP-R scores, R = .01, F (3, 111) = .41, ns. Measures of pain severity and pain sensitivity were entered in the second step of the analysis and contributed significant variance to the prediction of SOAPP-R scores, R2 change = .08, F (2, 109) = 4.5, p < .05. Measures of negative affect were entered in the third step of the analysis and contributed significant variance to the prediction of SOAPP-R scores, R2 change = .16, F (2, 107) = 11.0, p < .01. Catastrophizing (PCS) was entered in the final step of the analysis and contributed significant ‘unique’ variance to the prediction of SOAPP-R scores, R2 change = .04, F (1, 106) = 5.4, p < .05.
Table 5.
Hierarchical multiple regression analysis examining the unique influence of catastrophizing on risk for opioid misuse (SOAPP-R)
| B | R2 | R2 change | F change | |
|---|---|---|---|---|
| Step 1 | .01 | .01 | .41 | |
| Sex | .03 | |||
| Age | −.06 | |||
| Opioid | .07 | |||
| Step 2 | .09 | .08 | 4.5* | |
| BPI | .20* | |||
| TPTH | −.21* | |||
| Step 3 | .24 | .16 | 11.0** | |
| PASS | .36* | |||
| BOI | .16 | |||
| Step 4 | .28 | .04 | 5.4* | |
| PCS | .30* |
Note. Opioid, Opioid status; BPI, Brief Pain Inventory; TPThs, Thermal Pain thresholds; PASS, Pain Anxiety Symptoms Scale; BOI, Beck Depression Inventory; PCS, Pain Catastrophizing Scale; SOAPP-R, Screener and Opioid Assessment for Pain Patients-Revised
p < 05
p < 01
4. DISCUSSION
The primary purpose of the present study was to examine the factors that underlie the association between catastrophizing and heightened risk for prescription opioid misuse in patients with chronic pain. Consistent with previous research (Edwards et al., 2011a; Ferrari et al., 2012; Jamison et al., 2009; Morasco et al., 2013), we found that higher levels of catastrophizing were associated with higher scores on the SOAPP-R, a self-report questionnaire designed to identify patients at risk for prescription opioid misuse.
In the present study, we found a significant association between patients’ self-reports of pain severity and SOAPP-R scores. Patients who reported higher levels of pain scored higher on the SOAPP-R, which is consistent with the results of previous studies conducted among patients with chronic pain (Adams et al., 2004; Grattan et al., 2012; Jamison et al., 2009). It has been suggested that patients who report high levels of pain may, in an attempt to seek pain relief, inadvertently exhibit behaviors that fall within the spectrum of medication misuse or abuse (Jamison et al., 2011; Park et al., 2010).
Another finding consistent with previous studies is that heightened pain sensitivity (i.e., low pain thresholds) was associated with higher scores on the SOAPP-R. This finding corroborates the work of Edwards et al. (2011), who also found a significant association between pain sensitivity and SOAPP-R scores. Research suggests that heightened pain sensitivity might result from dysfunctions in peripheral or central pain processing, or from dysfunctions in opioid-mediated endogenous pain inhibitory systems (Bruehl et al., 2009; Edwards et al., 2005b; Millan, 1986; Pertovaara et al., 2006). Interestingly, dysfunctions in endogenous opioidergic activity have been found to play a role in the experience of craving among patients with various forms of substance use problems (Gianoulakis and deWaele, 1994; Koob and Le Moal, 2001; Williams et al., 2007, 2009; Zubieta et al., 1996). Applied to the context of pain, it is thus possible that dysfunctions in endogenous opioid systems may, at least in some patients, increase the risk of opioid craving, which in turn might increase the risk for prescription opioid misuse.
In the present study, we found that higher levels of negative affect (i.e., pain-related anxiety, depressive symptoms) were associated with higher scores on the SOAPP-R. The association between measures of negative affect and risk for prescription opioid misuse is well documented, and has been reported in patients with a variety of chronic pain conditions (Becker et al., 2008; Grattan et al., 2012; Morasco et al., 2013; Trafton et al., 2011; Turk et al., 2008; Wilsey et al., 2008). This finding parallels those from the addiction literature showing that negative affect is associated with an increased likelihood of drug abuse (for reviews, see Conner et al., 2007, 2008; Conway et al., 2006; Grant et al., 2004; Sinha, 2001).
Of particular interest in the present study was to examine the factors that might underlie the association between catastrophizing and heightened risk for prescription opioid misuse. In our study, higher levels of catastrophizing were associated with higher levels of self-reported pain severity, pain sensitivity, pain-related anxiety, and depressive symptoms. A series of mediational analyses were conducted to examine the potential role of these variables in mediating the association between catastrophizing and risk for opioid misuse. Analyses revealed that patients’ levels of pain severity, pain sensitivity, and depressive symptoms were not significant mediators of the PCS-SOAPP-R association. Analyses, however, revealed that patients’ levels of pain-related anxiety partially mediated the association between the PCS and SOAPP-R. These results suggest that anxiety might represent one of the mechanisms by which catastrophizing confers increased risk for prescription opioid misuse. It has been argued that anxiety may alter patients’ beliefs about their medication needs, which may result in opioid misuse or abuse (Scheiffer et al., 2005). It has also been argued that patients with high levels of anxiety may tend to overuse opioid medication as a way to control or alleviate their psychological distress (Ballantyne and LaForge, 2007; Hasin et al., 2002; Jamison et al., 2011; Passik et al., 2011; Wasan et al., 2007).
Interestingly, results of a hierarchical multiple regression analysis revealed that catastrophizing contributed significant ‘unique’ variance to the prediction of SOAPP-R scores, even after controlling for patient demographics, the BPI, TPThs, and measures of negative affect (i.e., PASS, BDI). This set of findings parallel those of Morasco et al (2013), who found that catastrophizing was a significant unique predictor of risk for prescription opioid misuse in patients with pain, even after controlling for a number of demographic, pain, and psychological variables. Our results, together with those of Morasco et al. (2013), raise further questions concerning the factors that might place high catastrophizers at increased risk for prescription opioid misuse. Although speculative, it is possible that high catastrophizers present certain psychological characteristics independent of negative affect that increase their risk for prescription opioid misuse. For example, studies have shown that catastrophizing is associated with low self-efficacy beliefs and poor pain coping skills (Keefe et al., 1997, 2004; Nicholas et al., 2007; Shelby et al., 2008; Sullivan et al., 2001), two variables that have been found to be associated with reduced medication compliance in patients with other health-related conditions (Catz et al., 2000; Heckman et al., 2004; Vyavaharkar et al., 2007). Low self-efficacy beliefs and poor coping skills might decrease catastrophizers’ ability to cope with pain without the use of medication, and might place them at increased risk for prescription opioid misuse. Similarly, it is possible that high catastrophizers hold preexisting personality traits that are associated with an increased risk for opioid misuse. For example, catastrophizing has been associated with heightened impulsivity and sensation seeking (D’Acremont and Van der Linden, 2007), two personality traits that have been associated with an increased likelihood of substance abuse in patients with substance use problems (Ball et al., 1994; Franques et al., 2003; McKay et al., 1999; Moeller et al., 2002; Rosenthal et al., 1990).
A number of limitations must be considered when interpreting the findings of the present study. First, the cross-sectional nature of our study design precludes any firm conclusions regarding the directionality of associations between study variables. Second, we did not assess important medication use variables such as the duration of opioid therapy or the specific types and/or doses of opioid analgesics taken by patients. In our study, patients who were taking opioids did not differ significantly from patients who were not taking opioids in terms of pain interference and negative affect, which contrasts with some previous studies in this area (e.g., Braden et al., 2009; Sullivan et al., 2005; Webster et al., 2007). This might be due, in part, to the specific types and/or doses of opioid analgesics taken by patients. Third, patients with SUDs were excluded from the present study; future research is needed to examine whether our findings are generalizable to sub-populations of patients presenting with chronic pain and a comorbid SUD. Finally, the SOAPP-R was not designed to assess patients’ actual misuse of opioids and, as such, high scores on the SOAPP-R are not necessarily indicative of ongoing opioid misuse.
Despite these limitations, our study provides new insights into the mechanisms that might underlie the association between catastrophizing and heightened risk for prescription opioid misuse. Our findings suggest that catastrophizing might confer an increased risk for opioid misuse due, in part, to patients’ heightened levels of pain-related anxiety. Perhaps the most important finding of our study, however, is that catastrophizing remained significantly associated with an increased risk for opioid misuse even when controlling for patients’ levels of pain severity, pain sensitivity, and measures of negative affect such as anxiety and depressive symptoms. Taken together, these findings might have implications for the management of patients who are being considered for opioid therapy. Our findings suggest that complementary interventions designed to reduce patients’ levels of anxiety and catastrophizing might contribute to decreasing risks for prescription opioid misuse in patients with chronic pain. Further research will be needed to examine the psychological factors that are associated with an increased risk for prescription opioid misuse among patients with chronic pain conditions. Advances in this domain could ultimately lead to more effective management of patients who are being prescribed opioid analgesics, and to reduced rates of prescription opioid misuse in patients with chronic pain.
Acknowledgments
Funding
Funding for this study was provided by the National Institutes of Health (NIH): Grants AG034982 and CA 120500. NIH had no further role, whether in terms of study design, data collection, data analysis, or data interpretation. NIH was neither involved in the writing of the manuscript, nor in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
RR Edwards designed the study. MO Martel conducted statistical analyses, interpreted study data, and wrote the major part of the manuscript. RR Edwards, AD Wasan, and RN Jamison all contributed to data interpretation. They also contributed and approved the final manuscript.
Conflict of Interest
The authors have no financial interests in the results of this research, and all authors declare that they have no conflicts of interest.
REFERENCES
- Adams LL, Gatchel RJ, Robinson RC. Development of a self-report screening instrument for assessing potential opioid medication misuse in chronic pain patients. J. Pain Symptom Manage. 2004;27:440–459. doi: 10.1016/j.jpainsymman.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Akbik H, Butler SF, Budman SH, Fernandez K, Katz NP, Jamison RN. Validation and clinical application of the Screener and Opioid Assessment for Patients with Pain (SOAPP) J. Pain Symptom Manage. 2006;32:287–293. doi: 10.1016/j.jpainsymman.2006.03.010. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: 1994. [Google Scholar]
- Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J. Pain. 2009;10:556–572. doi: 10.1016/j.jpain.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Ball SA, Carroll KM, Rounsaville BJ. Sensation seeking, substance abuse, and psychopathology in treatment-seeking and community cocaine abusers. J. Consult. Clin. Psychol. 1994;62:1053–1057. doi: 10.1037//0022-006x.62.5.1053. [DOI] [PubMed] [Google Scholar]
- Ballantyne JC, LaForge KS. Opioid dependence and addiction during opioid treatment of chronic pain. Pain. 2007;129:235–255. doi: 10.1016/j.pain.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Ballantyne JC. Opioid controls: regulate to educate. Pain Med. 2010;11:480–481. doi: 10.1111/j.1526-4637.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- Banta-Green CJ, Merrill JO, Doyle SR, Boudreau DM, Calsyn DA. Opioid use behaviors, mental health and pain--development of a typology of chronic pain patients. Drug Alcohol Depend. 2009a;104:34–42. doi: 10.1016/j.drugalcdep.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta-Green CJ, Merrill JO, Doyle SR, Boudreau DM, Calsyn DA. Measurement of opioid problems among chronic pain patients in a general medical population. Drug Alcohol Depend. 2009b;104:43–49. doi: 10.1016/j.drugalcdep.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Becker WC, Sullivan LE, Tetrault JM, Desai RA, Fiellin DA. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008;94:38–47. doi: 10.1016/j.drugalcdep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Braden JB, Sullivan MD, Ray GT, Saunders K, Merrill J, Silverberg MJ, Rutter CM, Weisner C, Banta-Green C, Campbell C, Von Korff M. Trends in long-term opioid therapy for noncancer pain among persons with a history of depression. Gen. Hosp Psychiatry. 2009;6:564–570. doi: 10.1016/j.genhosppsych.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehl S, Burns JW, Chung OY, Chont M. Pain-related effects of trait anger expression: neural substrates and the role of endogenous opioid mechanisms. Neurosci. Biobehav. Rev. 2009;33:475–491. doi: 10.1016/j.neubiorev.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SF, Budman SH, Fernandez K, Jamison RN. Validation of a screener and opioid assessment measure for patients with chronic pain. Pain. 2004;112:65–75. doi: 10.1016/j.pain.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Butler SF, Fernandez K, Benoit C, Budman SH, Jamison RN. Validation of the revised screener and opioid assessment for patients with pain (SOAPP-R) J. Pain. 2008;9:360–372. doi: 10.1016/j.jpain.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SF, Budman SH, Fernandez KC, Fanciullo GJ, Jamison RN. Cross-validation of a screener to predict opioid misuse in chronic pain patients (SOAPP-R) J. Addict. Med. 2009;3:66–73. doi: 10.1097/ADM.0b013e31818e41da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TA. Patterns, correlates and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychol. 2000;19:124–133. [PubMed] [Google Scholar]
- Chabal C, Erjavec MK, Jacobson L, Mariano A, Chaney E. Prescription opioid abuse in chronic pain patients: clinical criteria, incidence, and predictors. Clin. J. Pain. 1997;13:150–155. doi: 10.1097/00002508-199706000-00009. [DOI] [PubMed] [Google Scholar]
- Compton P. Should opioid abusers be discharged from opioid-analgesic therapy? Pain Med. 2008;9:383–390. doi: 10.1111/j.1526-4637.2008.00462.x. [DOI] [PubMed] [Google Scholar]
- Conner KR, Pinquart M, Duberstein PR. Meta-analysis of depression and substance use and impairment among intravenous drug users (IDUs) Addiction. 2007;103:524–534. doi: 10.1111/j.1360-0443.2007.02118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner KR, Pinquart M, Holbrook AP. Meta-analysis of depression and substance use and impairment among cocaine users. Drug Alcohol Depend. 2008;98:13–23. doi: 10.1016/j.drugalcdep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry. 2006;67:247–257. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- D'Acremont M, Van der Linden M. How is impulsivity related to depression in adolescence? Evidence from a french validation of the cognitive emotion regulation questionnaire. J. Adolesc. 2007;30:271–282. doi: 10.1016/j.adolescence.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Dersh J, Mayer TG, Gatchel RJ, Polatin PB, Theodore BR, Mayer EA. Prescription opioid dependence is associated with poorer outcomes in disabling spinal disorders. Spine. 2008;33:2219–2227. doi: 10.1097/BRS.0b013e31818096d1. [DOI] [PubMed] [Google Scholar]
- Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum. Mol. Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007a;129:355–362. doi: 10.1016/j.pain.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Edlund MJ, Sullivan M, Steffick D, Harris KM, Wells KB. Do users of regularly prescribed opioids have higher rates of substance use problems than nonusers? Pain Med. 2007b;8:647–656. doi: 10.1111/j.1526-4637.2006.00200.x. [DOI] [PubMed] [Google Scholar]
- Edlund MJ. Chronic opioid therapy for chronic noncancer pain in the United States: long day's journey into night? Gen. Hosp. Psychiatry. 2011;33:416–418. doi: 10.1016/j.genhosppsych.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Sarlani E, Wesselmann U, Fillingim RB. Quantitative assessment of experimental pain perception: multiple domains of clinical relevance. Pain. 2005a;114:315–319. doi: 10.1016/j.pain.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Edwards RR. Individual differences in endogenous pain modulation as a risk factor for chronic pain. Neurology. 2005b;65:437–443. doi: 10.1212/01.wnl.0000171862.17301.84. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Bingham CO, Bathon J, Haythornthwaite JA. Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis Rheum. 2006;55:325–332. doi: 10.1002/art.21865. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Kronfli T, Haythornthwaite JA, Smith MT, McGuire L, Page GG. Association of catastrophizing with interleukin-6 responses to acute pain. Pain. 2008;140:135–144. doi: 10.1016/j.pain.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RR, Wasan AD, Michna E, Greenbaum S, Ross E, Jamison RN. Elevated pain sensitivity in chronic pain patients at risk for opioid misuse. J. Pain. 2011a;12:953–963. doi: 10.1016/j.jpain.2011.02.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RR, Cahalan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nat. Rev. Rheumatol. 2011b;7:216–224. doi: 10.1038/nrrheum.2011.2. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Capraro M, Visentin M. Risk factors in opioid treatment of chronic noncancer pain: a multidisciplinary assessment. In: Gabor B, Noe CE, editors. Pain Management: Current Issues and Opinions. Rijeka: Intech; 2012. pp. 419–459. [Google Scholar]
- Fillingim RB, Lautenbacher S. The importance of quantitative sensory testing in the clinical setting. In: Lautenbacher S, Fillingim RB, editors. Pathophysiology of Pain Perception. New York: Kluwer Academic; 2004. pp. 215–227. [Google Scholar]
- Fillingim RB, Ness TJ, Glover TL, Campbell CM, Price DD, Staud R. Experimental pain models reveal no sex differences in pentazocine analgesia in humans. Anesthesiology. 2004;100:1263–1270. doi: 10.1097/00000542-200405000-00031. [DOI] [PubMed] [Google Scholar]
- Franques P, Auriacombe M, Piquemal E, Verger M, Brisseau-Gimenez S, Grabot D, Tignol J. Sensation seeking as a common factor in opioid dependent subjects and high risk sport practicing subjects. A cross sectional study. Drug Alcohol Depend. 2003;69:121–126. doi: 10.1016/s0376-8716(02)00309-5. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, de Waele JP. Genetics of alcoholism: role of the endogenous opioid system. Metab. Brain Dis. 1994;9:105–131. doi: 10.1007/BF01999765. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann. Fam. Med. 2012;10:304–311. doi: 10.1370/afm.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, Knott C, Mulkey F, Rothwell R, Maixner W. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. J. Pain. 2011;12:61–74. doi: 10.1016/j.jpain.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D, Liu X, Nunes E. Effects of major depression on remission and relapse of substance dependence. Arch. Gen. Psychiatry. 2002;59:375–380. doi: 10.1001/archpsyc.59.4.375. [DOI] [PubMed] [Google Scholar]
- Heckman BD, Catz SL, Heckman TG, Miller JG, Kalichman SC. Adherence to antiretroviral therapy in rural persons living with HIV disease in the United States. AIDS Care. 2004;16:219–230. doi: 10.1080/09540120410001641066. [DOI] [PubMed] [Google Scholar]
- Holmbeck GN. Toward terminological, conceptual, and statistical clarity in the study of mediators and moderators: examples from the child clinical and pediatric psychology literatures. J. Consult. Clin Psychol. 1997;65:599–610. doi: 10.1037//0022-006x.65.4.599. [DOI] [PubMed] [Google Scholar]
- Ives TJ, Chelminski PR, Hammett-Stabler CA, Malone RM, Perhac JS, Potisek NM, Shilliday BB, DeWalt DA, Pignone MP. Predictors of opioid misuse in patients with chronic pain: a prospective cohort study. BMC Health Serv. Res. 2006;6:46. doi: 10.1186/1472-6963-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison RN, Link CL, Marceau LD. Do pain patients at high risk for substance misuse experience more pain? A longitudinal outcomes study. Pain Med. 2009;10:1084–1094. doi: 10.1111/j.1526-4637.2009.00679.x. [DOI] [PubMed] [Google Scholar]
- Jamison RN, Ross EL, Michna E, Chen LQ, Holcomb C, Wasan AD. Substance misuse treatment for high-risk chronic pain patients on opioid therapy: a randomized trial. Pain. 2010;150:390–400. doi: 10.1016/j.pain.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison RN, Serraillier J, Michna E. Assessment and treatment of abuse risk in opioid prescribing for chronic pain. Pain Res Treat. 2011;12:1–12. doi: 10.1155/2011/941808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe FJ, Kashikar-Zuck S, Robinson E, Salley A, Beaupre P, Caldwell D, Baucom D, Haythornthwaite J. Pain coping strategies that predict patients' and spouses' ratings of patients' self-efficacy. Pain. 1997;73:191–199. doi: 10.1016/S0304-3959(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Lefebvre JC, Egert JR, Affleck G, Sullivan MJ, Caldwell DS. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: the role of catastrophizing. Pain. 2000;87:325–334. doi: 10.1016/S0304-3959(00)00296-7. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Lipkus I, Lefebvre JC, Hurwitz H, Clipp E, Smith J, Porter L. The social context of gastrointestinal cancer pain: a preliminary study examining the relation of patient pain catastrophizing to patient perceptions of social support and caregiver stress and negative responses. Pain. 2003;103:151–156. doi: 10.1016/s0304-3959(02)00447-5. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Affleck G, France CR, Emery CF, Waters S, Caldwell DS, Stainbrook D, Hackshaw KV, Fox LC, Wilson K. Gender differences in pain, coping, and mood in individuals having osteoarthritic knee pain: a within-day analysis. Pain. 2004;110:571–577. doi: 10.1016/j.pain.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Khan RS, Ahmed K, Blakeway E, Skapinakis P, Nihoyannopoulos L, Macleod K, Sevdalis N, Ashrafian H, Platt M, Darzi A, Athanasiou T. Catastrophizing: a predictive factor for postoperative pain. Am. J. Surg. 2011;201:122–131. doi: 10.1016/j.amjsurg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Zayfert C, Gross RT. The pain anxiety symptom scale: development and validation of a scale to measure fear of pain. Pain. 1992;50:67–73. doi: 10.1016/0304-3959(92)90113-P. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Dhingra LA. Short version of the pain anxiety symptoms scale (PASS-20): preliminary development and validity. Pain Res. Manag. 2002;7:45–50. doi: 10.1155/2002/517163. [DOI] [PubMed] [Google Scholar]
- McKay JR, Alterman AI, Mulvaney FD, Koppenhaver JM. Predicting proximal factors in cocaine relapse and near miss episodes: clinical and theoretical implications. Drug Alcohol Depend. 1999;56:67–78. doi: 10.1016/s0376-8716(99)00013-7. [DOI] [PubMed] [Google Scholar]
- Michna E, Ross EL, Hynes WL, Nedeljkovic SS, Soumekh S, Janfaza D, Palombi D, Jamison RN. Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. J. Pain Symptom Manage. 2004;28:250–258. doi: 10.1016/j.jpainsymman.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Michna E, Jamison RN, Pham LD, Ross EL, Janfaza D, Nedeljkovic SS, Narang S, Palombi D, Wasan AD. Urine toxicology screening among chronic pain patients on opioid therapy: frequency and predictability of abnormal findings. Clin. J. Pain. 2007;23:173–179. doi: 10.1097/AJP.0b013e31802b4f95. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Multiple opioid systems and pain. Pain. 1986;27:303–347. doi: 10.1016/0304-3959(86)90158-2. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Oderinde V, Mathias CW, Harper RA, Swann AC. Increased impulsivity in cocaine dependent subjects independent of antisocial personality disorder and aggression. Drug Alcohol Depend. 2002;68:105–111. doi: 10.1016/s0376-8716(02)00106-0. [DOI] [PubMed] [Google Scholar]
- Morasco BJ, Dobscha SK. Prescription medication misuse and substance use disorder in VA primary care patients with chronic pain. Gen. Hosp. Psychiatry. 2008;30:93–99. doi: 10.1016/j.genhosppsych.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Morasco BJ, Turk DC, Donovan DM, Dobscha SK. Risk for prescription opioid misuse among patients with a history of substance use disorder. Drug Alcohol Depend. 2013;127:193–199. doi: 10.1016/j.drugalcdep.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur. J. Pain. 2007;11:153–163. doi: 10.1016/j.ejpain.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Park J, Lavin R. Risk factors associated with opioid medication misuse in community-dwelling older adults with chronic pain. Clin. J. Pain. 2010;26:647–655. doi: 10.1097/AJP.0b013e3181e94240. [DOI] [PubMed] [Google Scholar]
- Passik SD, Lowery A. Psychological variables potentially implicated in opioid-related mortality as observed in clinical practice. Pain Med. 2011;12:36–42. doi: 10.1111/j.1526-4637.2011.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertovaara A, Almeida A. Descending inhibitory systems. In: Cervero F, Jensen TS, editors. Handbook of Clinical Neurology. Amsterdam: Elsevier; 2006. pp. 179–192. [DOI] [PubMed] [Google Scholar]
- Peters ML, Vlaeyen JW, Weber WE. The joint contribution of physical pathology, pain-related fear and catastrophizing to chronic back pain disability. Pain. 2005;113:45–50. doi: 10.1016/j.pain.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Poole H, Bramwell R, Murphy P. Factor structure of the Beck Depression Inventory-II in patients with chronic pain. Clin. J. Pain. 2006;22:790–798. doi: 10.1097/01.ajp.0000210930.20322.93. [DOI] [PubMed] [Google Scholar]
- Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev. Neurother. 2009;9:745–758. doi: 10.1586/ERN.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs J, McCracken L, Peters ML, Crombez G, van Breukelen G, Vlaeyen JW. Psychometric evaluation of the Pain Anxiety Symptoms Scale (PASS) in chronic pain patients. J. Behav. Med. 2004;27:167–183. doi: 10.1023/b:jobm.0000019850.51400.a6. [DOI] [PubMed] [Google Scholar]
- Rosenthal TL, Edwards NB, Ackerman BJ, Knott DH, Rosenthal RH. Substance abuse patterns reveal contrasting personal traits. J. Subst. Abuse. 1990;2:255–263. doi: 10.1016/s0899-3289(05)80060-4. [DOI] [PubMed] [Google Scholar]
- Schieffer BM, Pham Q, Labus J, Baria A, Van Vort W, Davis P, Davis F, Naliboff BD. Pain medication beliefs and medication misuse in chronic pain. J. Pain. 2005;6:620–629. doi: 10.1016/j.jpain.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Sehgal N, Manchikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Physician. 2012;15:67–92. [PubMed] [Google Scholar]
- Shelby RA, Somers TJ, Keefe FJ, Pells JJ, Dixon KE, Blumenthal JA. Domain specific self-efficacy mediates the impact of pain catastrophizing on pain and disability in overweight and obese osteoarthritis patients. J. Pain. 2008;9:912–919. doi: 10.1016/j.jpain.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacol. (Berl.) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol. Assess. 1995;7:524–532. [Google Scholar]
- Sullivan MJL, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspectives on the relation between catastrophizing and pain. Clin. J. Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- Sullivan MJL, Stanish WD. Psychologically based occupational rehabilitation: the Pain-Disability Prevention Program. Clin. J. Pain. 2003;19:97–104. doi: 10.1097/00002508-200303000-00004. [DOI] [PubMed] [Google Scholar]
- Sullivan MJL, Tanzer M, Stanish W, Fallaha M, Keefe FJ, Simmonds M, Dunbar M. Psychological determinants of problematic outcomes following Total Knee Arthroplasty. Pain. 2009;143:123–129. doi: 10.1016/j.pain.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Sullivan MD, Edlund MJ, Steffick D, Unützer J. Regular use of prescribed opioids: association with common psychiatric disorders. Pain. 2005;119:95–103. doi: 10.1016/j.pain.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: The TROUP Study. Pain. 2010;150:332–339. doi: 10.1016/j.pain.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the brief pain inventory for nonmalignant pain. J. Pain. 2004;5:133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Cucciare MA, Lewis E, Oser M. Somatization is associated with non adherence to opioid prescriptions. J. Pain. 2011;12:573–580. doi: 10.1016/j.jpain.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin. J. Pain. 2008;24:497–508. doi: 10.1097/AJP.0b013e31816b1070. [DOI] [PubMed] [Google Scholar]
- Vowles KE, Gross RT, Sorrell JT. Predicting work status following interdisciplinary treatment for chronic pain. Eur. J. Pain. 2004;8:351–358. doi: 10.1016/j.ejpain.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Vyavaharkar M, Moneyham L, Tavakoli A, Phillips KD, Murdaugh C, Jackson K, Meding G. Social support, coping, and medication adherence among HIV-positive women with depression living in rural areas of the southeastern United States. AIDS Patient Care. 2007;21:667–680. doi: 10.1089/apc.2006.0131. [DOI] [PubMed] [Google Scholar]
- Wasan AD, Butler SF, Budman SH, Benoit C, Fernandez K, Jamison RN. Psychiatric history and psychological adjustment as risk factors for aberrant drug-related behavior among patients with chronic pain. Clin. J. Pain. 2007;23:307–315. doi: 10.1097/AJP.0b013e3180330dc5. [DOI] [PubMed] [Google Scholar]
- Wasan AD, Butler SF, Budman SH, Fernandez K, Weiss RD, Greenfield SF, Jamison RN. Does report of craving opioid medication predict aberrant drug behavior among chronic pain patients? Clin. J. Pain. 2009;25:193–198. doi: 10.1097/AJP.0b013e318193a6c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine. 2007;32:2127–2132. doi: 10.1097/BRS.0b013e318145a731. [DOI] [PubMed] [Google Scholar]
- Williams TM, Daglish MR, Lingford-Hughes A, Taylor LG, Hammers A, Brooks DJ, Grasby P, Myles JS, Nutt DJ. Brain opioid receptor binding in early abstinence from opioid dependence: positron emission tomography study. Br. J. Psychiatry. 2007;191:63–69. doi: 10.1192/bjp.bp.106.031120. [DOI] [PubMed] [Google Scholar]
- Williams TM, Davies SJ, Taylor LG, Daglish MR, Hammers A, Brooks DJ, Nutt DJ, Lingford-Hughes A. Brain opioid receptor binding in early abstinence from alcohol dependence and relationship to craving: an [11C] diprenorphine PET study. Eur. Neuropsychopharmacol. 2009;19:740–748. doi: 10.1016/j.euroneuro.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Wilsey BL, Fishman SM, Tsodikov A, Ogden C, Symreng I, Ernst A. Psychological comorbidities predicting prescription opioid abuse among patients in chronic pain presenting to the emergency department. Pain Med. 2008;9:1107–1117. doi: 10.1111/j.1526-4637.2007.00401.x. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D, Pud D. Quantitative sensory testing. In: Binnie C, Cooper R, Mauguiere F, Osselton JW, Prior P, Tedman B, editors. Clinical Neurophysiology. Revised and enlarged edition. EMG, Nerve Conduction and Evoked Potentials. Amsterdam: Elsevier; 2004. pp. 309–336. [Google Scholar]
- Zubieta JK, Gorelick DA, Stauffer R, Ravert HT, Dannals RF, Frost JJ. Increased mu opioid receptor binding detected by PET in cocaine-dependent men is associated with cocaine craving. Nat. Med. 1996;2:1225–1229. doi: 10.1038/nm1196-1225. [DOI] [PubMed] [Google Scholar]


